Abstract
The successful nitrogen-fixing symbiosis between the Gram-negative soil bacterium Sinorhizobium meliloti and its leguminous plant host alfalfa (Medicago sativa) requires the bacterial exopolysaccharide succinoglycan. Succinoglycan and flagellum production, along with the ability to metabolize more than 20 different carbon sources and control the expression of a large number of S. meliloti genes, is regulated by the ExoR–ExoS/ChvI signalling pathway. The ExoR protein interacts with and suppresses the sensing activities of ExoS, the membrane-bound sensor of the ExoS/ChvI two-component regulatory system. Here we show that exoR expression is clearly upregulated in the absence of any functional ExoR protein. This upregulation was suppressed by the presence of the wild-type ExoR protein but not by a mutated ExoR protein lacking signal peptide. The levels of exoR expression could be directly modified in real time by changing the levels of total ExoR protein. The expression of exoR was also upregulated by the constitutively active sensor mutation exoS96, and blocked by two single mutations, exoS* and exoSsupA, in the ExoS sensing domain. Presence of the wild-type ExoS protein further elevated the levels of exoR expression in the absence of functional ExoR protein, and reversed the effects of exoS96, exoS* and exoSsupA mutations. Altogether, these data suggest that ExoR protein autoregulates exoR expression through the ExoS/ChvI system, allowing S. meliloti cells to maintain the levels of exoR expression based on the amount of total ExoR protein.
INTRODUCTION
Bacterial sensing plays an essential role in the establishment of nitrogen-fixing symbiosis between the Gram-negative soil bacterium Sinorhizobium meliloti and its leguminous plant partner alfalfa (Medicago sativa) (Gage, 2004; Jones et al., 2007). The sensing of plant signalling molecules – flavonoids – leads to the production of nodulation factor by S. meliloti, which induces the formation of curled root hairs that are colonized by S. meliloti cells (Brewin, 1991; Gibson et al., 2008; Long, 1989, 2001; van Rhijn & Vanderleyden, 1995). The success of the next step of the symbiosis, the formation of infection threads in the colonized curled alfalfa root hairs, requires the presence of the S. meliloti exopolysaccharide succinoglycan, which is regulated by the S. meliloti ExoR–ExoS/ChvI signal-transduction pathway (Cheng & Walker, 1998a, b; Doherty et al., 1988; Krol & Becker, 2009; Leigh & Walker, 1994; Pellock et al., 2000). These infection threads serve as the entryway for S. meliloti cells to colonize and establish nitrogen-fixing symbiosis inside pink alfalfa root nodules (Gibson et al., 2008; Jones et al., 2007).
The S. meliloti exoR gene, encoding a 268-amino-acid ExoR protein, was initially discovered with the isolation of the exoR95 : : Tn5 mutant (Doherty et al., 1988; Reed et al., 1991). This loss-of-function mutant overproduces succinoglycan and terminates the production of flagella by modulating the expression of biosynthesis genes (Wells et al., 2007; Yao et al., 2004). The exoR95 mutant also shows a 70 % reduction in its ability to nodulate alfalfa (Yao et al., 2004). A suppressive mutation, exoS*, of the exoR95 mutation was isolated from a pink nodule on alfalfa plants inoculated with the exoR95 mutant and was mapped genetically to the S. meliloti genomic region containing the exoS gene (Ozga et al., 1994). The presence of the exoS* mutation suppressed the succinoglycan-overproduction phenotype of the exoR95 mutant (Ozga et al., 1994),
The S. meliloti exoS gene, which encodes the ExoS sensor with the periplasmic sensing domain and cytoplasmic kinase domain of the ExoS/ChvI two-component regulatory system, was discovered with the isolation of the exoS96 : : Tn5 mutant (Cheng & Walker, 1998a; Doherty et al., 1988; Osteras et al., 1995). The mutant ExoS96 protein resulting from the Tn5 insertion appeared to have lost a large part of its first transmembrane domain and to have become a constitutively active sensor, leading to continuous activation or suppression of ExoS/ChvI-regulated genes (Cheng & Walker, 1998a). These changes were also reflected in succinoglycan overproduction and loss of flagella in the exoS96 mutant (Yao et al., 2004). Interestingly, the exoS96 mutation showed little effect on symbiosis (Yao et al., 2004). Efforts to delete the exoS genes from the S. meliloti genome were unsuccessful until recently, with the use of a merodiploid-facilitated strategy: the complete loss of ExoS affected the growth of S. meliloti cells on 21 different carbon sources (Belanger et al., 2009). This is consistent with other findings showing that the ExoS/ChvI system regulates the expression of hundreds of S. meliloti genes (Chen et al., 2009; Wang et al., 2010; Wells et al., 2007).
Recent biochemical and genetic analyses of both ExoR and the ExoS/ChvI system have placed them into one signal-transduction pathway (Chen et al., 2008; Wells et al., 2007). ExoR is exported into the periplasm, losing its signal peptide in the process, so that the ExoR protein can be found in two different forms, ExoRp, the full-length precursor form, and ExoRm, the mature form without its signal peptide (Chen et al., 2008). The ExoRm protein may interact directly with the ExoS protein to form an ExoRm–ExoS protein complex, which keeps ExoS in the off state (Chen et al., 2008). Thus, the current hypothesis is that the amount of ExoRm protein in the periplasm modulates the status of ExoS, enabling ExoR to indirectly modulate the expression of hundreds of S. meliloti genes regulated by the ExoS/ChvI two-component system. This hypothesis led us to focus on mechanisms regulating exoR gene expression.
In this work, we characterized exoR expression in different exoR and exoS genetic backgrounds using an exoR promoter–gfp fusion. We were able to uncover the regulatory mechanism of exoR expression and its effect on S. meliloti cells' ability to regulate the expression of a large number of genes regulated by the ExoS/ChvI two-component regulatory system, including succinoglycan and flagellum-biosynthesis genes.
