Abstract
Background:
Upper gastrointestinal bleeding (UGIB), a potentially fatal occurrence, can sometimes follow coronary artery bypass graft (CABG) surgery. However, little has been published about its prevalence, risk factors, and outcomes.
Aim:
This study aimed to determine the rate, etiologies, predisposing factors, and outcomes of UGIB following CABG.
Method:
The authors conducted a retrospective chart review of all UGIBs which followed CABGs performed at the University of Alberta Hospital from January 1, 1998 to December 31, 2002.
Results:
During the study period, 4,502 CABGs were performed at the UAH. Eighteen patients (0.4%) had a documented major UGIB (defined as evidence of melena, red or coffee-grounds emesis, blood per NG tube, or a decrease of Hgb by > 20 g/l and requiring a confirmation by endoscopy or radiological study). Two of these 18 patients (11%) had a past history of peptic ulcer disease, and one of these patients had had previous UGIB. Three patients (17%) had been taking proton pump inhibitors (PPI) before the UGIB occurred. At the time of UGIB, PPIs were prescribed for 16 patients (89%), and the PPIs achieved effective hemostasis as a single agent for 10 (62.5%). Of the 18 patients, 16 (89%) underwent upper GI endoscopy. Bleeding was found to be due to duodenal ulceration in 9 (56%), esophagitis in 4 (22%) and gastritis in 6 cases (33%); fifty percent of these patients had multiple sites of bleeding. Endoscopic therapeutic intervention was needed by 6 patients (37.5%), and successful hemostasis was achieved for 5 of these patients (83%). One patient had a recurrence of bleeding and required surgery. One patient underwent surgery as the primary hemostatic therapy after a diagnostic endoscopy. The overall surgical rate was 11.1% for this patient cohort. In this cohort, three patients died, two from multi-organ failure, and the third, a surgically managed patient, had a cardiac arrest 72 hours post-surgery. The number of complication increased as both cardiopulmonary bypass and cross clamp time increased. There were no endoscopy-related complications.
Conclusions:
UGI bleeding following CABGs is relatively infrequent, occurring at a rate of 0.4% in this study. Upper gastrointestinal bleeding post-CABG is most frequently related to a duodenal ulcer, though 50% of the patients had multiple bleeding sites. prolonged bypass and cross clamp time associated with more complications.
Keywords: Upper gastrointestinal bleeding, Coronary artery bypass surgery, Post-operative complications
Introduction
Significant upper gastrointestinal (UGI) hemorrhage is an infrequent but potentially lethal complication associated with coronary artery bypass grafting (CABG). This operation has now become one of the most common major procedures done in many hospitals; hence, the numerical importance of this significant complication is also rising. At present, however, there is no truly recent data available related to the incidence or outcome of UGI bleeds in North America, and no Canadian data has been published at all. Table 1 outlines the published studies which have addressed gastrointestinal bleeding post-CABG during the last 35 years. These studies report the frequency of UGI bleeding following CABGs to lie somewhere between nil and 11%. The studies with the highest reported rates of bleeding post-surgeries are those conducted in the 1970s and 1980s, prior to the implementation of what are now routine preventive measures and to the use of interventional endoscopic therapy.
Table 1. Previous Reports.
