Abstract
Both Mycoplasma hominis and Trichomonas vaginalis utilize arginine as an energy source via the arginine dihydrolase (ADH) pathway. It has been previously demonstrated that M. hominis forms a stable intracellular relationship with T. vaginalis; hence, in this study we examined the interaction of two localized ADH pathways by comparing T. vaginalis strain SS22 with the laboratory-generated T. vaginalis strain SS22-MOZ2 infected with M. hominis MOZ2. The presence of M. hominis resulted in an approximately 16-fold increase in intracellular ornithine and a threefold increase in putrescine, compared with control T. vaginalis cultures. No change in the activity of enzymes of the ADH pathway could be demonstrated in SS22-MOZ2 compared with the parent SS22, and the increased production of ornithine could be attributed to the presence of M. hominis. Using metabolic flow analysis it was determined that the elasticity of enzymes of the ADH pathway in SS22-MOZ2 was unchanged compared with the parent SS22; however, the elasticity of ornithine decarboxylase (ODC) in SS22 was small, and it was doubled in SS22-MOZ2 cells. The potential benefit of this relationship to both T. vaginalis and M. hominis is discussed.
INTRODUCTION
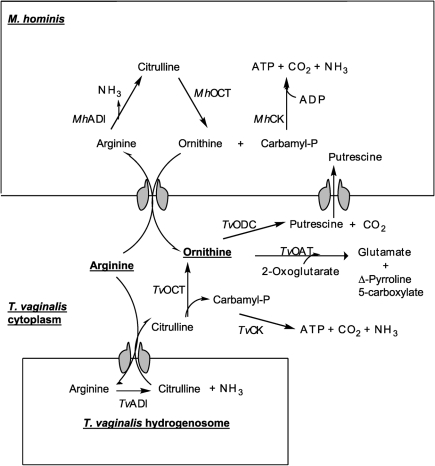
The arginine dihydrolase (ADH) pathway catalyses the conversion of arginine to ornithine and ammonia via the enzymes arginine deiminase (ADI), catabolic ornithine carbamyltransferase (cOCT) and carbamate kinase (CK) (Fig. 1). Cumulatively, the pathway removes nitrogen from amino acids with the generation of ATP, performing a function analogous to that of the urea cycle of vertebrates. The ADH pathway is present in some protists such as Trichomonas vaginalis (Linstead & Cranshaw, 1983) and Giardia intestinalis (Schofield et al., 1990), as well as some Gram-positive (Streptococcus spp.; Griswold et al., 2004) and Gram-negative bacteria (Pseudomonas spp.; Lu et al., 2004), and some Mollicutes (Mycoplasma hominis, Mycoplasma arginini; Fenske & Kenny, 1976; Das et al., 2004), in which it has been proposed to function as an alternative ATP-generating mechanism. The Giardia lamblia ADI is proposed to have multiple functions, including a peptidylarginine deiminase that converts protein bound arginine to citrulline (Touz et al., 2008). No such activity has been demonstrated for T. vaginalis, in which the pathway is proposed to function solely for energy generation, contributing about 10 % of the total energy requirements (Yarlett et al., 1996). In T. vaginalis, the first and rate-limiting enzyme, ADI, is localized to a subcellular fraction, possibly the hydrogenosome, whereas the other enzymes of the pathway are present in the cytoplasmic fraction (Yarlett et al., 1994).
Fig. 1.
Enzymes of the ADH pathway. Enzyme reactions that occur in the hydrogenosome are indicated in the lower box; enzyme reactions that occur in the intracellular M. hominis are indicated in the upper box. M. hominis and T. vaginalis enzymes are prefixed Mh and Tv, respectively.
T. vaginalis and several Mycoplasma species (M. hominis, Mycoplasma genitalium, Ureaplasma urealyticum) are common urogenital parasites of vertebrates. In addition, M. hominis is commonly found as an intracellular parasite of T. vaginalis (Rappelli et al., 1998; Dessì et al., 2005), but not of Tritrichomonas foetus (Dessì et al., 2005). In a study of 35 patients with trichomoniasis, M. hominis was isolated from 33 patients by in vitro culture of T. vaginalis; no other Mycoplasma species was detected (Dessì et al., 2005). The exact nature of this intimate relationship between T. vaginalis and M. hominis is unknown, but it has been proposed to be saprophytic (Rappelli et al., 1998). This study attempts to clarify the metabolism of arginine in T. vaginalis infected with M. hominis, and to determine whether this relationship benefits T. vaginalis and/or M. hominis.
METHODS
Cultures.
T. vaginalis SS22, T. vaginalis infected with M. hominis (SS22-MOZ2) and M. hominis (MOZ2) were cultured in tryptose–yeast extract–maltose medium supplemented with 10 % horse serum (Diamond, 1957). The presence of M. hominis in T. vaginalis SS22-MOZ2 was detected by PCR using the primers RNAH1 and RNAH2 for M. hominis (Blanchard et al., 1993), as previously described (Rappelli et al., 1998). The isogenic strain SS22-MOZ2 was obtained by infecting SS22 with M. hominis (MOZ2) isolated from a naturally occurring T. vaginalis strain, MPM-02, infected with M. hominis. Briefly, T. vaginalis MPM-02 was harvested during exponential growth by centrifugation at 350 g, and the supernatant was filtered through a 0.45 μm pore-size filter membrane. An aliquot of filtered supernatant containing 106 (calculated as c.f.u.) mycoplasma was added to a 10 ml culture of T. vaginalis SS22. Identical bacterial suspensions were inoculated both on 10 ml fresh Diamond's medium (Diamond, 1957) and on 10 ml T. vaginalis-conditioned medium (obtained from a filtered overnight culture of mycoplasma-free SS22). All samples were then daily passaged with 1 : 16 dilution for 30 days. Exponential-phase cells were harvested at 4000 g for 10 min at 4 °C in a Sorvall RC-2B centrifuge (DuPont) and washed in a buffer containing 30 mM sodium phosphate, 74 mM sodium chloride, 0.6 mM calcium chloride and 1.6 mM potassium chloride, pH 7.4. Washed cells were resuspended in 225 mM sucrose–10 mM Tris isotonic buffer, pH 7.4.