METHODS
Bacterial strains and growth media.
The bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli strains were grown in Luria–Bertani (LB) medium at 37 °C (Sambrook et al., 1989). S. meliloti was grown in LB medium supplemented with 2.5 mM MgSO4 and 2.5 mM CaCl2 (LB/MC) at 30 °C (Leigh et al., 1985). When required, IPTG was added to induce gene expression at a final concentration of 0.8 mM. To examine succinoglycan production on solid medium, calcofluor white M2R (CF, Fluorescent Brightener 28, Sigma) was added to a final concentration of 0.02 % (w/v) in LB/MC agar, which was buffered to pH 7.4 with 10 mM HEPES (Leigh et al., 1985). The following antibiotics were used at the concentrations indicated: chloramphenicol (Cm), 10 μg ml−1; neomycin (Nm), 200 μg ml−1; spectinomycin (Sp), 100 μg ml−1; streptomycin (Sm), 500 μg ml−1, and tetracycline (Tc), 10 μg ml−1.
Table 1.
Strains and plasmids
| Strain or plasmid | Relevant properties | References |
|---|---|---|
| E. coli | ||
| DH5α | General-purpose strain | Hanahan (1983) |
| MT616 | MT607, pRK600, CmR | Finan et al. (1986) |
| S. meliloti | ||
| Rm1021 | SU47, SmR | |
| Rm7095 | Rm1021 exoR95 : : Tn5, NmR | Doherty et al. (1988) |
| Rm7096 | Rm1021 exoS96 : : Tn5, NmR | |
| SmHC20 | exoR95exoS*, NmR | This work |
| SmHC21 | exoR95exoSsupA, NmR | This work |
| Plasmids | ||
| pMB393 | Cloning vector, SpR | Gage et al. (1996) |
| pHC77 | pMB393 carrying the exoX–exoY intergenic region and exoY : : gfp fusion | Cheng & Yao (2004) |
| pHC505 | pMB393 with the fusion of the exoR promoter (−20 to −1 region) and the gfp gene | This work |
| pHC501 | pMB393 with the fusion of the exoR promoter (−662 to −1 region) and the gfp gene | This work |
| pHC514 | pPexoRgfp, pMB393 with the fusion of the exoR promoter (−325 to −1 region) and the gfp gene | This work |
| pHC548 | pMB393 with the fusion of the exoR promoter (−662 to −353 and −20 to −1 region) and the gfp gene | This work |
| pSW213 | Cloning vector, IncP-derived, lacIQ, PlaclacZ, TcR | Mantis & Winans (1993) |
| pHC530 | pPlacexoR, pSW213 with the exoR gene expressed from the inducible lac promoter | This work |
| pHC556 | pPlacexoRΔsp, pSW213 with a mutated exoR lacking its signal peptide sequence expressed from the inducible lac promoter | This work |
| pHC560 | pPlacexoS, pSW213 with the exoS gene expressed from the inducible lac promoter | This work |
| pRK600 | Helper plasmid, CmR | Finan et al. (1986) |
| pJK19-1 | GFP(S65T), Apr | Gift from P. Silver |
Construction of ExoR-expressing plasmids.
An XhoI–KpnI DNA fragment containing the complete exoR ORF was obtained by PCR using S. meliloti Rm1021 genomic DNA as the template and two PCR primers: exoRf-20x and exoRr807k (see Supplementary Table S1, available with the online version of this paper). The PCR product was digested with XhoI and KpnI, and ligated with similarly digested vector pSW213 to generate plasmid pHC530 (labelled pPlacexoR), which expresses the wild-type exoR gene from an IPTG-inducible lac promoter. Similarly, an XhoI/KpnI DNA fragment containing part of the exoR gene without the signal-peptide-coding region was obtained by PCR using S. meliloti Rm1021 genomic DNA as the template and two PCR primers: exoRf91x and exoRr807k (Supplementary Table S1). This mutated exoR, exoRΔsp, was cloned into vector pSW213, giving plasmid pHC556 (labelled pPlacexoRΔsp) expressing exoRΔsp under the control of the same IPTG-inducible lac promoter. The ExoRΔsp should have one extra N-terminal methionine compared with ExoRm.
Construction of an ExoS-expressing plasmid.
A BamHI–KpnI DNA fragment containing the complete exoS ORF (1788 bp) was obtained by PCR using S. meliloti Rm1021 genomic DNA as the template and two PCR primers: exoSf1-atgb and exoSr1818k (Supplementary Table S1). The forward primer exoSf1-atgb introduced ATG as the exoS start codon, replacing the original TTG (predicted) in the genome. The PCR product was digested with BamHI and KpnI, and ligated with similarly treated vector pSW213 to generate plasmid pHC560 (labelled pPlacexoS), expressing the exoS gene from the IPTG-inducible lac promoter on the vector.
Determination of the exoS and chvI gene sequences.
The ORFs of exoS and chvI were amplified by PCR using primers listed in Supplementary Table S1 and sequenced at Albert Einstein College of Medicine using primers listed in Supplementary Table S2.
Construction of exoR promoter–gfp fusions.
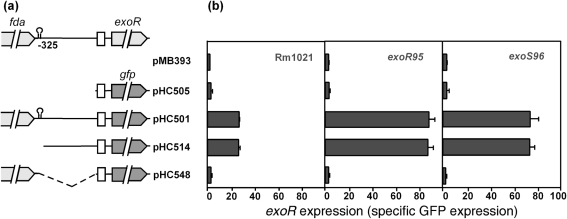
DNA fragments covering different exoR promoter regions were amplified from genomic DNA of S. meliloti Rm1021. The DNA fragment containing the gfp gene was amplified from plasmid pJK19-1 (a gift from P. Silver). Primers used are listed in Supplementary Table S1. DNA fragments containing exoR promoter–gfp fusions (PexoR–gfp) were constructed either by joining the exoR promoter fragment with the gfp gene fragment at a common NheI site or by recombinant PCR. Each of the PexoR–gfp fusions was digested with HindIII and XhoI, and ligated with similarly digested vector pMB393. This set of PexoR–gfp fusions, covering the exoR gene upstream regions of −20 to −1, −662 to −1, −325 to −1, and −20 to −1 fused with −662 to −353, was expressed from plasmids pHC505, pHC501, pHC514 and pHC548, respectively (Table 1, Fig. 1a). All plasmids were moved into S. meliloti strains through conjugation using MT616 as the helper.
