Abstract
Unilateral intrahippocampal injections of tetrodotoxin were used to temporarily inactivate one hippocampus during specific phases of training in an active allothetic place avoidance task. The rat was required to use landmarks in the room to avoid a room-defined sector of a slowly rotating circular arena. The continuous rotation dissociated room cues from arena cues and moved the arena surface through a part of the room in which foot-shock was delivered. The rat had to move away from the shock zone to prevent being transported there by the rotation. Unilateral hippocampal inactivations profoundly impaired acquisition and retrieval of the allothetic place avoidance. Posttraining unilateral hippocampal inactivation also impaired performance in subsequent sessions. This allothetic place avoidance task seems more sensitive to hippocampal disruption than the standard water maze task because the same unilateral hippocampal inactivation does not impair performance of the variable-start, fixed hidden goal task after procedural training. The results suggest that the hippocampus not only encodes allothetic relationships amongst landmarks, it also organizes perceived allothetic stimuli into systems of mutually stable coordinates. The latter function apparently requires greater hippocampal integrity.
The rodent hippocampus is a key neural system for processing information about the current spatial arrangement of stimuli and events (1–4), but it is still unclear what spatial computations the hippocampus actually does. One direction of the current experimental effort in this area is studying what aspects of spatial information the hippocampal network stores (5–12); a second is studying what spatial computations an intact hippocampal system is necessary for (13–19).
It is crucial for this latter approach that there are behavioral paradigms, with clearly defined demands, that subjects must solve by using a limited set of potential solutions. The standard, variable-start, fixed hidden goal place navigation task in the water maze (20) has been invaluable because it allows the optimal solution to be readily distinguished from other less efficient strategies. The optimal strategy is for the rat to learn the allothetic relationships between distant, typically visual stimuli and the position of the escape platform. The absence of stable stimuli on the liquid substrate, like visual and tactile marks and odor cues, and the use of variable start locations make it difficult after standard training for the rat to solve the task optimally by using beacon-guided, praxis, or route-following strategies.
Since the early eighties, place learning in the water maze has been used in lesion studies to elucidate the role of the hippocampus in spatial cognition (21, 22). Selective bilateral lesions of the dentate gyrus and CA3-CA4 were found to severely impair water maze performance (23). However, in that study, unilateral dentate gyrus lesions, but not CA3-CA4 lesions disturbed water maze performance.
One difficulty in interpreting the results of experiments using permanent lesions is that neural reorganization during recovery may occur to the extent that different strategies and even different brain areas are able to compensate for the initial deficit. Acute reversible lesions that only temporarily disturb the function of a structure have the advantage that the behavioral effects of the inactivation cannot be readily compensated, and thus effects are typically more pronounced (24).
The standard water maze task was used in combination with reversible hippocampal inactivation to learn that one functioning hippocampus was sufficient for the optimal allothetic solution (18). Once the rats had learned the procedural aspects of the task, learning in the water maze during unilateral hippocampal inactivation caused the memory for the location of the escape platform to be lateralized to the hemisphere of the intact hippocampus (18, 25). Subsequent experiments even showed that the lateralized place navigation engram could be made available to the naive, previously inactivated side after only a brief exposure to the environment with both hippocampi intact (19, 25). These experiments provide strong evidence that rats can encode and retrieve allothetic spatial memories with only one functioning hippocampus.
We investigate what information the hippocampus encodes by studying how allothetic and idiothetic information controls hippocampal electrophysiology and the rat's concurrent, purposeful behavior. We have thus developed dry arena navigation tasks that allow us to control the information indispensable for the solution of the task (26–30). In one of these paradigms, the “active allothetic place avoidance” task, the rat is in a continuously concentrically rotating cylindrical arena with an electrifiable grid floor. The arena surface moves through a fixed room-defined arena sector where shocks are administered. The rat must use distant landmarks stable in the experimental room to avoid the shock by moving away from the punished area whenever the arena rotation brings it close to the shock sector (31). Successful avoidance has been shown, as in the water maze (25), to depend on the integrity of the dorsal hippocampal formation, because bilateral tetrodotoxin (TTX) inactivation abolishes the avoidance (32). In the present experiments, we asked whether, like in the standard water maze task, the rats would be unimpaired by inactivating only one hippocampus with TTX.
Surprisingly, inactivating one hippocampus prevented the acquisition, retrieval, and even the consolidation of this active allothetic avoidance memory. The results indicate that this task is extremely sensitive to impairment of hippocampal function, more so than the variable-start, fixed hidden goal water maze task. This enhanced sensitivity to hippocampal disruption suggests that the role of the hippocampus in spatial navigation and cognition is not merely in encoding allothetic relationships amongst environmental stimuli, but rather in the organization of the perceived allothetic stimuli into systems sharing mutually stable coordinates.
Materials and Methods
Subjects.
Twenty-eight 3-month-old male rats of the Long Evans strain were obtained from the breeding colony of the Institute of Physiology, Prague and housed two per cage in a vivarium at 20–21°C with natural lighting. Food and water were freely available. Experiments were run between 10:00 and 19:00. All procedures were in accordance with Institutional, Czech, and National Institutes of Health guidelines for the treatment of laboratory animals. A within-subjects design was used to minimize the number of animals in the study.
