Abstract
Background
Atrial fibrillation (AF) is the most common sustained arrhythmia and has a substantial heritable component. Numerous associations between single nucleotide polymorphisms (SNPs) and AF have been described, but few have been replicated.
Objective
We sought to systematically replicate SNPs reported to be associated with AF in two large study samples of European descent.
Methods
We searched PubMed for studies reporting associations between SNPs and AF published before July 1st, 2007. SNPs were genotyped in two independent case control samples from Germany and the United States. Associations between SNPs and AF were assessed using logistic regression models adjusting for age, sex and hypertension. A meta-analysis of the results from the two studies was performed.
Results
We identified 21 SNPs and the ACE I/D polymorphism that were reported to be associated with AF in the literature. Nine of these genetic variants were not represented on common genome-wide SNP arrays. We successfully genotyped 21 of these 22 variants in 2,145 cases with AF from the German Competence Network for Atrial Fibrillation and 4,073 controls from the KORA S4 study, and 16 variants in 790 cases and 1,330 controls from the Massachusetts General Hospital. None of the SNPs replicated in independent populations with AF.
Conclusion
Our results suggest that previously reported associations to AF were likely false-positives, and highlight the need for systematic replication of genetic associations in large, independent cohorts to accurately detect variants associated with disease.
Keywords: Atrial fibrillation, arrhythmia, genetics, single nucleotide polymorphism, replication, meta-analysis
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and a cause of major morbidity and mortality.1 Numerous risk factors for AF have been established, including age, sex, heart failure, valve disease and hypertension.2 Additionally, growing evidence supports a heritable component underlying AF.
Family studies have revealed chromosomal loci and gene mutations that underlie rare Mendelian forms of AF.3 In addition, there is evidence of genetic susceptibility to more common forms of AF. Family studies have reported an increased risk for AF among offspring of affected parents, with a risk increase in first-degree relatives ranging from 1.77 (95% confidence interval 1.67–1.88) for AF observed in the community to between 3–5 fold for early-onset AF.4–6 Genome-wide association studies (GWAS) have identified loci on chromosomes 4q25,7, 8 16q229 and 1q2110 associated with AF. Despite these findings, a substantial proportion of the heritability underlying common AF remains unexplained.
Numerous previous reports suggested associations between common genetic variants and AF.11–28 However, relative to current study designs, the sample sizes were typically small, multiple subgroups were compared to identify significant associations, and the identified variants were not replicated in independent populations.29–32 We therefore sought to systematically replicate the previously reported associations between genetic variants and AF in two large cohorts from the German Competence Network for Atrial Fibrillation (AFNET) and the Massachusetts General Hospital, Boston, MA (MGH).
Methods
Candidate gene selection
We searched PubMed and other scientific databases for original and review articles published before the publication of the first GWAS on atrial fibrillation (July 1st, 20077) that reported significant associations between genetic variants and AF. Database search terms included: “atrial fibrillation” or “AF” and “gene”, “genetic”, “snp”, “single nucleotide polymorphism” or “variant”. In order to enhance the power of our analysis, we limited our replication genotyping to variants reported to be significantly associated with AF (P<0.01) or studies that included at least 150 case subjects.
Study populations
The first sample of subjects was derived from the German Competence Network for Atrial Fibrillation (AFNET), a national registry of AF patients. DNA samples were collected from patients with AF in whom onset occurred before 60 years of age. A total of 2,145 AF cases were included. Samples available from the registry (n=1,249) were enriched with AF patients collected at the Medical Department I of the University Hospital Munich, Campus Grosshadern of the Ludwig-Maximilians University Munich (n=241) and the Medical Department I of the Technical University Munich Hospital and the Deutsches Herzzentrum München (n=655). Patients were included if AF was present on a 12-lead electrocardiogram and confirmed by a trained physician. Patients with heart failure greater than New York Heart Association class II in severity, moderate to severe valve disease (greater than grade II of IV), or hyperthyroidism were excluded. No case subjects had cardiac surgery within 30 days of inclusion. There was no known familial relationship between AF cases.
The control sample originated from the population-based, epidemiological KORA S4 survey of persons living in or near the city of Augsburg, Southern Germany.33 This study was conducted between 1999 and 2001. Study participants were identified through the registration office. A sample of 6,640 participants was drawn with ten strata of equal size according to sex and age and 4261 individuals (66.8%) agreed to participate. Exclusion criteria for control probands were reported history of AF, signs or symptoms of AF on physical examination, or absence of sinus rhythm on a resting 12-lead electrocardiogram that all probands received. After application of exclusion criteria a total of 4,073 subjects were included.