Fig. 1.
(a) Schematic representation of the exoR gene region in S. meliloti. Solid bars indicate the exoR promoter regions in the constructs and the dashed line represents the region that was not included. The box indicates the RBS and the hairpin indicates the transcription terminator. (b) exoR promoter activities of different constructs in the wild-type Rm1021 and in exoR95 and exoS96 mutants. Specific GFP expression was determined by normalizing GFP fluorescence intensity to cell density (OD600) and used to represent exoR promoter activity. Data are means±ranges from two independent experiments.
Measurement of the exo gene promoter activities.
GFP fluorescence intensity was used to represent the exoR promoter activities in S. meliloti cells expressing the PexoR–gfp fusions or the exoY promoter activities in cells expressing the PexoY–gfp fusions as previously described (Cheng & Yao, 2004). Briefly, bacterial cultures were collected, washed, and resuspended in 0.85 % sterile NaCl solution to OD600 of about 0.1. Equal volumes of the diluted cultures (100 μl) were transferred to wells of a black 96-well microplate and a transparent 96-well microplate. The cultures in the black microplate were used to determine the intensities of GFP fluorescence using a fluorescence microplate reader (SpectraMax Gemini XS, Molecular Devices). The cultures in the transparent microplate were used to determine cell densities (OD600) using an absorbance microplate reader (SpectraMax 340PC, Molecular Devices). The intensity of GFP fluorescence of each culture was normalized to its corresponding cell density and used to represent the exoR or the exoY promoter activities.
Alfalfa nodulation assays.
Alfalfa nodulation was carried out on plates as previously described (Leigh et al., 1985). Briefly, alfalfa seeds were surface-sterilized in 50 % (v/v) freshly diluted bleach for 10 min, washed in sterile distilled water four times, spread on 0.8 % (w/v) agar, wrapped in aluminium foil, and placed in a plant growth chamber (26 °C) for 40 h for germination. A set of seven seedlings (each about 2.5 cm long) was placed on Jensen's nitrogen-free agar medium in square Petri dishes. S. meliloti cells were collected from overnight cultures in LB/MC medium, washed, and diluted with sterile distilled water to OD600 0.03. Cell suspension (1 ml) was spread evenly over the seedlings in each square Petri dish. The Petri dishes were left standing for a few hours to absorb the liquid and then wrapped with aluminium foil on three sides to cover the roots. The Petri dishes with alfalfa plants were placed in the plant growth chamber for 4 weeks. The number of nodules and nodule colour were examined to determine overall symbiosis efficiency.
Isolation of exoR suppressor mutation.
The suppressor mutation of the exoR95 mutation was isolated as described previously (Ozga et al., 1994). Briefly, nodules were removed from alfalfa inoculated with the exoR95 mutant, surface-sterilized by 2 min incubation in 50 % (v/v) Clorox bleach, washed six times in sterile distilled water, and crushed in 100 μl LB/MC with 5.4 % (w/v) glucose inside the wells of a 96-well microplate. The suspensions were diluted 1 : 100 in the same medium and plated on LB/MC/CF agar plates with appropriate antibiotics. Dim colonies, which indicated a reduction in succinoglycan production, were further characterized as having the suppressor mutation for the exoR95 mutation.
Cell motility.
Cell motility was examined using swimming plates as described previously (Yao et al., 2004). Briefly, fresh bacterial cultures were prepared, diluted to an OD600 of 0.1, and 2 μl aliquots were pipetted onto LB/MC soft agar (0.3 %) plates and incubated for 3 days.
RESULTS
Analysis of the exoR promoter region
To analyse exoR expression, a set of fusions of the gfp gene to different exoR promoter regions was constructed (Fig. 1a). Levels of exoR expression were first examined in the wild-type Rm1021 background using specific GFP fluorescence (Fig. 1b), which was generated by normalizing GFP fluorescence intensities to the optical densities of the cultures. A background level of specific GFP fluorescence of 1.99±0.17 was determined using Rm1021 with the expression vector pMB393 without gfp (Fig. 1b). Levels of exoR expression from the region containing the putative ribosome-binding site (RBS) in plasmid pHC505 and the region upstream of the putative terminator in plasmid pHC548 were 2.81±1.38 and 2.94±0.76, respectively. Levels of exoR expression from the −1 to −662 (pHC501) and −1 to −325 (pHC514) regions upstream of the exoR gene were 26.95±0.41 and 26.35±1.33, respectively. The latter plasmid, named pPexoRgfp, was used to measure exoR expression throughout the study. Taken together, these data suggest that the exoR promoter is located within the −17 to −325 region upstream of the exoR gene, hereafter referred to as the exoR promoter region.
exoR expression is upregulated in the exoR95 and exoS96 mutant backgrounds
Levels of exoR expression from different fusions were also determined in exoR95 and exoS96 mutants (Fig. 1b). The exoR95 and exoS96 mutants carrying the putative RBS (pHC505) or the region upstream of the putative terminator (pHC548) showed no exoR expression. Interestingly, both exoR95 and exoS96 mutants carrying the exoR promoter region in plasmid pPexoRgfp showed significantly higher levels of exoR expression: 87.56±4.40 and 74.07±3.88, respectively. Both exoR95 and exoS96 mutants carrying the exoR promoter region and the region upstream of the putative terminator in pHC501 showed similarly upregulated levels of exoR expression: 88.32±4.92 and 74.38±7.32, respectively. Compared with Rm1021 carrying the same plasmids, these data clearly suggest that exoR expression is upregulated about threefold in the exoR95 and exoS96 mutants, raising the possibility that the ExoS/ChvI two-component regulatory system is involved in regulating exoR expression.