Surgery.
Stainless steel, 22-gauge guide cannulae were implanted bilaterally to allow intrahippocampal injections of TTX. The rats were mounted in a stereotaxic frame under ketamine (50 mg/kg i.p.) and xilazynum (20 mg/kg i.m.), anesthesia. Two holes were drilled in the exposed skull for implanting the 10-mm-long guide cannulae. The cannulae tips were positioned above the dorsal hippocampus 2 mm below the skull, 4 mm caudal from bregma, and 2.7 mm lateral from the midline (33). An anchoring bolt was fixed in the bone caudal to one of the guides and cemented with dental acrylate to the bone along with the cannulae. The skin was sutured around the implant and the cannulae were closed with paraffin oil smeared mandrels. Training started 1 week later.
Intrahippocampal Injections.
Unilateral control injections of 1 μl saline, or inactivating injections of 5 ng of TTX in 1 μl saline were made through a guide cannula. The rat was restrained by hand, the mandrels were removed, and a 30-gauge injection needle was inserted into one guide cannula so that the needle protruded 2 mm from the guide into the dorsal hippocampus. The needle was attached to a 5-μl Hamilton syringe by a short piece of polyethylene tubing. The 1-μl solution was continuously injected over 60 s. The needle was left in place for another 90 s before it was slowly removed, and the mandrels were replaced.
Apparatus.
The apparatus was described in detail elsewhere (31). Briefly, it was located in a rectangular room (4 m × 3 m) with many extramaze landmarks. The arena was a 48-cm-diameter cylinder with a 40-cm-high transparent wall and an electrifiable grid floor. The cylinder floor was a square grid (50 cm side) formed by parallel metal rods (4 mm in diameter, 15 mm gap) that provided an internal structure to the arena surface. The even and odd rods were connected to a 50 Hz sine wave generated by a PC-controlled digital to analog converter through a custom circuit that limited the current across the poles to 1 mA. The cylinder was centered on a 90-cm-high, circular turntable that could be rotated about its center at 1 rpm by an electric motor.
The computer controlled the rotation and recorded the rat's position by using an infrared TV camera mounted along with six infrared light-emitting diodes (LEDs), 1.2 m above the arena. The TV signal was analyzed by a custom spot-tracker in the PC to find the location of an infrared reflector that was held between each rat's shoulders by a latex harness. A similar reflector marked a spot on the outer part of the arena, and its location. The rat and arena spots were detected every 100 ms to track the rat and rotation of the maze and thereby to calculate the rat's movement relative to the arena surface.
Basic Training.
The rats were handled for 10 min each day for 3 days. They were then habituated to the arena by being allowed to explore it for 10 min while it was stable and there was no shock. A day before the avoidance training began, they received a unilateral, habituating injection of TTX into the right hippocampus and were returned to the home cage.
For avoidance training, the rats were put on the arena across from the 60° shock sector defined by the PC-system. The arena began to rotate and whenever the rat entered the shock sector for more than 0.2 s, a mild (0.3–0.8 mA) electric foot shock lasting 0.3 s was delivered. If the rat did not leave the punished sector, an additional shock was given every 1.4 s until the rat left the shock sector. Sessions lasted 20 min. In the first session the shock current for each rat was increased from 0.3 mA in 0.06 mA steps until the shock elicited a reaction in the rat and it actively moved from the shock zone. Once set, the shock level was the same on all training days for an individual rat. Only one rat was in the experimental room at a time and it was returned to the home cage immediately after training. The time series of the room frame positions of the rat and arena marker, as well as of shock occurrence, were stored for off-line analysis.
Experimental Design and Analyses.
Avoidance was assessed by studying the rat position and the shock occurrence time-series in the room-frame. The number of entrances to the shock sector (N), the maximum time between entrances to the shocked area (MAX), and the latency to enter the shock area for the first time (T) are reported (average ± SEM). These performance measures were evaluated, when appropriate, by paired t test, or by repeated measures ANOVA followed by a Newman–Keul's post hoc test. Significance was accepted at P < 0.05.
The effect of a unilateral hippocampal inactivation was evaluated in three experiments. The same basic training and inactivation procedures were used for all experiments. The principal difference between the three experiments was at which stage of training the injections were given.
Experiment 1 was designed to test (i) the effect of unilateral inactivation on the retrieval of avoidance of the northeast (NE) sector of the arena that was well learned with both hippocampi intact, and (ii) the effect of unilateral hippocampal inactivation on learning to avoid the opposite 60° southwest (SW) sector of the arena.
Twelve rats were first trained with functioning hippocampi to avoid a stable room-defined NE sector of the arena for 5 consecutive days (ne1–ne5). Four hours after the ne5 session, the animals received their habituating TTX injection in the right hippocampus. The effect of the unilateral inactivation on retrieving the learned avoidance of the NE was tested on the following day (ne6-TTX) by injecting TTX into the right hippocampus 1 h before training. The effect of the injection itself was evaluated the next day (ne7-SAL) by injecting saline into the right hippocampus.
