Abstract
NarL and NarP are paralogous response regulators that control anaerobic gene expression in response to the favoured electron acceptors nitrate and nitrite. Their DNA-binding carboxyl termini are in the widespread GerE–LuxR–FixJ subfamily of tetrahelical helix–turn–helix domains. Previous biochemical and crystallographic studies with NarL suggest that dimerization and DNA binding by the carboxyl-terminal domain (CTD) is inhibited by the unphosphorylated amino-terminal receiver domain. We report here that NarL-CTD and NarP-CTD, liberated from their receiver domains, activated transcription in vivo from the class II napF and yeaR operon control regions, but failed to activate from the class I narG and fdnG operon control regions. Alanine substitutions were made to examine requirements for residues in the NarL DNA recognition helix. Substitutions for Val-189 and Arg-192 blocked DNA binding as assayed both in vivo and in vitro, whereas substitution for Arg-188 had a strong effect only in vivo. Similar results were obtained with the corresponding residues in NarP. Finally, Ala substitutions identified residues within the NarL CTD as important for transcription activation. Overall, results are congruent with those obtained for other GerE-family members, including GerE, TraR, LuxR and FixJ.
INTRODUCTION
The helix–turn–helix (HTH) is a widespread DNA-binding domain. One variation, the tetrahelical HTH superfamily (Aravind et al., 2005), includes one family defined initially through sequence similarity to the Vibrio fischeri LuxR quorum sensor, response regulators such as Sinorhizobium meliloti FixJ, and the single-domain GerE regulator from Bacillus subtilis (Henikoff et al., 1990). This family is annotated in domain databases as GerE (pfam00196), HTH_LuxR (smrt00421) and LuxR_C_like (cd06170) (Marchler-Bauer et al., 2009). Approximately one quarter of all DNA-binding response regulators have the GerE-family carboxyl-terminal domain (CTD) (Galperin, 2006), including Escherichia coli NarL, which mediates nitrate-responsive transcriptional regulation (Stewart & Rabin, 1995).
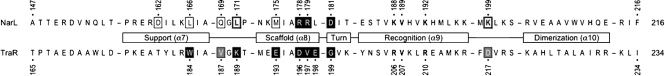
NarL has been analysed by X-ray crystallography of both the unphosphorylated monomeric protein (Baikalov et al., 1996) and the isolated dimeric CTD in complex with DNA (Maris et al., 2005, 2002). The CTD comprises scaffold and DNA-recognition helices (α8 and α9, respectively), which form the HTH per se, as well as support and dimerization helices (α7 and α10, respectively; Fig. 1). Superimposable structures have been determined for other GerE-family proteins, including GerE (Ducros et al., 2001), FixJ (Kurashima-Ito et al., 2005) and Agrobacterium tumefaciens TraR (Vannini et al., 2002; Zhang et al., 2002), a LuxR homologue (Nasser & Reverchon, 2007; Pappas et al., 2004). In full-length NarL, the recognition helix is blocked by the receiver, whereas the dimerization helix is packed against the interdomain linker. Receiver phosphorylation results in domain rearrangement to relieve this inhibition of DNA binding (Eldridge et al., 2002).
Fig. 1.
NarL and TraR CTD sequences. The four α-helices include the central HTH element. Results for TraR are from Qin et al. (2009) and White & Winans (2005). Boxed residues indicate phenotypes for Ala substitutions: black, PC; white, functional; bold type and outline, deficient. Grey-shaded boxes indicate positions where substitution with residues other than Ala results in the PC phenotype. Residues in bold type are implicated in direct recognition of DNA (Maris et al., 2005; White & Winans, 2007). The TraR-CTD sequence shown is from plasmid pTiR10 (White & Winans, 2005); the TraR-CTD sequence from plasmid pTiC58 differs at three positions (Val-168, Met-189 and Val-194) (Qin et al., 2009).
The NarL CTD binds as an antiparallel dimer to inverted repeat DNA sequences (Maris et al., 2005, 2002) termed 7–2–7 heptamer pairs. Heptamers have a consensus of 5′-TACYYMT-3′, where Y=C or T and M=A or C (Darwin et al., 1997). The two nucleotides separating the heptamers are usually A or T to accommodate dimerization over the minor groove (Maris et al., 2005). In cocrystals, protein–DNA interaction occurs through primary base-pair recognition by residues Lys-188, Val-189 and Lys-192, as well as secondary DNA backbone interactions (Maris et al., 2005, 2002). TraR interacts similarly with its DNA targets (White & Winans, 2007).
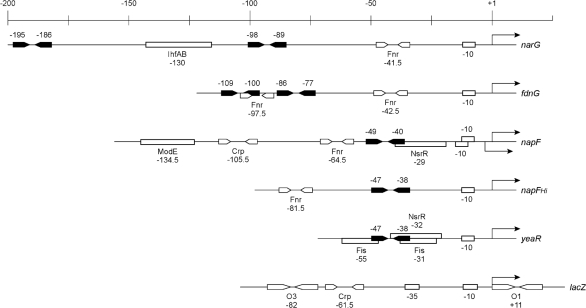
NarL and its paralogue NarP regulate transcription from target operon control regions with various binding-site architectures (Stewart & Rabin, 1995) (Fig. 2). Most of these are activated by Fnr, which functions during anaerobiosis (Browning et al., 2002; Kiley & Beinert, 1998). Control regions are classified according to the location of the activator binding site (Barnard et al., 2004). Nar class II control regions, exemplified by the napF and yeaR operons (Lin et al., 2007; Squire et al., 2009; Stewart & Bledsoe, 2005), have a single Nar-binding site immediately adjacent to the promoter. Nar class I control regions, exemplified by the narG and fdnG operons, have two Nar-binding sites upstream of the promoter (Stewart & Rabin, 1995). For the nirB and nrfA control regions, the single upstream Nar-binding site functions in remodelling an inhibitory nucleoprotein complex (Barnard et al., 2004). Finally, NarL and NarP repress transcription from several control regions, exemplified by synthetic constructs in which a Nar 7–2–7 binding site has replaced the lacZ operon primary operator O1 (Stewart & Bledsoe, 2003).
Fig. 2.
Control regions used in this study. The scale is in nucleotides. Nar 7–2–7 heptamer pairs are depicted as black inverted arrows, whereas sites for other proteins are depicted as white boxes or inverted arrows. Numbers show positions of binding-site centres relative to the transcription initiation point.
GerE functions as a direct transcriptional activator (Zheng et al., 1992), demonstrating that the GerE-family domain can make activating contacts with RNA polymerase. Likewise, the isolated LuxR (Choi & Greenberg, 1991) and FixJ (Kahn & Ditta, 1991) CTDs function as class II activators at some promoters. Residues required for activation have been identified in GerE (Crater & Moran, 2002), LuxR (Egland & Greenberg, 2001), TraR (Costa et al., 2009; Qin et al., 2009; White & Winans, 2005) and FixJ (Ton-Hoang et al., 2001).