Subcellular fractionation.
Concentrated cell pellets were broken by 35 strokes in a Potter–Elvehjem tissue homogenizer at 4 °C. The broken cells were diluted with 225 mM sucrose–10 mM Tris, pH 7.4, containing 1 mM calcium chloride and 1 mM magnesium chloride, and centrifuged successively at 400 g for 10 min, 2200 g for 10 min and 28 000 g for 30 min, yielding nuclear-enriched, hydrogenosome-enriched and lysosome-enriched pellets, respectively.
Density-gradient centrifugation.
Self-generating gradients were prepared using 50 % (w/v) Percoll containing 225 mM sucrose–10 mM Tris, pH 7.4, 1 mM calcium chloride and 1 mM magnesium chloride. Fractions (1 ml, 12–18 mg protein) were layered onto 10 ml Percoll mixture and centrifuged at 46 000 g for 45 min at 4 °C in a 6×12 ml swing-out rotor (Beckman OTD 95). Fractions were collected by removing 1 ml aliquots. The density of fractions was calculated from determining the weight of 1 ml volumes. Chemicals and reagents were supplied by Sigma.
For enzyme analysis, T. vaginalis SS22, SS22-MOZ2 and M. hominis MOZ2 were cultured as described above in 1 l of medium. Cells were harvested by centrifugation at 5000 g for 10 min, washed twice in PBS, and resuspended in 10 mM phosphate buffer, pH 7.4, containing 1 mg each of aprotinin, leupeptin and N-α-tosyl-l-lysinyl-chloromethylketone (TLCK) ml−1. Cells were stored at −70 °C until used.
Enzyme assays.
The integrity of the organelles was confirmed by performing all enzyme assays in isotonic buffered solutions (225 mM sucrose), to which 0.05 % Triton X-100 was added to demonstrate latency of activity. ADI (EC 3.5.3.6) was determined by measuring the colorimetric formation of citrulline at 37 °C. The assay contained 0.5–2.5 mM l-arginine, 40 mM MES, pH 8.0, and 0.07 mg protein in a final volume of 1.0 ml. After 30 min, the reaction was stopped by the addition of 0.075 ml 100 % (w/v) TCA, and the citrulline formed determined using diacetyl monoxime, as described by Boyde & Rahmatullah (1980). cOCT (EC 2.1.3.3) was determined by measuring 14CO2 release from l-[14C-carbamyl] citrulline. The reaction mixture contained 40 mM Tricine, pH 6.0, 0.1 mM l-citrulline, 0.1–1.0 mM l-[14C-carbamyl] citrulline [57.7 mCi mmol−1 (2135 MBq mmol−1)] (DuPont, N.E.N. Research Products) and 0.07 mg protein in a final volume of 1 ml. After incubation at 37 °C for 1 h, the reaction was stopped with 1 ml 40 % TCA and incubated for a further 30 min, and the CO2 was trapped using benzethonium hydroxide-soaked filter paper. In the anabolic direction, anabolic ornithine carbamyltransferase (aOCT) was determined by measuring citrulline formation with 10 mM ornithine in 0.1 M Tris buffer, pH 8.0, and 0.07 mg protein; the reaction was started by the addition of 10 mM carbamyl phosphate and incubated for 15 min at 37 °C. The reaction was stopped by the addition of 6 % TCA, and the amount of citrulline formed was determined as described by Boyde & Rahmatullah (1980). CK (EC 2.7.2.2) activity was determined in incubations containing 0.1–1.0 mM ADP, 20 mM MgSO4, 0.15 mM luciferin, 1 mg firefly lantern extract and 1 mM carbamyl phosphate, in 50 mM potassium phosphate buffer, pH 7.6. ATP formation was determined by monitoring luminescence using a photomultiplier tube. Ornithine decarboxylase (ODC; EC 4.1.1.17) was determined by measuring the release of 14CO2 from 0.05–1.0 mM 1-[14C]ornithine [42.5 mCi mmol−1 (1602.25 MBq mmol−1)] in 0.1 M acetate buffer, pH 6.5, containing 60 μM pyridoxal phosphate, as previously described (Yarlett et al., 1993). Malic enzyme (EC 1.1.1.39) was assayed using 6 mM triethanolamine, pH 6.8, containing 1 mM NAD, 0.66 mM MnCl2 and 0.1 % Triton X-100; the change at A340 was monitored upon the addition of 33 mM neutralized sodium malate (Dolezal et al., 2004). Determination of the pH optima of enzyme activities was performed using the above assay methods with pHs varying from 5.0 to 7.5 with 40 mM MES (pKa 6.15 at 20 °C) and from 7.0 to 9.0 with 40 mM HEPPS (pKa 8.0 at 20 °C). Protein concentrations were determined using the Lowry method.
HPLC.
The intracellular and extracellular amine content of T. vaginalis SS22, SS22-MOZ2 and M. hominis MOZ2 was determined by centrifugation of 24 h cultures at 5000 g for 10 min. Pellets were washed once with PBS, pH 7.4, and proteins removed by addition of 6 % TCA. Culture medium was mixed with 10 % (v/v) of 60 % TCA. Samples were microfuged at 14 000 g for 1 min, and amines were separated by reverse-phase HPLC using a series LC 410 pump (Perkin–Elmer) coupled to a C-18 10 μm particle size column (4.5×250 mm) at a flow rate of 1 ml min−1. The method employed a 70 min discontinuous gradient starting with 90 % (v/v) buffer A: 0.1 M sodium phosphate monobasic, pH 2.65, containing 8 mM octane sulfonic acid and 0.05 mM EDTA. Buffer B consisted of HPLC grade acetonitrile. Separation of amines used a 35 min discontinuous gradient switching to 80 % A at 15 min and 60 % A at 25 min, followed by a 10 min recycle time to regenerate the start conditions. Standards and samples were post-column derivatized by mixing with two parts 1.5 mM o-phthalaldehyde dissolved in 3 ml methanol and made up to 1 l with 0.5 M boric acid containing 0.43 M KOH and 0.014 M 2-mercaptoethanol, pH 10.4. The derivatized compounds were analysed using a fluorescence monitor (λexcit 320 nm, λem 455 nm). Areas under the peaks were determined using β-RAM computer software (IN/US Systems), version 1.62.