The second sample of subjects was derived from the Massachusetts General Hospital (MGH), where subjects with AF were identified from two separate cohorts. The first cohort consisted of patients with AF referred to the Cardiac Arrhythmia Service since June 2001. Inclusion criteria were AF documented by electrocardiogram before 66 years of age. In order to limit population stratification, recruitment of patients was limited to those reporting European ancestry. Exclusion criteria included any history of structural heart disease, rheumatic heart disease, hyperthyroidism, myocardial infarction, or heart failure before the onset of AF. The second MGH cohort included subjects with AF documented by electrocardiogram or history and admitted to the MGH Stroke service between January 1998 and July 2006 with an acute ischemic or hemorrhagic stroke as confirmed by CT or MRI. Patients were excluded for primary subarachnoid hemorrhage and for intracerebral hemorrhage secondary to head trauma, tumor, vascular malformation, or vasculitis. Controls without AF were recruited from a large, primary care practice of greater than 18,000 patients serving the MGH catchment area as well as the local Anticoagulation Management Unit. All subjects, or a family informant, were interviewed prospectively regarding medical history, medications and social and family history. Absence of AF was prospectively documented through interview and from review of medical records including all available electrocardiograms.
Variants examined for replication were directly genotyped in the AFNET and MGH samples, with the exception of the angiotensin converting enzyme insertion/deletion (ACE I/D) polymorphism owing to incompatibilities with the genotyping platform (see below). Thus, the ACE I/D polymorphism was tested for in silico replication in subsets of the AFNET and MGH case subjects using preexisting genome-wide data. The German sample consisted of 468 cases from AFNET and 438 controls derived from the KORA S4 survey. In MGH, 375 cases were included, and referent subjects (n=1108) were recruited from the population-based Framingham Heart Study.34–36 These two subsets have previously been described in detail.10
All case and control subjects gave their written informed consent prior to participation in our study. The study was approved by the Ludwig-Maximilians University Medical Ethics Committee and by the Institutional Review Boards at MGH and the Framingham Heart Study, and was performed according to the declarations of Helsinki and Somerset West.
DNA extraction and genotyping
DNA was extracted from EDTA anticoagulated blood using a salting out procedure37 (AFNET) or a commercially available kit (Gentra Puregene Blood Kit™, Qiagen, Valencia, CA, USA) (MGH). SNPs were genotyped using PCR, iPlex single base primer extension and matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry in a 384-well-format (Sequenom, San Diego, CA, U.S.A.) according to the manufacturer’s instructions as previously described.38 Systematic assessment of different genotyping methods for the ACE I/D variant revealed that assessment of SNPs in linkage disequilibrium with the polymorphism provides reliable results.39 However, none of the possible SNPs produced interpretable results on our MALDI-TOF platform, and therefore the ACE I/D variant was assessed using rs4351 as a proxy (r2=0.87)10, 40 in the aforementioned subsets with available genome wide data.
Statistical analysis
We examined associations between each SNP and AF using multivariable logistic regression, assuming an additive genetic model. AF is influenced by several factors. To minimize confounding of genetic association results by the most common factors, multivariable analyses were performed adjusting for age, sex and hypertension. Separate analyses were conducted for each study site and the results of both our samples were meta-analyzed by summing the z-scores and weighting by study size for each center as recently described.41 We used a Bonferroni adjusted significance threshold to account for multiple comparisons. Twentyone variants were analyzed, thus p-values below 0.0024 (0.05/21) were considered statistically significant. Statistical analyses were performed with STATA SE 8.0 (AFNET) and PLINK v1.05 42 (MGH).
Power was estimated using the CaTS power calculator for genetic studies assuming a one-stage design.43 We modeled a minor allele frequency of 0.28, which represents roughly the mean minor allele frequency in Europeans of the 21 variants under investigation.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
We identified 18 studies through July 1, 2007 reporting associations between genetic variants and AF that met our selection criteria, yielding 21 SNPs and the ACE I/D polymorphism that we attempted to replicate in our study samples.11–28 Detailed information for these variants is available in Table 1. Two SNPs in the angiotensinogen gene reported to be associated with AF, rs699 and rs5051, were in strong linkage disequilibrium (D′ = 0.967, r2 = 0.929). Therefore, only rs5051 was genotyped.
Table 1.
Single nucleotide polymorphisms associated with atrial fibrillation selected for the replication.
