Abstract
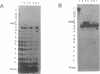
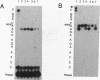
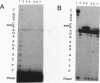
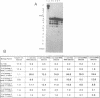
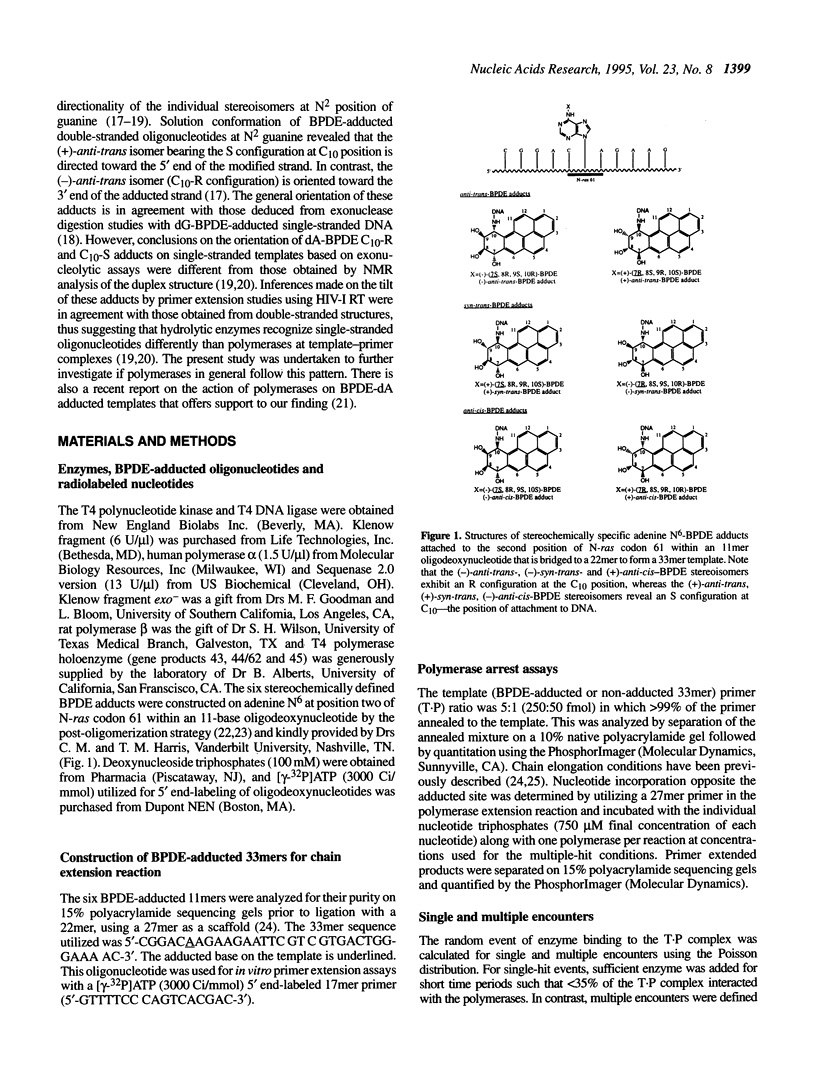
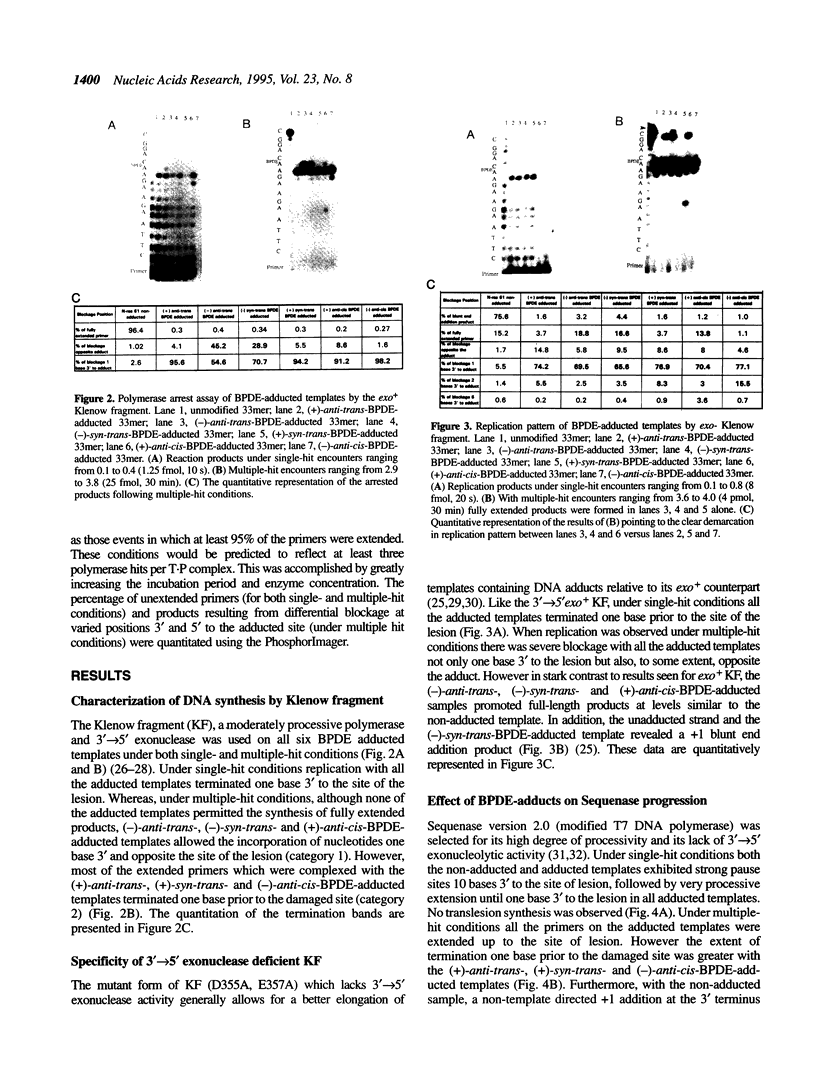
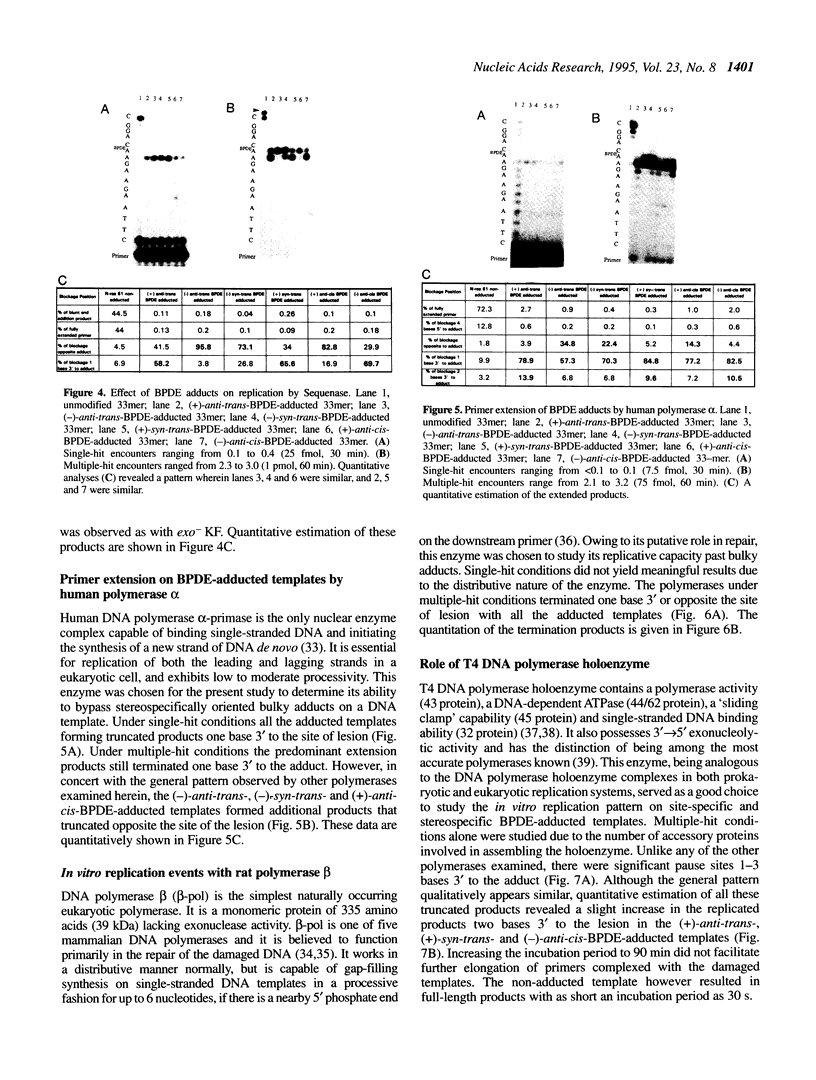
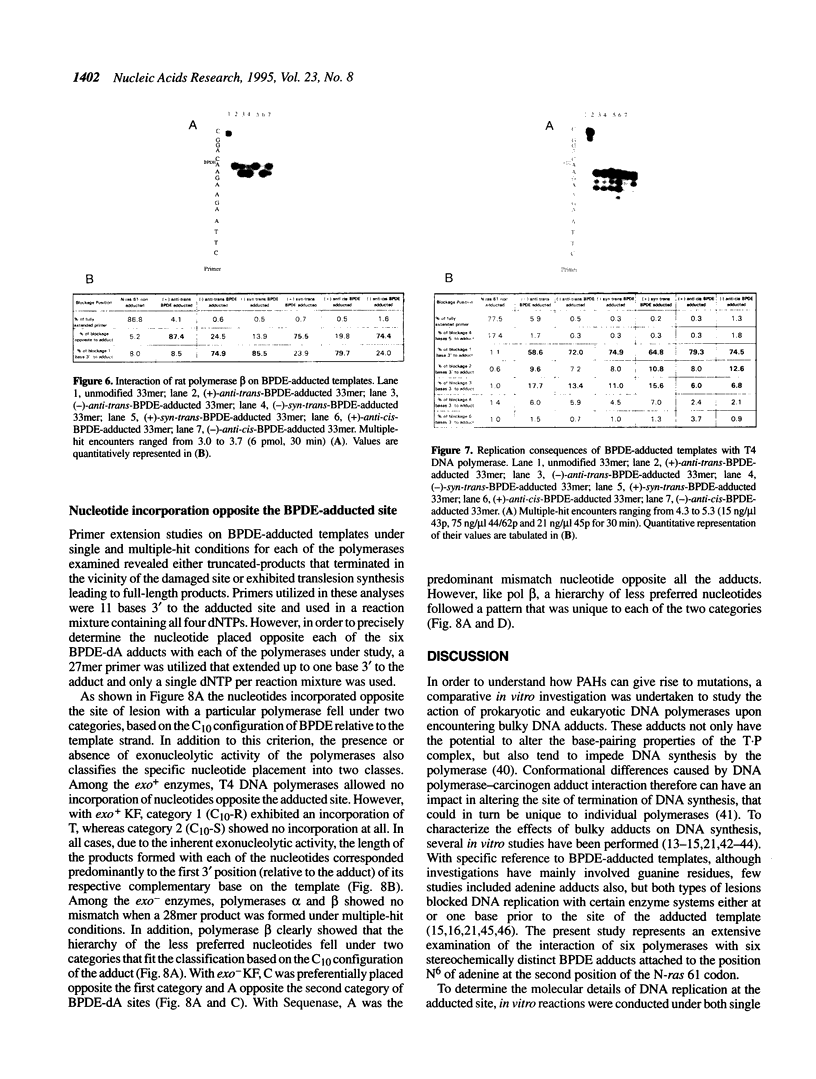
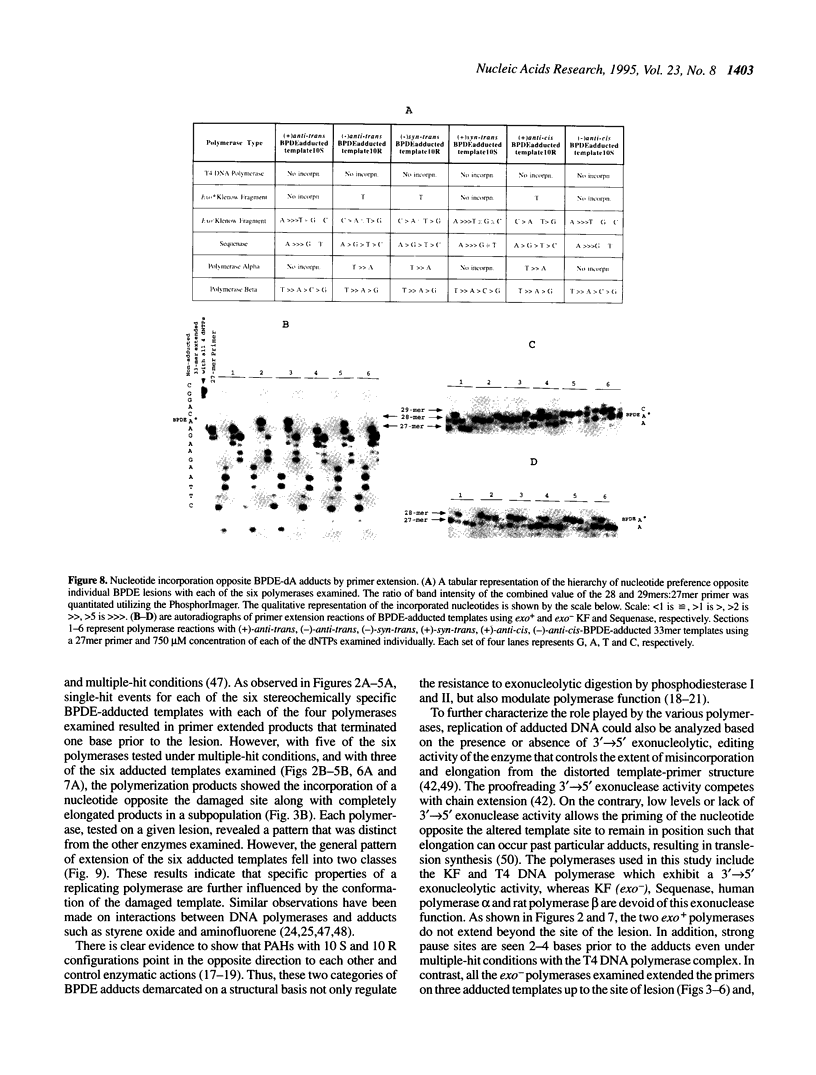
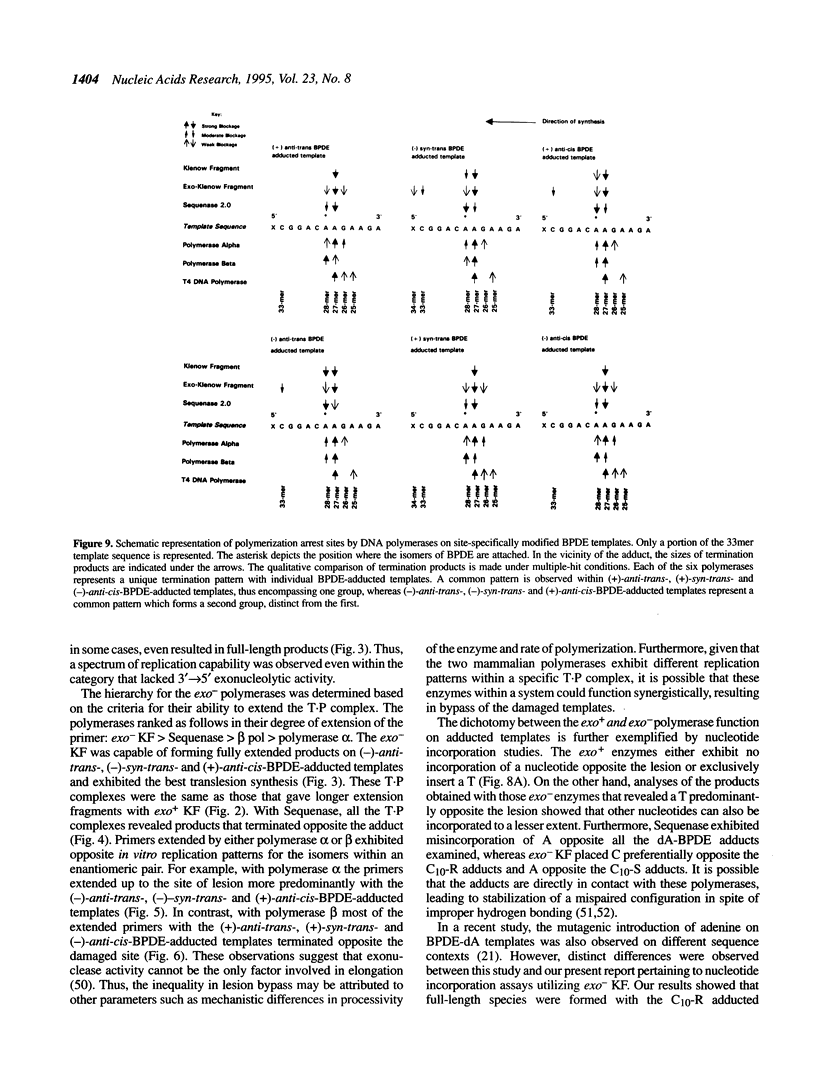
DNA adducts of the environmental carcinogen benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide (BPDE) interact stereospecifically with prokaryotic and eukaryotic polymerases in vitro. Toward understanding the capacity to replicate past different diastereomers of BPDE at specific sites in DNA, six deoxyoligonucleotides, each 33 bases long, were constructed with stereochemically defined BPDE adducts on adenine N6 at position two of the human N-ras codon 61. Four polymerases that were studied under single encounters with the template-primer complex terminated synthesis one base 3' to the lesion with all the adducted templates. When multiple encounters between polymerase and substrate were permitted, each of the polymerases analyzed revealed a unique pattern for a given adducted template. The general replication pattern was encompassed under two categories, reflecting the significance of the R and S configurations of C10 of the pyrenyl ring attached to the single-stranded DNA template. Furthermore, within each of these categories, every polymerase demonstrated distinct quantitative differences in product accumulation at a given site, for the various adducted templates. Among the polymerases utilized in this study, exonuclease-deficient Klenow fragment of polymerase I (exo- KF) exhibited the most efficient translesion synthesis resulting in approximately 16% full-length products with the modified templates bearing adducts with C10-S configuration. In contrast, chain elongation with bacteriophage T4 DNA polymerase bearing an active 3'-->5' exonucleolytic activity was most strongly inhibited by all six BPDE-adducted templates. Misincorporation of A opposite the adduct occurred in all the templates when polymerized with Sequenase, whereas exo- KF preferentially incorporated C opposite the C10-R BPDE adducts and A opposite the C10-S BPDE adducts.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu A. K., Hanrahan C. J., Malia S. A., Kumar S., Bizanek R., Tomasz M. Effect of site-specifically located mitomycin C-DNA monoadducts on in vitro DNA synthesis by DNA polymerases. Biochemistry. 1993 May 11;32(18):4708–4718. doi: 10.1021/bi00069a004. [DOI] [PubMed] [Google Scholar]
- Basu A. K., Hanrahan C. J., Malia S. A., Kumar S., Bizanek R., Tomasz M. Effect of site-specifically located mitomycin C-DNA monoadducts on in vitro DNA synthesis by DNA polymerases. Biochemistry. 1993 May 11;32(18):4708–4718. doi: 10.1021/bi00069a004. [DOI] [PubMed] [Google Scholar]
- Basu A. K., Wood M. L., Niedernhofer L. J., Ramos L. A., Essigmann J. M. Mutagenic and genotoxic effects of three vinyl chloride-induced DNA lesions: 1,N6-ethenoadenine, 3,N4-ethenocytosine, and 4-amino-5-(imidazol-2-yl)imidazole. Biochemistry. 1993 Nov 30;32(47):12793–12801. doi: 10.1021/bi00210a031. [DOI] [PubMed] [Google Scholar]
- Belguise-Valladier P., Maki H., Sekiguchi M., Fuchs R. P. Effect of single DNA lesions on in vitro replication with DNA polymerase III holoenzyme. Comparison with other polymerases. J Mol Biol. 1994 Feb 11;236(1):151–164. doi: 10.1006/jmbi.1994.1125. [DOI] [PubMed] [Google Scholar]