Enzyme kinetics.
Kinetic analysis of the ADH pathway in T. vaginalis SS22, SS22-MOZ2 and M. hominis MOZ2 was performed by varying the substrate for each enzyme and measuring the rate of product formation. Km and Vmax values were determined from straight-line Hanes–Woolf plots ([S]/v versus [S]). The specificity constant V/Km is derived from the initial slope of the graph of velocity versus substrate concentration for a reaction that obeys the Michaelis–Menten equation (Cornish-Bowden, 2004). This constant is therefore the second-order rate constant for the reaction at low substrate concentration, which is typically true for intracellular environments where substrate concentrations are below the Km concentration. The elasticity (ε), which is defined as the fractional change in rate of the enzyme for a fractional change in substrate concentration, was determined for each enzyme in the pathway from a plot of log(ν) versus log[S] (Fell, 1997). The intracellular concentration around which the elasticity was required was determined by HPLC of the intracellular amine concentration and the ν at this substrate concentration determined from the Hanes–Woolf plot. The enzyme elasticity was calculated from the slope of the double log plot ranging from 95 % of [S] to 105 % of [S]. Using the connectivity theorem (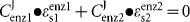 ), it is possible to express the ratio of the flux control coefficients for pairs of enzymes in the pathway under different conditions (
), it is possible to express the ratio of the flux control coefficients for pairs of enzymes in the pathway under different conditions (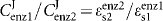 ).
).
Bioinformatic analyses.
The amino acid sequence of G. intestinalis (strain WB) ADI (accession no. XP_001705755) was used as the query for a blast search in the T. vaginalis (strain G3) genome database of Eukaryotic Pathogen Database Resources (http://eupathdb.org/eupathdb/). Three sequences with significant similarity to G. intestinalis ADI (TVAG_467820, TVAG_344520 and TVAG_183850) were aligned with M. hominis (accession no. D13314 0.1) and M. arginini (accession no. CAA38210) using clustal_x (Thompson et al., 1997) and manually edited with BioEdit software (Hall, 1999). Putative mitochondrial targeting sequences and cellular localization were predicted using the program psort II (http://psort.hgc.jp/).
Quantitative RT-PCR (RT-qPCR) of ADI.
To determine whether the elevated ADI activity in T. vaginalis SS22-MOZ2 compared with the parent SS22 was related to the additive effect of intracellular M. hominis or to upregulation of expression of T. vaginalis ADI, an RT-qPCR was designed to compare the mRNA expression of all three ADIs identified in the T. vaginalis genomic library. The three sets of primers were designed on the basis of sequence differences between M. hominis and T. vaginalis ADI. In particular, we used the following pairs of primers: TvADI 423-cDNA-92185, Tv92185F (5′-TTCGTCCAACCTTCAACTCAAGAAG-3′) and Tv92185R (5′-CTTTATCTGGATCTGGTGGTTTTTCATAG-3′), amplicon length 111 bp; TvADI-485-cDNA-96423, Tv96423F (5′-CGCGCATCATCAAGTTTTCGC-3′) and Tv96423R (5′-CTTTTTGGGATTCGGTGGGTGC-3′), amplicon length 119 bp; TvADI-185-cDNA-86485, Tv86485F (5′-GCTCTGTTCAATTCAACGCAG-3′) and Tv86485R (5′-GAATTGTGTGGCAGCTGTTGGTGG-3′), amplicon length 82 bp. These sequences were used to probe the M. hominis-infected (SS22-MOZ2) and -uninfected (SS22) strains of T. vaginalis. Results were compared with the expression of the actin gene (housekeeping gene) in M. hominis-infected and -uninfected T. vaginalis. RNA from cells was harvested by centrifugation and extracted with TRIzol reagent (Invitrogen), followed by double DNase I digestion for subsequent RT-qPCR with specific primers. Relative gene expression was calculated by the 2−ΔΔCt method, by using actin as housekeeping gene. Experiments were performed in triplicate, and variation in gene expression was quantified by calculating 2−ΔΔCt M. hominis-infected T. vaginalis/2−ΔΔCt : M. hominis-uninfected T. vaginalis ratios. The expression of all ADI genes was evaluated in triplicate in three different experiments.
RESULTS
Enzyme activities and metabolic flow through the ADH pathway of T. vaginalis with and without M. hominis
We have previously observed significant variations in the activities of the ADH enzymes, particularly ADI and cOCT, between different strains of T. vaginalis, and that was borne out by the variations in enzyme activities observed for strain SS22 in this study and by earlier data with strain C1 (Table 1 and Supplementary Table S1). The parent mycoplasma-free T. vaginalis strain (SS22) was infected in vitro with M. hominis (MOZ2), isolated from a naturally occurring T. vaginalis isolate, MPM02, infected with M. hominis (MOZ2) (Dessì et al., 2005). Typically, about 5–10 mycoplasma were observed in these strains (Fig. 2). Whole-cell extracts prepared from T. vaginalis SS22, T. vaginalis infected with M. hominis SS22-MOZ2 and M. hominis MOZ2 were examined for the kinetic properties of the enzymes of the ADH pathway. The enzymes ornithine carbamyltransferase (OCT), CK and ODC demonstrated minimal differences in kinetic properties between T. vaginalis infected with M. hominis SS22-MOZ2 and the parent uninfected T. vaginalis SS22 (Table 1). In contrast, the first and rate-limiting enzyme of the ADH pathway, ADI, demonstrated significant differences in Vmax (Table 1). The T. vaginalis infected with M. hominis SS22-MOZ2 had an average sixfold increased Vmax compared with the uninfected parent SS22 (Table 1). Overall, the T. vaginalis strain SS22-MOZ2 had a 19-fold increased specificity (Vmax/Km) for ADI compared with the parent strain SS22. The kinetic constants for ADI of M. hominis (MOZ2) indicated that the Vmax and Km for ADI were similar to those obtained for T. vaginalis strain SS22-MOZ2, suggesting that the increased specificity for this enzyme was the result of the presence of M. hominis. Kinetic analysis of ADH enzymes from M. hominis revealed that the specificity of ADI was 23-fold greater than that for T. vaginalis SS22; likewise, kinetic analysis of cOCT revealed a 227-fold greater specificity than that of T. vaginalis strain SS22 (Table 1); however, the ATP-forming enzyme CK was fivefold more efficient in T. vaginalis SS22 compared with M. hominis (Table 1). The combination of both ADH pathways in T. vaginalis SS22-MOZ2 had a higher overall specificity for conversion of arginine to ATP and ornithine, which may be beneficial to T. vaginalis and M. hominis. The elasticity (ε) of the enzymes of the pathway was determined using metabolic flow analysis (Fell, 1997). The only enzyme of the pathway with increased specificity was ODC (Table 2).