| SNP | Chr | Genomic position | Band | Alleles | Gene | Role | Genomic change | Amino acid change | Reference |
|---|---|---|---|---|---|---|---|---|---|
| rs11552588 | 1 | 145,712,007 | 1q21.1 | A/G | GJA5 | 5′ UTR | −61a>g | - | 11 |
| rs35594137 | 1 | 145,712,121 | 1q21.1 | A/G | GJA5 | Promoter | −44a>g | - | 11 |
| rs1800872 | 1 | 205,013,030 | 1q32.1 | A/C | IL10 | Promoter | −592a>c | - | 12 |
| rs699 | 1 | 228,912,417 | 1q42.2 | C/T | AGT | Exon | 916c>t | M268T | 13 |
| rs5051 | 1 | 228,916,495 | 1q42.2 | C/T | AGT | 5′ UTR | −6c>t | - | 13 |
| rs5049 | 1 | 228,916,706 | 1q42.2 | A/G | AGT | Promoter | −217a>g | - | 13 |
| rs12621643 | 2 | 223,626,227 | 2q36.1 | G/T | KCNE4 | Exon | 435g>t | E145D | 14 |
| rs1805124 | 3 | 38,620,424 | 3p22.2 | A/G | SCN5A | Exon | 1867a>g | H558R | 15 |
| rs1800795 | 7 | 22,733,170 | 7p15.3 | C/G | IL6 | Promoter | −174g>c | - | 16 |
| rs1805123 | 7 | 150,276,467 | 7q36.1 | A/C | KCNH2 | Exon | 2690a>c | K897T | 44 |
| rs2070744 | 7 | 150,321,012 | 7q36.1 | C/T | NOS3 | Intron | −786t>c | - | 17 |
| rs1799983 | 7 | 150,327,044 | 7q36.1 | G/T | NOS3 | Exon | 894g>t | E298D | 17 |
| rs1799963 | 11 | 46,717,631 | 11p11.2 | A/G | F2 | Promoter | 20210a>g | - | 18 |
| rs583362 | 11 | 107,083,931 | 11q22.3 | C/G | SLN | 5′ UTR | −65c>g | - | 19 |
| rs5443 | 12 | 6,825,136 | 12p13.31 | C/T | GNB3 | Exon | 825c>t | S275S | 20 |
| rs243865 | 16 | 54,069,307 | 16q12.2 | C/T | MMP2 | Promoter | −1306c>t | - | 12 |
| rs708272 | 16 | 55,553,789 | 16q13 | C/T | CETP | Intron | 55,553,789c>t | - | 21 |
| rs5882 | 16 | 55,573,593 | 16q13 | A/G | CETP | Exon | 1394a>g | V422I | 21 |
| ACE I/D (rs4351) | 17 | 58,923,464 | 17q23.3 | A/G | ACE | Intron | 15276a>g | - | 13, 25–28 |
| rs1805128 | 21 | 34,743,550 | 21q22.12 | A/G | KCNE1 | Exon | 878a>g | D85N | 14 |
| rs1805127 | 21 | 34,743,691 | 21q22.12 | A/G | KCNE1 | Exon | 737a>g | S38G | 17, 22, 23 |
| rs17003955 | X | 108,754,809 | Xq22.3 | A/G | KCNE1L | Exon | 97a>g | P33T | 24 |
Abbreviations: Chr: chromosome, 5′UTR: 5′ untranslated region, amino acids are in single letter code.
A total of 2,935 subjects with AF and 5,403 control subjects without AF were included in for this analysis. The clinical characteristics of the study subjects are shown in Table 2. In both samples, subjects with AF were predominantly male. In AFNET, subjects with AF had an older mean age than control subjects (61.0 ± 11.6 yrs vs. 49.2 ± 13.9 yrs, p<0.0001). In the MGH sample, subjects with AF on average were younger than control subjects (63.4 ± 14.6 yrs vs. 66.4 ± 12.8 yrs, p<0.0001).
Table 2.
Clinical characteristics of the study cohorts.
| Study site | Munich | MGH | ||
|---|---|---|---|---|
| AF status | AF | No AF | AF | No AF |
| Number | 2,145 | 4,073 | 790 | 1,330 |
| Age in years (mean ± SD) | 61.0 ± 11.6 | 49.2 ± 13.9* | 63.4 ± 14.6 | 66.4 ± 12.8* |
| Male | 1,564 (72.9) | 2,005 (49.2)* | 547 (69.2) | 712 (53.5)* |
| Hypertension | 1,148 (53.5) | 730 (17.9)* | 383 (48.5) | 677 (50.9) |
Data is shown as mean ± standard deviation or number (percentage) where applicable.
p value < 0.0001 for comparison between cases and controls.
Abbreviations: SD: standard deviation.
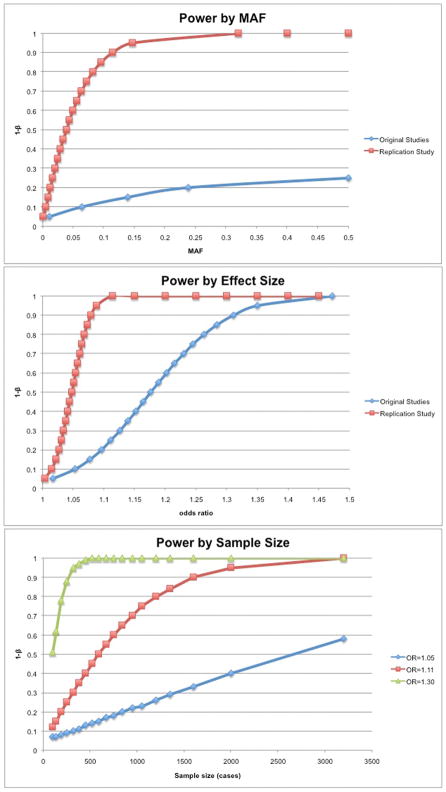
Based on our power calculation, we estimated that we would have at least 80% power to detect relative risks of 1.10 for risk alleles with frequencies of 28%, and 1.20 for risk alleles with frequencies of 5%, assuming an additive genetic model and a two-sided, adjusted significance level of 0.0024. The power depending on minor allele frequency, effect size and sample size is illustrated in Figure 1.