The next day (EXT), training continued with the shock switched off to extinguish the avoidance of the NE sector. On the next 3 days (sw1-TTX, sw2-TTX, sw3-TTX), TTX was injected into the left hippocampus before training the rats to avoid the opposite, SW sector.
Experiment 2 tested the effect of unilateral hippocampal blockade on initial acquisition. Nine naive rats (Initial-TTX) were trained from the start to do the task during unilateral hippocampal inactivation. The day after the habituation procedures were finished, the right hippocampus was inactivated and the rats were trained to avoid the shock sector in the NE. Performance of this group was compared with the Initial-intact performance of the Experiment 1 rats on day 1 (ne1) because that was the first day of intact training. Performance under TTX was also compared with the Trained-TTX performance of the Experiment 1 rats on day 6 (ne6-TTX) because on that day the rats had received TTX for the first time after they were familiar with the task. Because these two comparisons were made between groups, independent t tests were used for their evaluation.
Experiment 3 tested the effect of unilateral hippocampal inactivation on the consolidation of the allothetic place avoidance memory. Seven naive animals were used. The day after the habituation procedures, avoidance of the NE shock sector was trained for 7 days. After finishing the session on days 1 to 4, the right hippocampus was inactivated. After finishing the sessions on days 5 and 6 saline was injected into the right hippocampus. The learning curve was compared with that of the first five days of Experiment 1 (ne1–ne5) when naive animals learned the task with intact hippocampi.
After the experiments, the animals were deeply anesthetized with thiopental (100 mg/kg) and perfused transcardially with saline followed by 10% formalin. The brains were removed, fixed in formalin, and embedded in paraffin. Forty-micrometer histological slices were cut and stained with cresyl violet and the locations of the injection tracks were all verified to be in the dorsal hippocampus and within 0.7 mm of the target.
Results
Experiment 1: Retrieval and Learning to Avoid a Different Place.
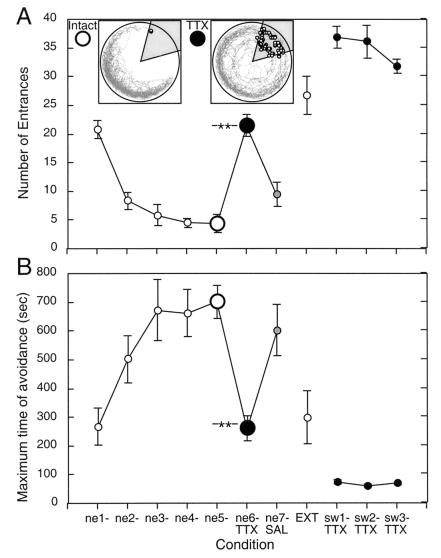
Fig. 1A shows the performance across all phases of Experiment 1 as measured by N. The rats learned rapidly; performance was already improved in the second session, and reached the performance asymptote by day 3 (31, 32). The TTX injection disturbed the avoidance, whereas the saline injection did not. After the intact extinction session, the inactivation was even more disturbing for acquisition of the new to-be-avoided opposite sector.
Figure 1.
Performance during Experiment 1 measured by N (A) and MAX (B). Avoidance of the room-defined NE sector was rapidly learned with both hippocampi intact (ne1–ne5). Performance was asymptotic by day 3. Retrieval of the avoidance was disrupted by unilateral hippocampal inactivation (ne6-TTX). Good avoidance was observed when on the next day, only saline was injected (ne7-SAL). The room frame track and shocks (open circles in the shaded punished sector) during experiments from a representative rat are shown as Insets. Asymptotic performance (ne5) is shown in the Left Inset. Performance on the next day (ne6-TTX) during TTX inactivation is shown in the Right Inset. Notice that the rat entered only once during the intact session (at 298 s), but entered 18 times (from 42 to 948 seconds) under TTX. During the TTX session it often reacted to the shock not by escaping, but by actively moving deeper into the shock area. On day 8 (EXT), the shock was turned off to extinguish the avoidance. The following three days (sw1-TTX–sw3-TTX), avoidance of the opposite, SW sector was trained during TTX inactivation of the left hippocampus. The rats were unable to learn this new avoidance. Performance was worse than when under TTX a previously learned avoidance could be remembered (ne6-TTX). The number of entrances increased to >50% more than what would occur if the rat sat immobile because after escaping a short distance from shock, the rats often sat motionless and were thus soon transported into the punished area again. Labels: Sessions during which the hippocampi were both functional are marked in the plots by open circles. Black and gray circles mark the sessions during TTX inactivation and following saline injection, respectively. Asterisks mark the P < 0.001 significance of the decrement in performance caused by TTX inactivation relative to the asymptotic performance on the previous day.
These impressions were confirmed by the repeated measures ANOVA on the training conditions (11 days). The effect of condition was significant (F10,110 = 42.4, P < 0.0001). According to the post hoc test, N decreased during intact training (ne1 > ne2 > ne3 = ne4 = ne5). During inactivation (ne6-TTX), N was larger relative to ne2 through ne5 and ne7-SAL. The disturbance was reversible, because after the saline injection, N reached a value statistically similar to the intact, asymptotic day 5 level (ne5 = ne7-SAL). In the extinction session, N increased to the initial level of day 1 and the level after TTX inactivation on day 6 (EXT = ne1 = ne6-TTX). Training to avoid the newly punished SW sector with the other, left hippocampus caused N to reach its highest values.