The upregulation of exoR expression was compared with that of the exoY gene, which is the best-known regulatory target of the ExoS/ChvI system. exoY expression was measured using a fusion of the gfp gene to the exoY promoter and part of the exoY gene on plasmid pHC77 (pPexoYgfp). The levels of exoY expression in the exoR95 mutant was increased sixfold compared with the levels in the wild-type Rm1021 strain while the expression of exoR was increased threefold in the side-by-side comparison (Fig. 2). These results suggest that exoR expression is upregulated in the absence of functional ExoR protein but the level of upregulation is less than that of exoY expression.
Fig. 2.
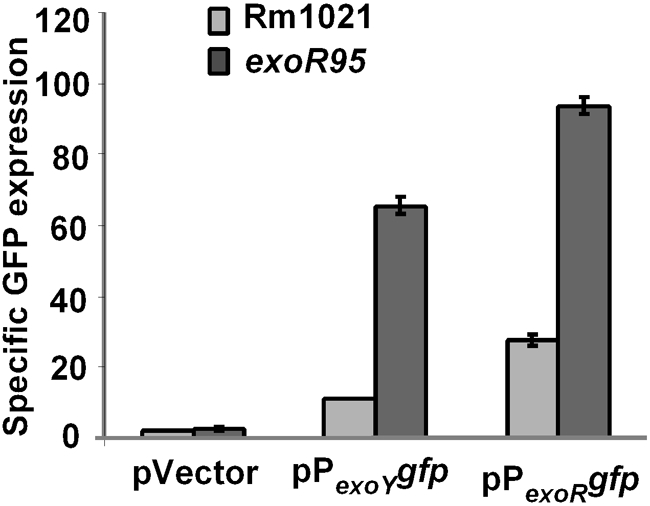
Comparison of exoY and exoR promoter activities in wild-type Rm1021 and the exoR95 mutant using fusion of the gfp gene to the exoY promoter on plasmid pHC77/ pPexoY gfp and to the exoR promoter on pHC514/pPexoR gfp. The results are means±ranges of two independent experiments.
Characterization of two exoS mutations
To examine the possible involvement of the ExoS protein in regulating exoR expression, two exoS mutations, exoS* and exoSsupA, were further characterized. The exoS* mutation has been isolated and mapped genetically (Ozga et al., 1994). The exoSsupA mutation was isolated recently in our lab using the same approach as that used to isolate the exoS* mutation.
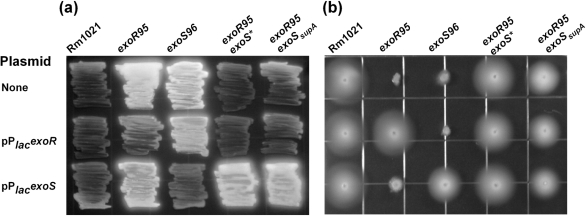
The presence of either the exoS* or the exoSsupA mutation suppressed the phenotypes of the exoR95 mutant, so that both exoR95exoS* and exoR95exoSsupA double mutants showed wild-type levels of succinoglycan, motility (Fig. 3) and symbiosis with alfalfa (Table 2).
Fig. 3.
Abilities of S. meliloti cells to produce succinoglycan (a) and to swim (b) were examined on plates containing LB/MC/CF/IPTG medium and LB/MC/IPTG with 0.3 % agar, respectively. Intensity of CF fluorescence represents level of succinoglycan production (a). The exoR gene was expressed from the lac promoter on plasmid pPlacexoR/pHC530. The exoS gene was expressed from the lac promoter on plasmid pPlacexoS /pHC560.
Table 2.
Nodulation efficiency of different S. meliloti strains
Percentage of pink nodules and average number of nodules per plant were determined using seven 4-week-old alfalfa plants for each of the six bacterial strains. The results are means±sd of three independent repeats.
| Strain | Pink nodules (%) | Nodules per plant |
|---|---|---|
| Rm1021 | 94.15±5.58 | 4.79±0.38 |
| exoY210 | 0.00±0.00 | 5.43±0.65 |
| exoR95 | 15.74±4.03 | 7.00±0.65 |
| exoS96 | 79.33±6.51 | 5.93±1.17 |
| exoR95exoS* | 95.96±7.00 | 5.43±1.08 |
| exoR95exoSsupA | 95.63±5.12 | 4.43±0.76 |
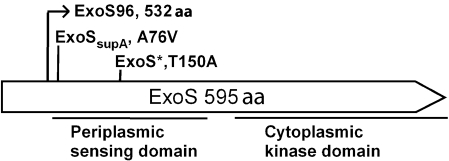
Our sequencing results showed that the exoS* mutation is a single A-to-G base substitution resulting in a threonine-to-alanine (T150A) change at position 150 of ExoS (Fig. 4). The exoSsupA mutation is a single C-to-T base substitution resulting in an alanine-to-valine (A76V) change at position 76 of ExoS (Fig. 4). Both exoS* and exoSsupA mutations are located in the sensing domain of ExoS, which suggests that these mutations alter the sensing function or status of the ExoS protein to a constant low level, resulting in suppression of succinoglycan overproduction and of other phenotypes of the exoR95 mutant.
Fig. 4.
Schematic diagram of ExoS protein showing the periplasmic sensing and cytoplasmic kinase domains. The starting position of the ExoS96 mutant protein (532 aa), and the positions of two spontaneous mutations, exoS* and exoSsupA, are indicated.