Figure. Statistical power depending on the study design.
A: Power according to MAF. The red line represents results from our replication study underlying our joint sample size of 2935 cases and 5403 controls. The blue line represents the original reporting studies with an average of 233 cases and 380 controls. Both distributions assume the genetic variant associated with AF has an OR of 1.11. B: Power according to effect size. The results of the replication study are displayed in red and the original reporting studies are in blue, assuming an average MAF of 0.28 and the sample sizes described above (see A). C: Power according to sample size. We assumed a minor allele frequency of 0.28. Different power distributions were modeled for different ORs 1.05, 1.11 and 1.30, respectively. Abbreviations: MAF: minor allele frequency; OR: odds ratio.
All 21 variants were genotyped in the AFNET cohort. SNPs rs35594137, rs1799983, rs1800795 and 708272 technically failed genotyping in the MGH sample and are thus available from the AFNET sample only. The genotyping of all remaining variants appeared to be of high quality as reflected in the call rate, lack of deviation from Hardy-Weinberg equilibrium, and minor allele frequencies in good correspondence with public data bases. Details of the SNP genotyping are provided in Supplemental Table 1.
In the AFNET sample the SNPs most significantly associated with AF were rs1800872/IL10 (OR 0.87, CI 0.78 – 0.96, P=0.007) and rs12621643/KCNE4 (OR 0.89, 95% CI 0.81 – 0.98, P=0.02), neither of which were below the adjusted significance threshold (p<0.0024). Similarly, in the MGH study, the SNP most strongly associated with AF was rs1805128/KCNE1 (OR 1.73, 95% CI 1.01 – 2.97, P=0.047), but was not significant after accounting for multiple comparisons (Table 3).
Table 3.
Association results for SNPs in the AFNET and MGH samples and the meta-analysis of both.
| SNP | Gene | AFNET |
MGH |
Meta-analysis |
||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR 95% CI | P | Weighted z-score | OR (95% CI) | P | ||
| rs11552588 | GJA5 | 0.95 (0.86–1.06) | 0.38 | 0.88 (0.75–1.03) | −1.5170 | 0.93 (0.83–1.05) | 0.13 | |
| rs35594137 | GJA5 | 0.98 (0.88–1.08) | 0.64 | - | - | −0.4700 | 0.98 (0.88–1.08) | 0.64 |
| rs1800872 | IL10 | 0.87 (0.78–0.96) | 0.01 | 0.97 (0.84–1.13) | 0.69 | −2.5668 | 0.89 (0.79–1.00) | 0.01 |
| rs5051 | AGT | 1.07 (0.98–1.16) | 0.16 | 1.12 (0.98–1.28) | 0.09 | 2.0661 | 1.08 (0.98–1.19) | 0.04 |
| rs5049 | AGT | 1.10 (0.95–1.28) | 0.20 | 1.06 (0.85–1.31) | 0.62 | 1.3413 | 1.09 (0.92–1.29) | 0.18 |
| rs12621643 | KCNE4 | 0.89 (0.81–0.98) | 0.02 | 1.12 (0.97–1.29) | 0.12 | −1.3020 | 0.95 (0.85–1.05) | 0.19 |
| rs1805124 | SCN5A | 1.05 (0.95–1.17) | 0.35 | 1.17 (0.99–1.39) | 0.07 | 1.7216 | 1.08 (0.96–1.22) | 0.09 |
| rs1800795 | IL6 | 1.02 (0.93–1.12) | 0.69 | - | - | 0.4000 | 1.02 (0.93–1.12) | 0.69 |
| rs1805123 | KCNH2 | 1.37 (1.17–1.59) | 0.0001 | 1.09 (0.93–1.28) | 0.29 | 4.0141 | 1.30 (1.11–1.51) | 0.0001 |
| rs2070744 | NOS3 | 0.92 (0.83–1.02) | 0.06 | 1.10 (0.96–1.26) | 0.18 | −0.7357 | 0.97 (0.86–1.08) | 0.46 |
| rs1799983 | NOS3 | 0.98 (0.89–1.08) | 0.73 | - | - | −0.3500 | 0.98 (0.89–1.08) | 0.73 |
| rs1799963 | F2 | 0.71 (0.50–1.01) | 0.36 | 1.01 (0.59–1.72) | 0.97 | −1.6277 | 0.78 (0.52–1.18) | 0.10 |
| rs583362 | SLN | 0.96 (0.88–1.05) | 0.56 | 1.10 (0.96–1.25) | 0.16 | −0.1068 | 0.99 (0.90–1.10) | 0.91 |
| rs5443 | GNB3 | 0.98 (0.88–1.08) | 0.66 | 1.00 (0.87–1.15) | 0.96 | −0.3518 | 0.99 (0.88–1.10) | 0.73 |
| rs243865 | MMP2 | 1.08 (0.98–1.20) | 0.12 | 1.00 (0.86–1.17) | 0.96 | 1.3668 | 1.06 (0.95–1.19) | 0.17 |
| rs708272 | CETP | 0.95 (0.87–1.04) | 0.29 | - | −1.0600 | 0.95 (0.87–1.04) | 0.29 | |
| rs5882 | CETP | 0.93 (0.84–1.02) | 0.11 | 1.11 (0.96–1.27) | 0.15 | −0.6663 | 0.97 (0.87–1.08) | 0.51 |
| ACE I/D (rs4351) | ACE | 1.00 (0.79–1.26) | 0.95 | 1.17 (0.96–1.43) | 0.13 | 1.0549 | .11 (0.90–1.35) | 0.29 |
| rs1805128 | KCNE1 | 1.04 (0.15–7.41) | 0.97 | 1.73 (1.01–2.97) | 0.05 | 1.0108 | 0.45 (0.36–6.34) | 0.31 |
| rs1805127 | KCNE1 | 0.98 (0.90–1.08) | 0.73 | 0.98 (0.84–1.14) | 0.78 | −0.4423 | 0.98 (0.89–1.09) | 0.66 |
| rs17003955 | KCNE1L | 0.98 (0.78–1.22) | 0.83 | 1.17 (0.88–1.55) | 0.28 | 0.3279 | 1.02 (0.80–1.30) | 0.74 |
In each sample, associations were adjusted for age, sex and hypertension. The meta-analysis z-scores were calculated summing the respective z-scores from both samples weighted for the size of each study. All p-values are unadjusted. Threshold for significance after Bonferroni correction is p<0.0024.