| Study | Total Cohart | UGI Bleed(%) | Mortality% | Gender% |
|---|---|---|---|---|
| Mead 1 | 246 | 5 (2%) | - | - |
| Lebovics13 | 4892 | 18 (0.4) | 11.1 | 89% M |
| Norton25 | 10,573 | 55 (.5) | 1.8 | 91%M |
| Welsh3 | 7,333 | 16 (.22) | 81 | 50%M |
| Katz2 | 100 | 8 (8) | - | 73%M |
| Rosen18 | 9,199 | 25 (.27) | 28 | 65.4%M |
| Hanks5 | 5080 | 19 (.37) | 38 M -33 S | 74%M |
| Welling9 | 1596 | 1 (.06) | 0 | 100%M |
| Spotnitz23 | 1831 | 16 (1) | 31 | |
| Moneta8 | 2,428 | 2 (.08) | - | 86% |
| Ohri14 | 4629 | 20 (.4) | 20 | 75%M |
| Huddy16 | 4473 | 20 (.45) | 55 | 80%Ma |
| Christenson19 | 3493 | 13 (.4) | 7.7 | 85%Ma |
| Taylor4 | 5000 | 38 (.76) | 23.60 | 76%Ma |
| Egleston20 | 8559 | 22 (.26) | 22.7 | 57%Ma |
| Johnston17 | 5438 | 36 (.66) | 16.6 | 71%Ma |
| Tsiotos21 | 19,246 | 44 (.23) | 20 | 74%Ma |
| Perugini24 | 1477 | 20 (1.35) | 15 | 68.4%Ma |
| Krasna12 | 1279 | 5 (.39) | 33 | 60%M |
| Pinson6 | 5682 | NIL | ||
| Heikkinen11 | 1686 | 17 (.976) | 53 | 79%Ma |
| Aranha7 | 5719 | 24 (.42) | 0 | 72.6%Ma |
| Mercado 22 | 4923 | 26 (.52) | 50M–67S | 64% Ma |
| Leitman10 | 6,4 52 | 20(.3) | 45 | 53%M |
| Jayaprakash27 | 2274 | 20(0.9%) | 15% | 70%M |
| Simic26 | 4288 | 10(0.2%) | 10% | 56%M |
UGI upper gastrointestinal
M Male
Ma Gender percent for the whole study
Methods
The University of Alberta Hospital is a university teaching center and a tertiary care referral hospital located in Edmonton, Alberta. It serves a catchment area of over 1.8 million people from central and northern Alberta, northwestern Saskatchewan, northern British Columbia and the Northwest Territories. CABGs required by those in this catchment area are only performed at the University Hospital in Edmonton, where there are six cardiac surgeons who specialize in adult care. Approximately 800–1000 of these CABGs are performed annually. All CABGs performed at the University Hospital between January 1, 1998 and December 31, 2002 were evaluated. The University of Alberta Hospital uses the international classification of disease (ICD) coding, on a prospective basis, to identify procedures and diagnoses for all patients encountered. Previous reports have shown that most gastrointestinal bleeding associated with CABGs occurs within 40 days of the CABG procedure [5–26]. All incidents of gastrointestinal bleeding within 40 days post-CABG were therefore isolated using the code descriptions listed in Table 2.
Table 2. ICD Code descriptions.
|
Procedures Bypass coronary artery one vessel Bypass coronary artery two vessels Bypass coronary artery three vessels Bypass coronary artery four vessels Endoscopy esophagus Endoscopy jejunum Endoscopy stomach Biopsy gastroesophageal junction Biopsy duodenum endscopic Biopsy duodenum brush Biopsy esophagus Biopsy jejunum closed Biopsy stomach closed |
|
Diagnosis Unspecified esophagitis Other esophagitis Ulcer of esophagitis Esophageal haemorrhage Mallory Weiss syndrome Acute gastric ulcer with haemorrhage +/– perforation Chronic or unspecified gastric ulcer with haemorrhage +/– perforation Acute duodenal ulcer with haemorrhage +/– perforation Chroni/unspecified duodenal ulcer with haemorrhage +/– perforation Acute peptic ulcer with haemorrhage +/– perforation Chronic/unspecified peptic ulcer with haemorrhage +/– perforation Acute gastroesophageal ulcer with haemorrhage +/– perforation Chronic/unspecified gastroesophageal ulcer with haemorrhage +/– perforation Acute gastritis/duodenitis Unspecified gastric and duodenitis Dieulafoy’s lesion |
The appropriate patient records were then retrieved and hand-searched to confirm major gastrointestinal bleeding according to the following criteria: a) One or more of the following events: bright red hematemesis, malena, coffee-grounds emesis or an acute decrease in the hemoglobin level by >20 g/l, and b) Confirmation by endoscopy or radiological study. The data was extracted to identify patient demographics, including types of bypass procedures, cardiac bypass times, cross-clamp times, nature of the surgery (emergency or elective), comorbidity, ASA or NASIDs use, use of anticoagulants, evidence of previous peptic ulcer disease, previous bleeding, previous endoscopies and other investigations and therapies, smoking histories, and time intervals between the procedure and the bleeding incident. Diagnoses, as well as medical, surgical, and endoscopic therapies, were recorded. Finally, the outcome, including mortality, was noted. The medical therapy included IV proton pump inhibitors (omeprazole, pentaprazole, lanzoprazole). Therapeutic endoscopy includes the injection of adrenaline or cautery therapy. The average time between surgery and discharge for this patient’s cohort was 5–15 days, but all patients who were still in the hospital and experienced a UGI bleed up to 40 days post-op were included in the study.