Evaluating MAX produced a similar pattern of results (Fig. 1B). Performance differed across days (F10,110 = 18.08, P < 0.001). MAX increased significantly from day 1 to the asymptote on day 3 (ne1 < ne2 < ne3 = ne4 = ne5). Inactivation caused MAX to drop to the level of day 1 (ne6-TTX = ne1). Performance after the saline injection was at the asymptote (ne7-SAL = ne3 = ne4 = ne5), but as expected, during the extinction session MAX decreased to the first day (ne1) and inactivation day (ne6-TTX) levels of performance (EXT = ne1 = ne6-TTX).
Because the experiment was designed specifically to learn the effect of unilateral inactivation on retrieval and then acquisition of a new avoidance, these effects were evaluated with more specific analyses. The effect of the unilateral hippocampal inactivation on retrieval of the avoidance that was learned with intact hippocampi was evaluated by comparing performance during the inactivation (ne6-TTX) with the performance on the preceding (ne5) intact session (Fig. 1A, Inset). Avoidance was abolished during inactivation (N: t11 = 7.6; P < 0.0001; MAX: t11 = 6.1; P < 0.0001); on average the rat entered the shock area about once per min, and at best avoided entering the shocked area for about 260 s.
The inactivation and change of the to-be-avoided place caused the rats to enter the new shock area often, more than in any other condition (N ≈ 34). Consequently, MAX decreased to ≈67 s, well below the level in all of the other conditions. The repeated measures ANOVA comparing se1, se2, and se3 showed they were similar (N: F2,22 = 2.15; P > 0.05; MAX: F2,22 = 3.2; P > 0.05).
In summary, unilateral hippocampal inactivation impaired retrieval of the place avoidance memory that was learned during intact training. Strikingly, the inactivation also prevented the rats from learning a new place avoidance, even though they were familiar with the procedural aspects of the task.
Experiment 2: Initial Acquisition.
One explanation of the disrupted retrieval of bilaterally learned avoidance by unilateral blockade of hippocampus is that the avoidance memory was encoded in a distributed manner across all of the available hippocampal tissue (15). Because this was the whole hippocampus during intact learning (ne1–ne5), the inactivation would have permitted only partial, insufficient access to the distributed engram. If only one hippocampus was available during encoding, the memory would be encoded in the restricted functional network, and thus available for recall during subsequent unilateral inactivations of the same hippocampus. According to this interpretation, the rats should have been able to learn the new avoidance (sw1–sw3). However, despite the intervening extinction, this SW avoidance is essentially a reversal task for the functioning hippocampus, and because these may be more difficult to learn, the unilateral inactivation was sufficient to block it. Another possibility is that the first inactivation caused a state-dependent learned helplessness (i.e., inactivation is accompanied by inescapable shock) and so the rats did not even try to avoid shock on days sw1–sw3.
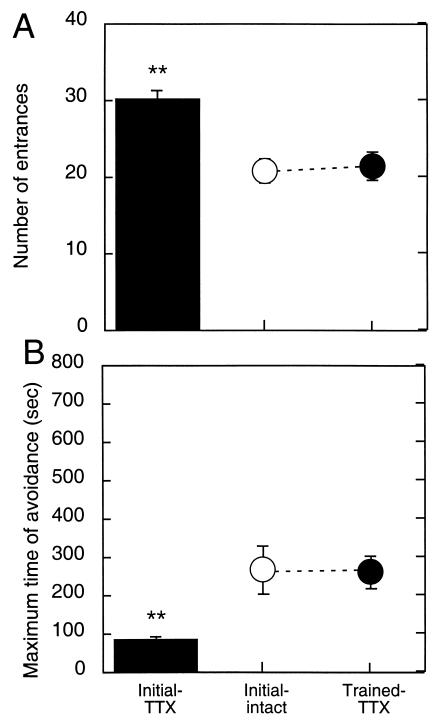
As in Experiment 1, the Initial-TTX rats could not avoid the shock sector (Fig. 2 A and B); they entered the shock sector on average 1.5 times per min, more than if they were immobile. Their performance was so poor, and similar to days se1–se3 of Experiment 1 (Fig. 2), that we considered it inappropriate to continue the avoidance training. The number of entrances during Initial-TTX was higher than in the Initial-intact group (t19 = 4.37; P = 0.0003). It was also higher than in the animals that were injected with TTX for the first time after they were trained (t19 = 3.52; P = 0.002). Similarly, during Initial-TTX performance, MAX was lower than during both the Initial-intact (t19 = 2.56; P = 0.02) and the Trained-TTX sessions (t19 = 3.62; P = 0.002). Therefore, the inactivation also disturbed the rats' ability to acquire this task, independent of prior avoidance learning and experience with apparently “inescapable” shock.
Figure 2.
