Abstract
Background:
Fetal sexual differentiation relies on the translation of chromosomal sex established at fertilization into gonadal sex and somatic sex as development proceeds. In cases where chromosomal, gonadal, and somatic sex are incongruent in human infants and children, rapid establishment of the diagnosis and implementation of medical and surgical management is of paramount importance, since the gender identity is so important to the psychological well-being throughout life.
Objective:
This work was done in order to test the value of PCR technique for rapid sex determination compared to classic cytogenetic technique.
Methods:
Subjects included 20, cases including 10 neonates with ambiguous genitalia, 2 adult females with delayed puberty and 8 adult males with infertility, in addition to 20 normal infants of both sexes as a control group. The diagnosis of sex was attempted through examination, cytogenetic study, ultrasonography, gonadal biopsy and hormonal analysis, in addition to PCR amplification for the detection of SRY and ATL1 gene loci on Y and X chromosomes respectively.
Results:
Four neonates were diagnosed as partial testicular feminization showed both positive bands for the Y and X chromosomes and a karyogram of 46/XY. Three neonates were diagnosed as true hermaphrodites showed positive amplification for both Y and X chromosomes with a mosaic karyogram 46,XX/XY. Three neonates were diagnosed as cases of adrenogenital syndrome showed positive amplification of only the Xchromosome and had a karyogram of 46/XX. One of the two adult females was diagnosed as turner syndrome showed positive amplification of the X chromosome and a karyogram of 45/XO; the other one was diagnosed as complete testicular feminization had a positive amplification of X and Y chromosomes and a karyogram of 46/XY. The 8 adult males with infertility showed a positive amplification of X and Y chromosome and a karyogram of 47/XXY (Klinefelter syndrome) in 7 cases and 46/XY gonadal dysgenesis in one case.
Conclusion:
We concluded that PCR as a simple, rapid and reliable technique can complement and also confirm cytogenetic studies in the diagnosis of sex in cases of sex chromosome disorders.
Keywords: PCR, Sex, Intersex, Ambiguous genitalia
Introduction
Fetal sexual differentiation relies on the translation of chromosomal sex established at fertilization into gonadal sex and somatic sex as development proceeds. In cases where chromosomal, gonadal, and somatic sex are incongruent in human infants and children, rapid establishment of the diagnosis and implementation of medical and surgical management is of paramount importance, since the gender identity is so important to the psychological well-being throughout life [1]. It has been established that the SRY gene is necessary and sufficient for the sex determining function of the Y chromosome in mammals [2].
Currently, most methods for genotypic sex determination use the detection of Y chromosome in males. The cytogenetic analysis is a conventional method not only to identify sex, but also to detect other chromosomal aberrations. It is a non-invasive, inexpensive and rapid method, but it lacks the reliability and ability to detect mosaicism as well as other chromosomal abnormalities [3].
Until the pre-implantation genetic diagnosis of X-linked disorders was developed, a large number of techniques have been utilized for a rapid and reliable sex determination, i.e. non-radioactive in situ hybridization, fluorescence in situ hybridization (FISH), and primed in situ (PRINS) labeling technique [4]. These methods were performed by the detection of the Y chromosome in the inter-phase or meta-phase nuclei. In addition, sex determination using DNA analysis, i.e. dot blot hybridization and polymerase chain reaction (PCR), have also been reported [5].
The PCR-based sex determination identified by the presence (male) or absence (female) of the SRY gene has already been described. This method needs to have an internal control to verify the absence of the SRY gene from the failure of PCR amplification [6].
In this study, we will use the ATL1 locus as an internal control. The ATL1 is the sequence in the FMR1 gene located on the long arm of the X chromosome [7].
Subjects
Subjects were in the form of 20 cases including 10 neonates with ambiguous genitalia, 2 adult females with delayed puberty and 8 adult males with infertility, in addition to 20 normal infants of both sexes as a control group. These cases were referred to the Genetics Unit, Mansoura University Children Hospital, Egypt for the assessment of sex chromosomes. An informed consent was obtained from all adult subjects and from the guardian of neonatal and infantile cases as well. These experiments were also acknowledged from the university scientific and ethical committees.
