Abstract
In Neisseria meningitidis, iron-responsive gene regulation is mediated primarily by the ferric uptake regulator (Fur) protein. When complexed with iron, Fur represses gene expression by preventing transcription initiation. Fur can also indirectly activate gene expression via the repression of regulatory small RNAs (sRNA). One such Fur- and iron-regulated sRNA, NrrF, was previously identified in N. meningitidis and shown to repress expression of the sdhA and sdhC genes encoding subunits of the succinate dehydrogenase complex. In the majority of Gram-negative bacteria, sRNA-mediated regulation requires a cofactor RNA-binding protein (Hfq) for proper gene regulation and stabilization. In this study, we examined the role of Hfq in NrrF-mediated regulation of the succinate dehydrogenase genes in N. meningitidis and the effect of an hfq mutation on iron-responsive gene regulation more broadly. We first demonstrated that the stability of NrrF, as well as the regulation of sdhC and sdhA in vivo, was unaltered in the hfq mutant. Secondly, we established that iron-responsive gene regulation of the Fur-regulated sodB gene was dependent on Hfq. Finally, we demonstrated that in N. meningitidis, Hfq functions in a global manner to control expression of many ORFs and intergenic regions via iron-independent mechanisms. Collectively these studies demonstrate that in N. meningitidis, iron- and NrrF-mediated regulation of sdhC and sdhA can occur independently of Hfq, although Hfq functions more globally to control regulation of other N. meningitidis genes primarily by iron-independent mechanisms.
INTRODUCTION
In the Gram-negative pathogen Neisseria meningitidis, a portion of the genome is regulated in response to iron (∼10 %) (Grifantini et al., 2003) and the majority of this regulation is mediated by the iron-responsive ferric uptake regulator (Fur) (Delany et al., 2006; Grifantini et al., 2003). In an iron-replete environment, Fur binds to DNA regulatory sequences known as Fur boxes repressing transcription (Desai et al., 1996; Escolar et al., 1998a, b; Stojiljkovic & Hantke, 1995). In Escherichia coli, Fur has also been demonstrated to positively regulate gene expression via a Fur-dependent regulatory small RNA (sRNA) (Masse & Gottesman, 2002). Regulatory sRNAs are non-coding RNA molecules that act at the post-transcriptional level to mediate their effects. While a small number of sRNAs function by modulating protein activity, the most common class of regulatory sRNAs act by base-pairing with target mRNAs over short regions of complementarity. Base-pairing between an sRNA and an mRNA generally leads to repression of mRNA translation (Urban & Vogel, 2007), although in some instances, sRNA : mRNA interactions can increase mRNA translation (Repoila et al., 2003).
In E. coli, the Fur-regulated sRNA RyhB is repressed under iron-replete conditions and, when active, functions as a post-transcriptional repressor of genes involved in iron homeostasis (Masse et al., 2003, 2005). Recently, our group and others identified a Fur-regulated sRNA, NrrF, in N. meningitidis (Mellin et al., 2007). NrrF regulates components of the succinate dehydrogenase complex as do Fur-regulated sRNAs in other organisms, and fulfils a functionally analogous role to these other Fur-regulated sRNAs.
For nearly all sRNAs studied in E. coli and other Gram-negative species, the interaction with a target mRNA in vivo requires a cofactor RNA chaperone protein named Hfq (Gottesman, 2004; Majdalani et al., 2005; Urban & Vogel, 2007). Hfq is a homohexameric ring-shaped protein first described as a host factor required for replication of the Qβ phage in E. coli (Franze de Fernandez et al., 1968). It is a member of the Sm family of proteins associated with RNA splicing events in eukaryotes and is widely conserved in bacterial species. Nearly all of the sRNAs examined from E. coli and other Gram-negative bacteria were shown to require Hfq as an essential cofactor for regulating mRNA targets, as well as for sRNA stability (Christiansen et al., 2006; Gottesman, 2004; Majdalani et al., 2005). However, recent studies have identified Hfq-independent sRNAs in the Gram-positive organisms Staphylococcus aureus and Bacillus subtilis (Bohn et al., 2007; Heidrich et al., 2006, 2007). In B. subtilis, the Fur-regulated sRNA FsrA requires three proteins hypothesized to be RNA chaperones which act in place of Hfq (Gaballa et al., 2008).
In this report, we examined the requirement for Hfq in N. meningitidis iron-responsive gene regulation. We demonstrate that Hfq is dispensable for NrrF-mediated in vivo regulation of the succinate dehydrogenase genes. We also established that iron-responsive regulation of the Fur-regulated sodB gene was dependent on Hfq. Finally, we demonstrate that in N. meningitidis, Hfq influences the expression of several ORFs as well as intergenic (IG) regions via iron-independent mechanisms.
METHODS
Bacterial strains, media and growth conditions.
N. meningitidis MC58 was the wild-type strain used in this study. An hfq mutant was generated by transforming wild-type MC58 with pSLhfq-KO, a derivative of pSLfur-C1 (Delany et al., 2003) constructed with hfq-flank primers (Table 1) to generate strain JM23. Complementation of JM23 was by transformation with pSLhfq-C1, a derivative of pSLfur-C1 (Delany et al., 2003) constructed with hfqcomp primers (Table 1). JM30 carries the hfq gene in the IG region between NMB1074 and NMB1075. Strain JM44 was generated by transforming a derivative of the pLES94 plasmid (Silver & Clark, 1995) into wild-type MC58 (Supplementary Fig. S1, available with the online version of this paper). The plasmid contains the sdhC promoter region from −277 to +14 with respect to the translational start site. Strains JM45 and JM46 were constructed in the same manner, but using JM23 and JM30 in place of the wild-type, respectively. Strain JM47 was generated by transforming a derivative of the pLES94 plasmid containing the sodB promoter into wild-type MC58. This fusion contains the sodB promoter region from −166 to +13. Strains JM48 and JM49 were constructed in a similar manner, but using JM23 and JM30 in place of the wild-type, respectively. All DNA manipulations were carried out as described previously (Delany et al., 2003) or by following the manufacturer's instructions; correct insertion of the lacZ transcriptional fusion as well as all other constructs was verified by PCR.
Table 1.