- Bridgewater L. C., Manning F. C., Woo E. S., Patierno S. R. DNA polymerase arrest by adducted trivalent chromium. Mol Carcinog. 1994 Mar;9(3):122–133. doi: 10.1002/mc.2940090304. [DOI] [PubMed] [Google Scholar]
- Brookes P., Osborne M. R. Mutation in mammalian cells by stereoisomers of anti-benzo[a] pyrene-diolepoxide in relation to the extent and nature of the DNA reaction products. Carcinogenesis. 1982;3(10):1223–1226. doi: 10.1093/carcin/3.10.1223. [DOI] [PubMed] [Google Scholar]
- Chary P., Latham G. J., Robberson D. L., Kim S. J., Han S., Harris C. M., Harris T. M., Lloyd R. S. In vivo and in vitro replication consequences of stereoisomeric benzo[a]pyrene-7,8-dihydrodiol 9,10-epoxide adducts on adenine N6 at the second position of N-ras codon 61. J Biol Chem. 1995 Mar 10;270(10):4990–5000. doi: 10.1074/jbc.270.10.4990. [DOI] [PubMed] [Google Scholar]
- Christner D. F., Lakshman M. K., Sayer J. M., Jerina D. M., Dipple A. Primer extension by various polymerases using oligonucleotide templates containing stereoisomeric benzo[a]pyrene-deoxyadenosine adducts. Biochemistry. 1994 Nov 29;33(47):14297–14305. doi: 10.1021/bi00251a043. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Joyce C. M., Beardsley G. P. Novel blunt-end addition reactions catalyzed by DNA polymerase I of Escherichia coli. J Mol Biol. 1987 Nov 5;198(1):123–127. doi: 10.1016/0022-2836(87)90462-1. [DOI] [PubMed] [Google Scholar]
- Comess K. M., Burstyn J. N., Essigmann J. M., Lippard S. J. Replication inhibition and translesion synthesis on templates containing site-specifically placed cis-diamminedichloroplatinum(II) DNA adducts. Biochemistry. 1992 Apr 28;31(16):3975–3990. doi: 10.1021/bi00131a013. [DOI] [PubMed] [Google Scholar]
