Abstract
Background:
Breast cancer continues to be a major cause of morbidity and mortality throughout the world. The behavior of breast cancer varies widely. Several parameters have been investigated to predict the prognosis in breast cancer. But still there is no single parameter that can predict prognosis in an individual patient. Among the novel prognostic markers is E-cadherin; a calcium-dependent epithelial cell adhesion molecule. Its loss has been associated with metastases, thereby providing evidence for its role as an invasion suppressor.
The objective of the present study was to assess the prognostic value of E-cadherin expression in breast cancer cases, and its correlations with the other studied prognostic parameters.
Methods:
The study comprised 54 breast cancer patients admitted at King Fahd Specialist Hospital, Qassim during the period 2001–2006. The median tumor size was 3cms. Fifty cases (92.6%) had invasive ductal carcinoma, four cases had lobular carcinomas, and most were grade II (82%), stage II (48%), and the majority of cases had positive axillary lymph nodes (70.3%). Representative sections from formalin-fixed paraffin embedded tissue blocks were taken from the 54 cases of breast cancer, and were stained for E-cadherin expression by immunohistochemical technique (monoclonal E-cadherin (NCL-E-cad), Novocastra). All the lobular carcinoma cases were negative for membranous expression of E-cadherin while 72% of invasive ductal carcinomas were positive for the marker.
Results:
A significant correlation was found between strong E-cadherin expression and node negative cases. Node negative cases were found to be an independent predictor of strong E-cadherin expression while node positive cases predicted negative expression of E-cadherin (P = 0 .026). Also loss of E-cadherin was noted in advanced stages of breast cancer supporting the view that loss of E-cadherin expression is a marker of aggressiveness. However, there was no correlation between the E-cadherin and other prognostic parameters as tumor size, tumor grade, ER, PR, and HER-2 expression.
Conclusion
A significant correlation was found between strong E-cadherin expression and node negative cases.
Keywords: breast cancer, E-cadherin, immunohistochemistry, immunostaining, prognostic factors
Introduction
Breast cancer continues to be a major cause of morbidity and mortality throughout the world. Although it had once been presumed that the incidence of breast cancer in Saudi Arabia was low, yet more recent data have indicated the contrary. Breast cancer is the most common malignant neoplasm in Saudi females, as documented by Saudi Arabia cancer registry. (1–6)
The outcome for women with breast cancer varies widely. Some women have the same life expectancy as women without breast cancer. Other women have only a 13% probability of being alive in five years. (7)
Several parameters have been investigated to predict the prognosis of breast cancer, such as lymph node status, tumor size, histologic type, tumor grade, hormonal receptor status, ploidy, and proliferating markers (7–11). Different regions of Saudi Arabia have studied the pattern and prevalence of breast cancer, but virtually no data on the prognostic factors of breast cancer are available for the Qassim region (1–6, 12).
Despite the numerous prognostic indicators currently in use or under investigation, it is impossible to predict the outcome in an individual patient. For this reason there is continous search for better or more refined biological markers of prognosis and more effective treatment modalities.
The major prognostic factors include the invasive carcinoma versus in situ carcinoma, distant metastasis, lymph node metastases, tumor size, locally advanced disease, and inflammatory carcinoma. Minor prognostic factors include histologic subtype, tumor grade, estrogen and progesterone receptors, HER-2 neu expression, proliferative activity, and DNA content. (7–12)
One of the recently employed prognostic factors is E-cadherin.(13,14) E-Cadherin is a calcium-dependent epithelial cell adhesion molecule expressed at adherens junctions. Loss of E-cadherin may result in a poorly differentiated phenotype of tumor cells. E-cadherin mutations, affecting the extra cellular domain, have been observed to alter cell shape toward a less epithelioid morphology, and also to interfere with adhesion. Further more, cells with mutated E-cadherin demonstrate increased motility and altered organization of their actin cytoskeleton. Loss of E-cadherins has also been associated with metastases, thereby providing evidence for its role as an invasion suppressor. Loss of E-cadherin has been observed in many types of human cancer, e.g. lobular carcinoma of the breast. (13–17)
Studies showed that E-cadherins are associated with aggressive behavior. (15–17) This data indicates that E-cadherin can provide an accurate determination of the aggressiveness of cancer. This information can be used to identify breast cancer patients who will benefit by more aggressive treatment, thus increasing chances of survival.