Strains, plasmids and oligonucleotides used in this study
All primers are given in 5′–3′ orientation.
| Strain, plasmid or oligonucleotide | Genotype, phenotype or sequence | Description/reference |
|---|---|---|
| N. meningitidis strains | ||
| MC58 | Clinical isolate; sequenced strain | Tettelin et al. (2000) |
| JM23 | Mutant derivative of MC58 carrying a kanamycin insertion/deletion of the hfq locus | This study |
| JM30 | Complemented hfq mutant in which the hfq gene was cloned into the IG region between NMB1074 and NMB1075 under the control of its own promoter | This study |
| JM44 | MC58 with the sdhC : lacZ transcriptional fusion inserted into the proA/B genes | This study |
| JM45 | JM23 with the sdhC : lacZ transcriptional fusion inserted into the proA/B genes | This study |
| JM46 | JM30 with the sdhC : lacZ transcriptional fusion inserted into the proA/B genes | This study |
| JM47 | MC58 with the sodB : lacZ transcriptional fusion inserted into the proA/B genes | This study |
| JM48 | JM23 with the sodB : lacZ transcriptional fusion inserted into the proA/B genes | This study |
| JM49 | JM30 with the sodB : lacZ transcriptional fusion inserted into the proA/B genes | This study |
| Plasmids | ||
| pSL-hfqKO | KanR AmpR | This study |
| pSL-hfq-C1 | KanR AmpR ErmR | This study |
| pLES94 | AmpR ChlorR | Silver & Clark (1995) |
| Primers (for cloning)* | ||
| hfq-5′flankF | GGAACTAGTAGCCACAATCCCGTAAACAG | Amplifies a 893 bp SpeI/XhoI fragment |
| hfq-5′flankR | GACTCGAGGCACGAAGCATGACGTGT | |
| hfq-3′flankF | GGACGGCCGATTTTTAACTCCGTTATTATGATTGTG | Amplifies a 744 bp EagI/SalI fragment |
| hfq-3′flankR | GGAGTCGACTGGACATGAACGAAACCGT | |
| hfqcompF | ACAGGATCCAAAGATATGACACGTCATGCTTC | Amplifies a 568 bp EagI/BamHI fragment |
| hfqcompR | ACACGGCCGCAACCCGTTACCATCGTATTG | |
| tonBflankF | ATGCGGCCGCAACCGCCTAATTCAATTCAA | Amplifies a 901 bp EagI/SalI fragment |
| tonBflankR | ATGGTCGACTTGTAAAAATAGGTTGCAAATAGGAA | |
| exbBflankF | ATGACTAGTCAGCTATCCTTTTGATTAAGCAGG | Amplifies a 676 bp SpeI/XhoI fragment |
| exbBflankR | ATGCTCGAGGTGTTGGAATCATTATGAATTTGAAA | |
| Oligonucleotides used for EMSA | ||
| SdhC | UUUUUGAGUCGAUAUAUAACUACAGAGGAAUUGACUAUGUCUGCC | Cy-5 labelled 45 bp RNA probe |
| NrrF | GGAAGCCGUCCGUUCUAUACGAGACAUACAUUCCCUUUUUAUAUAUCAGAUAC | Cy3-labelled 53 bp RNA probe |
| Primers (for qRT-PCR) | ||
| NMB0382F | GTTACGATGCTGCGGATTTT | Amplifies a 407 bp fragment of the rmp gene |
| NMB0382R | GAAACCATTTCCCTGTCTGC | |
| NMrrnaA16SF | CATACCGTGGTAAGCGGACT | Amplifies a 236 bp fragment of the 16S rRNA |
| NMrrnaA16SR | TGGTCGGTACAGAGGGTAGC | |
| NMB0950F | ATTACCACGGCGAAGTTGTC | Amplifies a 288 bp fragment of the sdhA gene |
| NMB0950R | ATGCATCAACGTTTTCACCA | |
| NMB0948F | GGGGTCGGGCTGTTTATTAT | Amplifies a 151 bp fragment of the sdhC gene |
| NMB0948R | GGTGCAGATAAGCCCACAAT | |
| NMB0884F | GGCGTTGGAAGTAGAAACCA | Amplifies a 277 bp fragment of the sodB gene |
| NMB0884R | CCTGCCTTTGGAAGATTG | |
| Probes for Northern blots | ||
| Nrrf | GTATGTCTCGTATATGCCGACTCCAAGTGTGAAAGTGATGATGGGGAAAT | |
| 5S rRNA | TTTCACGGTCCTGTTCGGGATGGGAAGGCGTGGGACCAACTCGCTATGGC | |
*Restriction sites are underlined. F, forward primer, R, reverse primer
For all growth experiments, bacteria were plated from glycerol stocks onto GCB agar plates and grown overnight. Cells were then inoculated into chemically defined media (CDM) (Morse & Bartenstein, 1980) to an OD600 of 0.06–0.08 using a Genesys 20 spectrophotometer and cuvettes with a pathlength of 10 mm. For experiments in which RNA was to be isolated, cells were grown to an OD600 of 0.4–0.5 before being divided and inoculated with either 100 μg ferric nitrate ml−1 or 100 μg desferal ml−1 (an iron chelator) to constitute iron-replete or iron-depleted conditions, respectively. Cultures were grown for an additional 30 min before RNA was harvested. When appropriate, antibiotics were used at the following concentrations: 100 μg kanamycin ml−1, 5 μg erythromycin ml−1, 5 μg chloramphenicol ml−1.
RNA isolation and quantitative RT-PCR (qRT-PCR).
Total RNA was isolated using Trizol reagent (Invitrogen) according to the manufacturer's instructions. RNA was treated with DNase I and successful removal of DNA contamination was verified by PCR. RNA integrity was confirmed on precast 5 % TBE–urea ready polyacrylamide gels (Bio-Rad). For qRT-PCR experiments, 25 μl reactions were prepared with Quantitect SYBR green RT-PCR reagents (Qiagen). Total RNA (25 ng) was added to each reaction and primers were used at a final concentration of 2 ng μl−1. Reactions were cycled in an ABI Prism 7000 cycler and analysed with the SDS 1.2.3 software suite (ABI). Transcript quantities were calculated using a standard curve and normalized to the 16S transcript. Two biological replicates were analysed and fold changes were calculated as an average of the two values.
β-Galactosidase assays.
N. meningitidis strains harbouring the sdhC promoter fusion or sodB promoter fusion were grown to an OD600 of 0.4–0.5 before the addition of iron or desferal. Cells were then collected by centrifugation and β-galactosidase assays were carried out as described previously (Miller, 1972). The optical density was determined at 420 nm and again at 550 nm to correct for light scattering. Miller units were then calculated using the following equation: Miller units=1000×[OD420−(1.75×OD550)]/(V×T×OD600), where V is the volume in ml and T is the time in min.
Half-life experiments.