In a weighted meta-analysis of the results from both AFNET and MGH, two SNPs were weakly associated with AF: rs1800872/IL10 (OR 0.88, 95% CI 0.79 – 1.00, P=0.01) and rs5051/AGT (−6 g>a, OR 1.08, 95% CI 0.98 – 1.19, P=0.04). However, despite the increased power to detect associated variants in a combined sample, neither was significantly associated with AF after accounting for multiple testing. The full results of the meta-analysis are depicted in Table 3.
The previously reported SNP rs1805123/KCNH2 remained formally significant in the AFNET sample (OR 1.37, 95% CI 1.17 – 1.59, P=0.0001). This association was not significant in the MGH sample alone, but was in the meta-analysis (OR 1.30, 95% CI 1.11 – 1.51, P=0.0001).
Discussion
We identified 22 SNPs from 15 candidate genes previously reported to be associated with AF, and attempted to replicate these associations in two large case control samples. Using a total of 2,935 cases with AF and 5,403 controls from Germany and the United States, we could not reliably replicate associations between any of the previously reported SNPs and AF after accounting for multiple testing.
The meta-analyzed associations between some SNPs and AF in our study were of borderline significance (rs5051, P=0. 04 and rs1800872, P=0.01) and several SNPs demonstrated trends toward association in one but not both samples. Our Bonferroni-adjusted significance threshold may be considered conservative for a replication study, and we acknowledge that SNPs with relatively low p-values may confer some degree of association with AF.
The association between rs1805123 in KCNH2 and AF was previously established by our group.44 However, in the current analysis, a significant association was detected only in AFNET and not in the MGH sample. The association in the MGH sample tended into the same direction, but did not reach significance. Thus, the significant finding in the meta-analysis of both samples is driven primarily by the AFNET sample. It is commonly the case that even strong associations with a phenotype do not replicate in all other cohorts. Similarly, despite a strong association between the chromosome 4q25 locus and AF, this association does not replicate in all attempts.45 In conclusion, further replication in additional, independent populations will be necessary to delineate the relation between rs1805123 and AF.
We had excellent power to replicate associations with the originally published effect sizes. Given the results of our study, it is thus likely that previously reported associations were false positives. The previous case-control studies for AF had, on average, 233 case and 380 control subjects. Consequently, their power to reliably detect associations was markedly smaller. Our study was approximately ten-fold larger compared to the average size of the original reporting studies and was sufficiently powered to detect associations with odds ratios as small as 1.10 for more common alleles and 1.20 for relatively rare SNPs (Figure 1). The original reports may merely be examples of the ‘winner’s curse’.46
It is possible that there may be distinct forms of AF, such as postoperative AF, that have different genetic backgrounds from the AF studied in our samples. As a consequence, it is possible that our lack of replication could be due to the fact that some of the original publications examined selected populations. The suitability of our samples to determine genetic variants associated with AF is supported by the fact that both cohorts have replicated the most widely reported genetic variant associated with AF in the chromosome 4q25 region; the MGH cohort was used in the initial publication7 and the AFNET cohort was involved in a subsequent large-scale replication of this finding.8
Non-genetic factors are likely to modify the associations between the genetic variants and AF. We thus adjusted our results for age, sex and hypertension. Although interactions that modify genetic associations with AF have not been reliably demonstrated, we acknowledge that such factors have the potential to lead to heterogeneity in the associations observed between different study samples. Indeed, in a recent GWAS, the association between variants and AF at a novel disease susceptibility locus in ZFHX3 did not exceed the significance threshold in all of the samples.9 While the heterogeneity in the associations between samples remains unexplained, it underscores the principle that the lack of replication in one cohort is not definitive, just the way replication in one study is not definitive.