The objective of the present study was to determine the clinical utility of E-cadherin as a novel prognostic marker of breast cancer, and a potential to predict which patients will experience more aggressive forms of the disease. This information can be used to reduce morbidity and mortality in breast cancer patients.
Methods
The study comprised 54 breast cancer cases registered in the pathology department of King Fahd Specialist Hospital, Buraida (Qassim), during the period 2001–2006. The cases were selected according to the availability of the paraffin blocks.
Haematoxylin and eosin stained sections from all the cases were reviewed by the authors to confirm the diagnosis and the different histopathological prognostic pararmeters as histologic type, tumor grade, lymphatic vascular invasion, lymph nodes involvement, and extranodal extension. Pathology reports of the patients were reviewed to collect the data needed as tumor size, number of lymph nodes involved, and whether resection margins were involved by the tumor or not.
Data on immunohistochemical (IHC) stain for estrogen and progesterone receptors status and HER-2 neu oncogene expression were taken from the pathology records of the patients.
Representative sections from formalin-fixed paraffin embedded tissue were taken from the 54 cases of breast cancer, and immunohistochemical stain of E-cadherin was performed.
Methodology of IHC stain of E-cadherin
A mouse monoclonal antibody E-cadherin (NCL-E-cad) Novocastra, and peroxidase detection system (Novocastra) was used. IHC technique was performed according to the manufacturer’s protocol but with some modifications as follows:
Sections were incubated in xylene, rehydrated by grades of alcohol, immersed in distilled water, then immersed in antigen retrieval solution (pH 6) in microwave (1400W) for 5 minutes, then washed in deionized water. Endogenous peroxidase was neutralized by using peroxidase block for 5 minutes; incubated with protein block for 5 minutes; incubated with the primary antibody for 90 minutes (dilution 1:50); incubated with secondary antibody for 60 minutes; then with streptavidin HRP for 60 minutes. Between incubations, sections were washed two times in phosphate buffered saline for 5 minutes each. The brown colour reaction was developed by using DAB working solution for 5 minutes, counterstained with haematoxylin for 15 minutes, washed in running water, dehydrated and mounted. All steps are performed at room temperature (25 °C)
Quality control
Normal breast tissue in the cut sections was used as internal positive control with each run together with a negative control to exclude the non-specific staining.
Interpretation of the results
Sections were scored for IHC as follows: Negative, 1+ stands for weak staining, and less than 10 % of tumor cells show positive reaction for E-cadherin, 2 + stands for moderate staining, and more than 10 % show positive reaction for E-cadherin, and 3+ reflects strong staining in most of the tumor cells. Stain was considered positive when it was membranous or membranous and cytoplasmic.
Statistical analysis
Univariate analyses were performed to identify associations between E-cadherin and the variables; tumor size, histologic grades, number of involved lymph nodes, extranodal extension, involved resection margins, disease stage, and estrogen and progesterone receptor status and HER s-2 neu expression. Each ordinal variable was dichotomized on the basis of number of subjects in various categories. This yielded the following binary values for analysis: E-cadherin (0 if e-cad= 0, 1+, 1 if E-cad = 2+ or 3+). Stage (0 if stage is less than or equal to II, 1 if stage is III or IV), Grade 0 if tumor is less than grade III, 1 if equal to grade III, ER 0 if ER< 2, 1 if ER ≥2, PR 0 if PR< 2, 1 if PR ≥2, Her-2 0 if < 2, 1 if ≥ 2.