N. meningitidis strains were grown in CDM to an OD600 of 0.4, inoculated with 100 μg desferal ml−1 to induce nrrF transcription and grown for an additional 15 min. Cells were then treated with rifampicin (500 μg ml−1) and nalidixic acid (40 μg ml−1) to halt further transcription and DNA synthesis. Aliquots were removed at subsequent time points and were mixed 1 : 1 with a frozen slurry of stop buffer (10 mM Tris pH 7.2, 5 mM MgCl2, 0.4 mg chloramphenicol ml−1 and 20 mM sodium azide). Cells were harvested by centrifugation and RNA was isolated using Trizol reagent (Invitrogen). Total RNA (4 μg) was then run on precast 5 % TBE–urea ready polyacrylamide gels (Bio-Rad), transferred to a Hybond-N+ nytran membrane (Amersham Biosciences), UV cross-linked and prehybridized for 1 h at 42 °C in ULTRAHyb Oligo hybridization solution (Ambion). Membranes were subsequently probed overnight at 42 °C with a γ-32P-labelled DNA oligonucleotide probe (106 c.p.m. ml−1) complementary to nrrF, then stripped and reprobed with a probe to the 5S rRNA. The intensity of the bound probe was quantified using a phosphoimager and normalized to 16S rRNA intensity.
Microarray procedures.
DNA microarrays were designed by Nimblegen using the N. meningitidis MC58 genome as a reference (GenBank accession no. NC_003112.2) (Tettelin et al., 2000). Arrays were designed using the Nimblegen 4-plex format consisting of four arrays on each individual chip. Each array contained 72 000 probes, for a total of 385 000 probes per chip. For interrogation of each target ORF, ten unique corresponding 60-mer synthetic DNA oligonucleotide probes were synthesized directly onto Nimblegen 4-plex arrays, and each probe was printed in duplicate. One hundred per cent of identified ORFs were represented on the arrays. Additionally, 60-mer tiling probes were designed to both strands of the IG regions providing complete coverage of IG regions. Intergenic probes were also printed on each array in duplicate. Total RNA (30 μg) for each sample was converted to double-stranded cDNA using the SuperScript double-stranded cDNA conversion kit (Invitrogen). Samples were labelled and hybridized according to Nimblegen's protocols. Data were normalized using the method described by Bolstad et al. (2003). Expression values were determined as described previously (Irizarry et al., 2003). Data were further analysed using the Arraystar 2.0 software suite (dnastar). For each strain and condition, three biological replicates were analysed for a total of 18 arrays. Student's t-test was used to calculate P-values. The GEO accession no. for the microarray experiments carried out in this paper is GSE20294.
RESULTS
Growth of the hfq mutant is attenuated in vitro
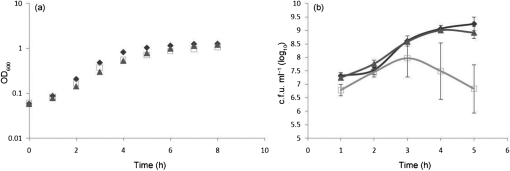
To examine the role that Hfq plays in general meningococcal physiology, we constructed a N. meningitidis hfq mutant (JM23) and a complemented hfq (JM30) strain. When grown in CDM, growth of the N. meningitidis hfq mutant strain was slightly attenuated when compared with the wild-type or complemented hfq strain, as determined by OD600 readings, and was more severely attenuated as determined by c.f.u. measurements (Fig. 1). The N. meningitidis hfq mutant exhibited approximately a 1 log reduction in c.f.u. compared with the N. meningitidis wild-type and complemented hfq strains when examined at 3 h, and a 2.5 log reduction when examined at 5 h (Fig. 1b). Additionally, hfq mutant colonies were smaller in size compared with wild-type and complemented mutant strains when grown on GCB agar plates overnight (data not shown). The attenuated growth of the N. meningitidis hfq mutant was largely corrected in the complemented strain (Fig. 1).
Fig. 1.
N. meningitidis wild-type, hfq deletion mutant and complemented hfq strains were grown in CDM (Morse & Bartenstein, 1980), growth was monitored by optical density (OD600) (a) and colony forming unit (b) measurements. Results represent the average of three experiments and error bars represent sd. Wild-type, ⧫; hfq mutant, □; complemented hfq,▴.
Hfq is dispensable for NrrF-mediated regulation of the succinate dehydrogenase genes
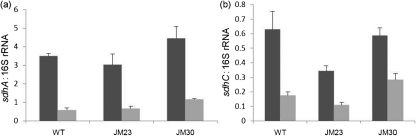
We previously reported that expression of the sdhA and sdhC genes was increased under iron-replete conditions and was repressed under iron-depleted conditions in an NrrF-dependent manner (Mellin et al., 2007). Therefore, we next examined the expression of the sdhA and sdhC genes by qRT-PCR in N. meningitidis wild-type (MC58), hfq mutant (JM23) and complemented hfq mutant (JM30) strains grown in CDM under either iron-replete or -depleted conditions. As expected, in N. meningitidis wild-type cultures grown in iron-replete versus -depleted conditions, we observed a 6.13-fold increase in the expression of the sdhA transcript (Fig. 2a). In N. meningitidis, JM23 and JM30 cultures grown in iron-replete versus -depleted conditions, we observed a 4.68- and 3.83-fold increase, respectively, in the expression of the sdhA transcript. Similar results were obtained when we examined the expression of the sdhC transcript in N. meningitidis wild-type, JM23 and JM30 cultures grown under iron-replete conditions compared with iron-depleted conditions (Fig. 2b). Thus, repression of both the sdhA and sdhC genes under iron-depleted growth conditions was not altered in the hfq mutant.
Fig. 2.
Effect of the hfq mutation on regulation of the succinate dehydrogenase genes. Expression of sdhA (a) and sdhC (b) transcripts under iron-replete (dark grey bars) versus iron-depleted (light grey bars) conditions was evaluated by qRT-PCR. Transcript levels were normalized to the 16S rRNA transcript which is not regulated in response to iron. Primers amplified a 151 bp fragment of the sdhC gene, a 288 bp fragment of the sdhA gene, and a 236 bp fragment of the 16S rRNA gene. Results represent the average of two independent experiments and error bars represent sd.
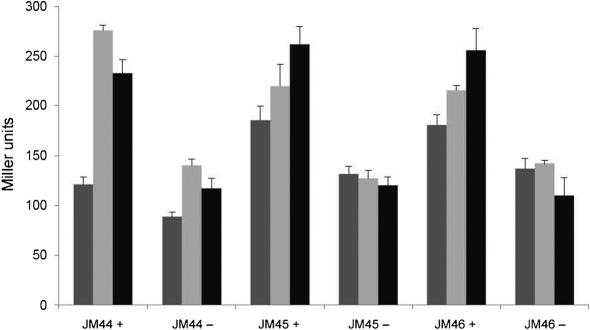
To further support the results obtained by qRT-PCR analysis, we created N. meningitidis lacZ translational fusions in strains MC58, JM23 and JM30 with the promoter of sdhC, the first gene of the succinate dehydrogenase operon. We observed similar iron-dependent and Hfq-independent regulation of the sdhC : lacZ fusion strains (Fig. 3). These results indicate that Hfq is dispensable for iron-mediated regulation of the sdhC gene.
Fig. 3.