Here we report results from experiments designed to examine DNA binding and transcriptional activation by NarL and NarP. The findings provide context for understanding NarL structure in relation to its functions.
METHODS
Mutants and their analysis
Strain construction.
Strains and plasmids are listed in Table 1. Mutant alleles were transferred between strains by bacteriophage P1-mediated generalized transduction (Miller, 1972). For some strains, att80-integrated alleles were transferred by selection for the adjacent trp+ marker. Standard methods were used for restriction endonuclease digestion, ligation, transformation and PCR amplification (Maloy et al., 1996). Oligonucleotide-directed site-specific mutagenesis followed the QuikChange protocol (Stratagene Cloning Systems), as described previously (Lin et al., 2007).
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype | Source or reference |
|---|---|---|
| E. coli K-12 strains | ||
| BW25113 | hsdR lacIQ Δ(lacZ) Δ(araBAD) Δ(rhaBAD) | Datsenko & Wanner (2000) |
| VJS632 | F−λ− prototroph | Stewart & Parales (1988) |
| VJS676 | As VJS632 but Δ(argF–lacIZYA)U169 | Stewart & Parales (1988) |
| VJS8364 | As VJS632 but ΔlacZ | Lin et al. (2007) |
| Derivatives of VJS676 | ||
| VJS2197 | λΦ(narG–lacZ) | Rabin & Stewart (1992) |
| VJS4147 | λΦ(fdnG–lacZ) [Δ313; Fnr 2−] | Li & Stewart (1992) |
| VJS6317 | λ[O1-nirB lacZ+ Y+ A+] narX+ narL215 : : Tn10 narQ+ narP253 : : Tn10d(Cm) | Stewart & Bledsoe (2003) |
| VJS6906 | λΦ(napFHi–lacZ) [Δ260] | Stewart & Bledsoe (2005) |
| VJS6990 | λΦ(napF–lacZ) [Δ146; P2−] | Stewart et al. (2003) |
| VJS7449 | λ−Δ(attλ-lom) : : bla [O1-napF lacZ+] | Stewart & Bledsoe (2003) |
| VJS7489 | λ−Δ(attλ-lom) : : bla [O1-nrfA lacZ+] | Stewart & Bledsoe (2003) |
| VJS7623 | λ−Δ(attλ-lom) : : bla [O1-nirB −74/−74 lacZ+] | This work |
| VJS9284 | λΦ(napFHi–lacZ) [Δ260] narX+ ΔnarL261 narQ+ ΔnarP262 | This work |
| VJS9719 | λ−Δ(attλ-lom) : : bla [O1-napF lacZ+] narX+ ΔnarL261 narQ+ ΔnarP262 | This work |
| VJS10030 | λ[O1-nirB lacZ+ Y+ A+] narX+ narL215 : : Tn10 ΔnarQ264 : : aph narP253 : : Tn10d(Cm) | This work |
| VJS10054 | λ−Δ(attλ-lom) : : bla [O1-nirB −74/−74 lacZ+] narX+ ΔnarL261 : : aph ΔnarQ264 ΔnarP262 trpB : : Tn10 | This work |
| VJS10247 | λΦ(napFHi–lacZ) [Δ260] narX+ ΔnarL261 ΔnarQ264 ΔnarP262 | This work |
| VJS10248 | λΦ(fdnG–lacZ) [Δ313; Fnr2−] narX+ ΔnarL261 ΔnarQ264 ΔnarP262 | This work |
| VJS10258 | λΦ(narG–lacZ) narX+ ΔnarL261 : : aph ΔnarQ264 ΔnarP262 | This work |
| VJS10461 | λ−Δ(attλ-lom) : : bla [O1-nrfA lacZ+] narX+ ΔnarL261 : : aph ΔnarQ264 ΔnarP262 trpB : : Tn10 | This work |
| VJS10649 | λΦ(napF–lacZ) [Δ146; P2–] narX+ ΔnarL261 : : aph ΔnarQ264 ΔnarP262 | This work |
| VJS10665 | λ−Δ(attλ-lom) : : bla [O1-napF lacZ+] narX+ ΔnarL261 : : aph ΔnarQ264 ΔnarP262 | This work |
| Derivatives of VJS8364 | ||
| VJS9565 | λ−Δ(attλ-lom) : : bla {Φ(yeaR–lacZ) [Δ175]} (NsrR−) | Lin et al. (2007) |
| VJS10983 | λ−Δ(attλ-lom) : : bla {Φ(yeaR–lacZ) [Δ175]} (NsrR−) narX+ ΔnarL261 narQ+ ΔnarP262 | This work |
| VJS11159 | λ−Δ(attλ-lom) : : bla {Φ(yeaR–lacZ) [Δ175]} (NsrR−) narX+ ΔnarL261 narQ+ ΔnarP262 trpB : : Tn10 | This work |
| Plasmids | ||
| LITMUS 39 | Apr; ori pMB9 | New England Biolabs |
| pACYC184 | Cmr, Tcr; ori P15A | Chang & Cohen (1978) |
| pAH69 | Apr; int HK022; ori pSC101(Ts) | Haldimann & Wanner (2001) |
| pAH123 | Apr; int Φ80; ori pSC101(Ts) | Haldimann & Wanner (2001) |
| pAH144 | Smr; att HK022; ori R6Kγ | Haldimann & Wanner (2001) |
| pAH153 | Gmr; att Φ80; ori R6Kγ | Haldimann & Wanner (2001) |
| pCP20 | Apr, Cmr; Flp+; ori pSC101(Ts) | Datsenko & Wanner (2000) |
| pKD13 | Apr, Kmr; ori R6Kγ | Datsenko & Wanner (2000) |
| pKD46 | Apr; Red+; ori pSC101(Ts) | Datsenko & Wanner (2000) |
| pQE30 | Apr; ori pMB9 | Qiagen |
| pQE32 | Apr; ori pMB9 | Qiagen |
| pSU18 | Cmr; ori P15A; pUC18 polylinker | Bartolomé et al. (1991) |
| pSU19 | Cmr; ori P15A; pUC19 polylinker | Bartolomé et al. (1991) |
| pVJS2288 | Apr; His6-narP in pQE32 | Noriega et al. (2010) |
| pVJS2294 | Apr; His6-narL in pQE30 | Noriega et al. (2010) |
| pVJS4095 | Cmr; narL† in pSU19 | This work |
| pVJS4098 | Cmr; narP† in pSU19 | This work |
| pVJS4505 | Cmr; narL-CTD in pSU18 | This work |
| pVJS4506 | Cmr; narP-CTD in pSU18 | This work |
| pVJS5259 | Apr; His6-narP-CTD in pQE30 | This work |
| pVJS5265 | Apr; His6-narL-CTD in pQE30 | This work |
Null alleles.