Results
As shown in Table 3, a total of 4,502 CABGs were performed in the 5 years between January 1998 and December 2002. Fifty-six records were initially isolated for review based on the search criteria outlined above. After a hand-search through all 56 retrieved cases, 18 of these records were found to belong to patients who had had upper gastrointestinal bleeding post-CABG. This number represents 0.4% of the total number of patients who underwent CABGs in the same period. On average, the bleeding occurred 13 days after the bypass surgery. Part of the routine protocol at the University Hospital is to give patients Aspirin (81 mg 6 hours post-surgery) and Ranitidine (150 mg 1 day before surgery and again after surgery). Four patients were fully anticoagulated at the time of haemorrhage (22%).
Table 3. Number of upper gastrointestinal bleeds.
| Procedures | Patients |
|---|---|
| Total coronary bypass surgeries | 4,502 |
| Upper gastrointestinal bleeding post-CABG | 18 |
| Total deaths | 3 |
| Medical management | 16 |
| Surgical management | 2 |
CABG: Coronary Artery Bypass Surgery.
Table 4 outlines the results which show that 11% of 18 patients (n2) had been previously diagnosed with peptic ulcer disease, and 3 patients (16%) were taking proton pump inhibitors.
Table 4. Patient characteristics.
| Cases | Age (Years) | CPT Min | CCT Min | P OP Days | Diagnosis | Method | Endosc1 | Endosc2 | Therapy | ICU | Compli | Disch |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | 120 | 83 | 17 | Esophagitis, gastritis | Melena | Diagnostic | Medical | 48 | 146 | Died | |
| 2 | 71 | 230 | 113 | 7 | GU, esophagitis | Melena | Diagnostic | Medical | 3 | 1 | Discharged | |
| 3 | 65 | 78 | 50 | 3 | Hematemesis | 1 | 7 | Died | ||||
| 4 | 69 | 240 | 63 | 8 | Hematemesis | Medical | 13 | 1,345 | Died | |||
| 5 | 66 | 111 | 69 | 9 | DU | Drop of Hgb | Therapeutic | Medical & surgical | 5 | 6 | Discharged | |
| 6 | 68 | 137 | 46 | 26 | GU, esophagitis, gastritis | Coffee-ground emesis & drop of Hgb | Therapeutic | Therapeutic | Medical | 30 | 145 | Discharged |
| 7 | 67 | 86 | 55 | 8 | DU | Drop of Hgb | Therapeutic | Medical | 1 | Discharged | ||
| 8 | 68 | 237 | 188 | 14 | Gastritis | Drop of Hgb | Diagnostic | Diagnostic | Medical | 18 | 156 | Discharged |
| 9 | 58 | 90 | 51 | 6 | DU | Drop of Hgb | Diagnostic | Medical | 5 | Discharged | ||
| 10 | 71 | 127 | 72 | 4 | GU, esophagitis | Melena | Diagnostic | Medical | 1 | Discharged | ||
| 11 | 73 | 131 | 61 | 26 | DU | Melena & drop of Hgb | Diagnostic | Diagnostic | Medical & surgical | 1 | 46 | Discharged |
| 12 | 79 | 196 | 172 | 5 | DU, GU | Hematemesis, melena & drop of Hgb | Therapeutic | Diagnostic | Medical | 4 | Discharged | |
| 13 | 49 | 52 | 28 | 21 | DU, gastritis | Hematemesis, melena & drop of Hgb | Diagnostic | Medical | 1 | Discharged | ||
| 14 | 77 | 100 | 54 | 1 | DU, GU | Hematemesis | Therapeutic | Diagnostic | Medical | 1 | 456 | Discharged |
| 15 | 71 | 79 | 44 | 5 | Gastritis | Melena & drop of Hgb | Diagnostic | Medical | 1 | Discharged | ||
| 16 | 78 | 80 | 47 | 10 | DU | Melena & drop of Hgb | Therapeutic | Diagnostic | Medical | 30 | 6 | Discharged |
| 17 | 71 | 155 | 100 | 29 | Mucosal oozing | Melena & drop of Hgb | Diagnostic | Medical | 2 | 56 | Discharged | |