Unilateral TTX inactivation blocked initial place avoidance learning. The number of entrances (A) was higher and the maximum time between entrances was lower (B) than during Experiment 1, when the rats first experienced the shock with intact hippocampi (Initial-intact) or when they first experienced the TTX inactivation after reaching the performance asymptote.
Experiment 3: Consolidation.
Experiments 1 and 2 demonstrated that unilateral inactivation of the hippocampus impairs not only the retrieval of the previously acquired place avoidance but also acquisition of a new avoidance. The inactivation may have disturbed the “online” processing underlying the allothetic avoidance by some noncognitive effect interfering with performance. To examine this possibility, in Experiment 3, place avoidance was trained and its retrieval tested in rats with functioning hippocampi. We asked whether the unilateral hippocampal inactivation elicited immediately after training disturbs allothetic learning by interfering only with the “offline” processing of the encoded information or with consolidation of the place avoidance memory.
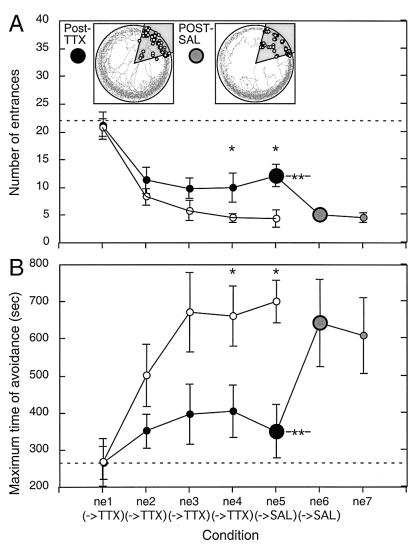
The posttraining unilateral inactivations caused a severe learning deficit. Performance improved from day 1 to day 2 when N was similar to that observed after intact training in Experiment 1. However, although the performance of intact rats was improved by further training, the posttraining inactivations prevented this subsequent improvement and by day 3 performance was asymptotic around N ≈ 10 and MAX ≈ 370 s (Fig. 3). Note that, nonetheless, performance was better than that observed during unilateral hippocampal inactivation in Experiments 1 and 2 (N > 22; MAX < 262 s).
Figure 3.
Inactivating one hippocampus after training (filled circles) disrupted place avoidance measured by the number of entrances (A) and the maximum time between entrances (B). Asymptotic performance was reached by day 3 (ne3→TTX), but the level of performance was worse than in Experiment 1 (open circles). Significantly worse performance according to the Newman-Keul's test is marked by a single asterisk. The posttraining inactivations caused a milder impairment compared with the inactivation given just before training in Experiment 1 (indicated by the broken horizontal lines). After training on day 5, the rats were injected with saline (ne5→SAL). The next day, performance was already improved relative to the previous day (double asterisk). The improvement was to the level of the intact asymptote and this was maintained on the next day following another posttraining saline injection. Representative performance during the last posttraining TTX (Left Inset) and the first posttraining saline (Right Inset) sessions are shown. The rat entered the shock sector for the first and last times at 54 s and 1,193 s during the posttraining TTX session, but only in the first half of the posttraining saline session (136 s to 582 s, first and last entrance times, respectively).
Saline was injected after training on days 5 and 6 and performance immediately (i.e., on day 6) improved to the intact asymptotic level. There was a significant effect of days (F6,36 = 10.7, P < 0.001). Compared with day 1, N was lower but did not change from days 2 to 5 following the posttraining inactivations. Once saline only was injected after training, the performance improved to a new stable asymptote (day 5 > day 6 = day 7).
The same analysis of MAX revealed an effect of days (F6,36 = 3.23, P < 0.01). The Newman–Keul's test showed that MAX was stable from days 1 to 5, and only improved on days 6 and 7 compared with day 1. We also calculated the latency to enter for the first time (T) in each session (Table 1). The ANOVA revealed there was a significant difference between days (F6,36 = 4.26, P < 0.003), and the post hoc test showed this was significantly longer on days 6 and 7 compared with days 1–5.
Table 1.
Latencies to the first entrance (T) in Experiment 3
| Condition | ne1→TTX | ne2→TTX | ne3→TTX | ne4→TTX | ne5→SAL | ne6→SAL | ne7 |
|---|---|---|---|---|---|---|---|
| Avg T (s) | 25.3 | 49.0 | 27.3 | 85.4 | 88.3 | 169.9* | 260.7* |
| SEM (s) | 3.9 | 10.3 | 7.8 | 39.4 | 26.0 | 56.0 | 83.0 |
T did not reliably increase until training in session ne6 (
). The preceding session (ne5→SAL) was followed by a unilateral hippocampal saline injection, for the first time instead of TTX. Thus, ne6 was the first session after which the bilaterally learned avoidance could be consolidated with both hippocampi functioning. T was also increased on ne7 (*) after only saline was injected following training in session ne6.
The performance was compared with the intact learning in Experiment 1 by two-way ANOVA (groups × days) with repeated measures on days. For the number of entrances, there was a significant effect of group (F1,17 = 5.2, P < 0.05) and days (F4,68 = 30.1, P < 0.001), but no interaction (F4,68 = 1.78, P > 0.05). The groups differed on days 4 and 5 because the intact animals entered the shock area less frequently, as also confirmed by t tests (t17 = 2.49, P < 0.05; t17 = 3.07, P < 0.007, respectively; Fig. 3A).