Table 1.
Kinetic properties of ADH enzymes in the parent T. vaginalis SS22, T. vaginalis infected with M. hominis (SS22-MOZ2) and M. hominis MOZ2
Enzyme assays were performed as described in Methods using whole-cell extracts. Vmax is expressed as μM min−1 using 1 mg protein. The Vmax and Km values are expressed as mean±sd for four determinations. nd, Not detected.
| Enzyme | T. vaginalis SS22 | T. vaginalis SS22-MOZ2 | M. hominis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Vmax (μM min−1) | Km (μM) | Vmax/Km | Vmax (μM min−1) | Km (μM) | Vmax/Km | Vmax (μM min−1) | Km (μM) | Vmax/Km | |
| ADI | 76.6±7.4 | 200±36 | 0.38 | 433±14.0 | 60±10 | 7.2 | 516±24 | 60±7.1 | 8.6 |
| cOCT | 40.1±4.5 | 138±18 | 0.29 | 48.0±12.1 | 125±23 | 0.38 | 11864±432 | 180±12 | 65.9 |
| aOCT | 1938±210 | 3400±170 | 0.57 | 1624±180 | 2900±240 | 0.56 | 75964±9258 | 4200±295 | 18.1 |
| CK | 3900±480 | 60±3.8 | 65.0 | 4274±390. | 83±12.0 | 51.5 | 1274±94 | 95±12 | 13.4 |
| ODC | 1.10±0.2 | 18±3.0 | 0.06 | 1.15±0.3 | 16±2.0 | 0.07 | nd | nd | – |
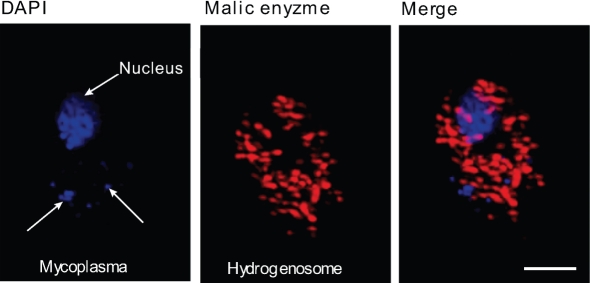
Fig. 2.
M. hominis in experimentally infected T. vaginalis strain SS22-MOZ 2. M. hominis was visualized by DNA staining using 4′,6-diamidino-2-phenylindole (DAPI; blue). Hydrogenosomes were stained for malic enzyme using rabbit polyclonal anti-malic enzyme antibody and Alexa Fluor 546-conjugated donkey anti-rabbit immunoglobulin (red). Bar, 10 μm.
Table 2.
Flux control coefficients for enzymes of the ADH pathway in T. vaginalis strain SS22 and T. vaginalis infected with M. hominis (SS22-MOZ2)
Enzyme elasticities and flux control coefficients are expressed as 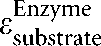 and
and 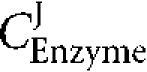 , respectively.
, respectively.
| T. vaginalis strain | |||||
|---|---|---|---|---|---|
| SS22 | 1.07 | 1.01 | 0.04 | 0.94 | 0.035 |
| SS22-MOZ2 | 1.00 | 0.98 | 0.09 | 0.98 | 0.10 |
The pH optima of the enzymes from T. vaginalis SS22 and SS22-MOZ2 were compared with those of M. hominis (Supplementary Fig. S1). The optimum pH for cOCT was 6.0 for SS22, SS22-MOZ2 and MOZ2; however, aOCT was found to have an optimal pH of 7.0 for MOZ2 and 8.0 for T. vaginalis SS-22 and SS22-MOZ2. By comparison, ADI was found to have an optimal pH of 6.5 for M. hominis MOZ2 and T. vaginalis SS22-MOZ2, whereas the parent uninfected T. vaginalis SS22 had an optimal pH of 8.0 for ADI, further supporting the view that the M. hominis ADI is responsible for the enhanced ADI kinetics in T. vaginalis SS22-MOZ2.
ADI-coding genes and localization of the gene products in T. vaginalis
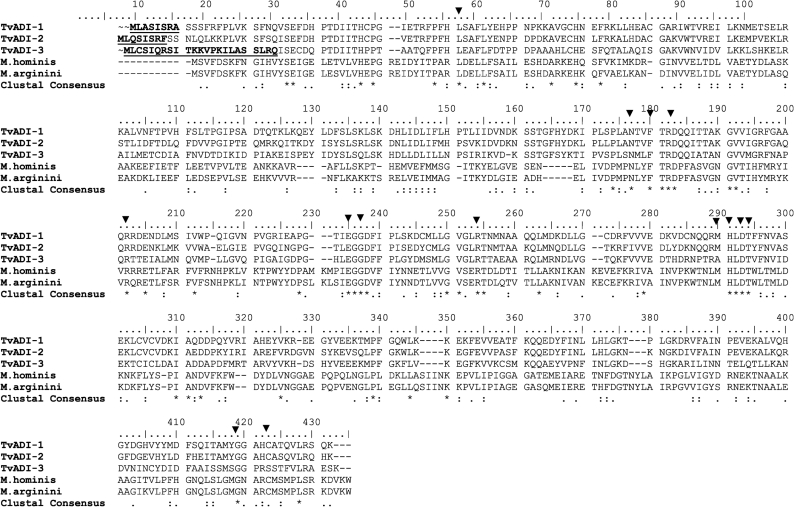
A blast search of the T. vaginalis genome database revealed the presence of three copies of the ADI gene (TvADI-1, TvADI-2 and TvADI-3) encoding proteins with a calculated molecular mass of 46 000–47 000 Da. Alignment with M. arginini, for which a crystal structure has been determined (Das et al., 2004), revealed the presence of conserved residues involved in substrate binding and/or the enzyme active site (Fig. 3). The positional equivalent of the catalytic triad Cys397, His268, Glu213 determined in M. arginini ADI (Das et al., 2004) is conserved in TvADI-1 (Cys408, His283, Glu230) and TvADI-2 (Cys405, His281, Glu228), while Cys405 is replaced by Ser405 in TvADI-3.