Upregulation of exoR expression is blocked by exoS* and exoSsupA mutations
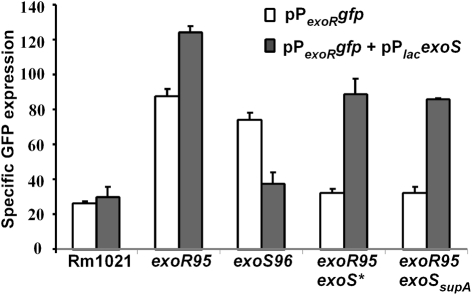
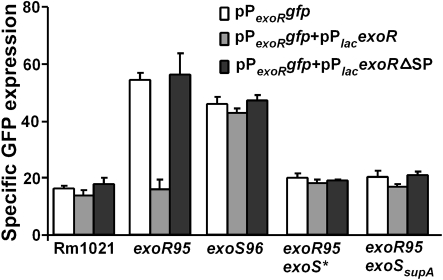
The effects of the exoS* and exoSsupA mutations on the upregulation of exoR expression observed in mutants exoR95 and exoS96 were further studied to determine whether the ExoS/ChvI two-component regulatory system is involved in regulating exoR expression. Levels of exoR expression in different strains were determined using the exoR promoter–gfp fusion on plasmid pPexoRgfp. Levels of exoR expression in exoR95exoS*(pPexoRgfp) and exoR95exoSsupA (pPexoRgfp) mutants were 32.26±2.59 and 32.57±3.48, respectively, similar to that of Rm1021(pPexoRgfp) (Fig. 5). These data suggested that both exoS* and exoSsupA mutations block the exoR95-induced upregulation of exoR expression.
Fig. 5.
exoR expression in different genetic backgrounds with or without the presence of the wild-type exoS gene. The exoR promoter was fused to the gfp gene and expressed from plasmid pPexoRgfp (pHC514). The wild-type exoS gene was expressed using the E. coli lac promoter from plasmid pPlacexoS (pHC560). Data represent means±ranges from two independent experiments.
To further confirm this finding, both exoS* and exoSsupA mutations were complemented with wild-type exoS carried on a compatible plasmid, pPlacexoS. The presence of extra copies of exoS showed no apparent effect on exoR expression in wild-type Rm1021, further elevated exoR expression in the exoR95 mutant background, and decreased exoR expression in the exoS96 mutant to wild-type levels (Figs 3 and 5), which is consistent with the exoS96 mutation being recessive (Cheng & Walker, 1998a). Most importantly, ExoS brought levels of exoR expression in exoR95exoS*(pPexoRgfp, pPlacexoS) and exoR95exoSsupA(pPexoRgfp, pPlacexoS) close to the level of exoR expression in the exoR95(pPexoRgfp) single mutant (87.56±4.40). Taken together, these data show that levels of exoR expression are clearly affected by the functional status of the ExoS protein, suggesting that exoR expression is most likely regulated through the ExoS/ChvI two-component regulatory system.
exoR expression is only autoregulated by full-length ExoR protein
The loss of functional ExoR protein in the exoR95 mutant led to upregulation of exoR expression. This finding and previous reports of ExoR–ExoS interactions (Chen et al., 2008) raised the possibility that ExoR is involved in regulating its own expression through an interaction with the ExoS sensor in the periplasm. To test this, a mutated form of ExoR without its conserved signal peptide, ExoRΔSP, was constructed and expressed from plasmid pPlacexoRΔsp, which is compatible with plasmid pPexoRgfp. We have previously found that the ExoRΔSP protein remains in the cytoplasm (unpublished) so it should not be able to interact with the periplasmic ExoS sensing domain. When both ExoR and ExoRΔSP were expressed in Rm1021, and in exoR95, exoS96, exoR95exoS* and exoR95exoSsupA mutants, the only significant difference in exoR expression was found in the exoR95 mutant (Fig. 6). These data suggest that the wild-type ExoR, but not ExoRΔSP, is able to suppress exoR gene expression. Taken together, these results imply that ExoR regulates its own expression through the ExoS/ChvI two-component regulatory system.
Fig. 6.
exoR expression in different genetic backgrounds with or without the wild-type ExoR and mutated ExoR without its native signal peptide (ΔSP). The exoR promoter–gfp fusion, PexoRgfp, was expressed from plasmid pPexoRgfp (pHC514) to monitor levels of exoR expression. The wild-type ExoR protein was expressed from the lac promoter on plasmid pPlacexoR (pHC530) and the mutated ExoRΔSP was expressed from the lac promoter on plasmid pPlacexoRΔsp (pHC556). Data represent means±ranges from two independent experiments.
Kinetic analysis of ExoR autoregulation
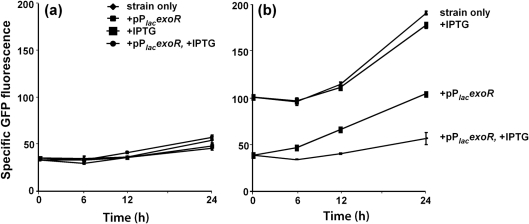
To examine ExoR autoregulation in real time, the exoR gene expressed from the E. coli lac promoter on plasmid pPlacexoR was used to examine the link between the amount of intracellular ExoR protein and levels of exoR expression. The exoR promoter–gfp fusion on plasmid pPexoRgfp was used to monitor levels of exoR expression. Overnight cultures of Rm1021(pPexoRgfp), Rm1021(pPexoRgfp, pPlacexoR), exoR95(pPexoRgfp) and exoR95(pPexoRgfp, pPlacexoR) were prepared in the presence of IPTG to ensure high levels of intracellular ExoR. Cells from half of each culture were washed and resuspended in the same medium without IPTG. Levels of exoR expression were determined at 6, 12 and 24 h after removal of IPTG. exoR expression by Rm1021(pPexoRgfp) and Rm1021(pPexoRgfp, pPlacexoR) was not affected by the removal of IPTG (Fig. 7a). exoR expression remained at high levels in the exoR95(pPexoRgfp) mutant with or without IPTG (Fig. 7b); it remained at low levels in the exoR95(pPexoRgfp, pPlacexoR) mutant in the continuous presence of IPTG, and in the exoR95(pPexoRgfp, pPlacexoR) mutant it increased after the removal of IPTG (Fig. 7b). This latter increase in exoR expression was detectable 6 h after IPTG removal and expression reached a level below that of exoR95(pPexoRgfp) after 24 h (Fig. 7b). This could be because exoR95(pPexoRgfp, pPlacexoR) had a low level of ExoR protein expressed from the leaky lac promoter, which cannot be completely shut off, even in the presence of LacIQ expressed from the same pPlacexoR plasmid. These data showed that a reduction in the amount of ExoR protein results in increased exoR expression, providing real-time evidence of ExoR's negative regulation of its own expression.