- Cosman M., de los Santos C., Fiala R., Hingerty B. E., Singh S. B., Ibanez V., Margulis L. A., Live D., Geacintov N. E., Broyde S. Solution conformation of the major adduct between the carcinogen (+)-anti-benzo[a]pyrene diol epoxide and DNA. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1914–1918. doi: 10.1073/pnas.89.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echols H., Goodman M. F. Mutation induced by DNA damage: a many protein affair. Mutat Res. 1990 Sep-Nov;236(2-3):301–311. doi: 10.1016/0921-8777(90)90013-u. [DOI] [PubMed] [Google Scholar]
- Eger B. T., Kuchta R. D., Carroll S. S., Benkovic P. A., Dahlberg M. E., Joyce C. M., Benkovic S. J. Mechanism of DNA replication fidelity for three mutants of DNA polymerase I: Klenow fragment KF(exo+), KF(polA5), and KF(exo-). Biochemistry. 1991 Feb 5;30(5):1441–1448. doi: 10.1021/bi00219a039. [DOI] [PubMed] [Google Scholar]
- Feig D. I., Loeb L. A. Oxygen radical induced mutagenesis is DNA polymerase specific. J Mol Biol. 1994 Jan 7;235(1):33–41. doi: 10.1016/s0022-2836(05)80009-9. [DOI] [PubMed] [Google Scholar]
- Hruszkewycz A. M., Canella K. A., Dipple A. DNA polymerase-mediated nucleotide incorporation adjacent to hydrocarbon-deoxyadenosine and hydrocarbon-deoxyguanosine adducts. Carcinogenesis. 1991 Sep;12(9):1659–1663. doi: 10.1093/carcin/12.9.1659. [DOI] [PubMed] [Google Scholar]
- Hruszkewycz A. M., Canella K. A., Peltonen K., Kotrappa L., Dipple A. DNA polymerase action on benzo[a]pyrene-DNA adducts. Carcinogenesis. 1992 Dec;13(12):2347–2352. doi: 10.1093/carcin/13.12.2347. [DOI] [PubMed] [Google Scholar]