Fig. 3.
Genome sequence of ADI from T. vaginalis. Sequences were obtained by blast search in the T. vaginalis genome database (strain G3) of Eukaryotic Pathogen Database Resources (http://eupathdb.org/eupathdb/) using G. intestinalis (strain WB) ADI (accession no. XP_001705755) as query. Three sequences with significant similarity to G. intestinalis ADI (TVAG_467820, TVAG_344520 and TVAG_183850) were aligned with M. hominis (accession no. D13314 0.1) and M. arginini (accession no. CAA38210) using clustal_x (Thompson et al., 1997) and manually edited with BioEdit software (Hall, 1999). Putative mitochondrial targeting sequences (underlined) were predicted using the program psort II (http://psort.hgc.jp/). Triangles indicate residues involved in substrate binding and reaction mechanism (Das et al., 2004). Asterisk, fully conserved residue; colon, ‘strong’ group; period, conserved ‘weaker’ group according to clustal x.
All three putative TvADI sequences contained a mitochondrion-like N-terminal targeting presequence with a predicted cleavage site for a processing peptidase (Fig. 3), and a high probability of mitochondrial localization estimated by psort II (56–65 %) and TargetP (mTP values 0.500–0.710). These predictions suggested the localization of ADI in T. vaginalis hydrogenosomes, an anaerobic form of mitochondria in these parasites. The T. vaginalis ADI sequences group within the Eukaryota, suggesting that the ADI-encoding genes were present in a common ancestor, which does not support horizontal gene transfer from a prokaryotic source (Supplementary Fig. S2).
Kinetics of ADI in hydrogenosomal fractions from M. hominis-infected and -uninfected T. vaginalis
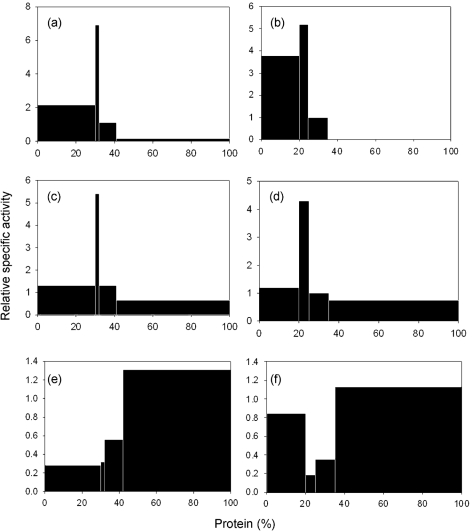
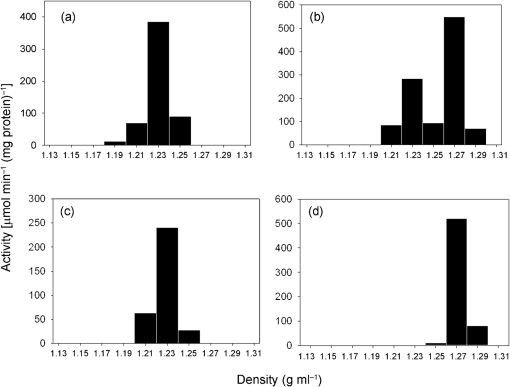
Subcellular fractionation of T. vaginalis SS22 and the M. hominis-infected SS22-MOZ2 had typical distribution profiles for hydrogenosomes based upon the data for malic enzyme, a marker enzyme for the organelle (Fig. 4a, b). The relative specific activity plot for ADI mirrored that for malic enzyme in extracts prepared from SS22 (Fig. 4c). However, the SS22-MOZ2 fractions typically had a second band of ADI activity which co-sedimented with the nuclear fraction, suggesting that M. hominis sediments with the nuclei and only minor contamination of the hydrogenosome fraction occurred (Fig. 4d). OCT, which in aerobic eukaryotes localizes to the mitochondrion, was found predominantly in the non-sedimentable fraction in both SS22 and SS22-MOZ2 (Fig. 4e, f). Kinetic analysis of ADI using the hydrogenosome-enriched fractions from T. vaginalis SS22 and SS22-MOZ2 revealed minimal differences in the Vmax (65.8±1.2 and 79.8±2.8 μM min−1, respectively) and Km (180±16 and 187±8.4 μM, respectively), suggesting that the observed increase in ADI kinetics for whole-cell studies of M. hominis-infected T. vaginalis was due to the contribution of M. hominis and not overexpression of host cell ADI. Further purification of hydrogenosome-enriched fractions by Percoll gradients revealed a single fraction with maximum ADI activity from SS22 and an isopycnic density of 1.23 g ml−1 (Fig. 5a), which overlays malic enzyme, a marker for hydrogenosomes (Fig. 5c). When the hydrogenosome-enriched fraction obtained from SS22-MOZ2 was used, the Percoll gradient purification resulted in two fractions with ADI activity, the hydrogenosomal fraction at 1.24 g ml−1 and a second fraction at 1.27 g ml−1 (Fig. 5b). The activity in the 1.27 g ml−1 fraction overlaid M. hominis ADI (Fig. 5d).
Fig. 4.
Subcellular localization of ADH enzymes in T. vaginalis SS22, T. vaginalis infected with M. hominis (SS22-MOZ2) and M. hominis MOZ2. Distribution and percentage recovery for each enzyme after subcellular fractionation of T. vaginalis (a) SS22 malic enzyme, 93 %; (b) SS22-MOZ2 malic enzyme, 88 %; (c) SS22 ADI, 87 %; (d) SS22-MOZ2 ADI, 96 %; (e) SS22 cOCT, 98 %; and (f) SS22-MOZ2 cOCT, 104 %.