All pairwise associations among dichotomous variables were assessed with Fisher exact test. Multivariate logistic regression was then utilized to determine which variables were independent predictors of E-cadherin expression. Logistic regression could predict the presence of positive lymph nodes. Pearson chi square test was also used.
Results
Clinico-Pathological data
Representative sections of tumors from 54 breast cancer patients were evaluated. The patients median age was 46 years (range 29–75 years). The median tumor size was 3cm. Fifty cases were invasive ductal carcinoma (92.6 %), and four cases were invasive lobular carcinoma. Most of the cases were grade II (82 %), stage II (48%). ER was positive in 63 % of cases while PR was positive in 57.4 % of cases, and HER-2 was positive in 37% of the studied cases.
Immunohistochemical results
In this study, immunostaining was performed on a total number of 54 cases; 4 lobular carcinomas, and 50 ductal carcinomas with variable grades. The staining was membranous in all the positive cases, with additional cytoplasmic staining in 40% of cases.
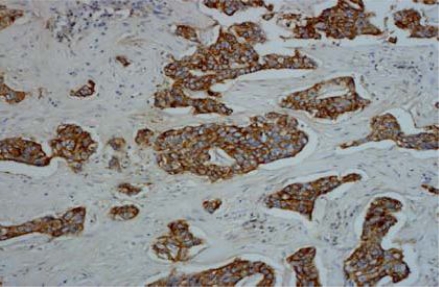
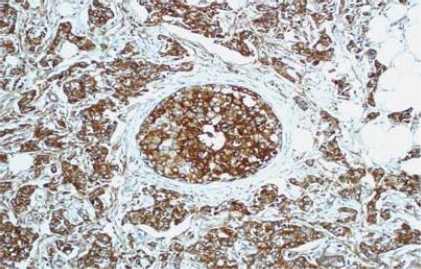
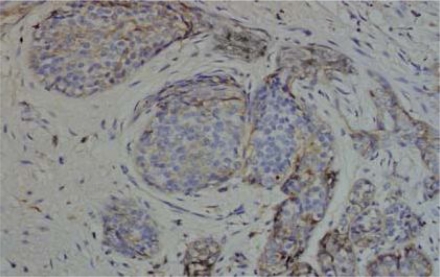
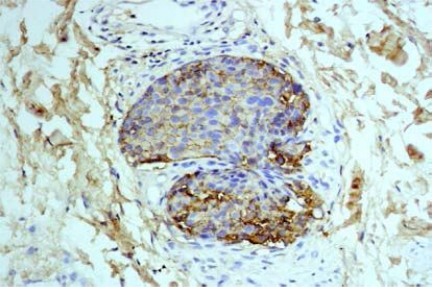
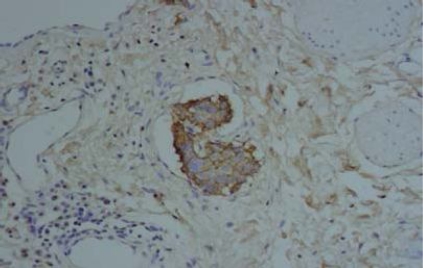
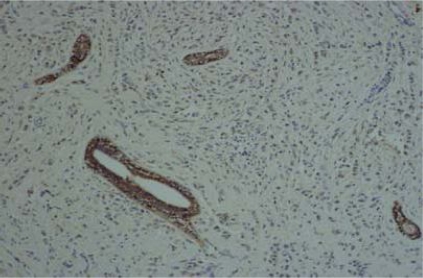
IHC results of the 54 cases showed positive staining in 36 out of 50 (72%) of IDC, and negative membranous staining in all the studied lobular carcinomas. The positive cases showed variable degrees of expression whereas 13 cases (27%) showed strong membranous staining (Fig 1). The invasive tumors showed variable in situ component ranging from 10% to 90% of the tumor tissue. All the foci of ductal carcinoma in situ showed membranous staining (Fig. 2) while foci of lobular carcinoma in situ were negative (Fig 3). The invasive component of the neoplasms showed variable degrees of E-cadherin expression (Fig. 4). The foci of lymphatic vascular invasion showed well defined membranous staining (Fig 5), all the cases of lobular carcinomas showed negative membranous staining for E-cad expression (Fig 6).
