Abstract
In the present study, the cause of suspected false-positive (anomalous) values for CK-MB activity, in Indian patients investigated for ACS. Total serum CK and CK-MB activity, serum Troponin I were measured and CK-MB as a percentage of the total CK activity (%CK-MB) calculated. CK-MB was also estimated using densitometry and CK-MB mass assay. Anomalous specimens were tested for the presence of CK isoenzymes. In 22 healthy subjects, 11 male and female, the %CK-MB ranged from 3.6 to 30.2. In 11 male patients, with proven ACS, the %CK-MB was from 4.0 to 17.5. The cut off for anomalous CK-MB activity values was set as >33.0%. In 35 patients with anomalies, total CK values ranged from 39 to 231 U/L, CK-MB from 30 to 161 U/L. Investigation of CK isoenzymes, showed 10 patients had a CK-BB band, 14 an intermediate band between CK-MM and CK-MB (macro-CK type 1), 7 had a cathodal band (macro-CK type 2), and 3 had a band intermediate between CK-MB and CK-BB. This later band does not seem to have been previously reported. Against the CK-MB mass assay, the activity assay showed no correlation, in 43 patients (19 M, 24 F), Pearson coefficient (R2) was 0.006. The CK-MB immunoinhibition assay is better described as measuring “non-CK-MM activity.” A %CK-MB activity >6% as a marker of ACS is not valid in our patient population. Laboratories should not use only CK-MB activity as a biochemical marker of ACS.
Keywords: CK-MB isoenzymes, Macro-creatine kinase, Anomalies, Biochemical markers, Acute coronary syndrome
Introduction
Cardiovascular disease is the leading cause of death in the world. Although mortality from cardiovascular disease has declined in the Western countries, it has increased in most Asian countries [1]. Mortality from acute coronary syndrome (ACS) is on the rise and this makes the correct diagnosis of myocardial infarction (MI) a vital necessity in Asian countries, including India.
There are over 100,000 private and government laboratories providing diagnostic analytical services in India with less than 200 of them being subject to an accreditation process [2]. Although a number of the above mentioned labs undertake measurement of biochemical markers for management of cardiac disease, most do not follow the recommended guidelines produced by the National Academy of Clinical Biochemistry (NACB) for use of cardiac markers in ACS. Specifically, the guidelines of the NACB recommend the use of cardiac troponin (cTn) as the preferred marker for diagnosis of myocardial infarction (MI) with CK-MB concentration, as measured by an immunoassay (mass assay) as an acceptable alternative when cardiac troponin is not available. The NACB guidelines allow the use of total CK or CK-MB activity as an acceptable alternative for evaluating cardiac injuries in institutions where cTn or CK-MB mass assays are not available or feasible [3, 4]. Few medical laboratories in India have the resources and specialized analytical equipment to measure CK-MB mass, instead the vast majority of laboratories only measure the serum activity of CK-MB isoenzyme as their biochemical cardiac marker and do not use a ratio of CK-MB to total CK activity as a guide to the validity of the CK-MB result.
We are a very large teaching and charitable hospital in India, performing around 60 investigations for suspected ACS daily. Originally the only tests used for MI diagnosis in our institution were serum CK-MB activity and serum Troponin I. With this testing strategy, we had a high false-positive rate for serum CK-MB activity values reported to us by our requesting clinicians. Next, we introduced the use of the ratio of CK-MB to total CK activity following the kit insert guidelines for CK-MB and Western guidelines for myocardial infarction (MI) diagnosis: serum total CK activity >upper limit of reference range for male or female as appropriate, serum CK-MB activity >24 U/L, CK-MB 6–25% total CK [5].
These guidelines were found by us to not be applicable in our patients. Some patients even had a normal total CK with the CK-MB activity value greater than the total CK activity. Our first response was to question the quality of the CK and CK-MB activity kits we were using. However, we have made the same observations with kits from three different reputable manufacturers. We have also received reports of this problem from other Indian laboratories. This study is an attempt to identify the cause of these suspected anomalous CK-MB values.