The ΔnarL261 allele was constructed through λRed-mediated recombineering (Datsenko & Wanner, 2000), using PCR primers LLC1287 and LLC1288 (5′-ATGGCACCAGATATCACCGTGGTTGGCGAAGCGAGTgtgtaggctggagctgcttc-3′ and 5′-CATTTTCTTCAGCATGTGCTTGACGTGCACTTTTACattccggggatccgtcgacc-3′, respectively) with plasmid pKD13 as template (sequence complementary to pKD13 is shown in lower case). This results in deletion of codons Asn-40 to Thr-186 (216 codons in total).
The ΔnarP262 allele was constructed by using primers LLC1281 and LLC1282 (5′-ATGCCTGAAGCAACACCTTTTCAGGTGATGATTGTGgtgtaggctggagctgcttc-3′ and 5′-TTATTGTGCCCCGCGTTGTTGCAGGAACAGAATGGTattccggggatccgtcgacc-3′, respectively). This results in deletion of codons Asp-13 to Ala-204 (215 codons in total).
The ΔnarQ264 allele was constructed by using primers LLC1279 and LLC1280 (5′-GTGATTGTTAAACGACCCGTCTCGGCCAGTCTGGCCgtgtaggctggagctgcttc-3′ and 5′-TTACATTAACTGACTTTCCTCACCCTCCGCAGAGCGattccggggatccgtcgacc-3′, respectively). This results in deletion of codons Arg-13 to Phe-555 (566 codons in total). For all three of these alleles, the residual scar sequence following excision of the aphA gene includes an in-frame nonsense codon.
The ΔnarX263 allele, designed to mimic the previously characterized ΔnarX242 allele (Egan & Stewart, 1990), was constructed by using primers LLC1285 and LLC1286 (5′-ATGGCGATGCTTGGAACTGCGTTGAACAATATGTCTattccggggatccgtcgacc-3′ and 5′-GCGGAATGTGGTGAGCAATTCACGCAACTGCGCCCgtgtaggctggagctgcttc-3′, respectively). This results in deletion of codons Ala-221 to Ser-441 (598 codons in total). The residual scar sequence does not contain any in-frame nonsense codons. Thus, this deletion does not have polar effects on expression of the overlapping narL gene (Egan & Stewart, 1990).
narL† and narP† alleles.
The narL gene is autoregulated, whereas the narP gene is not. Thus, to ensure equivalent expression levels, the narP upstream transcription control region and Shine–Dalgarno sequence were fused to the narL initiation codon. This was accomplished by introducing NdeI restriction endonuclease sites overlapping the initiation codons for narL (CCC ATG changed to CAT ATG) and narP (ACT ATG changed to CAT ATG), and then subcloning the narL gene from NdeI to a downstream BamHI site into the narP plasmid, replacing the corresponding narP sequence.
These NdeI-modified alleles were engineered to contain two additional restriction endonuclease sites. NgoMIV sites were introduced near the end of the receiver domain-coding regions (helix α5; narL codons 125–126, GCT GGC changed to GCC GGC; narP codons 124–125, GCG AAA changed to GCC GGC), and XhoI sites were introduced near the beginning of the CTD-coding regions (helix α7; narL codons 164–165, CTC AAG changed to CTC GAG; narP codons 162–163, CTG CAC changed to CTC GAG). These sites, which result in missense substitutions (NarL, Lys-165 to Glu; NarP, Lys-125 to Gly and His-163 to Glu), were designed for a separate study to analyse properties of NarL–NarP chimerae. Control experiments established that these missense substitutions did not influence regulatory phenotypes.
These modified genes are denoted as narL† and narP† to distinguish them from the wild-type. The narL† and narP† genes were recloned, from the EcoRI site 490 nt upstream of the narP initiation codon to the downstream BamHI site, into the moderate-copy-number plasmid pSU19. The cloned inserts are in opposite orientation to that of the vector lacZ promoter.
Monocopy narL† and narP† alleles.
Conditional-replication, integration and modular (CRIM) plasmids of Haldimann & Wanner (2001) were used to place modified narL and narP alleles at the chromosomal prophage attachment site for Φ80 (centisome 28; plasmid pAH153). Chromosomal integration and PCR analysis to confirm the resulting strains were performed essentially as described by Haldimann & Wanner (2001).
narL-CTD and narP-CTD alleles.
SphI sites were introduced in the interdomain linker coding regions (narL codons 147–148, GCC ACT changed to GCA TGC; narP codons 146–147, GCG GAA changed to GCA TGC). Fragments (SphI–HindIII) were cloned into plasmid LITMUS 39, and then recloned (SalI–HindIII) into plasmid pSU18. The resulting plasmids were designed to express NarL-CTD and NarP-CTD with vector-derived amino-terminal extensions of 20 residues (MTMITNSSSVPGDPLESTAC), corresponding to the LacZ amino terminus and polylinker.
Culture media and conditions.
Defined, complex and indicator media for genetic manipulations were used as described previously (Maloy et al., 1996). Defined medium to grow cultures for enzyme assays was buffered with MOPS, as described previously (Stewart & Parales, 1988). Medium for overnight cultures arrested in the mid-exponential phase contained glucose (6 mM) or glucose plus NaNO3 (4 and 10 mM, respectively) as indicated (Stewart & Bledsoe, 2003).
Plasmid-bearing strains were cultured in tryptone yeast extract glucose (TYEG) medium, which contains 0.8 % tryptone, 0.5 % yeast extract, 0.5 % NaCl, Vogel–Bonner phosphate-buffered salts (Maloy et al., 1996) and 10 mM glucose. NaNO3 (40 mM) was added as indicated.
Cultures were grown at 37 °C to the mid-exponential phase, about 35–40 Klett units. Culture densities were monitored with a Klett–Summerson photoelectric colorimeter (Klett Manufacturing) equipped with a number 66 (red) filter. Anaerobic cultures were grown in screw-capped tubes, as described previously (Stewart & Parales, 1988).
Transcription reporters.
Control region output was measured from lacZ fusions (Table 1) integrated at the chromosomal prophage attachment site for λ (centisome 17). The Φ(narG–lacZ) and Φ(fdnG–lacZ) reporters are activated by NarL but only weakly by NarP (Stewart & Rabin, 1995). Activities (Miller units) after growth in the absence and presence of nitrate were 37 and 2610 (narG), and 22 and 490 (fdnG).
The Φ(napFEc–lacZ) and Φ(napFHi–lacZ) reporters, from E. coli and Haemophilus influenzae, respectively, are activated by NarP. The Φ(napFHi–lacZ) reporter is also activated by NarL (Stewart & Bledsoe, 2005), as is the P2− promoter mutant version of the Φ(napFEc–lacZ) reporter (Stewart et al., 2003). The Φ(yeaR–lacZ) reporter is an Fnr-independent Nar class II control region; the version used here lacks the binding site for the nitric oxide-responsive NsrR repressor (Lin et al., 2007). Activities (Miller units) after growth in the absence and presence of nitrate were 13 and 160 (napFHi), 88 and 1730 (napFEc P2−), and 200 and 8040 (yeaR).