A recent GWAS on the electrocardiographic PR interval has demonstrated the variant rs6800541 in SCN5A to be associated with AF.47 SNP rs6800541 and the variant in SCN5A that we attempted to replicate in our study (rs1805124) are not in linkage disequilibrium in individuals of European descent (r2=0.001, D′=0.051, HapMap 3 CEU panel).40 This supports the interpretation that both loci are inherited independently, despite the genomic proximity. In conclusion, it lends support to the fact that SCN5A is indeed involved in AF genetics, but highlights that the selection of single SNPs based on a priori consideration has a low probability of yielding reliable associations.
Most variants investigated in our study were common. The mean minor allele frequency was 28%. In contrast, mutations of relevance in familial forms of AF and other rare genetic variants (≪1% allele frequency) might be of importance also in explaining the genetic background of common AF.48 Such variants could not be studied in our current investigation. New sequencing technology will allow the identification of rare genetic variants in larger populations in the future.
Strengths and limitations
A major strength of our study is the large sample size. Standardized and validated methods were used for genotyping; analysis and results were generated at different centers experienced in the conduction of genetic association studies. Another strength is the comprehensiveness of our study.
While the number of GWAS is growing, it is appealing to use this unbiased approach to detect novel risk alleles for AF or other disease entities. It is notable that 9 of the 21 variants in our study are not represented on any commercially available genome-wide SNP array and have not been genotyped within the scope of the HapMap. However, even if all 21 variants considered in this study were represented on common genotyping arrays, the associations we observed were well above the significance threshold in a typical GWAS (P<~1×10−8).
Yet, there are several limitations to our study that merit discussion. We acknowledge that potential misclassification of referent subjects that will later develop AF may bias our results toward the null hypothesis. Furthermore, variants associated with severe forms of AF could affect sampling of case subjects, and thus result in null associations between these variants and AF.
In order to limit the possibility of population stratification, our study sample was limited to individuals of European ancestry only. Thus, the generalizability of our results to other races and ethnicities may be limited. While most variants were initially reported in whites, some were identified in subjects of Asian descent. The discovery of putative AF variants in Asian cohorts could serve as an explanation why we were not able to replicate some of the initially reported findings in our study.
Additionally, four SNPs were not genotyped in the MGH sample. The associations between these SNPs and AF in AFNET did not approach statistical significance. However, we concede that we cannot finally exclude false negative results, in particular if the effects conferred by these SNPs were modest.
Conclusion
In conclusion, we were unable to independently replicate the prior findings relating many common SNPs to AF. Future studies reporting an association between individual SNPs and disease should be supported by convincing replication in multiple independent cohorts. In addition, future studies exploring factors that underlie heterogeneity in associations between SNPs and AF in large study samples, such as interactions with other genes or environmental variables, are warranted.
Supplementary Material
Acknowledgments
Acknowledgement for financial support: This study was funded by grants from the LMU Research Grant FöFoLe 577/569 to Dr. Sinner, the German National Genome Research Network NGFN 01 GS 0499, 01 GS 0838, German Federal Ministry of research 01 EZ 0874, German Competence Network on AF (AF-Net) 01 GI 0204/N, Leducq Foundation 07-CVD 03 and LMU Excellence Initiative to Dr. Kääb, from the German National Genome Research Network NGFN 01GR0803 and the BMBF German Federal Ministry of research 01EZ0874 to Dr. Pfeufer, by grants to Dr. Ellinor from the Deane Institute for Integrative Research in Atrial Fibrillation and Stroke and grants from the NIH to Dr. Rosand (R01NS059727), Dr. Furie (5P50NS051343), Dr. Lubitz (T32HL007575) and Drs Ellinor and Benjamin (R01HL092577). The KORA platform is funded by the BMBF and by the State of Bavaria.