| 18 | 78 | 234 | 200 | 40 | DU, GU | Hematemesis | Diagnostic | Diagnostic | Medical | 11 | 46 | Discharged |
Four patients (22%) were being treated with NSAIDs. Fifty percent of these patients (n9) had a bleed caused by duodenal ulceration. Six patients bled due to gastritis (33%), six patients (33%) from gastric ulcer, and four from esophagitis (22%). These numbers add up to more than 100% because 50% of these patients (n9) received more than one diagnosis. At the time of bleeding, proton pump inhibitors were prescribed for 16 patients (89%), and this treatment achieved effective homeostasis as a single agent for 10 patients (62.5%). Eighty–nine percent of the 18 patients (n16) underwent endoscopy. Two patients did not undergo endoscopy because their conditions suddenly deteriorated. Therapeutic endoscopy was needed by six patients (37.5%), and successful homeostasis was achieved for five patients (83%). Endoscopy was repeated (as a second look) for seven patients out of 16 (43.7%), and one of these endoscopies was therapeutic. There were no endoscopy–related complications. In this group of patients, two required surgery: one patient had a recurrence of bleeding and required surgery, and one patient underwent surgery as primary haemostatic therapy after diagnostic endoscopy. The average ICU stay for this group was 9.7 days. In this cohort, 3 patients died (16.6%), two from multi-organ failure and the third patient from a cardiac arrest 72 hours post-surgery.
Discussion
These results indicate that UGI haemorrhage is a relatively infrequent complication of CABG surgery, occurring in 0.4% of this patient group. The last investigation in North America to address this subject took place in 1997 by Perugini et al. with an incidence of 1.3%.
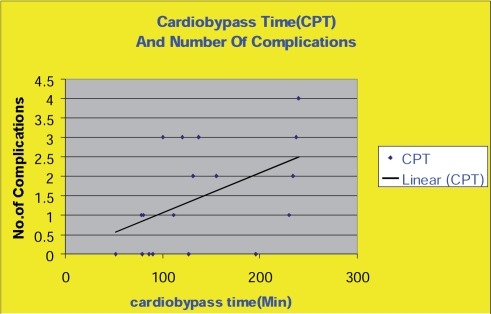
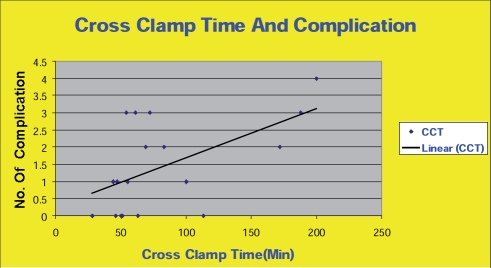
A few reports from Europe have recently come out with similar results to ours. Most of the studies on CABGs and UGI bleeding, however, were carried out in the 1970s and 1980s before the invention of new treatment modalities. These studies were also usually undertaken as investigations of whole GI complications [1–27]. The reported frequency of significant UGI bleeding post-CABG ranges from zero to 11.1% (Table 1) and mortality in this small group is reported to be as high as 81%. The incidence of milder UGI bleeding that does not require transfusion or intervention may be much higher [24]. Upper gastrointestinal heamorrhage due to stress ulceration is the most commonly identified mechanism [27]. Stress ulceration has been attributed to ischemia and/or reperfusion injury of the splanchnic territory and endotoxemia leading to the impairment of gastric and duodenal mucosal defense mechanisms [19, 27–34]. According to previous cohort studies, several factors increase the risk of bleeding in this setting, including the duration of the cardiopulmonary bypass time, cross clamp time, and the need for iontropes [22]. Prolonged mechanical ventilation is an independent risk factor [27, 35]. Our study showed a clear correlation between complications and prolonged cross clamp and cardiopulmonary bypass times (Figs. 1 and 2).