Fig. (1). Immunohistochemical stain of E-cadherin showing intense membranous expression in invasive ductal carcinoma, 200 X magnification.
Fig. (2). Intense membranous and cytoplasmic stain of E-cadherin in IDC and focus of ductal carcinoma in situ, 200 X magnification.
Fig. (3). Foci of lobular carcinoma insitu shows negative membranous stain for E-cadherin, 200 X magnification.
Fig. (4). An invasive cluster of IDC shows variable expression of E-cadherin stain, 200 X magnification.
Fig. (5). A tumor cluster of IDC shows lymphatic vascular invasion, and retains the membranous expression of E-cadherin, 200 X magnification.
Fig. (6). Invasive lobular carcinoma shows loss of membranous expression of E-cadherin, although it is retained in the membranes of epithelial cells in the normal ducts, 100 X magnification.
Statistical analysis
Table 1 shows associations between E-cadherin expression and the different clinicopathological variables as patient’s age, tumor size, histologic type, tumor grade, lymphatic vascular invasion, resection margins, and number of involved lymph nodes, extranodal extension and tumor stage.
Table (1). Frequency distribution of the studies samples according to the clinicopathological variables and E-cadherin expression.
| E- cadherin expression | |||||||
|---|---|---|---|---|---|---|---|
| Negative | Positive | Total = 54 | P-value | ||||
| Age in years | No. | % | No. | % | No. | % | 0.5060 |
| Less than 40 | 3 | 23.1 | 10 | 76.9 | 13 | 24.1 | |
| 40 & more | 15 | 36.6 | 26 | 63.4 | 41 | 75.9 | |
| Tumor Size | No. | % | No. | % | No. | % | 0.2889 |
| < 2cm. | 3 | 20.0 | 12 | 80.0 | 15 | 27.8 | |
| 2–5cms. | 10 | 38.5 | 16 | 61.5 | 26 | 48.1 | |
| > 5cms. | 5 | 38.5 | 8 | 61.5 | 13 | 24.1 | |
| Histologic type | No. | % | No. | % | No. | % | 0.0096* |
| 0 | 4 | 100.0 | 0 | 0.00 | 4 | 100.0 | |
| 1 | 14 | 8.0 | 36 | 72.0 | 50 | 92.6 | |
| Tumor grade | No. | % | No. | % | No. | % | 0.6510 |
| Low | 17 | 35.4 | 31 | 64.6 | 48 | 88.9 | |
| High | 1 | 16.7 | 5 | 83.3 | 6 | 11.1 | |
| Lymphatic vascular invasion | No. | % | No. | % | No. | % | 0.4072 |
| 0 | 11 | 29.7 | 26 | 70.3 | 37 | 68.5 | |
| 1 | 7 | 41.2 | 10 | 58.8 | 17 | 31.5 | |
| Resection margin | No. | % | No. | % | No. | % | 0.4609 |
| 0 | 14 | 31.1 | 31 | 68.9 | 45 | 83.3 | |
| 1 | 4 | 44.4 | 5 | 55.6 | 9 | 16.7 | |
| LN number | No. | % | No. | % | No. | % | 0.0128* |
| 0 | 1 | 6.3 | 15 | 93.8 | 16 | 29.7 | |
| 1–3 | 8 | 40.0 | 12 | 60.0 | 20 | 37.0 | |
| 4+ | 9 | 50.0 | 9 | 50.0 | 18 | 33.3 | |
| Extranodal exten. | No. | % | No. | % | No. | % | 1.000 |
| 0 | 15 | 32.6 | 31 | 67.4 | 46 | 85.2 | |
| 1 | 3 | 37.5 | 5 | 62.5 | 8 | 14.8 | |
| Stage | No. | % | No. | % | No. | % | 0.0143* |
| Stage 0-I-II | 8 | 22.2 | 28 | 77.8 | 36 | 66.7 | |
| Stage III,IV | 10 | 55.6 | 8 | 44.4 | 18 | 33.3 | |