Methods
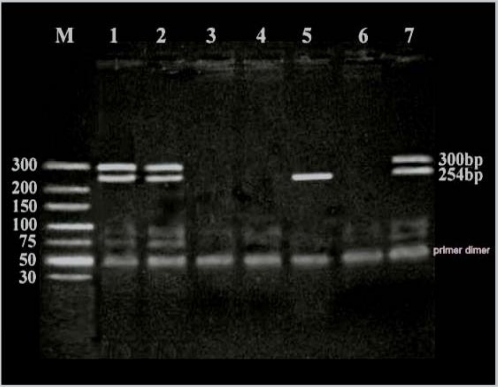
The diagnosis of sex was attempted through examination, cytogenetic study [8], ultrasonography, gonadal biopsy and hormonal analysis, in addition to PCR amplification for the detection of SRY and ALT1 gene loci on Y and X chromosomes respectively (Fig. 1).
Fig. 1. Photograph of electrophoresed PCR products showing band sized 254 bp for the SRY (Y chromosome) and band sized 300 bp for the ATL1 (X chromosome). Lanes 1, 2 and 7 show positive bands for both X and Y chromosomes, while lane 5 shows only a positive control band for the Y chromosome, and lanes 3, 4 and 6 are negative controls with no DNA.
The DNA extraction was performed. The PCR amplification and interpretation of the results were performed prior to obtaining the cytogenetic results.
Each 15 ul PCR reaction comprised 100 ng DNA, 1.5 ul of 10X buffer II (Perkin Elmer), 1 ul of 25 mmol dNTP, 1.5 mM MgCl2, 1 pmol each of SRY and ATL1 primers, and 0.25 unit Taq DNA polymerase. All PCR reactions were performed in a thermal cycler (Perkin Elmer 480) at 94°C (2 min) for initial DNA denaturation, followed by 35 cycles of 94°C (15 sec) for DNA denaturation, 65°C (20 sec) for primers annealing and 72°C (20 sec) for primer extension, with a final extension of the cycle at 72°C for 10 min. The amplified PCR products were separated on 2.5% agarose gels, ethidium bromide stained and visualized under the UV Transillumination. The sequences of oligonucleotide primers were 5’-CAT GAA CGC ATT CAT CGT GTG GTC-3’; and 5’-CTG CGG GAA GCA AAC TGC AAT TCT T-3’ for SRY [9]; and 5’-CCC TGA TGA AGA ACT TGT ATC TC-3’; and 5’-GAA ATT ACA CAC ATA GGT GGC ACT-3’ for ATL1 [10].
Results
Regarding the neonatal cases with ambiguous genitalia, four showed a positive PCR amplification for both the X and Y chromosomes and a karyogram of 46/XY. Pelvic ultrasonography showed no mullerian duct structures. The level of 17OH progesterone is not increased. Their gonads were testes. Therefore, they were diagnosed as having partial testicular feminization. Three neonates showed a positive amplification for both Y and X chromosomes with a mosaic karyogram 46,XX/XY; and internal organs suggestive of the presence of mullerian duct structures. Their gonads were in the form of ovitestes. They were diagnosed as true hermaphrodites.
Three neonates showed a positive amplification of only the X-chromosome and had a karyogram of 46/XX. Internal structures were suggested to mullerian duct derivatives and their 17OH progesterone level was elevated. They were diagnosed as cases of adrenogenital syndrome. One of the two adult females showed a positive amplification of the X chromosome and a karyogram of 45/XO confirming the diagnosis of turner syndrome, while the other one had a positive amplification of X and Y chromosomes and a karyogram of 46/XY with blind vagina and absent uterus and undescended testicular gonads was diagnosed as complete testicular feminization. The 8 adult males with infertility showed a positive amplification of X and Y chromosome and a karyogram of 47/XXY in 7 cases giving the diagnosis of Klinefelter syndrome and 46/XY in one case with a diagnosis of gonadal dysgenesis.