- Hruszkewycz A. M., Dipple A. Bypass of a hydrocarbon adduct in an oligonucleotide template mediated by mispairing adjacent to the adduct. Carcinogenesis. 1991 Nov;12(11):2185–2187. doi: 10.1093/carcin/12.11.2185. [DOI] [PubMed] [Google Scholar]
- Jennette K. W., Jeffrey A. M., Blobstein S. H., Beland F. A., Harvey R. G., Weinstein I. B. Nucleoside adducts from the in vitro reaction of benzo[a]pyrene-7,8-dihydrodiol 9,10-oxide or benzo[a]pyrene 4,5-oxide with nucleic acids. Biochemistry. 1977 Mar 8;16(5):932–938. doi: 10.1021/bi00624a019. [DOI] [PubMed] [Google Scholar]
- Joyce C. M. How DNA travels between the separate polymerase and 3'-5'-exonuclease sites of DNA polymerase I (Klenow fragment). J Biol Chem. 1989 Jun 25;264(18):10858–10866. [PubMed] [Google Scholar]
- Kunkel T. A. Exonucleolytic proofreading. Cell. 1988 Jun 17;53(6):837–840. doi: 10.1016/s0092-8674(88)90189-4. [DOI] [PubMed] [Google Scholar]
- Latham G. J., Lloyd R. S. Deoxynucleotide polymerization by HIV-1 reverse transcriptase is terminated by site-specific styrene oxide adducts after translesion synthesis. J Biol Chem. 1994 Nov 18;269(46):28527–28530. [PubMed] [Google Scholar]
- Latham G. J., Zhou L., Harris C. M., Harris T. M., Lloyd R. S. The replication fate of R- and S-styrene oxide adducts on adenine N6 is dependent on both the chirality of the lesion and the local sequence context. J Biol Chem. 1993 Nov 5;268(31):23427–23434. [PubMed] [Google Scholar]
- Lehman I. R., Kaguni L. S. DNA polymerase alpha. J Biol Chem. 1989 Mar 15;264(8):4265–4268. [PubMed] [Google Scholar]