P value is significant at ≤ 0.05
Table 2 shows the frequency distribution of the studied cases according to the ER, PR receptor status, Her-2, tumors with positive resection margins. A significant association was observed between E-cadherin membranous expression and the number of positive lymph nodes, where 15 out of 16 (94%) node negative cases were strongly positive for E-cadherin while 21 out of 38 (55%)of the node positive cases were positive, P= .0128). Also 28/36 (77.8%) of E-cadherin positive cases were stage II or less while 8/18 (44.4%) were stage III, and IV (P = 0.0143). However no association was found between E-cadherin expression and tumor size, tumor grade, lymphatic vascular invasion, positive resection margins, ER, PR and Her-2 expression.
Table (2). Frequency distribution of the studied samples according to the ER, PR, and HER-2 neu receptor status and E-cadherin expression.
| E- cadherin expression | |||||||
|---|---|---|---|---|---|---|---|
| Negative | Positive | Total | P-value | ||||
| ER | No. | % | No. | % | No. | % | 0.4254 |
| 0–1 | 8 | 40.0 | 12 | 60.0 | 20 | 37.0 | |
| 2–3 | 10 | 29.4 | 24 | 70.6 | 34 | 63.0 | |
| PR | No. | % | No. | % | No. | % | 0.1731 |
| 0–1 | 10 | 43.5 | 13 | 56.5 | 23 | 42.6 | |
| 2–3 | 8 | 25.8 | 23 | 74.2 | 31 | 57.4 | |
| HER-2 | No. | % | No. | % | No. | % | 0.6902 |
| 0–1 | 12 | 35.3 | 22 | 64.7 | 34 | 63.0 | |
| 2–3 | 6 | 30.0 | 14 | 70.0 | 20 | 37.0 | |
P value is significant at ≤ 0.05
Table 3 summarizes a multivariable logistic model to predict strong E-cadherin expression and number of positive lymph nodes, tumor grades, stage, tumor size, lymphatic vascular invasion, positive resection margins, extranodal extension, and patient’s age.
Table (3). Multivariate Logistic Regression model identifying independent predictors of negative E-cadherin expression.
| Significance | Adjusted Odds Ratio | |
|---|---|---|
| Positive nodes | 0.026 | 5.1 (1.4–3.6) |
| High grades | 0.389 | 3.7 (0.2–12.3) |
| Advanced stage | 0.171 | 3.9 (0.8– 1.8) |
| Large Tumor size | 0.274 | 3.3 (0.4– 27.8) |
| ER | 0.793 | 1.5 (0.3–11.6) |
| PR | 0.246 | 5.2 (0.3– 1.5) |
| HER-2 | 0.159 | 3.2 (0.8– 11.3) |
| Lymph.vascular invasion | 0.572 | 1.6 (0.3– 9.2) |
| Positive Resection margins | 0.881 | 1.2 (0.2– 7.5) |
| Extranodal extension | 0.971 | 1.1 (0.1–8.4) |
| Age | 0.881 | 1.2 (0.6– 8.7) |
Node negative cases were found to be an independent predictor of strong E-cadherin expression while node positive cases predicted negative expression of E-cadherin (P = 0.026).
Discussion
The association between loss or down regulation of E-cadherin and the progression of sporadic breast cancer has been extensively documented. Both irreversible and reversible mechanisms are at play and the prevalence of each is related to the histologic subtypes.