Effect of the hfq mutant on iron regulation of the sdhC promoter. JM44 (wild-type N. meningitidis with the lacZ fusion), JM45 (hfq mutant strain with the lacZ fusion) and JM46 (complemented hfq mutant strain with the lacZ fusion) cultures were grown under iron-replete or iron-depleted conditions for 30 (dark grey bars), 60 (light grey bars) or 120 (black bars) min. β-Galactosidase assays were carried out as described in Methods. Cultures grown under iron-replete and iron-depleted conditions are indicated by + and −, respectively. Assays were carried out in duplicate and results represent the average of three independent experiments. Error bars, sd.
NrrF stability is unchanged in the N. meningitidis hfq mutant
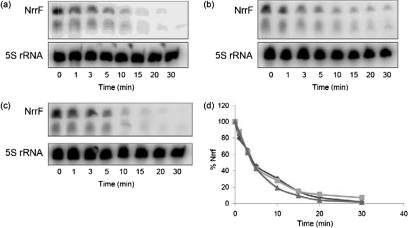
We next examined the stability of NrrF in the N. meningitidis wild-type, hfq and complemented hfq mutant strains. We observed that the NrrF sRNA was stable in the three N. meningitidis strains, as observed at 5 min, and the transcript levels persisted at low levels for 15 min (Fig. 4a–c). The calculated half-life of the NrrF transcript, as determined by densitometry analysis, was 5.0, 4.0 and 4.1 min in the N. meningitidis wild-type, hfq mutant and complemented hfq mutant strains, respectively (Fig. 4d). While we did observe an approximately 20 % difference in the half-life between the N. meningitidis wild-type and hfq mutant strains, this was not statistically significant. The small differences observed in the half-life of NrrF may not be directly related to the lack of Hfq, but rather to secondary effects of the mutant and complemented strains, as it was not corrected in the complemented mutant. Thus, while the hfq mutation had a minimal effect on NrrF stability, the results overall indicate that NrrF stability was not influenced by Hfq, since nearly all Hfq-dependent sRNAs are significantly less stable in an hfq null background (Christiansen et al., 2006; Moller et al., 2002).
Fig. 4.
Half-life of NrrF in the hfq mutant and hfq complemented strains. Cultures of wild-type (a), hfq (b) and complemented hfq (c) strains were grown in CDM to an OD600 of 0.4–0.5. Aliquots were taken at the time points indicated and RNA was isolated. Levels of bound probe were quantified using a phosphoimager. All blots were stripped and reprobed with a probe to the 16S rRNA to ensure equal levels of RNA were loaded onto each lane; levels of NrrF were then normalized to levels of 5S rRNA. (d) The decay of the probe over time. Wild-type, ⧫; hfq mutant,▪; complemented hfq, ▴.
Iron-responsive gene regulation of the Fur-regulated sodB gene is Hfq-dependent
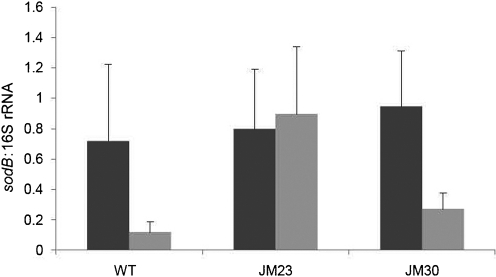
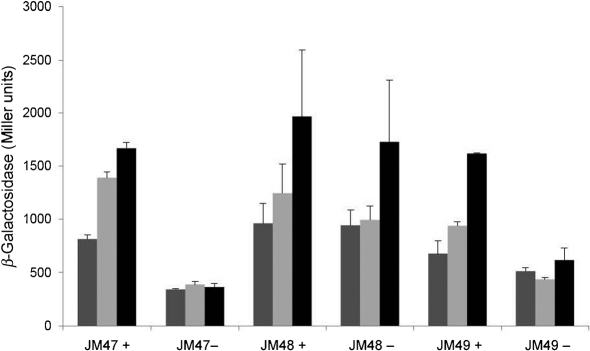
To determine whether Hfq played a role in the regulation of other iron- and Fur-responsive gene networks in N. meningitidis, we examined transcription of the iron- and Fur-activated gene, sodB, as it is the target of Fur-regulated sRNAs in other organisms (Večerek et al., 2003). Hfq-dependent regulation of sodB was first examined by qRT-PCR and expression was observed to be derepressed under iron-depleted conditions in the hfq mutant compared with the wild-type strain (Fig. 5). These results were further examined by the construction of translational fusions containing the promoter of sodB fused to lacZ. The sodB : : lacZ fusion showed similar derepression under iron-depleted conditions (Fig. 6). These results indicate that regulation of the sodB promoter was abolished in the N. meningitidis hfq mutant strain.
Fig. 5.
Effect of the hfq mutation on regulation of sodB. The expression of sodB under iron-replete conditions versus iron-depleted conditions was evaluated by qRT-PCR. RNA was isolated from wild-type (WT), hfq mutant (JM23) and complemented hfq mutant (JM30) strains grown under iron-replete (dark grey bars) and iron-depleted (light grey bars) conditions in CDM. Transcript levels were normalized to the 16S rRNA transcript which is not regulated in response to iron. Results represent the average of three independent experiments; error bars represent sd.
Fig. 6.
Effect of the hfq mutant on the iron regulation of the sodB promoter. JM47 (wild-type N. meningitidis with the lacZ fusion), JM48 (hfq mutant strain with the lacZ fusion) and JM49 (complemented hfq mutant strain with the lacZ fusion) cultures were grown under iron-replete or iron-depleted conditions for 30 (dark grey bars), 60 (light grey bars) or 120 (black bars) min. β-Galactosidase assays were carried out as described in Methods. Cultures grown under iron-replete and iron-depleted conditions are indicated by + and −, respectively. Assays were carried out in duplicate and results represent the average of three independent experiments. Error bars, sd.
Hfq functions globally to control gene expression in N. meningitidis via iron-independent mechanisms
To further evaluate the role of Hfq in N. meningitidis iron-dependent and -independent gene expression on a global level, we utilized a whole-genome tiling microarray to examine the transcriptional profiles of N. meningitidis wild-type, hfq and complemented hfq mutant strains grown under iron-replete and -depleted conditions. To identify genes that required Hfq for proper iron-responsive regulation, we analysed the microarray data for genes that exhibited iron-dependent regulation in the N. meningitidis wild-type and complemented hfq mutant strains (>2.5-fold) but little or no change in the hfq mutant (<1.5-fold). This analysis identified only one such gene meeting these criteria, sodB, further confirming the results presented above.
The observation that the N. meningitidis hfq mutant had an attenuated growth phenotype suggested that Hfq might serve a broad role in meningococcal physiology and gene regulation. Thus, we next analysed the microarray data to identify genes that required Hfq independently of iron (Table 2 and Supplementary Table S1). We identified 45 genes that were Hfq-regulated: 27 exhibited increased expression and 18 exhibited decreased expression in the hfq mutant relative to the wild-type strain. The majority of genes that exhibited increased expression in the N. meningitidis hfq mutant were classified as either hypothetical or uncharacterized genes. Of the known genes that exhibited increased expression in the N. meningitidis hfq mutant, several are involved in metabolism, including ackA-1, adhP, acnB, prpB, prpC and prpF.