O1-lac substitution reporters have Nar 7–2–7 binding sites in place of the primary operator. The O1-nirB, O1-napF and O1-nrfA versions have been described previously (Stewart & Bledsoe, 2003). Identical methods were used to construct the O1-nirB (−74/−74) (5′-AATACCCATATATGGGTATT-3′) version. Activities (Miller units) after growth in the absence and presence of nitrate were 1930 and 74 (O1-nirB), 1730 and 330 (O1-napF), 6360 and 150 (O1-nrfA), and 1240 and 200 (O1-nirB −74/−74).
LacZ assay.
β-Galactosidase activities were determined as described by Miller (1972). All cultures were assayed in duplicate, and reported values were averaged from at least two independent experiments. Relative activation or repression as percentages of the corresponding wild-type values were calculated as described elsewhere (Zhang et al., 1992).
Proteins and their analysis
Purification.
Isolation of His6-NarL and His6-NarP has been described previously (Noriega et al., 2010); essentially identical methods were used to prepare His6-NarL-CTD and His6-NarP-CTD. Expression constructs were made by cloning narL or narP sequence from the introduced SphI site (within the interdomain linker coding region) into plasmid pQE30. The amino termini were MRGSH6GSACTERD... for His6-NarL-CTD, and MRGSH6GSACEDPF... for His6-NarP-CTD (NarL and NarP sequence is in italic type). This His6-NarL-CTD protein is virtually identical to that used for X-ray analysis of NarL–DNA interaction, which has an amino terminus of MRGSH6GSATTERD... (Maris et al., 2002).
Electrophoretic mobility shift assays (EMSAs).
Templates were prepared from plasmid pVJS3253 constructs in which the lac operon primary operator O1-lac has been substituted with Nar-binding sites (Stewart & Bledsoe, 2003). Primers AVL2478 and AVL2479 (5′-GACGCCCGCCATAAACTGCCAGGAATTG-3′ and 5′-CGCCAGGGTTTTCCCAGTCACGACG-3′, respectively), which anneal upstream and downstream of O1-lac, respectively, generate 332 bp products. These yielded 296 bp fragments after digestion with EcoRI, which cleaves at a site introduced near the end of the lacI gene. The O1-lac substitutions are at the centre of the resulting fragments, which were end-labelled with [α-32P]dATP (Perkin Elmer) by using DNA polymerase I large fragment (Klenow) (New England Biolabs).
EMSA followed the procedure of Maris et al. (2002). Briefly, proteins were incubated with 2 nM 32P-labelled DNA for 10 min at room temperature in reaction buffer containing 10 mM Tris (pH 7.5), 50 mM KCl, 1 mM EDTA, 1 mM DTT, 5 mM MgCl2, 15 mg poly-dIdC ml−1 and 10 % glycerol (v/v). Acetyl phosphate at 100 mM was added for phosphorylation of His6-NarL and His6-NarP; control assays established that this concentration did not influence the EMSA. The reaction mixture was loaded immediately onto a 6 % non-denaturing polyacrylamide gel running at 100 V, and allowed to electrophorese for 1 h at 4 °C. Radiolabelled bands were analysed using a Storm PhosphorImager Scanner with ImageQuant Software (Molecular Dynamics).
RESULTS
NarL and NarP DNA binding
The affinities of NarL-CTD for several 7–2–7 target sites in vitro have been determined by using EMSA (Maris et al., 2005). We chose three of these sites to measure affinities for NarP-CTD, also by using EMSA. Target sequences were the native sites from the nirB and napF operons, and an artificial site consisting of two copies of nirB heptamer −74 in inverted orientation. Our version of the nirB (−74/−74) site (central AT) is subtly different from that used earlier (central TA) (Maris et al., 2005).
The KD for NarL-CTD binding to the nirB site (Table 2) was similar to that reported earlier: 0.45 μM (Maris et al., 2005). By constrast, we measured substantially higher KD values for the nirB (−74/−74) and napF sites, which were reported as 0.65 and 0.45 μM, respectively. The reason(s) for these differences is unknown. NarL-dependent repression at lac O1-substitution constructs was much stronger for the nirB site than for the nirB (−74/−74) and napF sites (Stewart & Bledsoe, 2003), so relative affinities in vitro and in vivo were broadly correlated.
Table 2.
DNA-binding affinities
| Protein | Affinity at site [KD (μM)]* | ||
|---|---|---|---|
| O1-nirB (−74/−74) | O1-nirB | O1-napF | |
| His6-NarL† | 1.0±0.05 | 0.3±0.1 | 3.2±0.08 |
| His6-NarL-CTD | 9±2 | 0.1±0.06 | 2.3±0.5 |
| His6-NarP-CTD | 4±1 | 0.2±0.06 | 6±1 |
| His6-NarP-CTD (K186A) | 1.0±0.2 | –‡ | – |
| His6-NarP-CTD (V187A) | >30 | – | – |
| His6-NarP-CTD (R190A) | >30 | – | – |
*EMSA with binding site amplified from the indicated O1-substitution constructs.
†Reaction performed with 100 mM acetyl phosphate.
‡–, Not determined.
Full-length NarL, phosphorylated by incubation with acetyl phosphate, exhibited affinities similar to those measured for NarL-CTD (Table 2), extending the observation made with the narG (−89/−89) site (Maris et al., 2002). By contrast, full-length NarP exhibited very weak binding even after incubation with a high concentration of acetyl phosphate (Lin, 2009). We hypothesize that acetyl phosphate is a relatively poor substrate for NarP autophosphorylation, but have not pursued this point further. Nevertheless, NarP-CTD exhibited strong binding to all three sites (Table 2). This confirms that the NarP receiver inhibits binding, similar to NarL, and that NarP-CTD binds target sites in vitro as avidly as NarL-CTD.
The NarL-CTD–DNA X-ray structure shows recognition helix residues Lys-188, Val-189 and Lys-192 in direct contact with major groove base pairs (Maris et al., 2005, 2002) (Fig. 1). We made Ala substitutions at each of these positions, and measured effects on NarL-CTD affinity for DNA in vitro and on NarL repression at O1-lac substitution constructs in vivo. The V189A substitution substantially decreased binding in both assays (Table 3). Similar results were obtained with the corresponding mutants TraR V207A in vivo and in vitro (White & Winans, 2007), and NarP V187A in vitro (Table 2).
Table 3.
Effects of recognition helix alterations on the NarL–DNA interaction
| narL allele* | Affinity [KD (μM)]† O1-nirB (−74/−74) | Percentage of wild-type repression‡ in strain§ | ||
|---|---|---|---|---|
| O1-nirB (−74/−74) | O1-nirB | O1-napF | ||
| None | – | 0 | 0 | 0 |
| Wild-type | 0.9±0.2 | 100 | 100 | 100 |
| K188A | 1.8±0.7 | 26 | 3.4 | 15 |
| V189A | 11±2 | 46 | 58 | 57 |
| K192A | >30 | <0.1 | 17 | 21 |
*Indicated His6-NarL-CTD protein or narL allele integrated at att Φ80.
†EMSA with binding site amplified from the indicated O1-substitution construct.