In the present study, E-cadherin expression was positive in 72% of infiltrating ductal carcinomas; the staining was strong linear at the cell borders of the well and moderately differentiated tumors but was heterogeneous and dotted over cell borders in the high grade tumors. All the lobular carcinomas were negative for E-cadherin expression, even for the lobular in situ components. These data indicate that loss of E-cadherin expression is an early event in the formation of the lobular type of breast carcinoma.
Our results are close to those of Moll et al (79% of E-cadherin positivity in IDC cases)(15), but less than those of Howard et al (84% positivity) (17) and Gamello et al (94% positivity). (18)
All the lobular carcinoma were negative for E-cadherin expression, which is consistent with most of the published data. (15,18,19) The absence of E-cadherin signifies a partial loss of epithelial differentiation and may account for the extended spread of lobular carcinoma in situ and the peculiar diffuse invasion mode of the ILC. Other factors are obviously involved during invasion of this carcinoma.
The loss of expression of E-cadherin in ILC result from loss of heterozygosity (LOH) at 6q22, involving the E-cadherin gene CDH1 (>50%), frequently in combination with mutation (∼ 50%) or epigenetic silencing of the remaining CDH1 allele. The diffuse growth patterns and loss of cellular coherence that characterize ILCs are in keeping with early studies on breast cancer cell lines, which correlated low E-cadherin expression with invasive properties, defining E-cadherin as an invasion suppression gene.(19) However, the tumor-suppressor role of E-cadherin is not completely clarified. In ductal carcinomas studies showed LOH at 16q also occurs in 50% of IDC. In contrast to ILC, ductal tumors lack mutations in the remaining CDH1 allele and show highly variable E-cadherin expression. Lowering of E-cadherin levels are caused by epigenetic silencing via promoter hypermethylation or transcriptional repression. (19)
Regarding the role of E-cadherins in development of lymphatic tumor emboli, of the 36 E-cadherin positive cases, 26 did not show lymph vascular invasion and 10 showed lymph vascular invasion. In the Lymph, vascular invasion positive cases the majority of tumor cells (including intralymphatic emboli) expressed E-cadherin with intensity similar to those of the normal lobules. In the negative cases the intensity varied; tumor cells at the tumor–stroma interface showed a more frequency and intensity of E-cadherin than did cells in the central region of the tumor. Emboli also exhibited high intensity expression. These findings suggest that E-cadherin plays an important role in tumor development and growth within the lymphatics, and challenges the hypothesis that loss of expression is necessary for metastases.
The persistence of E-cadherin expression in high grade tumors and large size tumors contrasts with most of the reports of E-cadherin in breast cancer which have described down regulation of this molecule in tumorigenesis. The significance of its expression is unclear at this point. Staining of E-cadherin may persist into late stages of breast carcinoma though it may be inactivated functionally. (20) A more likely scenario is a change in the functionality of E-cadherin molecule that promotes metastasis by enabling the tumor cells to adhere to the vascular epithelium, thus improving the capacity to metastasize. One hypothesis is that E-cadherin expression or functionality is transiently reduced, at which the malignant cells migrate into the vasculature and surrounding tissue. Once accomplished, E-cadherin is reintroduced and cells adhere to the vasculature and formulate tumor emboli. Another scenario is that only the complete E-cadherin/catenin complex is associated with no evidence of metastasis. It is possible that a defect in the E-cadherin/catenin complex without a change in its expression may be responsible for the malignant progression. (21)
In our study the node negative tumors showed association with the strong E-cadherin expression which is consistent with the results of Banklavi et al. (22) and in contrast with the findings of Howard et. al.(17) who found persistence of strong expression in cases with more lymph node positivity and proposed that increased expression of E-cadherin is necessary for tumor progression in patients with aggressive breast cancer.