- Mao B., Li B., Amin S., Cosman M., Geacintov N. E. Opposite stereoselective resistance to digestion by phosphodiesterases I and II of benzo[a]pyrene diol epoxide-modified oligonucleotide adducts. Biochemistry. 1993 Nov 9;32(44):11785–11793. doi: 10.1021/bi00095a006. [DOI] [PubMed] [Google Scholar]
- Michaels M. L., Reid T. M., King C. M., Romano L. J. Accurate in vitro translesion synthesis by Escherichia coli DNA polymerase I (large fragment) on a site-specific, aminofluorene-modified oligonucleotide. Carcinogenesis. 1991 Sep;12(9):1641–1646. doi: 10.1093/carcin/12.9.1641. [DOI] [PubMed] [Google Scholar]
- Moore P. D., Rabkin S. D., Osborn A. L., King C. M., Strauss B. S. Effect of acetylated and deacetylated 2-aminofluorene adducts on in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7166–7170. doi: 10.1073/pnas.79.23.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. D., Rabkin S. D., Strauss B. S. Termination of vitro DNA synthesis at AAF adducts in the DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4473–4484. doi: 10.1093/nar/8.19.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn M. M., Alberts B. M. The T4 DNA polymerase accessory proteins form an ATP-dependent complex on a primer-template junction. J Biol Chem. 1991 Oct 25;266(30):20024–20033. [PubMed] [Google Scholar]
- Rabkin S. D., Strauss B. S. A role for DNA polymerase in the specificity of nucleotide incorporation opposite N-acetyl-2-aminofluorene adducts. J Mol Biol. 1984 Sep 25;178(3):569–594. doi: 10.1016/0022-2836(84)90239-0. [DOI] [PubMed] [Google Scholar]
- Sawaya M. R., Pelletier H., Kumar A., Wilson S. H., Kraut J. Crystal structure of rat DNA polymerase beta: evidence for a common polymerase mechanism. Science. 1994 Jun 24;264(5167):1930–1935. doi: 10.1126/science.7516581. [DOI] [PubMed] [Google Scholar]