‡Percentage repression or activation relative to wild-type value during growth with nitrate (Zhang et al., 1992).
§VJS10054 [O1-nirB (−74/−74)], VJS10030 (O1-nirB) and VJS10665 (O1-napF).
The NarL K192A substitution abolished binding in both assays, as expected from the previous observation that the K192C substitution eliminates binding in vitro (Xiao et al., 2002). Similar results were obtained with the NarP R190A mutant in vitro, although the TraR R210A mutant exhibited 15–20 % of wild-type binding both in vivo and in vitro (White & Winans, 2007).
Finally, the NarL K188A and NarP K186A substitutions reduced binding in vitro by only two- to threefold (Tables 2 and 3). NarL residue Lys-188 contacts DNA positions 5, 6 and 7, which are less conserved in NarL heptamer sequences and therefore may be less critical for overall affinity. Nevertheless, the NarL K188A mutant exhibited very weak repression in vivo (Table 3), hinting that additional parameters (such as supercoiling) influence binding-site recognition. By contrast, the TraR R206A mutant displayed undetectable binding in both assays (White & Winans, 2007).
NarL-CTD and NarP-CTD transcription control
It has been reported that NarL-CTD ‘is not sufficient for transcriptional activation … (data not shown)’ (Maris et al., 2002). Because Nar-dependent control regions are complex and varied (Fig. 2), we wished to revisit this conclusion. Accordingly, we monitored target operon expression in narL narP double null strains expressing either full-length or CTD versions of NarL and NarP from medium-copy-number plasmids.
The NarL-CTD and NarP-CTD proteins activated Φ(napFHi–lacZ) transcription to the same extent as their full-length counterparts (Table 4). Similarly, NarP and NarP-CTD were equally effective activators of expression from the wild-type Φ(napFEc–lacZ) reporter (Lin, 2009), which is not activated by NarL (Stewart et al., 2003). Finally, the CTD proteins were about 20–25 % as effective as their full-length counterparts for stimulating Φ(yeaR–lacZ) expression. Thus, the CTD proteins were competent for activating transcription from Nar class II control regions.
Table 4.
Transcription control by NarL-CTD and NarP-CTD
| Plasmid | Activator | Percentage of wild-type regulation* in strain† | ||
|---|---|---|---|---|
| Repression O1-napF | Class II activation | |||
| Φ(napFHi–lacZ) | Φ(yeaR–lacZ) | |||
| pACYC184 | None | 0 | 0 | 0 |
| pVJS4095 | NarL | 100 | 100 | 100 |
| pVJS4505 | NarL-CTD | 66 | 98 | 21 |
| pVJS4098 | NarP | 99 | 155 | 76 |
| pVJS4506 | NarP-CTD | 63 | 154 | 20 |
*Percentage repression or activation relative to wild-type value during growth with nitrate (Zhang et al., 1992).
†VJS9284 [λΦ(napFHi–lacZ)], VJS10983 [λΦ(yeaR–lacZ)] and VJS9719 (O1-napF).
By contrast, the CTD proteins were very weak repressors of transcription from the lac O1-substitution constructs O1-napF (Table 4), O1-nirB and O1-nrfA (Lin, 2009), even though the CTD proteins bound these sites well in vitro (Table 2). Moreover, neither CTD protein activated Φ(narG–lacZ) or Φ(fdnG–lacZ) expression (Lin, 2009). These results imply that the receiver domain is important for these processes in vivo.
NarL positive control (PC) mutants
Specific side-chain determinants of transcription activation are identified by PC missense substitution alleles whose products display near-normal DNA binding but are defective in transcription activation (Browning et al., 2002). In order to identify NarL PC substitutions, we used site-specific mutagenesis to substitute Ala for nine different surface-exposed residues (Fig. 3), focusing on those identified as conferring the PC phenotype for TraR (Qin et al., 2009; White & Winans, 2005). (A tenth mutant, D180A, yielded conflicting results in different assays and so was excluded.)
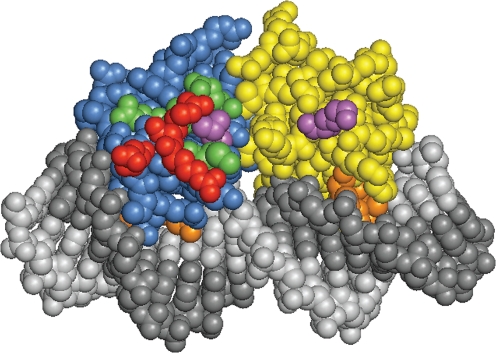
Fig. 3.
X-ray model of a NarL-CTD dimer in complex with its DNA-binding site from the nirB operon control region; from Maris et al. (2005). The two protomers are coloured blue and gold, and the two DNA strands are shaded light and dark grey. Residues are highlighted according to their phenotype: PC, red (Arg-178, Arg-179, Asp-181); functional, green (Asp-162, Leu-166, Gln-169, Met-175); deficient, violet (Leu-171, Lys-199); DNA-binding, orange (Lys-188, Val-189, Lys-192).
We assayed DNA binding in vivo by measuring repression at the lac O1-substitution constructs O1-nrfA and O1-napF, and we tested for activation from the class I Φ(narG–lacZ) and Φ(fdnG–lacZ) reporters, and from the class II Φ(napFEc–lacZ) and Φ(napFHi–lacZ) reporters (Table 5). Most of the mutants exhibited close to 100 % of the wild-type repression value for both of the O1-substitution constructs. The two exceptions were the L171A and K199A mutants, which exhibited less than 90 % repression with at least one construct (Table 5). These mutants also displayed the strongest defects in activation from both the class I and the class II reporters. Therefore, we classify these mutants as deficient for functions in addition to transcription activation.
Table 5.
Phenotypes of NarL missense mutants
| narL allele* | Percentage of wild-type regulation† in strain‡ | |||||
|---|---|---|---|---|---|---|
| Repression | Class I activation | Class II activation | ||||
| O1-nrfA | O1-napF | Φ(narG–lacZ) | Φ(fdnG–lacZ) | Φ(napFEc–lacZ) | Φ(napFHi–lacZ) | |
| None | 0 | 0 | 0 | 0 | 0 | 0 |
| Wild-type | 100 | 100 | 100 | 100 | 100 | 100 |
| Deficient | ||||||
| L171A | 85 | 68 | 1.2 | 24 | 48 | 56 |
| K199A | 94 | 86 | 1.0 | 26 | 40 | 43 |
| Functional | ||||||
| D162A | 96 | 97 | 70 | 52 | 52 | 86 |
| M175A | 100 | 101 | 86 | 92 | 103 | 185 |
| Q169A | 99 | 102 | 98 | 96 | 57 | 313 |
| L166A | 98 | 99 | 119 | 85 | 70 | 242 |
| Positive control | ||||||
| R178A | 101 | 99 | 18 | 33 | 86 | 86 |
| R179A | 100 | 101 | 61 | 52 | 226 | 100 |
| D181A | 98 | 102 | 52 | 41 | 146 | 93 |
| R178A+R179A | 101 | 105 | <0.1 | 5.5 | 8 | 28 |
| R179A+D181A | 101 | 81 | 39 | 14 | 20 | 31 |
*Indicated allele integrated at att Φ80.