The ANOVA on MAX also revealed significant effects of group (F1,17 = 6.54, P < 0.05) and days (F4,68 = 6.84, P < 0.001), but no interaction (F4,68 = 2.32, P > 0.05). t tests revealed that on days 4 and 5 the intact group avoided longer (t17 = 2.19, P < 0.05; t17 = 3.83, P < 0.002, respectively) than the injected group, but the difference was not significant on day 3 (t17 = 1.85, P > 0.05) (Fig. 3B).
Finally, the analysis of the latency to the first entrance in each session showed a significant effect of group (F1,17 = 11.5, P < 0.01), days (F4,68 = 7.01, P < 0.001), and the interaction (F4,68 = 3.89, P < 0.003). On sessions 4 and 5, from the beginning of the session, the intact rats avoided shock longer (day 4: T = 339.2 ± 104.1 s; day 5: T = 461.8 ± 89.5 s) than the posttraining inactivated group (day 4: T = 85.4 ± 39.4 s; day 5: T = 88.3 ± 26.0 s).
Discussion
The Inactivation Procedure.
Using reversible inactivation of the hippocampus allowed us to block the activity of one hippocampus at discrete times during training in an active, allothetic place avoidance task. The inactivations made it possible to train the animals with both hippocampi functioning and then to test the effect of the unilateral inactivation on retrieval after avoidance performance was stable (Experiment 1). It also made it possible to repeatedly block hippocampal activity selectively after training (Experiment 3). This post-trial inactivation also substantially impaired place avoidance performance, thus indicating that the disruption was not merely due to state-dependent learning or a general effect on performance variables like the rat's reaction to shock, or its ability to see or locomote. Incidentally, the total distance the rats actively moved in a session was not altered by the inactivations (data not shown).
Important considerations for interpreting the results from any reversible lesion are the type and temporal and spatial extents of the blockade. We have used TTX extensively and discussed these issues at length (18, 24, 34). TTX, a specific blocker of voltage-dependent sodium channels, interferes with both impulse and synaptic activity. Based on experiments to quantify and model the time course and spatial spread of the TTX (34), our inactivations should have affected a spherical region about 1.4 mm in diameter and lasted more than 3 h. Because TTX also blocks activity in fibers that pass through but do not originate or terminate in the affected region, the placement of the injection should have also blocked activity in the fimbria-fornix, which in particular would have disrupted the cholinergic and GABAergic innervation of the entire dorsal–ventral hippocampus. These projections arise in the septum and control the hippocampal theta rhythm (35). Thus, the inactivations in this study are expected to compromise hippocampal function, both in the dorsal hippocampus—where the injections were made—and in the ventral hippocampus.
Comparison with Place Learning in the Water Maze.
Our unilateral inactivations produced large and clear impairments. Place avoidance could not be learned and the avoidance learned with both hippocampi could not be retrieved after blockade of one of them. These results appear to contrast with an excellent set of studies showing that only a small, bilateral, ≈25% tissue section of the dorsal hippocampus is necessary for spatial learning in the water maze and that ventral lesions tend not to affect standard water maze performance as long as the lesions do not encroach on the dorsal hippocampus (14, 15, 36).
In addition, it was reported that the retrieval, but not the acquisition, of water maze place learning was impaired by lesions that removed 40% of the dorsal hippocampus bilaterally (15). These data provide evidence that stored spatial information is distributed across the neurons throughout the functioning hippocampus rather than in discrete hippocampal subregions. Bilateral, reversible inactivations using muscimol confirmed this interpretation in that the inactivation also blocked retrieval, but not acquisition, of water maze place learning.
Either differences in the lesions or differences in the tasks may reconcile these data with the present results. Each is considered in turn. The inactivations in the present study were extensive. On this basis, it is possible to conclude that the impairment was similar to the effect of the large dorsal hippocampal lesions that was seen by the Mosers and their colleagues (15, 36), assuming that a sufficient amount of hippocampal tissue must be available in both hippocampi to support place learning. This assumption, however, is not correct because we have shown that the same unilateral inactivations used in this study do not disturb standard water maze performance (18) once the water maze procedure is learned.
Assuming that the standard water maze task is an “easier” place learning task than the active allothetic place avoidance may explain why unilateral hippocampal inactivation disturbs the latter and not the former. It has been shown, for example, that standard water maze performance is not disturbed by N-methyl-d-aspartate (NMDA) receptor antagonists (37, 38), but that the delayed-matching-to place (DMP) task in the water maze is impaired at long (20 min) but not short (15 s) delays (16). If this explanation is correct, then temporary inactivation of one hippocampus should impair DMP at long delays too.
A Specific Role of the Hippocampus in Organizing Information into Coordinate Frames?