Fig. 1. Cardio bypass time and number of complications.
Fig. 2. Cross clamp time and complications.
According to Halm et al. [27], Helicobacter pylori does not appear to be associated with stress ulceration in critically ill patients. Proton pump inhibitors were the main agent used to achieve effective homeostasis as a single agent for 62% of those concerned, and the PPIs had a good safety profile. We are unable to comment on the specific drug efficacy of proton pump inhibitors as a class due to the small number of patients with UGI bleeding, and we could not compare the doses and methods of delivery due to the multiplicity of regimens. For this cohort, endoscopy was a safe procedure associated with a lack of complications, as has been previously reported [4, 12]. Interestingly, the need for surgery was very low, lower than the numbers reported in most studies [2, 18]. Because of the small number of therapeutic endoscopies (n6), we were unable to compare the two modalities of therapy (PPI and endoscopy).
Conclusion
UGI bleeding following CABGs is a relatively infrequent complication, occurring at a rate of 0.4% in this cohort of patients. Upper gastrointestinal bleeding post-CABG most frequently related to duodenal ulceration, though multiple sites of bleeding are reasonably common prolonged bypass and cross clamp time associated with more complications.
References
- [1].Mead J, Folk F. Gastrointestinal bleeding after cardiac surgery. N Eng Med. 1969;281:799. doi: 10.1056/NEJM196910022811420. [DOI] [PubMed] [Google Scholar]
- [2].Katz SE, Kornfeld DS, Harris PD, Yeoh CB. Acute gastrointestinal ulceration with open–heart surgery and aortic valve disease. Surgery. 1972;72(3):438–442. [PubMed] [Google Scholar]
- [3].Welsh GF, Bartholemew LG, Danielson GK. Gastrointestinal bleeding after open heart surgery. J Thor Cardio Surg. 1973;65:738–43. [PubMed] [Google Scholar]
- [4].Taylor PC, Loop FD, Hermann RE. Management of acute stress ulcer after cardiac surgery. Ann Surg. 1973;178:1–5. doi: 10.1097/00000658-197307000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hanks JB, Curtis SE, Hanks BB, Anderson DK, Cox JL, Scott JR. Gastrointestinal complications after cardiopulmonary bypass. Surgery. 1982;92:394–400. [PubMed] [Google Scholar]
- [6].Pinson CW, Alberty RE. General surgical complication after cardiopulmonary bypass surgery. The A J of Surgery. 1983;146:133–136. doi: 10.1016/0002-9610(83)90273-8. [DOI] [PubMed] [Google Scholar]
- [7].Aranha GV, Pickleman J, Pifarre R, Scanlon PJ, Gunnar R. The reason for gastrointestinal consultation after cardiac surgery. The Amer Surgeon. 1984;50:301–304. [PubMed] [Google Scholar]
- [8].Moneta G, Misbach G, Ivey T. Hyperfusion as a possible factor in the development of gastrointestinal complications after cardiac surgery. The Amer J of Surgery. 1984;149:648–650. doi: 10.1016/s0002-9610(85)80148-3. [DOI] [PubMed] [Google Scholar]
- [9].Welling R, Rath R, Albers JE, Glaser R. Gastrointestinal complications after cardiac surgery. Arch Surg. 1986;121:1178–1180. doi: 10.1001/archsurg.1986.01400100090017. [DOI] [PubMed] [Google Scholar]