We did not find a positive association between E-cadherin and Her-2. Previous studies have shown no association or inverse correlation between both markers. D’souza et al. (23) showed that C-erb-b2 over expression down-regulated E-cadherin expression at the transcriptional level in non-tumorigenic human mammary epithelial cell line. However, the opposite results of Howard et al argue for the role of C- erb-b2 as a transcriptional regulator of E-cadherin expression in breast cancer. (17) It seems that Epidermal Growth Factor Receptors (which includes Her2) result in disruption of the E-cadherin/ catenin complex without affecting the levels of expression.
In this study, E-cadherin expression was not associated with tumor grade similar to the work of Howard.(17) However, reduced E-cadherin expression has been associated with high histological grade in other studies.(24) The independence of E-cadherin expression supports the notion that it assists aggressive tumor growth by providing a support structure for cells to adhere and accelerates invasion and metastasis. (25)
The disparity between the results of different studies may be due to differences in the population under investigation, which might be indicative of the disparity in the biology of breast cancer in divergent populations.
Conclusion
From this study we conclude E-cadherin is a useful marker to differentiate between IDC and ILC where almost all lobular carcinomas are negative for E-cadherin expression. This marker is also important to confirm the diagnosis of carcinoma insitu of the breast with indeterminate features. Where positive cases will favor the diagnosis of ductal type and negative stain will favor the diagnosis of lobular carcinoma.The usefulness of E-cadherin expression as an independent prognostic indicator in ductal carcinomas of breast needs further investigation. One emerging opinion is that dynamic, reversible modulation of E-cadherin expression occurs during ductal carcinoma progression. Reduced E-cadherin expression favors dissemination, but regaining expression favors survival and reattachment of metastasis.
However, there is a strong association between node negative cases and strong E-cadherin expression. Moreover, E-cadherin showed diminished expression in more advanced stages supporting the view that loss of E-cadherin expression is a marker of aggressiveness. Whether this expression denotes functionality of the molecules or not is still debatable. Moreover, large scale studies are needed to clarify the prognostic value of E-cadherin.
Refrences
- 1.Chiedozi LC, El-Hag IA, Kollur SM. Breast diseases in the Northern region of Saudi Arabia. Saudi Med J. 2003 Jun;24(6):623–7. [PubMed] [Google Scholar]
- 2.El Hag IA, Katchabeswaran R, Chiedozi LC, Kollur SM. Pattern and incidence of cancer in Northern Saudi Arabia. Saudi Med J. 2002 Oct;23(10):1210–3. [PubMed] [Google Scholar]
- 3.Mansoor I. Profile of female breast lesions in Saudi Arabia. J Pak Med Assoc. 2001 Jul;51(7):243–7. [PubMed] [Google Scholar]
- 4.Archibong EI, Sobande AA, Sadek AA, Ajao OG, Khan AR, Fawehinmi O. The changing pattern of malignant neoplasms among females in Asir Region of Saudi Arabia. Saudi Med J. 2000 Sep;21(9):869–72. [PubMed] [Google Scholar]
- 5.Jamal AA. Pattern of Breast Diseases in a teaching hospital in Jeddah, Saudi Arabia. Saudi Med J. 2001 Feb;22(2):110–3. [PubMed] [Google Scholar]
- 6.Al-Idrissi HY. Pattern of Breast Cancer in Saudi females in eastern province of Saudi Arabia. Indian J Med Sci. 1991 Apr;45(4):85–7. [PubMed] [Google Scholar]
- 7.Kumar Vinay, Abbas Abul, Fausto Nelson. 7th ed. El Sevier Inc; 2005. Robbins and Cotran Pathologic Basis of Disease. [Google Scholar]