Fig. 5.
Isopycnic density-gradient centrifugation of the hydrogenosome-enriched fractions. Hydrogenosome-enriched fractions from T. vaginalis SS22, T. vaginalis infected with M. hominis SS22-MOZ2 and M. hominis MOZ2 obtained by differential centrifugation were purified using Percoll gradients. Fractions were assayed for ADI and malic enzyme as described in Methods. (a) Distribution of ADI in SS22 revealed a major peak of activity at a density of 1.23 g ml−1; (b) SS22-MOZ2 had two peaks of activity at densities of 1.23 and 1.27 g ml−1 for ADI; (c) SS22 had a single major peak of activity at 1.23 g ml−1 for the hydrogenosome marker enzyme malic enzyme; (d) MOZ2 had a single peak of activity for ADI at a density of 1.27 g ml−1. Percentage recoveries were 84 and 81 % for ADI and malic enzyme, respectively, for T. vaginalis SS22; 86 % for ADI with SS22-MOZ2; 93 % for ADI with MOZ2.
Intracellular and extracellular amine concentrations from T. vaginalis SS22 and SS22-MOZ2 cultures
The intracellular and extracellular concentrations of amines associated with the ADH pathway were determined by HPLC analysis of acid extracts. The intracellular concentration of arginine was not significantly different in the two strains (Table 3). However, arginine utilization was approximately 2.5-fold increased in T. vaginalis (SS22-MOZ2) cultures infected with M. hominis (Table 3). The increased flow through the ADH pathway of T. vaginalis SS22-MOZ2 was reflected by the increased intracellular ornithine concentration, which was 15-fold higher in SS22-MOZ2 than in the parent strain SS22 (Table 3). A similar fivefold increase was observed for the extracellular ornithine concentration. Also, intracellular and extracellular putrescine had approximately twofold higher intracellular and threefold higher extracellular concentrations in SS22-MOZ2 compared with the parent SS22. A duplicate set of data were obtained using the T. vaginalis C1/C1-MOZ2 pair (Supplementary Table S2).
Table 3.
Intracellular and extracellular concentrations of amines in the parent uninfected T. vaginalis strain SS22 and T. vaginalis infected with M. hominis (SS22-MOZ2)
The concentration of amines was determined by HPLC as described in Methods.
| Amine | T. vaginalis SS22 | T. vaginalis SS22-MOZ2 | ||
|---|---|---|---|---|
| Intracellular concentration (μM per 106 cells) | Extracellular concentration (mM) | Intracellular concentration (μM per 106 cells) | Extracellular concentration (mM) | |
| Arginine | 0.51 | 5.63 | 0.46 | 2.31 |
| Citrulline | 0.12 | 0.15 | 0.06 | 0.04 |
| Ornithine | 0.03 | 0.06 | 0.45 | 0.31 |
| Putrescine | 0.19 | 1.92 | 0.43 | 5.32 |
RT-qPCR ADI in M. hominis-infected and -uninfected T. vaginalis
The ratio of mRNA expression levels for ADI in SS22-MOZ2/SS22 (2−ΔΔCt infected T. vaginalis/2−ΔΔCt uninfected T. vaginalis) was 0.69 (TvADI-1), 0.6 (TvADI-2) and 1.29 (TvADI-3). These results indicate that TvADI-1 and TvADI-2 are 30 % downregulated, while TvADI-3 is 30 % upregulated in mycoplasma-infected trichomonads. Since a 30 % difference in mRNA expression, as evaluated by real-time RT-qPCR, is considered minor, it is concluded that an absence of upregulation or downregulation of the overall TvADI expression occurs in T. vaginalis cells infected with M. hominis.
DISCUSSION
ADI is the first step in the most widespread anaerobic route of arginine degradation via the ADH pathway. The pathway provides an important source of energy generation from amino acids in the absence of oxygen in some protists (Schofield et al., 1990; Yarlett et al., 1996), and T. vaginalis can potentially meet 10 % of its energy needs from this pathway (Yarlett et al., 1996). The ADH pathway is also present in M. hominis (Fenske & Kenny, 1976), a member of the Mollicutes, which has a symbiotic relationship with T. vaginalis (Dessì et al., 2005). Consequently, T. vaginalis infected with M. hominis contains two localized pathways competing for the same substrate, arginine. The effect of this endosymbiotic relationship on the ADH pathway of T. vaginalis has not previously been examined. We showed that cultures of T. vaginalis infected with M. hominis exhibit increased arginine consumption and a concomitant increase in ornithine and putrescine production. M. hominis has a minimal genome that lacks an ODC gene (Pereyre et al., 2009) and ODC enzyme activity (this study); hence, it is likely that the ornithine produced by M. hominis is exported, possibly via an arginine/ornithine transporter, as demonstrated in other prokaryotes that contain the ADH pathway (Driessen et al., 1987). M. hominis has a number of transporters that are capable of satisfying its nutritional requirement for several amino acids (Pereyre et al., 2009). In particular, MHO_5040 is proposed to encode a functional M. hominis arginine permease (Pereyre et al., 2009). The rapid removal of arginine from the cytoplasm of T. vaginalis by M. hominis and its replacement by exported ornithine via a putative arginine/ornithine transporter would result in an increased cytoplasmic ornithine concentration, which could then drive increased putrescine formation by the T. vaginalis ODC. Thus, this relationship would provide M. hominis with a constant supply of putrescine, which it is incapable of synthesizing. The benefit of this symbiotic relationship to T. vaginalis is not so clear; however, the increased scavenging of arginine may be significant, since it would reduce the amount of free arginine available for nitric oxide (NO) production by host macrophages. T. vaginalis has a flavorubredoxin-like-dependent NO reductase with a reported Km of 1.2 μM, which would effectively remove NO (Sarti et al., 2004). However, the concentration of arginine in vaginal fluid has been reported to be 210 μM (Chen et al., 1982), which is reduced to undetectable levels during infection; hence, the combined arginine sink in T. vaginalis infected with M. hominis would effectively reduce free arginine concentrations to levels below the Km of the host NO synthase. Depletion of arginine by ADI could therefore block NO production by macrophages and thereby block an important host defence mechanism (Dillon et al., 2002). A role for M. hominis ADI in suppression of NO production caused by macrophage-inducible NO synthase has been proposed (Noh et al., 2002). It has been proposed that G. intestinalis releases ADI in contact with host epithelial cells, which protects it from host NO production (Ringqvist et al., 2008). The G. intestinalis ADI has also been implicated in multiple functions, including antigenic switching and migration to the nucleus, where it has a regulatory role in gene expression during encystation (Touz et al., 2008). In T. vaginalis, however, ADI localizes predominantly to the hydrogenosomal fraction.