Table 2.
Hfq-regulated genes identified by microarray analysis
All genes showing at least 2.5-fold change in regulation between the wild-type strain and hfq mutant strains are listed. Fold change is defined as the average expression from three microarray replicates of the wild-type strain divided by the average expression of three microarray replicates of the hfq mutant strain or hfq complemented mutant strain. If the quotients were less than 1, the reciprocal was taken and a minus sign was added for ease of reading. All P-values of a fold change are below 0.02, and fold changes are complemented to between 60 and 140 % of wild-type expression. Because there were several genes with large changes, we also included those that showed a fourfold difference between the hfq mutant and complemented hfq mutant. WT, N. meningitidis MC58; JM23, hfq mutant; JM30, complemented hfq mutant. Hi, culture was grown under iron-replete conditions; Low, culture was grown under iron-depleted conditions. Genes in bold were also found in the study by either Pannekoek et al. (2009) or Fantappie et al. (2009).
| Gene | Name | Description | Fold change | |||
|---|---|---|---|---|---|---|
| WT vs JM23 Hi | WT vs JM23 Low | WT vs JM30 Hi | WT vs JM30 Low | |||
| Upregulated genes | ||||||
| Metabolism: | ||||||
| NMB0430 | prpB | 2-Methylisocitrate lyase | −20.35 | −15.32 | −1.18 | −1.85 |
| NMB0431 | pprC | Methylcitrate synthase | −23.87 | −19.69 | −1.19 | −1.82 |
| NMB0435 | ackA-1 | Acetate kinase | −13.65 | −10.26 | −1.75 | −2.43 |
| NMB0546 | adhP | Alcohol dehydrogenase | −1.71 | −3.09 | −1.15 | −1.09 |
| NMB1572 | acnB | Aconitate hydratase | −2.71 | −2.97 | 1.11 | 1.19 |
| Transport: | ||||||
| NMB0177 | Sodium/alanine symporter | −13.07 | −10.65 | 1.14 | 1.14 | |
| NMB0402 | putp | Sodium/proline symporter | −2.94 | −1.58 | −1.27 | −1.70 |
| NMB2136 | Peptide transporter | −3.65 | −2.54 | 1.51 | 1.51 | |
| Unclassified: | ||||||
| NMB0432 | Putative membrane protein | −5.86 | −4.11 | −1.36 | −1.65 | |
| NMB0752 | Putative ferredoxin | −1.20 | −2.88 | 1.32 | −1.33 | |
| NMB1472 | clpB | Heat stress response | −2.66 | −1.45 | 1.03 | 1.58 |
| NMB1552 | pivNM-1A | Pilin gene inverting protein | −2.42 | −2.59 | −1.04 | 1.00 |
| Hypothetical: | ||||||
| NMB0227 | Hypothetical protein | −3.28 | −2.63 | 1.22 | 1.14 | |
| NMB0625 | Hypothetical protein | −2.02 | −2.52 | 1.08 | 1.02 | |
| NMB0648 | Hypothetical protein | −4.24 | −2.07 | −1.83 | −1.07 | |
| NMB0649 | Hypothetical protein | −2.76 | −2.04 | −1.38 | −1.21 | |
| NMB0859 | Hypothetical protein | −3.21 | −8.85 | −1.16 | −2.04 | |
| NMB0861 | Hypothetical protein | −2.14 | −2.84 | −1.34 | −1.44 | |
| NMB0865 | Hypothetical protein | −5.89 | −3.10 | −1.15 | −1.40 | |
| NMB0866 | Hypothetical protein | −4.79 | −10.85 | −1.08 | −2.53 | |
| NMB1350 | Hypothetical protein | −3.28 | −2.96 | −1.06 | −1.81 | |
| NMB1406 | Hypothetical protein | −3.37 | −1.68 | −1.30 | −3.37 | |
| NMB1599 | Hypothetical protein | −17.04 | −10.64 | −2.89 | −2.11 | |
| NMB1600 | Hypothetical protein | −3.82 | −4.49 | −1.38 | −2.28 | |
| NMB1764 | Hypothetical protein | −3.35 | −1.93 | −1.34 | −1.23 | |
| NMB1766 | Hypothetical protein | −4.54 | −3.16 | −1.39 | −1.19 | |
| NMB1767 | Hypothetical protein | −2.59 | −2.54 | 1.27 | −1.18 | |
| Downregulated genes | ||||||
| Metabolism: | ||||||
| NMB0994 | Aceyl-CoA dehydrogenase | 8.22 | 2.97 | 2.59 | −2.41 | |
| NMB1044 | fpr-1 | Ferredoxin | 2.70 | 1.79 | 1.58 | 1.23 |
| Transport: | ||||||
| NMB0378 | Putative phosphate transporter | 1.70 | 3.18 | 1.29 | 1.36 | |
| NMB0543 | Putative l-lactate permease | 2.15 | 3.82 | 1.31 | 1.49 | |
| NMB0607 | secD | Type II protein secretion | 1.97 | 2.55 | 1.57 | 1.39 |
| NMB0787 | Amino acid ABC transporter | 2.16 | 3.01 | 1.22 | −1.17 | |
| NMB0788 | Amino acid ABC transporter | 1.90 | 2.64 | 1.30 | −1.10 | |
| NMB0881 | cysT | Sulfate ABC transporter | 7.29 | 4.69 | 1.93 | −3.05 |
| NMB1017 | sbp | Sulfate ABC transporter | 8.12 | 6.30 | 2.59 | −1.27 |
| NMB1315 | uraA | Uracil permease | 2.20 | 2.77 | −1.03 | −1.39 |
| NMB1362 | Putative transport protein | 1.98 | 2.70 | 1.55 | 1.63 | |
| Unclassified: | ||||||
| NMB0024 | pilS cassette | 2.83 | 2.85 | 1.34 | 1.00 | |
| NMB0748 | hfq | Host factor for Qβ phage | 289.26 | 837.89 | −1.10 | 1.63 |
| NMB0763 | cysK | Cysteine synthase | 2.72 | 2.65 | 1.66 | 1.15 |
| NMB0993 | Rubredoxin | ETC protein | 9.13 | 5.25 | 2.46 | −2.10 |
| NMB1617 | TehB | Methyltransferase | 2.98 | 2.65 | 1.65 | 1.56 |
| NMB1934 | atpD | ATP synthase | 1.79 | 3.11 | 1.04 | 1.11 |
| Hypothetical: | ||||||
| NMB0986 | Hypothetical protein | 2.3 | 2.5 | 1.4 | 1.2 | |
Of the genes that exhibited decreased expression in the hfq mutant relative to the wild-type strain, most were involved in transport. These included four ABC transport genes as well as genes encoding three proteins involved in the transport of lactate, phosphate and uracil. Such a global lack of proper metabolism and transport control in the hfq mutant strain could explain the attenuated growth phenotype we observed. Additionally, NMB0024, a member of the unexpressed pilS cassette was demonstrated to exhibit decreased expression in the hfq mutant relative to the wild-type strain. In agreement with the results obtained above, we did not observe differences in the expression of the sdhC and sdhA genes in the hfq mutant relative to the wild-type strain (Supplementary Table S2). Our tiling array was also designed to analyse IG regions and it detected several which showed reduced abundance in the hfq mutant strain compared with the wild-type or complemented hfq mutant strains (Table 3). Expression from IG regions is consistent with known sRNA transcriptional sites and most Hfq-dependent sRNAs exhibit reduced stability in the absence of Hfq (Christiansen et al., 2006; Moller et al., 2002). Therefore, it is plausible that IG regions that were identified as Hfq-regulated in our study had the potential to function as Hfq-dependent sRNAs in N. meningitidis. Consistent with previous data, the abundance of the NrrF IG transcript was not lowered in the hfq mutant strain, in fact it increased. In the wild-type strain the expression was 203 units, in the hfq mutant the expression was 537 units and in the complemented hfq strain, the expression was 182 units. The higher expression level in the hfq mutant is most likely the result of unknown downstream effects of the mutation.