- [10].Leitman IM, Paull DE, Barie PS, Isom OW, Shires GT. Intraabdominal complications of cardiopulmonary bypass operations. Surgery, Gyn & Obst. 1987;165:251–254. [PubMed] [Google Scholar]
- [11].Heikkinen LO, Ala-Kulju K. Abdominal complications following cardiopulmonary bypass in open–heart surgery. Scand J Thor Caridiovasc Surg. 1987;21:1–7. doi: 10.3109/14017438709116911. [DOI] [PubMed] [Google Scholar]
- [12].Krasna MJ, Flancbaum L, Trooskin SZ. Gastrointestinal complications after cardiac surgery. Surgery. 1988;104:773–80. [PubMed] [Google Scholar]
- [13].Lebovics E, Lee S, Dworkin BM, Heier S, Casellas A, Reed G, Rosenthal W. Upper gastrointestinal bleeding following open heart surgery. Digestive Diseases and Sciences. 36(6):757–760. doi: 10.1007/BF01311233. [DOI] [PubMed] [Google Scholar]
- [14].Ohri SK, Desai JB, Gear JAR, Roussak JB, Heshami M, Smith PLC, Taylor KM. Intraabdominal complications after cardiopulmonary bypass. Ann Thorac Surgery. 1991;52:826–31. doi: 10.1016/0003-4975(91)91219-l. [DOI] [PubMed] [Google Scholar]
- [15].Intraabdominal complications after cardiopulmonary bypass. Ann thorac Surgery. 1991;52:826–31. doi: 10.1016/0003-4975(91)91219-l. [DOI] [PubMed] [Google Scholar]
- [16].Huddy SPJ, Joyce WP, Pepper JR. Gastrointestinal complications in 4,473 patients who underwent cardiopulmonary bypass surgery. Br J Surg. 1991;78:293–296. doi: 10.1002/bjs.1800780309. [DOI] [PubMed] [Google Scholar]
- [17].Johnston G, Vitikainen K, Knight R, Annest L, Garcia C. Changing perspective on gastrointestinal complications in patients undergoing cardiac surgery. The Amer J of Surgery. 1992;163:525–529. doi: 10.1016/0002-9610(92)90402-d. [DOI] [PubMed] [Google Scholar]
- [18].Rosen H, Vlahakes GJ, Rattner D. Fulminant peptic ulcer disease in cardiac surgical patients: Pathognesis, prevention and management. 1992;20(3):354–359. doi: 10.1097/00003246-199203000-00011. [DOI] [PubMed] [Google Scholar]
- [19].Christenson JT, Schmuziger M, Maurice J, Simonet F, Velebit V. Postoperative visceral hypotension the common cause for gastrointestinal complications after cardiac surgery. Thorac Cardiovasc Surgeon. 1994;42:152–157. doi: 10.1055/s-2007-1016478. [DOI] [PubMed] [Google Scholar]
- [20].Egleston C, Wood A, Gorey T, McGovern E. Gastrointestinal complications after cardiac surgery. Ann of Royal Coll of Sur of England. 1993;75:52–56. [PMC free article] [PubMed] [Google Scholar]
- [21].Tsiotos GG, Mullany CJ, Zietlow S, van Heerden JA. Abdominal complications following cardiac surgery. The Amer J of Surg. 1994;167:553–557. doi: 10.1016/0002-9610(94)90096-5. [DOI] [PubMed] [Google Scholar]
- [22].Mercado P, Farid H, O’Connell TX, Sintek C, Pfeffer T, Khonsari S. Gastrointestinal complications associated with cardiopulmonary bypass procedures. The Amer Surg. 1994;60:789–792. [PubMed] [Google Scholar]
- [23].Spotnitz W, Sanders R, Hanks J, Nolan S, Tribble C, Bergin J, Zacour R, Abbott R, Kron I. Gerneral surgical complications can be predicted after cardiopulmonary bypass. Ann of Surg. 1995;221(5):489–497. doi: 10.1097/00000658-199505000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Perugini R, Orr R, Porter D, Dumas E, Maini B. Gastrointestinal complications following cardiac surgery. Arch Surg. 1997;132:352–357. doi: 10.1001/archsurg.1997.01430280026003. [DOI] [PubMed] [Google Scholar]