We have previously demonstrated that ADI is the step with the lowest velocity and is therefore proposed to be the rate-limiting step of the pathway (Yarlett et al., 1996). However, the actual change in flow through the pathway caused by the presence of M. hominis is more complex than this, and is best described by determination of the flux control coefficients and elasticity of the enzymes involved (Fell, 1997). Based on the kinetic data for the ADH enzymes and knowing the intracellular concentrations of the intermediates, the elasticity of each enzyme of the pathway can be determined (Table 2). From this it can be shown that the increase in ornithine has a significant effect upon the flux control of the pathway (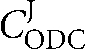 increases from 1/25 to 1/10). Based upon the data obtained in this study we propose that the additional ornithine present in the T. vaginalis cytosol is derived from M. hominis. Ornithine drives the formation of putrescine via a T. vaginalis ODC and can also be a substrate for the T. vaginalis lysine/ornithine aminotransferase (OAT; EC 2.6.1.13; TVAG 258770; Carlton et al., 2007), resulting in the formation of glutamate and Δ-pyrroline 5-carboxylate (Lowe & Rowe, 1986; Carlton et al., 2007). Glutamate, in turn, could drive the formation of cytosolic alanine by alanine aminotransferase (EC 2.6.1.2; Lowe & Rowe, 1986). Multiple copies of the alanine aminotransferase gene have been identified in the T. vaginalis genome (Carlton et al., 2007); based upon the presence of a hydrogenosomal targeting sequence, these genes encode alanine aminotransferases that localize to both the hydrogenosome and the cytosol (Carlton et al., 2007). The cytosolic alanine can enter the hydrogenosome, resulting in the production of hydrogenosomal pyruvate, and could therefore act as an alanine/pyruvate shuttle for provision of hydrogenosomal pyruvate and be involved in ATP generation by the T. vaginalis pyruvate : ferredoxin oxidoreductase-coupled succinate thiokinase pathway. We conclude that the association of M. hominis with T. vaginalis is an example of a mutually beneficial endosymbiotic relationship.
increases from 1/25 to 1/10). Based upon the data obtained in this study we propose that the additional ornithine present in the T. vaginalis cytosol is derived from M. hominis. Ornithine drives the formation of putrescine via a T. vaginalis ODC and can also be a substrate for the T. vaginalis lysine/ornithine aminotransferase (OAT; EC 2.6.1.13; TVAG 258770; Carlton et al., 2007), resulting in the formation of glutamate and Δ-pyrroline 5-carboxylate (Lowe & Rowe, 1986; Carlton et al., 2007). Glutamate, in turn, could drive the formation of cytosolic alanine by alanine aminotransferase (EC 2.6.1.2; Lowe & Rowe, 1986). Multiple copies of the alanine aminotransferase gene have been identified in the T. vaginalis genome (Carlton et al., 2007); based upon the presence of a hydrogenosomal targeting sequence, these genes encode alanine aminotransferases that localize to both the hydrogenosome and the cytosol (Carlton et al., 2007). The cytosolic alanine can enter the hydrogenosome, resulting in the production of hydrogenosomal pyruvate, and could therefore act as an alanine/pyruvate shuttle for provision of hydrogenosomal pyruvate and be involved in ATP generation by the T. vaginalis pyruvate : ferredoxin oxidoreductase-coupled succinate thiokinase pathway. We conclude that the association of M. hominis with T. vaginalis is an example of a mutually beneficial endosymbiotic relationship.
Acknowledgments
The authors thank Dr Thomas E. Gorrell for helpful suggestions. Parts of this study were supported by grants from the National Institutes of Health National Institute for Allergy and Infectious Diseases (NIH-NIAID) 49785 (N. Y.), and the Academy of Science of the Czech Republic IAA501110631 and MSM0021620858 (J. T.).
Abbreviations
ADH, arginine dihydrolase
ADI, arginine deiminase
aOCT, anabolic ornithine carbamyltransferase
CK, carbamate kinase
cOCT, catabolic ornithine carbamyltransferase
OAT, ornithine aminotransferase
OCT, ornithine carbamyltransferase
ODC, ornithine decarboxylase
Footnotes
Two supplementary figures and two supplementary tables are available with the online version of this paper.
References
- Blanchard, A., Yanez, A., Dybvyg, K., Watson, H. L., Griffiths, G. & Cassel, G. H. (1993). Evaluation of intraspecies genetic variation within the 16S rRNA gene of M. hominis and detection by PCR. J Clin Microbiol 31, 1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyde, T. R. & Rahmatullah, M. (1980). Optimization of conditions for the colorimetric determination of citrulline, using diacetyl monoxime. Anal Biochem 107, 424–431. [DOI] [PubMed] [Google Scholar]
- Carlton, J. M., Hirt, R. P., Silva, J. C., Delcher, A. L., Schatz, M., Zhao, Q., Wortman, J. R., Bidwell, S. L., Alsmark, U. C. & other authors (2007). Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science 315, 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, K. C., Amsel, R., Eschenbach, D. A. & Holmes, K. K. (1982). Biochemical determination of vaginitis: determination of diamines in vaginal fluid. J Infect Dis 145, 337–345. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden, A. (2004). Fundamentals of Enzyme Kinetics, 3rd edn. London. : Portland Press.