- 8.Mirza An, Mirza NQ. prognostic factors in node negative breast cancer. Ann Surg. 2001;235:10. doi: 10.1097/00000658-200201000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson Jf, Page DL. Prognostic value of histopathology in the breast. Semin Oncolo. 1992;19:254. [PubMed] [Google Scholar]
- 10.Hayes DF, Thor Ad. C-erbB2 in breast cancer: development of a clinically useful marker. Semin Oncolo. 2002;29:231. doi: 10.1053/sonc.2002.32899. [DOI] [PubMed] [Google Scholar]
- 11.Laura Medri, Volpi A, Silvestrini R. Prognostic relevance of mitotic activity in patients with node negative breast cancer. Modern Pathology. 2003;16:1067–75. doi: 10.1097/01.MP.0000093625.20366.9D. [DOI] [PubMed] [Google Scholar]
- 12.Al-Idrissi HY, Ibrahim EM, Kurashi NY, Sowayan SA. Breast cancer in a low-risk population. The influence of age and menstrual status on disease pattern and survival in Saudi Arabia. Int J Cancer. 1992 Aug 19;52(1):48–51. doi: 10.1002/ijc.2910520111. [DOI] [PubMed] [Google Scholar]
- 13.Bukholm Ik, Nesland JM, Boressen Dale AL. E-cadherin and Alpha, Beta, and gamma catenin protein in relation to metastases in human breast carcinoma. J of Pathol. 1998;185:265–266. doi: 10.1002/(SICI)1096-9896(199807)185:3<262::AID-PATH97>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 14.Takeichi M. Cadherins in cancer: Implications for invasion and metastases. Curr Opin Cell biol. 1993;5:806–11. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- 15.Moll R, Mitze M, Birchmeier W. Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas. American journal of pathology. 1993;143:1731–42. [PMC free article] [PubMed] [Google Scholar]
- 16.Berx G, Cleton-Jansen AM, Nollet F, de Leeuw WJ, van de Vijver M, Cornelisse C, van Roy F. E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. EMBO J. 1995;14:6107–6115. doi: 10.1002/j.1460-2075.1995.tb00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard EM, Lau AK, et al. Expression of E-cadherin in high risk breast cancer. J cancer res Clin Oncol. 2005;131:14–18. doi: 10.1007/s00432-004-0618-z. (2005). [DOI] [PubMed] [Google Scholar]
- 18.Gamello C, palacios J, Pizarro A, Cano A. Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. American Journal of pathology. 1993;142:987–993. [PMC free article] [PubMed] [Google Scholar]
- 19.Cowin P, Hastell S. Cadherins and catenins in breast cancer. Current opinion in cell biology. 2005;17(5):499–508. doi: 10.1016/j.ceb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Gillet Ce, Ryder K, et al. Retension of the expression of E-cadherin and catenins is associated with shorter survival in grade III ductal carcinoma 2001. J Pathol. 193:433–441. doi: 10.1002/path.831. [DOI] [PubMed] [Google Scholar]
- 21.Zschiese W, Schonborn I, et al. Expression of E-cadherin and catenins in invasive mammary carcinoma. Anticancer Res. 1997;17:561–567. [PubMed] [Google Scholar]
- 22.Banfalvi A, Terpe HJ, et al. Immunop-henotypic and prognostic analysis of E-cadherin and its relation and beta catenin expression during breast carcinogenesis and tumor progression 1999. Histopath. 34:25–34. doi: 10.1046/j.1365-2559.1999.00540.x. [DOI] [PubMed] [Google Scholar]
- 23.D’Souza B, Taylor J. overexpesion of Erbb2 in human mammary epithelial cells signals inhibition of e-cad gene. Proc Nath Acad Sci. 1994;91:7202–7206. doi: 10.1073/pnas.91.15.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker C, Robertson JF, et al. E-cadherin as a prognostic indicator in primary breast cancer. Br J of cancer. 2001;85:1958–1963. doi: 10.1054/bjoc.2001.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang WG, Mansel RE. E-cadherin complex and its abnormalities in human breast cancer. Surgical Oncology. 2000;9(4):151–171. doi: 10.1016/s0960-7404(01)00010-x. [DOI] [PubMed] [Google Scholar]