Fig. 7.
Effects of total ExoR protein reduction on exoR expression in wild-type Rm1021(pPexoRgfp) (a) and in the exoR95(pPexoRgfp) mutant background (b) with or without IPTG induction. exoR expression was monitored using a fusion of the exoR promoter and gfp, expressed from plasmid pPexoRgfp (pHC514). The amount of total ExoR protein was regulated using a fusion of the lacZ promoter and the exoR gene, expressed from plasmid pPlacexoR (pHC530). The curves are labelled for the strain alone or with additional plasmid pPlacexoR or IPTG or both. Data represent means±ranges from two independent experiments.
DISCUSSION
The periplasmic S. meliloti ExoR protein and ExoS/ChvI two-component regulatory system regulates the production of succinoglycan and flagella (Yao et al., 2004). ExoR is a negative regulator of the ExoS sensor (Chen et al., 2008). This would suggest that the amount of ExoR protein can affect the expression of hundreds of ExoS/ChvI-system-regulated genes. Thus, we focused on the regulation of exoR expression, which could lead to the discovery of the key factor(s) or regulator(s) functioning upstream of ExoR in this essential ExoR–ExoS/ChvI regulatory cascade.
To monitor exoR expression and identify its potential regulators, the maximum region upstream of the exoR gene which contains exoR promoter activities was identified using a set of nested deletions in the region. Interestingly, activities of the exoR promoter showed about a threefold increase in both exoR95 and exoS96 mutants. This threefold increase of exoR expression is smaller than the sixfold increase of the exoY gene in the same loss of ExoR function exoR95 mutant. This raised the possibility that exoR expression may be regulated by the ExoS/ChvI system directly or indirectly and that ExoR protein might be involved in regulating its own expression.
To directly characterize the link between the amount of total ExoR protein and exoR expression level, total ExoR production was regulated using an inducible E. coli lac promoter on plasmid pPlacexoR, which also expresses the LacIQ protein. Suppression of the lac promoter by LacIQ is not complete, which means that a small amount of total ExoR protein will be expressed from the lac promoter in the absence of inducer. We found that upregulation of exoR expression in the exoR95 mutant could be suppressed by the presence of plasmid pPlacexoR, with or without IPTG, suggesting that the presence of even small amounts of total ExoR can suppress exoR expression. In addition, when the amount of total ExoR was artificially reduced by removing IPTG, the level of exoR expression increased and stabilized at a new level, which was between those in the exoR95 mutant and in the wild-type Rm1021. These findings support the notion that higher levels of total ExoR protein will result in lower levels of exoR expression, suggesting that ExoR negatively regulates its own expression.
Because ExoR is the negative regulator of the ExoS sensor of the ExoS/ChvI system, ExoS could be involved in mediating ExoR autoregulation. Our initial finding of upregulation of exoR expression by the exoS96 mutant supports this possibility. In addition, we found that a mutated ExoR lacking its signal peptide, which could not be exported to the periplasm, was unable to affect exoR expression. Although other possibilities exist, these findings raise the possibility that ExoR can only autoregulate in the periplasm, via the ExoS/ChvI system.
If the ExoS/ChvI system is indeed involved in regulating exoR expression, mutations in the exoS gene should block the effects of ExoR protein amount on exoR expression. We analysed two spontaneous exoS mutations, exoS* and exoSsupA, that suppress succinoglycan overproduction, as well as other phenotypes of the exoR95 mutant. Both mutations are located in the ExoS periplasmic sensing domain and they may alter the structure of ExoS such that it remains in the inactive state in the absence of functional ExoR suppressor. Our data clearly show that the presence of either one of these two mutations suppresses upregulation of exoR expression in the exoR95 mutant background. The suppressive effects of exoS* and exoSsupA mutations on exoR95 succinoglycan overproduction were reversed by the presence of the wild-type exoS gene, which strongly suggest that the ExoS/ChvI system mediates ExoR autoregulation of exoR expression. Interestingly, the suppressive effects of exoS* and exoSsupA mutations on exoR95 motility were not reversed by the presence of wild-type exoS (Fig. 3). One possible explanation is that the activation of exo expression and the suppression of fla expression may require different levels of ExoS/ChvI activation. The ExoS sensor probably functions in dimeric form, so the exoR95exoS*(pPlacexoS) mutant should have ExoS*/ExoS*, ExoS/ExoS* and ExoS/ExoS dimers. Both ExoS*/ExoS* and ExoS/ExoS* dimers might not be active due to the presence of ExoS*, so the exoR95exoS*(pPlacexoS) mutant would have a small amount of ExoS/ExoS dimers. This could activate the ExoS/ChvI system enough to upregulate exo expression but not enough to suppress fla expression. The exoSsupA mutation may function in the same way. Both exoR95exoS*(pPlacexoS) and exoR95exoSsupA(pPlacexoS) mutants will be analysed in the future to further confirm the presence and the biological significance of such differential regulation.