- Das, K., Butler, G. H., Kwiatkowski, V., Clark, A. D., Jr, Yadav, P. & Arnold, E. (2004). Crystal structure of arginine deiminase with covalent reaction intermediates; implications for catalytic mechanism. Structure 12, 657–667. [DOI] [PubMed] [Google Scholar]
- Dessì, D., Delogu, G., Emonte, E., Catania, M. R., Fiori, P. L. & Rappelli, P. (2005). Long-term survival and intracellular replication of Mycoplasma hominis in Trichomonas vaginalis cells: potential role of the protozoon in transmitting bacterial infection. Infect Immun 73, 1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond, L. S. (1957). The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol 43, 488–490. [PubMed] [Google Scholar]
- Dillon, B. J., Holtsberg, F. W., Ensor, C. M., Bomalaski, J. S. & Clark, M. A. (2002). Biochemical characterization of the arginine degrading enzymes arginase and arginine deiminase and their effect on nitric oxide production. Med Sci Monit 8, BR248–BR253. [PubMed] [Google Scholar]
- Dolezal, P., Vánacová, S., Tachezy, J. & Hrdy, I. (2004). Malic enzymes of Trichomonas vaginalis: two enzyme families, two distinct origins. Gene 329, 81–92. [DOI] [PubMed] [Google Scholar]
- Driessen, A. J., Poolman, B., Kiewiet, R. & Konings, W. (1987). Arginine transport in Streptococcus lactis is catalyzed by a cationic exchanger. Proc Natl Acad Sci U S A 84, 6093–6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell, D. (1997). Measuring control coefficients. In Frontiers in Metabolism 2: Understanding the Control of Metabolism, pp. 135–195. Edited by Snell, K.. London. : Portland Press.
- Fenske, J. D. & Kenny, G. E. (1976). Role of arginine deiminase in growth of Mycoplasma hominis. J Bacteriol 126, 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold, A., Chen, Y. Y., Snyder, J. A. & Burne, R. A. (2004). Characterization of the arginine deiminase operon in Streptococcus rattus FA-1. Appl Environ Microbiol 70, 1321–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41, 95–98. [Google Scholar]
- Linstead, D. & Cranshaw, M. A. (1983). The pathway of arginine catabolism in the parasitic flagellate Trichomonas vaginalis. Mol Biochem Parasitol 8, 241–252. [DOI] [PubMed] [Google Scholar]
- Lowe, P. N. & Rowe, A. F. (1986). Aminotransferase activity in Trichomonas vaginalis. Mol Biochem Parasitol 21, 65–74. [DOI] [PubMed] [Google Scholar]
- Lu, X., Galkin, A., Herzberg, O. & Dunaway-Mariano, D. (2004). Arginine deiminase uses an active-site cysteine in nucleophilic catalysis of l-arginine hydrolysis. J Am Chem Soc 126, 5374–5375. [DOI] [PubMed] [Google Scholar]
- Noh, E. J., Kang, S. W., Shin, Y. J., Kim, D. C., Park, I. S., Kim, M. Y., Chun, B. G. & Min, B. H. (2002). Characterization of Mycoplasma arginine deiminase expressed in E. coli and inhibitory regulation of nitric oxide synthesis. Mol Cells 13, 137–143. [PubMed] [Google Scholar]
- Pereyre, S., Sirand-Pugnet, P., Bevan, L., Charron, A., Renaudin, H., Barre, A., Avenaud, P., Jacob, D., Couloux, A. & other authors (2009). Life on arginine for Mycoplasma hominis. Clues from its minimal genome and comparison with other human urogenital mycoplasmas. PLoS Genet 5, e1000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappelli, P., Addis, M. F., Carta, F. & Fiori, P. L. (1998). Mycoplasma hominis parasitism of Trichomonas vaginalis. Lancet 352, 1286. [DOI] [PubMed] [Google Scholar]
- Ringqvist, E., Palm, J. E., Skarin, H., Hehl, A. B., Weiland, M., Davids, B. J., Reiner, D. S., Griffiths, W. J., Eckmann, L. & other authors (2008). Release of metabolic enzymes by Giardia in response to interaction with intestinal epithelial cells. Mol Biochem Parasitol 159, 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarti, P., Fiori, P. L., Forte, E., Rappelli, P., Teixeira, M., Mastronicola, D., Sanciu, G., Giuffré, A. & Brunori, M. (2004). Trichomonas vaginalis degrades nitric oxide and expresses a flavorubredoxin-like protein: a new pathogenic mechanism? Cell Mol Life Sci 61, 618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield, P. J., Costello, M., Edwards, M. R. & O'Sullivan, W. J. (1990). The arginine dihydrolase pathway is present in Giardia intestinalis. Int J Parasitol 20, 697–699. [DOI] [PubMed] [Google Scholar]
- Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690. [DOI] [PubMed] [Google Scholar]
- Swofford, D. L. (1998). Phylogenetic analysis using parsimony (paup), version 4. Sunderland, MA: Sinauer Associates.
- Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F. & Higgins, D. G. (1997). The clustal_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25, 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touz, M. C., Ropolo, A. S., Rivero, M. R., Vranych, C. V., Conrad, J. T., Svard, S. G. & Nash, T. E. (2008). Arginine deiminase has multiple regulatory roles in the biology of Giardia lamblia. J Cell Sci 121, 2930–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarlett, N., Goldberg, B., Moharrami, M. A. & Bacchi, C. J. (1993). Trichomonas vaginalis: characterization of ornithine decarboxylase. Biochem J 293, 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarlett, N., Lindmark, D. G., Goldberg, B., Moharrami, M. A. & Bacchi, C. J. (1994). Subcellular localization of the enzymes of the arginine dihydrolase pathway in Trichomonas vaginalis and Tritrichomonas foetus. J Eukaryot Microbiol 41, 554–559. [DOI] [PubMed] [Google Scholar]
- Yarlett, N., Martinez, M. P., Moharrami, M. A. & Tachezy, J. (1996). The contribution of the arginine dihydrolase pathway to energy metabolism by Trichomonas vaginalis. Mol Biochem Parasitol 78, 117–125. [DOI] [PubMed] [Google Scholar]