The active allothetic avoidance task is learned as quickly as (see Fig. 1 and ref. 31), if not faster than, the standard water maze task. Thus, it is interesting to consider why the avoidance might be “more difficult.” It is well known that rats use distinct navigation strategies based on distinct classes of sensory cues to solve navigation problems (1, 39). In the present study, to avoid shock, the rats must use the room-based cues that define the shock sector. They cannot use substratal idiothesis (40) or the arena-based cues that support it because the rotation makes this arena-based coordinate frame unstable with respect to the to-be-avoided sector. Nonetheless, the rats are probably aware of this arena information because the discharge of some hippocampal place cells remains organized in the arena frame during rotation despite explicitly training the rat to solve a room-frame defined allothetic place preference task (11). A key difference between the active allothetic avoidance and the standard water maze tasks is that in the dry arena, but not the water maze, there is a supply of substrate-anchored olfactory and tactile marks as well as the idiothesis they support. To avoid the shock during rotation, the rat has to identify what landmarks and cues are mutually stable with the to-be-avoided sector. To avoid confusion, in the presence of stimuli that are moving in this coordinate system (but stable in another), the rat has to separate the perceived stimuli into their respective coordinate frames, which are composed of mutually stable elements.
When the arena is stable or the room is dark, the conflict between the room-frame and arena-frame bound stimuli is removed, and place learning is principally easier because any stable relations between the reinforced place and a landmark or idiothetic cue can be useful. In such conditions, when room and arena frame information are not dissociated, the rats readily learn to do a more complex, foraging-based place avoidance (26, 27) during unilateral hippocampal inactivation (unpublished observations). In the water maze, not only is the environment typically stable, the fluid substrate does not provide intramaze landmarks to support substratal idiothesis. Even so, a circulating current in the water caused increased escape latencies (41), perhaps because of the additional need for the rat to compensate for the passive rotation.
Necessity for Both Hippocampi or Sufficiently Many Cells?
The impairment of place avoidance by unilateral hippocampal inactivation may indicate that both hippocampi are needed for the input signal processing for separating and binding room frame and arena frame stimuli into their respective coordinate systems. Alternatively, it may indicate that more cells than are contained in one hippocampus are needed for these computations. Weaker unilateral and bilateral inactivations could be used to decide from among these alternatives.
Because both the hippocampi were functional during training in Experiment 3, and within-session performance improved, the observed impairment cannot be due to a failure of the online processing of input signals. Rather, it indicates that preventing the participation of both hippocampi in the posttraining processing of the avoidance task (by inactivating one) disturbed memory so much that subsequent intact brain retrieval showed a significantly weaker memory. Considered in the context of ref. 15, this may indicate that the distributed hippocampal network that initially encodes a memory must also be fully engaged during the off line processing and consolidation of the engram. Performance was only moderately impaired by the posttraining unilateral inactivation, possibly because the plastic changes underlying formation of the active place avoidance memory were to some extent consolidated in both hippocampi during the 20 min session.
In summary, unilateral hippocampal inactivation caused a robust impairment of allothetic place learning when the room and arena frames were continuously dissociated. The results suggest that in addition to encoding allothetic relations, the hippocampus may be even more crucial for the computations that allow the rat to organize allothetic stimuli into systems that share mutually stable coordinates.
Acknowledgments
We thank Dr. Edvard Moser for his constructive advice in revising an earlier draft, and Dr. Lynn Nadel and the University of Arizona for administering J. S. McDonnell Foundation Grant 98–38 CNS-QUA.05. M.W. was supported by a statutory grant from the State committee for Scientific Research (Poland) to the Nencki Institute. Additional support came from Granting Agency of the Czech Republic Grant 309/00/1656, and the 5th Framework Research and Technological Development Program of the European Commission (QLG3-CT-1999–00192).
Abbreviations
- TTX
tetrodotoxin
- NE
northeast
- SW
southwest
- N
number of entrances to the shock sector
- MAX
maximum time between entrances to the shocked area
- T
latency to enter the shock area for the first time
- EXT
extinction
References
- 1.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon; 1978. [Google Scholar]