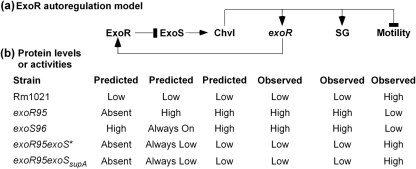
A model of ExoR autoregulation through the ExoS/ChvI system is proposed based on our new findings and previous understanding of ExoR and the ExoS/ChvI system (Fig. 8), suggesting the way in which total ExoR protein is regulated and how levels of total ExoR protein, especially mature ExoRm, in the periplasm can modulate all of the genes regulated by the ExoR–ExoS/ChvI pathway. In a wild-type Rm1021 cell, reduction in ExoRm will lead to activation of ExoS, direct or indirect upregulation of exoR expression, accumulation of ExoRp and ExoRm protein, and consequent suppression of ExoS. On the other hand, accumulation of ExoRm in the Rm1021 periplasm will lead to suppression of ExoS, suppression of exoR expression, reduction of ExoRm protein, and consequent activation of ExoS. This proposed system would enable S. meliloti cells to maintain a stable level of exoR expression based on the levels of total ExoR protein. Any disruptions in the pathway, such as loss of functional ExoR protein or constitutively active ExoS sensor, will disrupt ExoR regulation. As demonstrated here, the effects of such disruptions can be blocked by suppressor mutations in the exoS gene. Altogether, our new findings suggest that ExoR autoregulation may play a key role in regulating the activity levels of ExoS sensor.
Fig. 8.
(a) Schematic representation of the ExoR–ExoS/ChvI regulatory pathway and (b) levels of total ExoR protein, exoR expression, succinoglycan (SG) production and motility in different S. meliloti genetic backgrounds. The status of ExoS and ChvI proteins was predicted based on prior knowledge of the system.
ExoR autoregulation would also make it possible for S. meliloti cells to maintain the expression of a large number of ExoS/ChvI-regulated genes at relatively constant levels, and to return expression to those levels after any changes in ExoS activity. This would enable the bacterial cells to respond to the appearance of environmental stimuli by altering the expression of relevant genes, and quickly return their expression to ‘normal’ levels after disappearance of those stimuli. This might be the mechanism that allows S. meliloti cells to produce succinoglycan once they are trapped inside curled alfalfa root hairs and to terminate succinoglycan production upon entering the alfalfa root nodules. Loss of ExoR in the exoR95 mutant causes the mutant to remain in succinoglycan-overproducing mode and reduces its symbiotic efficiency, which further argues for the biological importance of ExoR autoregulation. We are currently researching ways to improve our ability to detect signals for the ExoS/ChvI system, which will greatly improve our understanding of the signalling between S. meliloti and alfalfa during nodulation.
Autoregulation, especially single-gene autoregulation, is quite common in S. meliloti, such as MucR autoregulation (Bahlawane et al., 2008), although fewer examples of autoregulation by periplasmic proteins are known. One such example is the E. coli periplasmic CpxP protein, which autoregulates through the CpxA/CpxR two-component regulatory system (Dong et al., 1993; Fleischer et al., 2007; Raivio & Silhavy, 1999; Wolfe et al., 2008). The CpxP protein normally forms a protein complex with CpxA, keeping the latter in the off state. Environmental stress, such as changes in pH, triggers the misfolding of periplasmic proteins. These misfolded proteins form complexes with CpxP, which are then degraded by DegP protease in the periplasm (Buelow & Raivio, 2005; Isaac et al., 2005). Thus released from the CpxP–CpxA complex, CpxA is activated and turns on the expression of all CpxA/CpxR-regulated genes, including cpxP, leading to CpxP suppression of CpxA (Buelow & Raivio, 2005; Isaac et al., 2005). Environmental stress conditions such as osmotic pressure and pH were able to modulate succinoglycan production (Hellweg et al., 2009). While the ExoS/ChvI pathway may be involved in sensing common stress conditions, it is also possible that the system is involved in sensing plant signals. Further study of the ExoR–ExoS/ChvI signalling pathway will provide new insights into bacterial signalling and sensing in microbe–plant interactions.
Acknowledgments
This work was supported by a grant (SGM081147) to H.-P. C. from US NIH. We thank John Leigh for providing the original exoS* mutant.
Abbreviations
CF, calcofluor white
References
- Bahlawane, C., McIntosh, M., Krol, E. & Becker, A. (2008). Sinorhizobium meliloti regulator MucR couples exopolysaccharide synthesis and motility. Mol Plant Microbe Interact 21, 1498–1509. [DOI] [PubMed] [Google Scholar]
- Belanger, L., Dimmick, K. A., Fleming, J. S. & Charles, T. C. (2009). Null mutations in Sinorhizobium meliloti exoS and chvI demonstrate the importance of this two-component regulatory system for symbiosis. Mol Microbiol 74, 1223–1237. [DOI] [PubMed] [Google Scholar]
- Brewin, N. J. (1991). Development of the legume root nodule. Annu Rev Cell Biol 7, 191–226. [DOI] [PubMed] [Google Scholar]
- Buelow, D. R. & Raivio, T. L. (2005). Cpx signal transduction is influenced by a conserved N-terminal domain in the novel inhibitor CpxP and the periplasmic protease DegP. J Bacteriol 187, 6622–6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, E. J., Sabio, E. A. & Long, S. R. (2008). The periplasmic regulator ExoR inhibits ExoS/ChvI two-component signaling in Sinorhizobium meliloti. Mol Microbiol 69, 1290–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, E. J., Fisher, R. F., Perovich, V. M., Sabio, E. A. & Long, S. R. (2009). Identification of direct transcriptional target genes of ExoS/ChvI two-component signaling in Sinorhizobium meliloti. J Bacteriol 191, 6833–6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, H. P. & Walker, G. C. (1998a). Succinoglycan production by Rhizobium meliloti is regulated through the ExoS–ChvI two-component regulatory system. J Bacteriol 180, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, H. P. & Walker, G. C. (1998b). Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J Bacteriol 180, 5183–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, H. P. & Yao, S. Y. (2004). The key Sinorhizobium meliloti succinoglycan biosynthesis gene exoY is expressed from two promoters. FEMS Microbiol Lett 231, 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty, D., Leigh, J. A., Glazebrook, J. & Walker, G. C. (1988). Rhizobium meliloti mutants that overproduce the R. meliloti acidic calcofluor-binding exopolysaccharide. J Bacteriol 170, 4249–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, J., Iuchi, S., Kwan, H. S., Lu, Z. & Lin, E. C. (1993). The deduced amino-acid sequence of the cloned cpxR gene suggests the protein is the cognate regulator for the membrane sensor, CpxA, in a two-component signal transduction system of Escherichia coli. Gene 136, 227–230. [DOI] [PubMed] [Google Scholar]
