Abstract
Apoptosis plays an important role in cellular homeostasis. In this study we have investigated whether apoptosis is a contributory factor to alcohol induced liver damage. Long term ethanol (1.6 g/kg body weight/day) exposure augmented liver apoptosis as reflected by high frequency of positive TUNEL staining nuclei and by an increased activity of caspase-3 and -8. Our study provides evidence that long-term ethanol consumption triggers apoptotic process in the liver.
Keywords: Apoptosis, Caspase, Ethanol, TUNEL
Introduction
Apoptosis plays an important role in cellular homeostasis. Apoptosis maintains the T cell repertoire and in deleting autoreactive lymphocytes, thus regulating the immune response [1–3]. Ethanol-associated endotoxaemia and subsequent release of inflammatory mediators may cause hepatic injury via oxyradical-dependent or -independent mechanisms [4]. In one study, ethanol-containing liquid diet feeding for 6 months caused oxidative stress, upregulated the mRNA levels of tumor necrosis factor (TNF)-α and caspase-3 activity in the liver of male adult 129S6 mice and developed hepatitis [5]. Other study has shown that TNF-α addition to ethanol-pretreated hepatocyte culture provokes the mitochondrial permeability transition, cytochrome c release, procaspase 3 activation, and apoptosis [6]. We have already shown that long-term ethanol induced liver damage is associated with oxidative challenges, immunomodulatory activity and angiogenesis processes [7–10]. In this study we have investigated the effects of long-term ethanol exposure on apoptosis in liver.
Materials and Methods
Chemicals
TUNEL assay kit from Calbiochem, Nottingham, UK; Caspase-3 (CPP32; Cat. K 106) and caspase-8 (Cat. K 113) kits from BioVision Research Products, Mountain View, CA, USA were used.
Animal Selection
Twenty-four male albino Wistar strain rats (16–18 weeks-old, 200–220 g) were housed in plastic cages inside a well-ventilated room. The room was maintained under standard husbandry condition. All animals had free access of standard diet [7–9] and water ad libitum. The animals were weighed daily and its general condition was recorded including their daily intake of liquid. A dose of 1.6 g ethanol/kg body weight/day was selected for administration to animals based on our previous dose-dependent study in male Wistar rats [7, 9, 11]. The rats were randomly divided into three ethanol treatment groups along with appropriate control. Ethanol was diluted with distilled water to get desired concentration and administered intragastrically for 4, 12 or 36 weeks. Control rats were fed isocaloric glucose solution instead of ethanol (1.6 g/kg body wt) per day. The Animal Ethics Committee of the Institution approved the procedures in accordance with the CPCSEA guideline.
Experimental Procedures
The rats were sacrificed after over-night fast at the end of each experimental schedule by administration of intraperitoneal Na-pentobarbital (Nembutal, 60 mg/kg body weight) (euthanasia) [12]. The liver tissues were collected, cleaned with ice-cold saline, blotted dry, and immediately preserved for further analysis.
Caspase activity—Fresh liver tissues were homogenized in chilled tissue homogenizer at 4°C with ice-cold extraction buffer [25 mM HEPES buffer, pH 7.4, containing 5 mM ethylenediaminetetraacetic acid (EDTA), 2 mM dithiothreitol and 0.1% CHAPS]. The homogenate was cold centrifuged at 20,000×g for 30 min. The supernatant was diluted with the assay buffer (50 mM HEPES, pH 7.2, 50 mM NaCl, 0.1% CHAPS, 10 mM EDTA, 5% glycerol and 10 mM dithiothreitol) and incubated at 37°C with 200 μM caspase-3 (Asp-Glu-Val-Asp-p-nitroanilide or Ac-DEVD-pNA) or caspase-8 (Ile-Glu-Thr-Asp-pNA or IETD-pNA) substrate. Release of p-nitroanilide from labelled substrates was monitored at 405 nm. Fold increase in activities was determined by comparing the results of treated samples with the level of the uninduced control.