Discussion
The sex chromosomes play a crucial role in the etiology of normal sexual development. The bipotential gonad requires at least two X chromosomes to developed into an ovary. Alternatively, in the presence of SRY gene located on the Y chromosome, a testis is developed. Discordance between chromosomal sex and the appearance of the external genitalia can lead to sexual ambiguity. In the case of sexual ambiguity, the patient may be either a true hermaphrodite or pseudohermaphrodite. Both the ovary and testes are present in the former, while both the gonads are either absent or only one of them is present in the latter [11].
The results of the present study demonstrated the usefulness of microsatellite markers of X and Y chromosomes in determining the sex of individuals with ambiguous genitalia. The positive amplification of Y chromosome confirmed the cytogenetic pattern of XX/XY mosaicism, XXY (Klinefelter syndrome) and XY gonadal dysgenesis. Likewise, a negative amplification of Y chromosome is beneficial in cases of Turner syndrome to exclude low level mosaicism that may warrant castration as a prophylactic step against the development of gonadal malignancy. It was also beneficial for the confirmation of the diagnosis of adrnogenital syndrome in females with ambiguous genitalia.
In fact, PCR is of great value in cases of 46,XX maleness, which is characterized by testicular development in subjects who have two X chromosomes but lack a normal Y chromosome that is sometimes translocated on one of the autosomes [12].
The case with normal 46/XY pattern and infertiligy can be explained by McElreavey et al. who reported mutations in the SRY-ORF in −20% of subjects with 46,XY complete gonadal dysgenesis. The mutations are clustered in sequences encoding the HMG domain of the molecule. As SRY is responsible for the primary step in testis determination, mutations in the SRY-ORF could be expected to completely block the testis formation [13].
Interestingly, a conventional nested PCR analysis of maternal plasma could be used for accurate fetal gender detection. The result was confirmed by the routine analysis of fetal tissue obtained by invasive procedure or examination of newborns after delivery. The result was completely concordant with the conventional fetal tissue analysis and the examination of the newborns after delivery [14].
Nonetheless, Rajender et al. have reported a case of SRY-negative XX male with complete masculinization but infertility. The patient had fully mature male genitalia with descended but small testes and no signs of undervirilization. PCR analysis for SRY, ZFY, Amelogenin, AZFa, AZFb, AZFc genes, a pair of primers from heterochromatic region and six Y-STRs showed the absence of any Y-chromosome-derived material. The absence of SRY gene was confirmed by three independent PCRs for each of the two sets of primers covering an increasing length of the gene [15].
References
- [1].Federman DD, Donahoe PK. Ambiguous genitalia: Etiology, diagnosis and therapy. Adv Endocrinol Metab. 1995;6:91–116. [PubMed] [Google Scholar]
- [2].Hawkins JR. Hum Mol Genet. 1994. Sex determination; pp. 1463–7. 3 Spec No. [DOI] [PubMed] [Google Scholar]
- [3].Macia Bobes C, Alonso Troncoso I, Botas Cervero P, Castano Fernandez G, Fau Cubero C. Clinical and genetic study of a 46,XX man with occult mosaicism. Arch Esp Urol. 2002 Oct;55(8):952–4. [PubMed] [Google Scholar]