- Finan, T. M., Kunkel, B., De Vos, G. F. & Signer, E. R. (1986). Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J Bacteriol 167, 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer, R., Heermann, R., Jung, K. & Hunke, S. (2007). Purification, reconstitution, and characterization of the CpxRAP envelope stress system of Escherichia coli. J Biol Chem 282, 8583–8593. [DOI] [PubMed] [Google Scholar]
- Gage, D. J. (2004). Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol Mol Biol Rev 68, 280–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage, D. J., Bobo, T. & Long, S. R. (1996). Use of green fluorescent protein to visualize the early events of symbiosis between Rhizobium meliloti and alfalfa (Medicago sativa). J Bacteriol 178, 7159–7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, K. E., Kobayashi, H. & Walker, G. C. (2008). Molecular determinants of a symbiotic chronic infection. Annu Rev Genet 42, 413–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan, D. (1983). Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166, 557–580. [DOI] [PubMed] [Google Scholar]
- Hellweg, C., Puhler, A. & Weidner, S. (2009). The time course of the transcriptomic response of Sinorhizobium meliloti 1021 following a shift to acidic pH. BMC Microbiol 9, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac, D. D., Pinkner, J. S., Hultgren, S. J. & Silhavy, T. J. (2005). The extracytoplasmic adaptor protein CpxP is degraded with substrate by DegP. Proc Natl Acad Sci U S A 102, 17775–17779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, K. M., Kobayashi, H., Davies, B. W., Taga, M. E. & Walker, G. C. (2007). How rhizobial symbionts invade plants: the Sinorhizobium–Medicago model. Nat Rev Microbiol 5, 619–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol, E. & Becker, A. (2009). Surface polysaccharides as fitness factors of rhizospheric nitrogen-fixing bacteria. In Bacterial Polysaccharides: Current Innovations and Future Trends, pp. 189–211. Edited by M. Ullrich. Norwich: Caister Academic Press.
- Leigh, J. A. & Walker, G. C. (1994). Exopolysaccharides of Rhizobium: synthesis, regulation and symbiotic function. Trends Genet 10, 63–67. [DOI] [PubMed] [Google Scholar]
- Leigh, J. A., Signer, E. R. & Walker, G. C. (1985). Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A 82, 6231–6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, S. R. (1989). Rhizobium–legume nodulation: life together in the underground. Cell 56, 203–214. [DOI] [PubMed] [Google Scholar]
- Long, S. R. (2001). Genes and signals in the Rhizobium–legume symbiosis. Plant Physiol 125, 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantis, N. J. & Winans, S. C. (1993). The chromosomal response regulatory gene chvI of Agrobacterium tumefaciens complements an Escherichia coli phoB mutation and is required for virulence. J Bacteriol 175, 6626–6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteras, M., Stanley, J. & Finan, T. M. (1995). Identification of Rhizobium-specific intergenic mosaic elements within an essential two-component regulatory system of Rhizobium species. J Bacteriol 177, 5485–5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozga, D. A., Lara, J. C. & Leigh, J. A. (1994). The regulation of exopolysaccharide production is important at two levels of nodule development in Rhizobium meliloti. Mol Plant Microbe Interact 7, 758–765. [DOI] [PubMed] [Google Scholar]
- Pellock, B. J., Cheng, H. P. & Walker, G. C. (2000). Alfalfa root nodule invasion efficiency is dependent on Sinorhizobium meliloti polysaccharides. J Bacteriol 182, 4310–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivio, T. L. & Silhavy, T. J. (1999). The σE and Cpx regulatory pathways: overlapping but distinct envelope stress responses. Curr Opin Microbiol 2, 159–165. [DOI] [PubMed] [Google Scholar]
- Reed, J. W., Glazebrook, J. & Walker, G. C. (1991). The exoR gene of Rhizobium meliloti affects RNA levels of other exo genes but lacks homology to known transcriptional regulators. J Bacteriol 173, 3789–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E. & Maniatis, T. (1989). Molecular Cloning: a Laboratory Manual, 2nd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
- van Rhijn, P. & Vanderleyden, J. (1995). The Rhizobium–plant symbiosis. Microbiol Rev 59, 124–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C., Kemp, J., Da Fonseca, I. O., Equi, R. C., Sheng, X., Charles, T. C. & Sobral, B. W. (2010). Sinorhizobium meliloti 1021 loss-of-function deletion mutation in chvI and its phenotypic characteristics. Mol Plant Microbe Interact 23, 153–160. [DOI] [PubMed] [Google Scholar]
- Wells, D. H., Chen, E. J., Fisher, R. F. & Long, S. R. (2007). ExoR is genetically coupled to the ExoS–ChvI two-component system and located in the periplasm of Sinorhizobium meliloti. Mol Microbiol 64, 647–664. [DOI] [PubMed] [Google Scholar]
- Wolfe, A. J., Parikh, N., Lima, B. P. & Zemaitaitis, B. (2008). Signal integration by the two-component signal transduction response regulator CpxR. J Bacteriol 190, 2314–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, S. Y., Luo, L., Har, K. J., Becker, A., Ruberg, S., Yu, G. Q., Zhu, J. B. & Cheng, H. P. (2004). Sinorhizobium meliloti ExoR and ExoS proteins regulate both succinoglycan and flagellum production. J Bacteriol 186, 6042–6049. [DOI] [PMC free article] [PubMed] [Google Scholar]