Detection of apoptosis-induced DNA strand breaks using terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL)—The Fragment End Labeling (FragEL) DNA fragmentation detection kit was used to detect apoptotic nuclei in paraffin-embedded tissue sections according to the manufacture’s instructions. Briefly, paraffin embedded tissue sections were deparaffinized, rehydrated with ethanol, washed with Tris buffered saline (TBS; 20 mM Tris, pH 7.6 with 140 mM NaCl), pretreated with proteinase K and H2O2, and incubated with the reaction mixture containing terminal deoxynucleotidyl transferase (TdT) and biotin-labeled or unlabeled deoxynucleotides (dUTP) in TdT buffer (1 M sodium cacodylate, 0.15 M Tris, 1.5 mg/ml BSA, 3.75 mM CoCl2, pH 6.6) for 1 h at 37°C. The reaction was terminated by using 0.5 M EDTA, pH 8.0; and blocked with 4% BSA in PBS. TdT binds with exposed 3P-OH ends of DNA fragments, which are generated in response to apoptotic signals and catalyze the addition of biotin-labeled and unlabeled deoxynucleotides. Biotinylated nucleotides are detected using a streptavidin horseradish peroxidase (HRP) conjugate with diaminobenzidine as chromogen. Methyl green counterstain aids in the morphological evaluation and characterization of normal and apoptotic cells. A mixture of HL60 cells incubated with actinomycin D to induce apoptosis and uninduced HL60 cells were used as control.
Statistical Analysis
All data were analyzed using the statistical package SPSS (version 11.0, SPSS Inc., Chicago, IL). Results were expressed as mean ± SD (standard deviation). The sources of variation for multiple comparisons were assessed by the analysis of variance (ANOVA), followed by Post-Hoc test with Bonferroni’s and Tukey’s multiple comparisons test. The difference were considered significant at P < 0.05.
Results
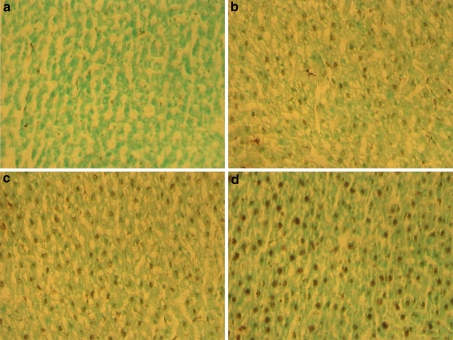
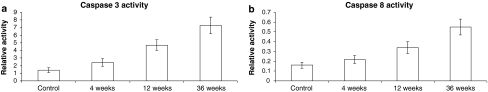
TUNEL analysis demonstrated an increase in apoptotic cell death following ethanol treatment. In comparison to the control rats (Fig. 1a), the staining intensity was different among the positive cells. Apoptotic bodies and cells appeared brown. Ethanol-treated rats showed positive staining in the liver (Fig. 1b–d) under light microscopy. These changes were accompanied with elevation of caspase-3 (Fig. 2a) and caspase-8 activities (Fig. 2b). Ethanol treatment for 4, 12, and 36 weeks increased caspase-3 activity by 1.71-, 3.36- and 5.2-fold, respectively (Fig. 2a), and caspase-8 activity by 0.38-, 2.12- and 3.43-fold, respectively (Fig. 2b).
Fig. 1.
TUNEL expression in liver tissues for a control group of rats, b 4 weeks, c 12 weeks, and d 36 weeks of ethanol exposed rats
Fig. 2.
Effect of increasing duration of ethanol exposure on a Caspase-3, and b Caspase-8 activities in liver tissues
Discussion
The caspases are a family of more than a dozen cysteine-aspartic acid proteases [13, 14]. These are synthesized as zymogens, and are themselves activated by cleavage at the carboxyl side of aspartate residues [15]. Initiator caspases (caspase-2, -8, -9, -and -10) cleave inactive pro-forms of effector caspases (apoptosis executioner) (caspase-3, -6, and -7), which in turn cleave other protein substrates within the cell to trigger the apoptotic process [16]. In our study, the progressive elevation of cell death was detected in the chronic ethanol-exposed rat livers by TUNEL assay (Fig. 1), and was confirmed by the increased expression of caspase-3 (Fig. 2a) and caspase-8 (Fig. 2b) activities with duration. These findings were in agreement with other reports [5, 17]. In other study, the amount of fragmented DNA and the number of apoptotic hepatocytes increased in vivo and in vitro, when hepatocytes from male Wistar rats were cultured in presence of 100 mM ethanol [16]. The caspases participate in cytokine maturation, apoptosis, necrosis and inflammation [13, 15]. Activation of caspase-3 and -8 are responsible for alcohol-induced liver apoptosis [14]. Our study thus provides evidence that long-term ethanol consumption triggers apoptotic process in the liver.
Acknowledgment
Financial assistance received from Kerala State Council for Science, Technology and Environment (KSCSTE), Government of Kerala, India and Van Slyke Foundation of American Association for Clinical Chemistry (AACC) is gratefully acknowledged.