- Schurter E. J., Yeh H. J., Sayer J. M., Lakshman M. K., Yagi H., Jerina D. M., Gorenstein D. G. NMR solution structure of a nonanucleotide duplex with a dG mismatch opposite a 10R adduct derived from trans addition of a deoxyadenosine N6-amino group to (-)-(7S,8R,9R,10S)-7,8-dihydroxy-9,10-epoxy-7,8,9,10- tetrahydrobenzo[a]pyrene. Biochemistry. 1995 Jan 31;34(4):1364–1375. doi: 10.1021/bi00004a031. [DOI] [PubMed] [Google Scholar]
- Shibutani S., Margulis L. A., Geacintov N. E., Grollman A. P. Translesional synthesis on a DNA template containing a single stereoisomer of dG-(+)- or dG-(-)-anti-BPDE (7,8-dihydroxy-anti-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene). Biochemistry. 1993 Jul 27;32(29):7531–7541. doi: 10.1021/bi00080a027. [DOI] [PubMed] [Google Scholar]
- Singhal R. K., Wilson S. H. Short gap-filling synthesis by DNA polymerase beta is processive. J Biol Chem. 1993 Jul 25;268(21):15906–15911. [PubMed] [Google Scholar]
- Slaga T. J., Bracken W. J., Gleason G., Levin W., Yagi H., Jerina D. M., Conney A. H. Marked differences in the skin tumor-initiating activities of the optical enantiomers of the diastereomeric benzo(a)pyrene 7,8-diol-9,10-epoxides. Cancer Res. 1979 Jan;39(1):67–71. [PubMed] [Google Scholar]
- Strauss B. S. In vitro mutagenesis and SOS repair. Ann Ist Super Sanita. 1989;25(1):177–189. [PubMed] [Google Scholar]
- Strauss B. S., Wang J. Role of DNA polymerase 3'----5' exonuclease activity in the bypass of aminofluorene lesions in DNA. Carcinogenesis. 1990 Dec;11(12):2103–2109. doi: 10.1093/carcin/11.12.2103. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrall B. D., Mann D. B., Smerdon M. J., Springer D. L. DNA polymerase, RNA polymerase and exonuclease activities on a DNA sequence modified by benzo[a]pyrene diolepoxide. Carcinogenesis. 1992 Sep;13(9):1529–1534. doi: 10.1093/carcin/13.9.1529. [DOI] [PubMed] [Google Scholar]