- 2.O'Keefe J. Hippocampus. 1999;9:352–364. doi: 10.1002/(SICI)1098-1063(1999)9:4<352::AID-HIPO3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 4.Muller R U, Poucet B, Fenton A A, Cressant A. Hippocampus. 1999;9:413–422. doi: 10.1002/(SICI)1098-1063(1999)9:4<413::AID-HIPO7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Skaggs W E, McNaughton B L. J Neurosci. 1998;18:8455–8466. doi: 10.1523/JNEUROSCI.18-20-08455.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Keefe J, Burgess N. Nature (London) 1996;381:425–428. doi: 10.1038/381425a0. [DOI] [PubMed] [Google Scholar]
- 7.Fenton A A, Csizmadia G, Muller R U. J Gen Physiol. 2000;116:191–210. doi: 10.1085/jgp.116.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenton A A, Csizmadia G, Muller R U. J Gen Physiol. 2000;116:211–222. doi: 10.1085/jgp.116.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro M L, Tanila H, Eichenbaum H. Hippocampus. 1997;7:624–642. doi: 10.1002/(SICI)1098-1063(1997)7:6<624::AID-HIPO5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 10.Save E, Nerad L, Poucet B. Hippocampus. 2000;10:64–76. doi: 10.1002/(SICI)1098-1063(2000)10:1<64::AID-HIPO7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 11.Zinyuk L, Kubik S, Kaminsky Yu, Fenton A A, Bures J. Proc Natl Acad Sci USA. 2000;97:3771–3776. doi: 10.1073/pnas.050576397. . (First Published March 14, 2000; 10.1073/pnas.050576397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood E R, Dudchenko P A, Robitsek R J, Eichenbaum H. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 13.Whishaw I Q, McKenna J E, Maaswinkel H. Curr Opinion Neurobiol. 1997;7:228–234. doi: 10.1016/s0959-4388(97)80011-6. [DOI] [PubMed] [Google Scholar]
- 14.Moser M-B, Moser E I, Forrest E, Andersen P, Morris R G M. Proc Natl Acad Sci USA. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moser M-B, Moser E I. J Neurosci. 1998;18:7535–7542. doi: 10.1523/JNEUROSCI.18-18-07535.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steele R J, Morris R G M. Hippocampus. 1999;9:118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Riedel G, Micheau J, Larn A G M, Roloff E L, Martin S J, Bridge H, De Hoz L, Poeschel B, McCulloch J, Morris R G M. Nature Neurosci. 1999;2:898–905. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- 18.Fenton A A, Bures J. Behav Neurosci. 1993;107:552–564. doi: 10.1037//0735-7044.107.4.552. [DOI] [PubMed] [Google Scholar]
- 19.Fenton A A, Arolfo M P, Nerad L, Bures J. Hippocampus. 1995;5:16–24. doi: 10.1002/hipo.450050104. [DOI] [PubMed] [Google Scholar]
- 20.Morris R G M. Learning and Motivation. 1981;12:239–260. [Google Scholar]
- 21.Morris R G M, Garrud P, Rawlins J N P, O'Keefe J. Nature (London) 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 22.Sutherland R J, Kolb B, Whishaw I Q. Neurosci Lett. 1982;31:271–276. doi: 10.1016/0304-3940(82)90032-5. [DOI] [PubMed] [Google Scholar]
- 23.Sutherland R J, Whishaw I Q, Kolb B. Behav Brain Res. 1983;7:133–153. doi: 10.1016/0166-4328(83)90188-2. [DOI] [PubMed] [Google Scholar]
- 24.Bures J, Buresova O. Concepts Neurosci. 1990;1:69–89. [Google Scholar]
- 25.Fenton A A, Bures J. Neurosci. 1994;58:481–491. doi: 10.1016/0306-4522(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 26.Bures J, Fenton A A, Kaminsky Yu, Rossier J, Sacchetti B, Zinyuk L. Philos Trans R Soc London B. 1997;352:1515–1524. doi: 10.1098/rstb.1997.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bures J, Fenton A A, Kaminsky Yu, Wesierska M, Zahalka A. Neuropharmacology. 1998;37:689–699. doi: 10.1016/s0028-3908(98)00031-8. [DOI] [PubMed] [Google Scholar]
- 28.Klement D, Bures J. Proc Natl Acad Sci USA. 2000;97:2946–2951. doi: 10.1073/pnas.040576197. . (First Published February 25, 2000; 10.1073/pnas.040576197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossier J, Kaminsky Yu, Schenk F, Bures J. Behav Neurosci. 2000;14:273–284. [PubMed] [Google Scholar]
- 30.Bures J, Fenton A A. News Physiol Sci. 2000;15:233–240. doi: 10.1152/physiologyonline.2000.15.5.233. [DOI] [PubMed] [Google Scholar]
- 31.Cimadevilla J M, Kaminsky Yu, Fenton A A, Bures J. J Neurosci Methods. 2000;102:155–164. doi: 10.1016/s0165-0270(00)00288-0. [DOI] [PubMed] [Google Scholar]
- 32.Cimadevilla J M, Fenton A A, Bures J. Neurosci Lett. 2000;285:53–56. doi: 10.1016/s0304-3940(00)01019-3. [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson M. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1986. [Google Scholar]
- 34.Zhuravin I A, Bures J. Exp Brain Res. 1991;83:687–690. doi: 10.1007/BF00229849. [DOI] [PubMed] [Google Scholar]
- 35.Buzsaki G, Leung L W, Vanderwolf C H. Brain Res. 1983;287:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- 36.Moser E, Moser M-B, Andersen P. J Neurosci. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bannerman D M, Good M A, Butcher S P, Ramsay M, Morris R G M. Nature (London) 1995;378:182–186. doi: 10.1038/378182a0. [DOI] [PubMed] [Google Scholar]
- 38.Saucier D, Cain D P. Nature (London) 1995;378:186–189. doi: 10.1038/378186a0. [DOI] [PubMed] [Google Scholar]
- 39.Gallistel C R. The Organization of Learning. Cambridge, MA: MIT Press; 1990. [Google Scholar]
- 40.Mittelstaedt M-L, Mittelstaedt H. Naturwissenschaften. 1980;67:566–567. [Google Scholar]
- 41.Moghaddam M, Bures J. Neurobiol Learn Mem. 1997;68:239–251. doi: 10.1006/nlme.1997.3800. [DOI] [PubMed] [Google Scholar]