References
- 1.Green DR, Scott DW. Activation-induced apoptosis in lymphocytes. Curr Opin Immunol. 1994;6:476–487. doi: 10.1016/0952-7915(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 2.Osborne BA. Apoptosis and the maintenance of homeostasis in the immune system. Curr Opin Immunol. 1996;8:245–254. doi: 10.1016/S0952-7915(96)80063-X. [DOI] [PubMed] [Google Scholar]
- 3.Nagata S. Apoptosis mediated by the Fas system. Prog Mol Subcell Biol. 1996;16:87–103. doi: 10.1007/978-3-642-79850-4_6. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Checha JC, Kaplowitz N, Colell A, Gracia-Ruiz C. Oxidative stress and alcoholic liver disease. Alcohol Health Res World. 1997;21:321–324. [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Z, Liu J, Song Z, McClain CJ, Kang YJ. Zinc supplementation inhibits hepatic apoptosis in mice subjected to a long-term ethanol exposure. Exp Biol Med (Maywood) 2009;233(5):540–548. doi: 10.3181/0710-RM-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koteish A, Yang S, Lin H, Huang X, Diehl AM. Chronic ethanol exposure potentiates lipopolysaccharide liver injury despite inhibiting Jun N-terminal kinase and caspase 3 activation. J Biol Chem. 2002;277(15):13037–13044. doi: 10.1074/jbc.M101632200. [DOI] [PubMed] [Google Scholar]
- 7.Das SK, Dhanya L, Varadhan S, Mukherjee S, Vasudevan DM. Effects of chronic ethanol consumption in blood: a time dependent study on rat. Indian J Clin Biochem. 2009;24(3):301–306. doi: 10.1007/s12291-009-0056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das SK, Varadhan S, Gupta G, Mukherjee S, Dhanya L, Rao DN, Vasudevan DM. Time dependent effects of ethanol on blood oxidative stress parameters and cytokines. Indian J Biochem Biophys. 2009;46(1):116–121. [PubMed] [Google Scholar]
- 9.Das SK, Mukherjee S, Vasudevan DM. Effects of long term ethanol consumption on adhesion molecules in the liver. Indian J Exp Biol. 2010;48(4):394–401. [PubMed] [Google Scholar]
- 10.Das SK, Mukherjee S, Gupta G, Rao DN, Vasudevan DM. Protective effect of resveratrol and vitamin E against ethanol-induced oxidative damage in mice: biochemical and immunological basis. Indian J Biochem Biophys. 2010;47(1):32–37. [PubMed] [Google Scholar]
- 11.Das SK, Vasudevan DM. Effect of ethanol on liver antioxidant defense systems: a dose dependent study. Indian J Clin Biochem. 2005;20(1):80–84. doi: 10.1007/BF02893047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fortunato F, Berger I, Gross ML, Rieger P, Buechler MW, Werner J. Immune-compromised state in the rat pancreas after chronic alcohol exposure: the role of peroxisome proliferators-activated receptor γ. J Pathol. 2007;213:441–452. doi: 10.1002/path.2243. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Billiar TR, Talanian RV, Kim YM. Nitric oxide reversibly inhibits seven members of the caspase family via S-nitrosylation. Biochem Biophys Res Commun. 1997;240(2):419–424. doi: 10.1006/bbrc.1997.7672. [DOI] [PubMed] [Google Scholar]
- 14.Lambert JC, Zhou Z, Kang YJ. Suppression of Fas-mediated signaling pathway is involved in zinc inhibition of ethanol-induced liver apoptosis. Exp Biol Med (Maywood) 2003;228(4):406–412. doi: 10.1177/153537020322800411. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9:459–470. doi: 10.1016/S1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]
- 16.Kurose I, Higuchi H, Miura S, Saito H, Watanabe N, Hokari R, Hirokawa M, Takaishi M, Zeki S, Nakamura T, Ebinuma H, Kato S, Ishii H. Oxidative stress-mediated apoptosis of hepatocytes exposed to acute ethanol intoxication. Hepatology. 1997;25(2):368–378. doi: 10.1002/hep.510250219. [DOI] [PubMed] [Google Scholar]
- 17.Cohen JI, Roychowdhury S, Dibello PM, Jacobsen DW, Nagy LE. Exogenous thioredoxin prevents ethanol-induced oxidative damage and apoptosis in mouse liver. Hepatology. 2009;49(5):1709–1717. doi: 10.1002/hep.22837. [DOI] [PMC free article] [PubMed] [Google Scholar]