Abbreviations
- AF
atrial fibrillation
- SNP
single nucleotide polymorphism
- GWAS
genome-wide association study
- AFNET
German Competence Network for Atrial Fibrillation
- MGH
Massachusetts General Hospital
Footnotes
Disclosures: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The anticoagulation and risk factors in atrial fibrillation (atria) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: Incidence, risk factors, and prognosis in the manitoba follow-up study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 3.Tsai CT, Lai LP, Hwang JJ, Lin JL, Chiang FT. Molecular genetics of atrial fibrillation. J Am Coll Cardiol. 2008;52:241–250. doi: 10.1016/j.jacc.2008.02.072. [DOI] [PubMed] [Google Scholar]
- 4.Ellinor PT, Yoerger DM, Ruskin JN, MacRae CA. Familial aggregation in lone atrial fibrillation. Hum Genet. 2005;118:179–184. doi: 10.1007/s00439-005-0034-8. [DOI] [PubMed] [Google Scholar]
- 5.Arnar DO, Thorvaldsson S, Manolio TA, et al. Familial aggregation of atrial fibrillation in iceland. Eur Heart J. 2006;27:708–712. doi: 10.1093/eurheartj/ehi727. [DOI] [PubMed] [Google Scholar]
- 6.Fox CS, Parise H, D’Agostino RB, et al. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291:2851–2855. doi: 10.1001/jama.291.23.2851. [DOI] [PubMed] [Google Scholar]
- 7.Gudbjartsson DE, Arnar DO, Helgadottir A, et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;448:353–357. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]
- 8.Kääb S, Darbar D, van Noord C, et al. Large scale replication and meta-analysis of variants on chromosome 4q25 associated with atrial fibrillation. Eur Heart J. 2009;30:813–819. doi: 10.1093/eurheartj/ehn578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benjamin EJ, Rice K, Arking DE, et al. Variants in ZFHX3 are associated with atrial fibrillation in individuals of european ancestry. Nat Genet. 2009;41:879–881. doi: 10.1038/ng.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellinor PT, Lunetta KL, Glazer N, et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet. 2010;42:240–244. doi: 10.1038/ng.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juang JM, Chern YR, Tsai CT, et al. The association of human connexin 40 genetic polymorphisms with atrial fibrillation. Int J Cardiol. 2007;116:107–112. doi: 10.1016/j.ijcard.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 12.Kato K, Oguri M, Hibino T, et al. Genetic factors for lone atrial fibrillation. Int J Mol Med. 2007;19:933–939. [PubMed] [Google Scholar]
- 13.Tsai CT, Lai LP, Lin JL, et al. Renin-angiotensin system gene polymorphisms and atrial fibrillation. Circulation. 2004;109:1640–1646. doi: 10.1161/01.CIR.0000124487.36586.26. [DOI] [PubMed] [Google Scholar]
- 14.Zeng Z, Tan C, Teng S, et al. The single nucleotide polymorphisms of i(ks) potassium channel genes and their association with atrial fibrillation in a chinese population. Cardiology. 2007;108:97–103. doi: 10.1159/000095943. [DOI] [PubMed] [Google Scholar]
- 15.Chen LY, Ballew JD, Herron KJ, Rodeheffer RJ, Olson TM. A common polymorphism in scn5a is associated with lone atrial fibrillation. Clin Pharmacol Ther. 2007;81:35–41. doi: 10.1038/sj.clpt.6100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaudino M, Andreotti F, Zamparelli R, et al. The −174g/c interleukin-6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication? Circulation. 2003;108(Suppl 1):II195–199. doi: 10.1161/01.cir.0000087441.48566.0d. [DOI] [PubMed] [Google Scholar]
- 17.Fatini C, Sticchi E, Genuardi M, et al. Analysis of mink and enos genes as candidate loci for predisposition to non-valvular atrial fibrillation. Eur Heart J. 2006;27:1712–1718. doi: 10.1093/eurheartj/ehl087. [DOI] [PubMed] [Google Scholar]
- 18.Poli D, Antonucci E, Cecchi E, et al. Thrombophilic mutations in high-risk atrial fibrillation patients: High prevalence of prothrombin gene g20210a polymorphism and lack of correlation with thromboembolism. Thromb Haemost. 2003;90:1158–1162. doi: 10.1160/TH03-04-0240. [DOI] [PubMed] [Google Scholar]
- 19.Nyberg MT, Stoevring B, Behr ER, Ravn LS, Mckenna W, Christiansen M. The variation of the sarcolipin gene (SLN) in atrial fibrillation, long qt syndrome and sudden arrhythmic death syndrome. Clin Chim Acta. 2007;375:87–91. doi: 10.1016/j.cca.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Schreieck J, Dostal S, von Beckerath N, et al. C825t polymorphism of the g-protein beta3 subunit gene and atrial fibrillation: Association of the tt genotype with a reduced risk for atrial fibrillation. Am Heart J. 2004;148:545–550. doi: 10.1016/j.ahj.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 21.Asselbergs FW, Moore JH, van den Berg MP, et al. A role for cetp taqib polymorphism in determining susceptibility to atrial fibrillation: A nested case control study. BMC Med Genet. 2006;7:39. doi: 10.1186/1471-2350-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai LP, Su MJ, Yeh HM, et al. Association of the human mink gene 38g allele with atrial fibrillation: Evidence of possible genetic control on the pathogenesis of atrial fibrillation. Am Heart J. 2002;144:485–490. doi: 10.1067/mhj.2002.123573. [DOI] [PubMed] [Google Scholar]