†Percentage repression or activation relative to wild-type value during growth with nitrate (Zhang et al., 1992).
‡VJS10461 (O1-nrfA), VJS9719 (O1-napF), VJS10649 [λΦ(napF–lacZ)], VJS10247 [λΦ(napFHi–lacZ)], VJS10258 [λΦ(narG–lacZ)] and VJS10248 [λΦ(fdnG–lacZ)].
Four other mutants, denoted as functional (D162A, M175A, Q169A and L166A), displayed 70 % or more of the wild-type activation value for at least one reporter from each class. The remaining three mutants, denoted as PC (R178A, R179A and D181A), exhibited 60 % or less of the wild-type value for both class I reporters (Table 5). However, all had 85 % or more of the wild-type value for both class II reporters.
To examine these mutants further, we made double substitutions. The R178A+D181A mutant yielded conflicting results in different assays, and so was excluded. The other two double mutants exhibited robust repression, but defective transcription activation of all reporters including Φ(napFEc–lacZ) and Φ(napFHi–lacZ). In particular, the R178A+R179A double mutant displayed a synergistic effect, with sharply reduced activation of the Φ(narG–lacZ) and Φ(fdnG–lacZ) reporters (Table 5).
The R178A mutant had the strongest PC phenotype. To examine this mutant further, we tested for activation of the Fnr-independent, class II Φ(yeaR–lacZ) reporter. Indeed, the R178A mutant exhibited a strong defect in transcription activation (29 %), whereas the R179A mutant was near-normal (79 %). Therefore, the class II PC phenotype of the R178A mutant apparently was masked by synergy with Fnr at the napF control regions.
DISCUSSION
The receiver domain of response regulators controls activity depending on its phosphorylation state. GerE-family CTDs define one of the three major categories of DNA-binding response regulators (Galperin, 2006), for which the NarL protein provides a well-studied model. This investigation evaluated NarL-CTD involvement in transcription control and DNA binding. Results are congruent with those for other GerE-family proteins, including the well-understood TraR regulator.
Transcription from most Nar-regulated operons is activated by Fnr, so NarL and NarP impose nitrate-responsive control on top of Fnr-dependent anaerobic induction. Activation is synergistic, because expression in wild-type strains is much higher than that in either fnr or nar null strains. Synergy is revealed further by studies of narG operon expression in fnr PC mutants (Lamberg & Kiley, 2000). Although basal anaerobic expression is low in these mutants, expression during growth with nitrate is near-normal. Thus, NarL bypasses the Fnr PC defect by providing additional contacts to Fnr and/or RNA polymerase (Barnard et al., 2004; Browning et al., 2002).
Liberated Nar CTDs activate transcription from Nar class II control regions
NarL-CTD and NarP-CTD, liberated from their receiver domains (Morrison & Parkinson, 1994) and expressed from plasmids, fully activated transcription from the Fnr-dependent napFHi control region, but they were less effective at the Fnr-independent yeaR operon control region (Table 4). This difference between the two control regions may reflect Fnr stabilization of CTD binding at the former, or it may result from inefficient nucleoprotein remodelling at the latter (Squire et al., 2009). Nevertheless, overall results demonstrate that NarL-CTD and NarP-CTD can activate transcription from class II control regions, consistent with prior studies of GerE, LuxR-CTD and FixJ-CTD (Choi & Greenberg, 1991; Kahn & Ditta, 1991; Zheng et al., 1992).
NarL PC mutants
Following the strategy employed by others (Crater & Moran, 2002; Egland & Greenberg, 2001; Qin et al., 2009; White & Winans, 2005), we substituted Ala for surface-exposed residues on NarL-CTD and evaluated the resulting mutants for PC phenotypes. All were studied in the context of full-length protein expressed from chromosomal monocopy constructs. We examined all of the residues that correspond to PC substitutions identified for TraR-CTD (Qin et al., 2009; White & Winans, 2005).
Ala substitutions at three positions (Arg-178, Arg-179 and Asp-181) yielded unambiguous PC phenotypes for expression from the Nar class I narG and fdnG operon control regions (Table 5, Fig. 1). These residues form a cluster around the end of the scaffold helix, and therefore are positioned appropriately for contact to RNA polymerase (Fig. 3).
Like TraR Gly-199 (Qin et al., 2009), Ala substitution for NarL Asp-181 resulted in a class I-specific PC phenotype. Ala substitutions for NarL Arg-178 and Arg-179 also appeared to be class I-specific PC mutants, unlike their TraR counterparts (Asp-196 and Val-197). However, the R178A+R179A and R179A+D181A double mutants exhibited strong class II PC phenotypes. Moreover, the R178A mutant exhibited a strong PC phenotype for activation from the Fnr-independent Nar class II yeaR operon control region (Lin, 2009). Therefore, Fnr–NarL synergy may obscure NarL PC phenotypes in addition to Fnr PC phentoypes (Lamberg & Kiley, 2000).
Substitutions at four other positions resulted in functional phenotypes. Two of these, Leu-166 and Met-175, correspond to TraR residues Trp-184 and Glu-193, for which Ala substitutions yielded PC phenotypes (Qin et al., 2009). The third NarL position, Gln-169, corresponds to TraR Val-187, for which Glu and Ile but not Ala substitution resulted in PC phenotypes (White & Winans, 2005).
Ala substitutions at Leu-171 and Lys-199 strongly affected transcription activation from all reporters tested (Table 5). However, these mutants also exhibited relatively weak repression, and therefore are classified as deficient.
Activation from class I and class II control regions involves interaction with RNA polymerase subunits α-CTD, and with α and σ, respectively (Barnard et al., 2004). However, since NarL works in synergy with Fnr, these NarL PC mutants might be defective in interaction with Fnr in addition to (or instead of) RNA polymerase. We attempted to study this by examining phenotypes of narL fnr double PC mutants. Initial results were inconclusive (Lin, 2009), so this point was not pursued further.
Possible roles for the NarL and NarP receiver domains
Both CTD and phosphorylated full-length Nar proteins bind DNA in vitro with equal affinities (Maris et al., 2005, 2002) (Table 2). We therefore were surprised to find that the CTD proteins mediated inefficient repression at lacO1-substitution constructs (Table 4) (Lin, 2009), because repression is a function of DNA-binding affinity (Schlax et al., 1995). This implies that, in vivo, the receiver domain stabilizes the CTD, enhances DNA binding or enables repression per se.
By contrast, we were not surprised to find that the CTD proteins did not activate transcription from the Nar class I control regions for the narG and fdnG operons (Lin, 2009), because we hypothesize that the NarL receiver mediates cooperative binding to the multiple sites in these control regions (Stewart & Bledsoe, 2008). Additionally, the receiver may make protein–protein contacts necessary for transcription activation, as shown by isolation of PC substitutions in the FixJ receiver and TraR amino-terminal ligand-binding domain (Costa et al., 2009; Qin et al., 2009; Ton-Hoang et al., 2001).