Table 3.
Hfq-regulated IG regions identified my microarray analysis
All IG regions showing at least 2.5-fold downregulation in the hfq mutant strain compared with the wild-type strain are listed. Fold change is defined as the average expression from two microarray replicates of the wild-type strain divided by the average expression of two microarray replicates of the hfq mutant strain or hfq complemented mutant strain. If the quotients were less than 1, the reciprocal was taken and a minus sign was added for ease of reading. For comparison, the fold change under iron-replete conditions versus iron-depleted conditions is also shown for each IG region. Because there was some variability between the two microarray replicates, IG regions were considered to be Hfq-regulated if sd was less than 0.33 of the fold change under either iron-replete or iron-depleted conditions. In addition, the fold change between the wild-type strain versus the complemented hfq mutant strain had to be between 0.6 and 1.4. Because some regions were very highly regulated, we also included IG regions where the fold change between the wild-type strain versus the hfq mutant strain was at least three times higher than the fold change between the wild-type strain versus the complemented hfq mutant strain. Hi, culture was grown under iron-replete conditions; Low, culture was grown under iron-depleted conditions.
| Fold change | |||||
|---|---|---|---|---|---|
| WT Hi vs low | WT vs JM23 Hi | WT vs JM23 Low | WT vs JM30 Hi | WT vs JM30 Low | |
| IG region (plus strand) | |||||
| 1 009 467–1 010 148 | 2.22 | 3.78 | 3.14 | 1.12 | −1.56 |
| 601 650–602 078 | −1.77 | 3.24 | 3.02 | 1.21 | 1.59 |
| 864 734–865 029* | 1.48 | 2.98 | 1.57 | −1.14 | −1.79 |
| 901 874–902 124 | 1.69 | 2.93 | 2.12 | 1.26 | −2.94 |
| 1 099 662–1 101 163 | −1.15 | 2.80 | 2.03 | 1.27 | 1.54 |
| 779 574–779 701 | −1.41 | 7.92 | 10.65 | 2.82 | 2.24 |
| 2 185 273–2 185 835* | −1.25 | 4.18 | 6.60 | 2.49 | 1.94 |
| 1 486 778–1 488 547 | −4.13 | −1.14 | 4.00 | 2.02 | 1.35 |
| 1 529 930–1 530 289* | −3.63 | 1.05 | 3.19 | 1.14 | 1.35 |
| 327 435–3 279 36* | −3.61 | −1.10 | 2.79 | 2.73 | 1.19 |
| 1 009 467–1 010 148 | 1.22 | 4.13 | 2.75 | 1.21 | −1.69 |
| IG region (minus strand) | |||||
| 1 009 467–1 010 148 | 1.26 | 4.72 | 4.06 | 1.25 | −1.86 |
| 601 650–602 078 | −1.62 | 3.31 | 2.52 | 1.22 | 1.45 |
| 2 091 429–2 092 651 | −40.09 | 1.42 | 3.24 | 1.32 | 1.23 |
| 639 542–639 763 | 1.14 | 2.71 | 2.88 | 2.01 | 1.38 |
| 601 650–602 078 | −1.56 | 2.65 | 2.64 | 1.24 | 1.29 |
| 1 599 520–1 600 541 | −1.94 | 1.66 | 2.57 | 1.31 | 1.19 |
*The IG region has a Rho-independent terminator.
DISCUSSION
In this study, we determined that Hfq does not play a major role in iron- and NrrF-mediated regulation of the sdhA and sdhC genes in N. meningitidis. We demonstrated that the regulation of sdhC and sdhA in vivo was unaltered in the hfq mutant. These results were confirmed using a translational lacZ fusion. We also demonstrated that the stability of NrrF was unaltered in the hfq mutant in contrast with the half-lives of Hfq-dependent sRNAs reported for hfq mutants constructed in other organisms (Christiansen et al., 2006; Moller et al., 2002). The contribution of Hfq to iron-responsive regulation in N. meningitidis was limited to the regulation of the sodB gene. While the sodB gene has been shown to be regulated by an sRNA in E. coli (Masse & Gottesman, 2002), we previously reported that sodB is regulated independently of NrrF in N. meningitidis (Mellin et al. 2007). How Hfq mediates sodB regulation via other sRNA-mediated mechanisms in N. meningitidis remains to be defined.
The majority of sRNA-mediated regulation systems in Gram-negative bacteria described to date have been shown to require Hfq as a cofactor (Gottesman, 2004; Majdalani et al., 2005; Moller et al., 2002; Murphy & Payne, 2007). Fur-regulated sRNAs of E. coli and Vibrio cholera, including RyhB, also require Hfq (Davis et al., 2005; Masse & Gottesman, 2002). Since sdhC is regulated in the absence of Hfq, it is possible that either another protein can function as a cofactor with NrrF or NrrF functions without a cofactor. The recent identification of an Hfq-independent, Fur-regulated sRNA in B. subtilis which is proposed to work in conjunction with three Fur-regulated chaperone proteins supports the idea that other sRNA cofactor proteins may exist in N. meningitidis. Our laboratory has previously identified a number of uncharacterized, Fur-regulated ORFs, and an effort to reexamine these proteins for potential protein cofactors of NrrF-mediated regulation is underway.