- [25].Norton ID, Pokorny CS, Baird DK, Selby WS. Upper gastrointestinal haemorrhage following coronary artery bypass grafting. Aust NZ Med. 1995;25:297–301. doi: 10.1111/j.1445-5994.1995.tb01893.x. [DOI] [PubMed] [Google Scholar]
- [26].Simic O, Strathausen S, Hess W, Ostermeyer J. Incidence and prognosis of abdominal complications after cardiopulmonary bypass. Cardiovasc Surg. 1999;4:419–24. doi: 10.1016/s0967-2109(99)00008-3. [DOI] [PubMed] [Google Scholar]
- [27].Jayaprakash Anthoor A, McGrath Christine B, McCullagh Emily A, Smith Frank B, Angelini Gianni C, Probert Christopher A. Upper gastrointestinal heamorrhage following cardiac surgery. Euro J of Gastro & Hepat. 2004;16(2):191–194. doi: 10.1097/00042737-200402000-00011. [DOI] [PubMed] [Google Scholar]
- [28].Halm U, Halm F, Thein D, Mohr FW, Mossner J. Helicobacter pylori infection: A risk factor for upper gastrointestinal bleeding after cardiac surgery? Crit Care Med. 2000;28:110–113. doi: 10.1097/00003246-200001000-00018. [DOI] [PubMed] [Google Scholar]
- [29].Van der Voort PH, Zandstra DF. Pathogenesis, risk factors, and incidence of upper gastrointestinal bleeding after cardiac surgery: Is specific prophylaxis in routine bypass procedures needed? J Cardiothorac Vasc Anesth. 2000;14:293–299. [PubMed] [Google Scholar]
- [30].Rochall TA, Logan RF, Devlin HB, Northfield TC. Incidence of and mortality from acute upper gastrointestinal heamorrhage in the United Kingdom. Br Med J. 1995;22:222–226. doi: 10.1136/bmj.311.6999.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pushparani P, Spitz L, Somerville J, de Leval MR. Profuse lower gastrointestinal heamorrhage as an indication for surgery in necrotizing enterocolitis. Pediatr Surg Int. 1999;15:405–406. doi: 10.1007/s003830050613. [DOI] [PubMed] [Google Scholar]
- [32].Sakorafas GH, Tsiotos GG. Intra-abdominal complications after cardiac surgery. Eur J Surg. 1999;165:820–827. doi: 10.1080/11024159950189285. [DOI] [PubMed] [Google Scholar]
- [33].Della Ratta RK, Corapi MJ, Horowitz BR, Calio AJ. Risk of postoperative upper gastrointestinal tract hemorrhage in patients with active peptic ulcer disease undergoing non-ulcer surgery. Arch Intern Med. 1993;153:2141–2144. doi: 10.1001/archinte.153.18.2141. [DOI] [PubMed] [Google Scholar]
- [34].Reath DB, Maull KI, Wolfgang TC. General surgical complications following cardiac surgery. Am Surg. 1983;49:11–14. [PubMed] [Google Scholar]
- [35].Chigot JP, Bitker M, Chalgadian R, Laroussinie G, Cabrol A, Gandjbakhch I, et al. Abdominal complications of heart surgery. Arch Mal Coeur Vaiss. 1981;74:665–673. (in French, with an English abstract). [PubMed] [Google Scholar]
- [36].Aouifi A, Piriou V, Bastien O, Joseph P, Blanc P, Chiari P, et al. Severe digestive complications after heart surgery using extra corporeal circulation. Can J Anaesth. 1999;46:114–121. doi: 10.1007/BF03012544. (in French, with English abstract). [DOI] [PubMed] [Google Scholar]