- Vousden K. H., Bos J. L., Marshall C. J., Phillips D. H. Mutations activating human c-Ha-ras1 protooncogene (HRAS1) induced by chemical carcinogens and depurination. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1222–1226. doi: 10.1073/pnas.83.5.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A. W., Chang R. L., Levin W., Yagi H., Thakker D. R., Jerina D. M., Conney A. H. Differences in mutagenicity of the optical enantiomers of the diastereomeric benzo[a]pyrene 7,8-diol-9,10-epoxides. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1389–1396. doi: 10.1016/s0006-291x(77)80133-2. [DOI] [PubMed] [Google Scholar]
- You M., Candrian U., Maronpot R. R., Stoner G. D., Anderson M. W. Activation of the Ki-ras protooncogene in spontaneously occurring and chemically induced lung tumors of the strain A mouse. Proc Natl Acad Sci U S A. 1989 May;86(9):3070–3074. doi: 10.1073/pnas.86.9.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. C., Reddy M. K., von Hippel P. H. Structure and function of the bacteriophage T4 DNA polymerase holoenzyme. Biochemistry. 1992 Sep 22;31(37):8675–8690. doi: 10.1021/bi00152a001. [DOI] [PubMed] [Google Scholar]
- van de Poll M. L., Lafleur M. V., van Gog F., Vrieling H., Meerman J. H. N-acetylated and deacetylated 4'-fluoro-4-aminobiphenyl and 4-aminobiphenyl adducts differ in their ability to inhibit DNA replication of single-stranded M13 in vitro and of single-stranded phi X174 in Escherichia coli. Carcinogenesis. 1992 May;13(5):751–758. doi: 10.1093/carcin/13.5.751. [DOI] [PubMed] [Google Scholar]