A recent study reported that NrrF-mediated regulation of sdhA and sdhC required Hfq (Metruccio et al., 2009). This study, as well as our laboratory's work, also examined the potential binding of Hfq to sdhC and NrrF riboprobes. Hfq has been shown to bind to both sdhC and NrrF in vitro and aid in the formation of an sdhC : NrrF duplex (Metruccio et al., 2009; Supplementary Fig. S2). However, this should not be taken as evidence that Hfq is the required cofactor of NrrF in vivo, as Hfq has been shown to bind to many species of RNA and DNA (Takada et al., 1997; Windbichler et al., 2008; Zhang et al., 1998), and thus it is difficult to find nucleic acids to act as non-specific controls in Hfq binding experiments. The study by Metruccio et al. (2009) also utilized an N. meningitidis hfq fur double mutant. Since Fur and Hfq are central regulatory proteins which cause significant growth defects when deleted individually, it is possible that the double knockout could have widespread and unknown effects on gene regulation and transcription. Finally, in contrast with Metruccio et al. (2009), our studies directly examined the role of Hfq in iron-dependent expression of the sdhA and sdhC genes. Furthermore, two recent proteomic studies did not identify sdhC as an Hfq-regulated gene (Fantappie et al., 2009; Pannekoek et al., 2009). Collectively, these results indicate that NrrF-mediated iron regulation of sdhA and sdhC can occur independently of Hfq.
While NrrF-mediated iron regulation was not dependent on Hfq, we demonstrated that Hfq has a broad role in the cellular physiology of N. meningitidis. First, the hfq mutant had significant effects on bacterial growth. Second, when gene expression profiles of wild-type bacteria were compared with those of the hfq mutant, numerous genes showed aberrant regulation irrespective of iron conditions, and these defects were largely corrected in the complemented hfq mutant. Over 40 genes were dysregulated in the hfq mutant compared with the wild-type and the complemented hfq mutant strains. Of these, 27 genes were upregulated. This was expected as it is known that Hfq mediates the binding of sRNAs to their mRNA targets, which in most cases leads to degradation of the mRNA. Therefore, the loss of Hfq would disrupt these sRNA circuits and stabilize their mRNA targets. Several genes encoding proteins of central metabolism pathways were upregulated in the absence of Hfq, as were genes encoding hypothetical uncharacterized proteins. Dysregulation of these pathways would be expected to have deleterious effects on growth. Microarray analysis also identified 18 genes that were downregulated in the hfq mutant, 53 % of which were classified as playing a role in transport. Thus, in addition to properly regulating central metabolism pathways, Hfq may also be involved in the regulation of genes required for efficient transport of proteins and molecules in and out of the cell.
In examining the gene expression profile of the hfq mutant, two general observations can be made. The first is that since the main function of Hfq is to act as a cofactor of sRNAs which downregulate their targets, Hfq/sRNA circuits directly affecting gene expression are most likely to be found when examining genes that are upregulated in an hfq mutant. Any of these transcripts has the potential of being directly controlled by an Hfq-dependent sRNA. Indirect regulation is also possible, as Hfq may negatively regulate an activator of these upregulated genes. The second observation is that genes which were downregulated in the hfq mutant are likely to be indirectly affected by Hfq and could result from Hfq negatively regulating a repressor protein. Since many of the downregulated genes identified are of only one type (transport) there may be a surprisingly small number of repressors under Hfq control. Several of the genes identified in our microarray analysis were also identified in two recent proteomic studies (Fantappie et al., 2009; Pannekoek et al., 2009) although our study identified additional genes under Hfq control including the secD, cysT, sbp, uraA, putP, clpB and pivNM-1A genes.
One important consideration regarding the widespread defects in the hfq mutant is that these were the result of disruption of other as-yet-unidentified Hfq-dependent sRNA regulatory circuits. The challenge lies in searching for these sRNAs and determining their mRNA targets. The tiling microarray used in our study examined not only N. meningitidis ORFs but also IG regions where most sRNA coding regions are found. To this end, our study proved useful in identifying not only iron-regulated intergenic transcripts but also numerous previously unknown transcripts which likely represent a plethora of novel sRNAs in N. meningitidis (Table 3). Work is currently underway in our laboratory to characterize these intergenic transcripts and determine if they are true non-coding sRNAs which have regulatory roles in N. meningitidis.
Acknowledgments
This study was supported by a grant to C. G. from the NIH-NIAID (AI048611). We acknowledge Virginia Clark and Kenneth Barth for critical review of the manuscript.
Abbreviations
CDM, chemically defined media
IG, intergenic
qRT-PCR, quantitative RT-PCR
sRNA, small RNA
Footnotes
The GEO accession no. for the microarray experiments carried out in this paper is GSE20294.
Two supplementary figures and two supplementary tables are available with the online version of this paper.
References
- Bohn, C., Rigoulay, C. & Bouloc, P. (2007). No detectable effect of RNA-binding protein Hfq absence in Staphylococcus aureus. BMC Microbiol 7, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad, B. M., Irizarry, R. A., Astrand, M. & Speed, T. P. (2003). A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19, 185–193. [DOI] [PubMed] [Google Scholar]
- Christiansen, J. K., Nielsen, J. S., Ebersbach, T., Valentin-Hansen, P., Sogaard-Andersen, L. & Kallipolitis, B. H. (2006). Identification of small Hfq-binding RNAs in Listeria monocytogenes. RNA 12, 1383–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, B. M., Quinones, M., Pratt, J., Ding, Y. & Waldor, M. K. (2005). Characterization of the small untranslated RNA RyhB and its regulon in Vibrio cholerae. J Bacteriol 187, 4005–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delany, I., Ieva, R., Alaimo, C., Rappuoli, R. & Scarlato, V. (2003). The iron-responsive regulator Fur is transcriptionally autoregulated and not essential in Neisseria meningitidis. J Bacteriol 185, 6032–6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delany, I., Grifantini, R., Bartolini, E., Rappuoli, R. & Scarlato, V. (2006). Effect of Neisseria meningitidis fur mutations on global control of gene transcription. J Bacteriol 188, 2483–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai, P. J., Angerer, A. & Genco, C. A. (1996). Analysis of Fur binding to operator sequences within the Neisseria gonorrhoeae fbpA promoter. J Bacteriol 178, 5020–5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escolar, L., Perez-Martin, J. & de Lorenzo, V. (1998a). Binding of the Fur (ferric uptake regulator) repressor of Escherichia coli to arrays of the GATAAT sequence. J Mol Biol 283, 537–547. [DOI] [PubMed] [Google Scholar]