Acknowledgments
We are indebted to Peggy Bledsoe and Li-Ling Chen for their substantial technical contributions to this study. We thank Michele Igo and Enoch Baldwin for helpful advice and guidance, Hui-Chung Wu for her early work to isolate narL PC mutants, Juan Parales for help and advice with protein purification, and Mary Berlyn, Yale University, New Haven, CT, USA, and Barry Wanner, Purdue University, West Lafayette, IN, USA, for strains and plasmids. This study was supported by a US Public Health Service Grant from the National Institute of General Medical Sciences (grant no. GM036877).
Abbreviations
CTD, carboxyl-terminal domain
EMSA, electrophoretic mobility shift assay
HTH, helix–turn–helix
PC, positive control
References
- Aravind, L., Anantharaman, V., Balaji, S., Babu, M. M. & Iyer, L. M. (2005). The many faces of the helix-turn-helix domain: transcription regulation and beyond. FEMS Microbiol Rev 29, 231–262. [DOI] [PubMed] [Google Scholar]
- Baikalov, I., Schröder, I., Kaczor-Grzeskowiak, M., Grzeskowiak, K., Gunsalus, R. P. & Dickerson, R. E. (1996). Structure of the Escherichia coli response regulator NarL. Biochemistry 35, 11053–11061. [DOI] [PubMed] [Google Scholar]
- Barnard, A., Wolfe, A. & Busby, S. (2004). Regulation at complex bacterial promoters: how bacteria use different promoter organizations to produce different regulatory outcomes. Curr Opin Microbiol 7, 102–108. [DOI] [PubMed] [Google Scholar]
- Bartolomé, B., Jubete, Y., Martinez, E. & de la Cruz, F. (1991). Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBR322 and its derivatives. Gene 102, 75–78. [DOI] [PubMed] [Google Scholar]
- Browning, D., Lee, D., Green, J. & Busby, S. (2002). Secrets of bacterial transcription initiation taught by the Escherichia coli FNR protein. In Signals, Switches, Regulons, and Cascades: Control of Bacterial Gene Expression, Society for General Microbiology Symposium series vol. 61, pp. 127–142. Edited by Hodgson, D. A. & Thomas, C. M.. Reading, UK: Society for General Microbiology.
- Chang, A. C. Y. & Cohen, S. N. (1978). Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol 134, 1141–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, S. H. & Greenberg, E. P. (1991). The C-terminal region of the Vibrio fischeri LuxR protein contains an inducer-independent lux gene activating domain. Proc Natl Acad Sci U S A 88, 11115–11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, E. D., Cho, H. & Winans, S. C. (2009). Identification of amino acid residues of the pheromone-binding domain of the transcription factor TraR that are required for positive control. Mol Microbiol 73, 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crater, D. L. & Moran, C. P., Jr (2002). Two regions of GerE required for promoter activation in Bacillus subtilis. J Bacteriol 184, 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin, A. J., Tyson, K. L., Busby, S. J. & Stewart, V. (1997). Differential regulation by the homologous response regulators NarL and NarP of Escherichia coli K-12 depends on DNA binding site arrangement. Mol Microbiol 25, 583–595. [DOI] [PubMed] [Google Scholar]
- Datsenko, K. A. & Wanner, B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97, 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducros, V. M., Lewis, R. J., Verma, C. S., Dodson, E. J., Leonard, G., Turkenburg, J. P., Murshudov, G. N., Wilkinson, A. J. & Brannigan, J. A. (2001). Crystal structure of GerE, the ultimate transcriptional regulator of spore formation in Bacillus subtilis. J Mol Biol 306, 759–771. [DOI] [PubMed] [Google Scholar]
- Egan, S. M. & Stewart, V. (1990). Nitrate regulation of anaerobic respiratory gene expression in narX deletion mutants of Escherichia coli K-12. J Bacteriol 172, 5020–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egland, K. A. & Greenberg, E. P. (2001). Quorum sensing in Vibrio fischeri: analysis of the LuxR DNA binding region by alanine-scanning mutagenesis. J Bacteriol 183, 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge, A. M., Kang, H. S., Johnson, E., Gunsalus, R. & Dahlquist, F. W. (2002). Effect of phosphorylation on the interdomain interaction of the response regulator, NarL. Biochemistry 41, 15173–15180. [DOI] [PubMed] [Google Scholar]
- Galperin, M. Y. (2006). Structural classification of bacterial response regulators: diversity of output domains and domain combinations. J Bacteriol 188, 4169–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldimann, A. & Wanner, B. L. (2001). Conditional-replication, integration, excision, and retrieval plasmid–host systems for gene structure–function studies of bacteria. J Bacteriol 183, 6384–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff, S., Wallace, J. C. & Brown, J. P. (1990). Finding protein similarities with nucleotide sequence databases. Methods Enzymol 183, 111–132. [DOI] [PubMed] [Google Scholar]
- Kahn, D. & Ditta, G. (1991). Modular structure of FixJ: homology of the transcriptional activator domain with the −35 binding domain of sigma factors. Mol Microbiol 5, 987–997. [DOI] [PubMed] [Google Scholar]
- Kiley, P. J. & Beinert, H. (1998). Oxygen sensing by the global regulator, FNR: the role of the iron–sulfur cluster. FEMS Microbiol Rev 22, 341–352. [DOI] [PubMed] [Google Scholar]
- Kurashima-Ito, K., Kasai, Y., Hosono, K., Tamura, K., Oue, S., Isogai, M., Ito, Y., Nakamura, H. & Shiro, Y. (2005). Solution structure of the C-terminal transcriptional activator domain of FixJ from Sinorhizobium meliloti and its recognition of the fixK promoter. Biochemistry 44, 14835–14844. [DOI] [PubMed] [Google Scholar]
- Lamberg, K. E. & Kiley, P. J. (2000). FNR-dependent activation of the class II dmsA and narG promoters of Escherichia coli requires FNR-activating regions 1 and 3. Mol Microbiol 38, 817–827. [DOI] [PubMed] [Google Scholar]
- Li, J. & Stewart, V. (1992). Localization of upstream sequence elements required for nitrate and anaerobic induction of fdn (formate dehydrogenase-N) operon expression in Escherichia coli K-12. J Bacteriol 174, 4935–4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, A. V. (2009). Factors influencing transcription activation by response regulators NarP and NarL of Escherichia coli. PhD dissertation, University of California, Davis, USA.