- [4].Delhanty JD, Griffin DK, Handyside AH, Harper J, Atkinson GH, Pieters MH, Winston RM. Detection of aneuploidy and chromosomal mosaicism in human embryos during preimplantation sex determination by fluorescent in situ hybridisation, (FISH) Hum Mol Genet. 1993 Aug;2(8):1183–5. doi: 10.1093/hmg/2.8.1183. [DOI] [PubMed] [Google Scholar]
- [5].Kao SM, Tang GC, Hsieh TT, Young KC, Wang HC, Pao CC. Analysis of peripheral blood of pregnant women for the presence of fetal Y chromosome-specific ZFY gene deoxyribonucleic acid sequences. Am J Obstet Gynecol. 1992 Mar;166(3):1013–9. doi: 10.1016/0002-9378(92)91381-j. [DOI] [PubMed] [Google Scholar]
- [6].Santos FR, Pandya A, Tyler-Smith C. Reliability of DNA-based sex tests. Nat Genet. 1998 Feb;18(2):103. doi: 10.1038/ng0298-103. [DOI] [PubMed] [Google Scholar]
- [7].Gunter C, Paradee W, Crawford DC, Meadows KA, Newman J, Kunst CB, Nelson DL, Schwartz C, Murray A, Macpherson JN, Sherman SL, Warren ST. Re-examination of factors associated with expansion of CGG repeats using a single nucleotide polymorphism in FMR1. Hum Mol Genet. 1998 Nov;7(12):1935–46. doi: 10.1093/hmg/7.12.1935. [DOI] [PubMed] [Google Scholar]
- [8].Ellaithi M, Gisselsson D, Nilsson T, Abd El-Fatah S, Ali T, Elagib A, Ibrahim ME, Fadl-Elmula I. A del(X) (p11) carrying SRY sequences in an infant with ambiguous genitalia. BMC Pediatr. 2006 Apr;4:6–11. doi: 10.1186/1471-2431-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cui KH, Warnes GM, Jeffrey R, Matthews CD. Sex determination of preimplantation embryos by human testis-determining-gene amplification. Lancet. 1994 Jan 8;343(8889):79–82. doi: 10.1016/s0140-6736(94)90815-x. [DOI] [PubMed] [Google Scholar]
- [10].Gunter C, Paradee W, Crawford DC, Meadows KA, Newman J, Kunst CB, Nelson DL, Schwartz C, Murray A, Macpherson JN, Sherman SL, Warren ST. Re-examination of factors associated with expansion of CGG repeats using a single nucleotide polymorphism in FMR1. Hum Mol Genet. 1998 Nov;7(12):1935–46. doi: 10.1093/hmg/7.12.1935. [DOI] [PubMed] [Google Scholar]
- [11].Sultan C, Paris F, Jeandel C, Lumbroso S, Galifer RB. Ambiguous genitalia in the newborn. Semin Reprod Med. 2002 Aug;20(3):181–8. doi: 10.1055/s-2002-35382. [DOI] [PubMed] [Google Scholar]
- [12].Fechner PY, Marcantonio SM, Jaswaney V, Stetten G, Goodfellow PN, Migeon CJ, Smith KD, Berkovitz GD, Amrhein JA, Bard PA, et al. The role of the sex-determining region Y gene in the etiology of 46,XX maleness. J Clin Endocrinol Metab. 1993 Mar;76(3):690–5. doi: 10.1210/jcem.76.3.8383144. [DOI] [PubMed] [Google Scholar]
- [13].McElreavey K, Vilain E, Barbaux S, Fuqua JS, Fechner PY, Souleyreau N, Doco-Fenzy M, Gabriel R, Quereux C, Fellous M, Berkovitz GD. Loss of sequences 3’ to the testis-determining gene, SRY, including the Y pseudoautosomal boundary associated with partial testicular determination. Proc Natl Acad Sci, USA. 1996 Aug 6;93(16):8590–4. doi: 10.1073/pnas.93.16.8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tungwiwat W, Fucharoen G, Ratanasiri T, Sanchaisuriya K, Fucharoen S. Non-invasive fetal sex determination using a conventional nested PCR analysis of fetal DNA in maternal plasma. Clin Chim Acta. 2003 Aug;334(1–2):173–7. doi: 10.1016/s0009-8981(03)00224-9. [DOI] [PubMed] [Google Scholar]
- [15].Rajender S, Rajani V, Gupta NJ, Chakravarty B, Singh L, Thangaraj K. SRY-negative 46,XX male with normal genitals, complete masculinization and infertility. Mol Hum Reprod. 2006 May;12(5):341–6. doi: 10.1093/molehr/gal030. [DOI] [PubMed] [Google Scholar]