- Escolar, L., Perez-Martin, J. & de Lorenzo, V. (1998b). Coordinated repression in vitro of the divergent fepA–fes promoters of Escherichia coli by the iron uptake regulation (Fur) protein. J Bacteriol 180, 2579–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantappie, L., Metruccio, M. M., Seib, K. L., Oriente, F., Cartocci, F., Ferlicca, M., Giuliani, M., Scarlato, V. & Delany, I. (2009). The RNA chaperone Hfq is involved in stress response and virulence in Neisseria meningitidis and is a pleiotropic regulator of protein expression. Infect Immun 77, 1842–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franze de Fernandez, M. T., Eoyang, L. & August, J. T. (1968). Factor fraction required for the synthesis of bacteriophage Qβ-RNA. Nature 219, 588–590. [DOI] [PubMed] [Google Scholar]
- Gaballa, A., Antelmann, H., Aguilar, C., Khakh, S. K., Song, K. B., Smaldone, G. T. & Helmann, J. D. (2008). The Bacillus subtilis iron-sparing response is mediated by a Fur-regulated small RNA and three small, basic proteins. Proc Natl Acad Sci U S A 105, 11927–11932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman, S. (2004). The small RNA regulators of Escherichia coli: roles and mechanisms. Annu Rev Microbiol 58, 303–328. [DOI] [PubMed] [Google Scholar]
- Grifantini, R., Sebastian, S., Frigimelica, E., Draghi, M., Bartolini, E., Muzzi, A., Rappuoli, R., Grandi, G. & Genco, C. A. (2003). Identification of iron-activated and -repressed Fur-dependent genes by transcriptome analysis of Neisseria meningitidis group B. Proc Natl Acad Sci U S A 100, 9542–9547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidrich, N., Chinali, A., Gerth, U. & Brantl, S. (2006). The small untranslated RNA SR1 from the Bacillus subtilis genome is involved in the regulation of arginine catabolism. Mol Microbiol 62, 520–536. [DOI] [PubMed] [Google Scholar]
- Heidrich, N., Moll, I. & Brantl, S. (2007). In vitro analysis of the interaction between the small RNA SR1 and its primary target ahrC mRNA. Nucleic Acids Res 35, 4331–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry, R. A., Hobbs, B., Collin, F., Beazer-Barclay, Y. D., Antonellis, K. J., Scherf, U. & Speed, T. P. (2003). Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264. [DOI] [PubMed] [Google Scholar]
- Majdalani, N., Vanderpool, C. K. & Gottesman, S. (2005). Bacterial small RNA regulators. Crit Rev Biochem Mol Biol 40, 93–113. [DOI] [PubMed] [Google Scholar]
- Masse, E. & Gottesman, S. (2002). A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc Natl Acad Sci U S A 99, 4620–4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse, E., Escorcia, F. E. & Gottesman, S. (2003). Coupled degradation of a small regulatory RNA and its mRNA targets in Escherichia coli. Genes Dev 17, 2374–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse, E., Vanderpool, C. K. & Gottesman, S. (2005). Effect of RyhB small RNA on global iron use in Escherichia coli. J Bacteriol 187, 6962–6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellin, J. R., Goswami, S., Grogan, S., Tjaden, B. & Genco, C. A. (2007). A novel Fur- and iron-regulated small RNA, NrrF, is required for indirect Fur-mediated regulation of the sdhA and sdhC genes in Neisseria meningitidis. J Bacteriol 189, 3686–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metruccio, M. M., Fantappie, L., Serruto, D., Muzzi, A., Roncarati, D., Donati, C., Scarlato, V. & Delany, I. (2009). The Hfq-dependent small noncoding RNA NrrF directly mediates Fur-dependent positive regulation of succinate dehydrogenase in Neisseria meningitidis. J Bacteriol 191, 1330–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, J. H. (1972). Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
- Moller, T., Franch, T., Hojrup, P., Keene, D. R., Bachinger, H. P., Brennan, R. G. & Valentin-Hansen, P. (2002). Hfq: a bacterial Sm-like protein that mediates RNA–RNA interaction. Mol Cell 9, 23–30. [DOI] [PubMed] [Google Scholar]
- Morse, S. A. & Bartenstein, L. (1980). Purine metabolism in Neisseria gonorrhoeae: the requirement for hypoxanthine. Can J Microbiol 26, 13–20. [DOI] [PubMed] [Google Scholar]
- Murphy, E. R. & Payne, S. M. (2007). RhyB, an iron-responsive small RNA molecule, regulates Shigella dysenteriae virulence. Infect Immun 75, 3470–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek, Y., Huis in 't Veld, R., Hopman, C. T., Langerak, A. A., Speijer, D. & van der Ende, A. (2009). Molecular characterization and identification of proteins regulated by Hfq in Neisseria meningitidis. FEMS Microbiol Lett 294, 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repoila, F., Majdalani, N. & Gottesman, S. (2003). Small non-coding RNAs, co-ordinators of adaptation processes in Escherichia coli: the RpoS paradigm. Mol Microbiol 48, 855–861. [DOI] [PubMed] [Google Scholar]
- Silver, L. E. & Clark, V. L. (1995). Construction of a translational lacZ fusion system to study gene regulation in Neisseria gonorrhoeae. Gene 166, 101–104. [DOI] [PubMed] [Google Scholar]
- Stojiljkovic, I. & Hantke, K. (1995). Functional domains of the Escherichia coli ferric uptake regulator protein (Fur). Mol Gen Genet 247, 199–205. [DOI] [PubMed] [Google Scholar]
- Takada, A., Wachi, M., Kaidow, A., Takamura, M. & Nagai, K. (1997). DNA binding properties of the hfq gene of Escherichia coli. Biochem Biophys Res Commun 236, 576–579. [DOI] [PubMed] [Google Scholar]
- Tettelin, H., Saunder, J., Heidelberg, J., Jeffries, A. C., Nelson, K. E., Eisen, J. A., Ketchum, K. A., Hood, D. W., Peden, J. F. & other authors (2000). Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287, 1809–1815. [DOI] [PubMed] [Google Scholar]
- Urban, J. H. & Vogel, J. (2007). Translational control and target recognition by Escherichia coli small RNAs in vivo. Nucleic Acids Res 35, 1018–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Večerek, B., Moll, I., Afonyushkin, T., Kaberdin, V. & Bläsi, U. (2003). Interaction of the RNA chaperone Hfq with mRNAs: direct and indirect roles of Hfq in iron metabolism of Escherichia coli. Mol Microbiol 50, 897–909. [DOI] [PubMed] [Google Scholar]
- Windbichler, N., von Pelchrzim, F., Mayer, O., Csaszar, E. & Schroeder, R. (2008). Isolation of small RNA-binding proteins from E. coli. RNA Biol 5, 30–40. [DOI] [PubMed] [Google Scholar]
- Zhang, A., Altuvia, S., Tiwari, A., Argaman, L., Hengge-Aronis, R. & Storz, G. (1998). The OxyS regulatory RNA represses rpoS translation and binds the Hfq (HF-I) protein. EMBO J 17, 6061–6068. [DOI] [PMC free article] [PubMed] [Google Scholar]