- Lin, H.-Y., Bledsoe, P. J. & Stewart, V. (2007). Activation of yeaR–yoaG operon transcription by the nitrate-responsive regulator NarL is independent of oxygen-responsive regulator Fnr in Escherichia coli K-12. J Bacteriol 189, 7539–7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy, S. R., Stewart, V. J. & Taylor, R. K. (1996). Genetic Analysis of Pathogenic Bacteria: a Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
- Marchler-Bauer, A., Anderson, J. B., Chitsaz, F., Derbyshire, M. K., DeWeese-Scott, C., Fong, J. H., Geer, L. Y., Geer, R. C., Gonzales, N. R. & other authors (2009). CDD: specific functional annotation with the Conserved Domain Database. Nucleic Acids Res 37, D205–D210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris, A. E., Sawaya, M. R., Kaczor-Grzeskowiak, M., Jarvis, M. R., Bearson, S. M., Kopka, M. L., Schröder, I., Gunsalus, R. P. & Dickerson, R. E. (2002). Dimerization allows DNA target site recognition by the NarL response regulator. Nat Struct Biol 9, 771–778. [DOI] [PubMed] [Google Scholar]
- Maris, A. E., Kaczor-Grzeskowiak, M., Ma, Z., Kopka, M. L., Gunsalus, R. P. & Dickerson, R. E. (2005). Primary and secondary modes of DNA recognition by the NarL two-component response regulator. Biochemistry 44, 14538–14552. [DOI] [PubMed] [Google Scholar]
- Miller, J. H. (1972). Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
- Morrison, T. B. & Parkinson, J. S. (1994). Liberation of an interaction domain from the phosphotransfer region of CheA, a signaling kinase of Escherichia coli. Proc Natl Acad Sci U S A 91, 5485–5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser, W. & Reverchon, S. (2007). New insights into the regulatory mechanisms of the LuxR family of quorum sensing regulators. Anal Bioanal Chem 387, 381–390. [DOI] [PubMed] [Google Scholar]
- Noriega, C. E., Lin, H.-Y., Chen, L.-L., Williams, S. B. & Stewart, V. (2010). Asymmetric cross-regulation between the nitrate-responsive NarX–NarL and NarQ–NarP two-component regulatory systems from Escherichia coli K-12. Mol Microbiol 75, 394–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas, K. M., Weingart, C. L. & Winans, S. C. (2004). Chemical communication in proteobacteria: biochemical and structural studies of signal synthases and receptors required for intercellular signalling. Mol Microbiol 53, 755–769. [DOI] [PubMed] [Google Scholar]
- Qin, Y., Keenan, C. & Farrand, S. K. (2009). N- and C-terminal regions of the quorum-sensing activator TraR cooperate in interactions with the alpha and sigma-70 components of RNA polymerase. Mol Microbiol 74, 330–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin, R. S. & Stewart, V. (1992). Either of two functionally redundant sensor proteins, NarX and NarQ, is sufficient for nitrate regulation in Escherichia coli K-12. Proc Natl Acad Sci U S A 89, 8419–8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlax, P. J., Capp, M. W. & Record, M. T., Jr (1995). Inhibition of transcription initiation by lac repressor. J Mol Biol 245, 331–350. [DOI] [PubMed] [Google Scholar]
- Squire, D. J., Xu, M., Cole, J. A., Busby, S. J. & Browning, D. F. (2009). Competition between NarL-dependent activation and Fis-dependent repression controls expression from the Escherichia coli yeaR and ogt promoters. Biochem J 420, 249–257. [DOI] [PubMed] [Google Scholar]
- Stewart, V. & Bledsoe, P. J. (2003). Synthetic lac operator substitutions to study the nitrate- and nitrite-responsive NarX–NarL and NarQ–NarP two-component regulatory systems of Escherichia coli K-12. J Bacteriol 185, 2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, V. & Bledsoe, P. J. (2005). Fnr-, NarP- and NarL-dependent regulation of transcription initiation from the Haemophilus influenzae Rd napF (periplasmic nitrate reductase) promoter in Escherichia coli K-12. J Bacteriol 187, 6928–6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, V. & Bledsoe, P. J. (2008). Substitutions at auxiliary operator O3 enhance repression by nitrate-responsive regulator NarL at synthetic lac control regions in Escherichia coli K-12. J Bacteriol 190, 428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, V. & Parales, J. (1988). Identification and expression of genes narL and narX of the nar (nitrate reductase) locus in Escherichia coli K-12. J Bacteriol 170, 1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, V. & Rabin, R. S. (1995). Dual sensors and dual response regulators interact to control nitrate- and nitrite-responsive gene expression in Escherichia coli. In Two-Component Signal Transduction, pp. 233–252. Edited by Hoch, J. A. & Silhavy, T. J.. Washington, DC: American Society for Microbiology.
- Stewart, V., Bledsoe, P. J. & Williams, S. B. (2003). Dual overlapping promoters control napF (periplasmic nitrate reductase) operon expression in Escherichia coli K-12. J Bacteriol 185, 5862–5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton-Hoang, B., Salhi, M., Schumacher, J., Da Re, S. & Kahn, D. (2001). Promoter-specific involvement of the FixJ receiver domain in transcriptional activation. J Mol Biol 312, 583–589. [DOI] [PubMed] [Google Scholar]
- Vannini, A., Volpari, C., Gargioli, C., Muraglia, E., Cortese, R., De Francesco, R., Neddermann, P. & Marco, S. D. (2002). The crystal structure of the quorum sensing protein TraR bound to its autoinducer and target DNA. EMBO J 21, 4393–4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, C. E. & Winans, S. C. (2005). Identification of amino acid residues of the Agrobacterium tumefaciens quorum-sensing regulator TraR that are critical for positive control of transcription. Mol Microbiol 55, 1473–1486. [DOI] [PubMed] [Google Scholar]
- White, C. E. & Winans, S. C. (2007). The quorum-sensing transcription factor TraR decodes its DNA binding site by direct contacts with DNA bases and by detection of DNA flexibility. Mol Microbiol 64, 245–256. [DOI] [PubMed] [Google Scholar]
- Xiao, G., Cole, D. L., Gunsalus, R. P., Sigman, D. S. & Chen, C. H. (2002). Site-specific DNA cleavage of synthetic NarL sites by an engineered Escherichia coli NarL protein-1,10-phenanthroline cleaving agent. Protein Sci 11, 2427–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., Zhou, Y., Ebright, Y. W. & Ebright, R. H. (1992). Catabolite gene activator protein (CAP) is not an “acidic activating region” transcription activator protein. J Biol Chem 267, 8136–8139. [PubMed] [Google Scholar]
- Zhang, R. G., Pappas, T., Brace, J. L., Miller, P. C., Oulmassov, T., Molyneaux, J. M., Anderson, J. C., Bashkin, J. K., Winans, S. C. & Joachimiak, A. (2002). Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA. Nature 417, 971–974. [DOI] [PubMed] [Google Scholar]
- Zheng, L., Halberg, R., Roels, S., Ichikawa, H., Kroos, L. & Losick, R. (1992). Sporulation regulatory protein GerE from Bacillus subtilis binds to and can activate or repress transcription from promoters for mother-cell-specific genes. J Mol Biol 226, 1037–1050. [DOI] [PubMed] [Google Scholar]