- 23.Prystupa A, Dzida G, Myśliński W, Małaj G, Lorenc T. Mink gene polymorphism in the pathogenesis of lone atrial fibrillation. Kardiologia polska. 2006;64:1205–1211. discussion 1212–1203. [PubMed] [Google Scholar]
- 24.Ravn LS, Hofman-Bang J, Dixen U, et al. Relation of 97t polymorphism in KCNE5 to risk of atrial fibrillation. Am J Cardiol. 2005;96:405–407. doi: 10.1016/j.amjcard.2005.03.086. [DOI] [PubMed] [Google Scholar]
- 25.Bedi M, McNamara D, London B, Schwartzman D. Genetic susceptibility to atrial fibrillation in patients with congestive heart failure. Heart Rhythm. 2006;3:808–812. doi: 10.1016/j.hrthm.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Fatini C, Sticchi E, Gensini F, et al. Lone and secondary nonvalvular atrial fibrillation: Role of a genetic susceptibility. Int J Cardiol. 2007;120:59–65. doi: 10.1016/j.ijcard.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 27.Gensini F, Padeletti L, Fatini C, Sticchi E, Gensini GF, Michelucci A. Angiotensin-converting enzyme and endothelial nitric oxide synthase polymorphisms in patients with atrial fibrillation. PACE. 2003;26:295–298. doi: 10.1046/j.1460-9592.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita T, Hayami N, Ajiki K, et al. Is ace gene polymorphism associated with lone atrial fibrillation? Jpn Heart J. 1997;38:637–641. doi: 10.1536/ihj.38.637. [DOI] [PubMed] [Google Scholar]
- 29.Morgan TM, Coffey CS, Krumholz HM. Overestimation of genetic risks owing to small sample sizes in cardiovascular studies. Clin Genet. 2003;64:7–17. doi: 10.1034/j.1399-0004.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- 30.Casas JP, Cooper J, Miller GJ, Hingorani AD, Humphries SE. Investigating the genetic determinants of cardiovascular disease using candidate genes and meta-analysis of association studies. Ann Hum Genet. 2006;70:145–169. doi: 10.1111/j.1469-1809.2005.00241.x. [DOI] [PubMed] [Google Scholar]
- 31.Thomas D, Xie R, Gebregziabher M. Two-stage sampling designs for gene association studies. Genet Epidemiol. 2004;27:401–414. doi: 10.1002/gepi.20047. [DOI] [PubMed] [Google Scholar]
- 32.Hirschhorn JN, Daly MJ. Genome-wide association studies for common diseases and complex traits. Nat Rev Genet. 2005;6:95–108. doi: 10.1038/nrg1521. [DOI] [PubMed] [Google Scholar]
- 33.Wichmann HE, Gieger C, Illig T. Kora-gen--resource for population genetics, controls and a broad spectrum of disease phenotypes. Gesundheitswesen. 2005;67 (Suppl 1):S26–30. doi: 10.1055/s-2005-858226. [DOI] [PubMed] [Google Scholar]
- 34.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: The framingham study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 36.Splansky GL, Corey D, Yang Q, et al. The third generation cohort of the national heart, lung, and blood institute’s framingham heart study: Design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 37.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfeufer A, Jalilzadeh S, Perz S, et al. Common variants in myocardial ion channel genes modify the qt interval in the general population: Results from the kora study. Circ Res. 2005;96:693–701. doi: 10.1161/01.RES.0000161077.53751.e6. [DOI] [PubMed] [Google Scholar]
- 39.Glenn KL, Du ZQ, Eisenmann JC, Rothschild MF. An alternative method for genotyping of the ACE I/D polymorphism. Mol Biol Rep. 2009;36:1305–1310. doi: 10.1007/s11033-008-9313-5. [DOI] [PubMed] [Google Scholar]
- 40.Johnson AD, Handsaker RE, Pulit SL, Nizzari MM, O’Donnell CJ, de Bakker PI. Snap: A web-based tool for identification and annotation of proxy snps using hapmap. Bioinformatics. 2008;24:2938–2939. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Bakker P, Ferreira M, Jia X, Neale B, Raychaudhuri S, Voight B. Practical aspects of imputation-driven meta-analysis of genome-wide association studies. Hum Mol Genet. 2008;17:R122–R128. doi: 10.1093/hmg/ddn288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Purcell S, Neale B, Todd-Brown K, et al. Plink: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skol AD, Scott LJ, Abecasis G, Boehnke M. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet. 2006;38:209–213. doi: 10.1038/ng1706. [DOI] [PubMed] [Google Scholar]
- 44.Sinner MF, Pfeufer A, Akyol M, et al. The non-synonymous coding ikr-channel variant KCNH2-K897T is associated with atrial fibrillation: Results from a systematic candidate gene-based analysis of KCNH2 (HERG) Eur Heart J. 2008;29:907–914. doi: 10.1093/eurheartj/ehm619. [DOI] [PubMed] [Google Scholar]
- 45.Lubitz SA, Ozcan C, Magnani JW, Kaab S, Benjamin EJ, Ellinor PT. Genetics of atrial fibrillation: Implications for future research directions and personalized medicine. Circ Arrhythm Electrophysiol. 2010;3:291–299. doi: 10.1161/CIRCEP.110.942441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young NS, Ioannidis JP, Al-Ubaydli O. Why current publication practices may distort science. PLoS Med. 2008;5:e201. doi: 10.1371/journal.pmed.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfeufer A, van Noord C, Marciante K, et al. Genome-wide association study of PR interval. Nat Genet. 2010;42:153–159. doi: 10.1038/ng.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lubitz S, Yi BA, Ellinor PT. Genetics of atrial fibrillation. Cardiol Clin. 2009;27:25–33. doi: 10.1016/j.ccl.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.