Materials and Methods
Patients
A series of 35 patients who presented over a 6 month period with query ACS were found to have anomalous CK-MB values (defined as >33.0% of the total CK activity). These samples were kept frozen at −70°C until analysis for CK isoenzymes could be performed. These are described in group 3. Group 1, 2 and 4 are various comparison groups. The detailed composition of the groups is described below.
Group 1 (healthy subjects) subjects were presumed-healthy laboratory staff (n = 26). This group comprised of 11 males (M), median age 34 years (range 25–54) and 11 females (F), median age 29 years (range 24–49 years). Four samples were excluded for reasons listed in the text, leaving 22 subjects. These specimens were only analysed for total CK and CK-MB activity.
Group 2 (definite ACS, CC) 11 males, median age 58 years (range 32–74 years) were randomly selected from the male patients who presented over the same 6 month period as group 3 with elevated CK > 200 U/L, CK-MB > 24 U/L, but less than 33% of total CK, and who had measurement of cTnI carried out. These samples were stored for CK isoenzyme electrophoresis. Individual diagnoses for all the groups are detailed in Appendix 1.
Group 3 (CK-MB anomalies) subjects (n = 35) were patients who had specimens sent for investigation of ACS over a 6 month period and who were found to have anomalous CK-MB values. Specimens were defined as anomalous on the basis of the percent of CK-MB of the total CK activity (>33% with a normal total CK). The clinical record of these patients was examined and if the diagnosis included ACS we defined them as CM (cardiac male with anomaly) and CF (cardiac female with anomaly). If there was no cardiac involvement they were named NCM (non-cardiac male with anomaly), and NCF (non-cardiac—female with anomaly).
those with definite cardiac involvement (n = 15). This group consisted of 6 males (CM), median age 56 years (range 47–77 years) and 9 females (CF), median age 51 years (range 42–77 years).
those with not-known cardiac involvement (n = 20). This group consisted of 8 males (NCM), median age 56 years (range 17–73 years) and 12 females (NCF), median age 54 years (range 19–72 years).
Only samples from all patients in group 2 and 3 were subject to investigation of CK isoenzymes. In addition three other control specimens i.e. non-anomalous, were also electrophoresed, see Appendix 1 for clinical details.
Group 4 (thoracic surgery) Samples were randomly chosen from 13 male thoracic surgery post-operative in patients. Their median age was 60 years (range 30–75 years). This group was chosen as representative patients who have had trauma to skeletal muscles, but usually do not to have any myocardial injury.
During and after the study period samples from another 30 patients with CK-MB anomalies (16 M, 14 F) were collected and the serum stored at −70°C until analyzed by the Roche CK-MB mass assay.
Requests for total CK and CK-MB analysis came primarily from Accident and Emergency department, medical ICU, Coronary Care unit, Respiratory Medicine unit and to a lesser extent, other units. Medical records were scrutinized at the end of the study.
Five deaths occurred among the patients, one CC, one CF, 2 NCM and 1 NCF patient.
Analytical Procedures
All specimens were collected in plain vacutainers, serum separated and analysed as STAT samples within 2 h of receipt by the laboratory. Analysis was done for CK, CK-MB and troponin I. Sera were frozen at −70°C for up to 6 months prior to electrophoresis.
Total CK was analysed by the CK NAC-activated procedure, Randox UK, on the Hitachi 912 Chemistry analyzer. The calibrator was CFAS (calibrator for automated systems) Roche/Hitachi systems, Roche Germany. Quality controls were Precinorm Roche/Hitachi systems and Bio-Rad Lyphochek Assayed Chemistry control level 1 and 2, Bio-Rad Laboratories, UK.
CK-MB was analysed by the Randox (NAC-activated) UV-method using their CK-MB calibrator and CK-MB control. QC values for between day precision were as follows: total CK, mean value 160 U/L, cv 5.0%; total CK mean 480 U/L, cv 5.6%; CK-MB mean 151, cv 3.65%.
Troponin I was analysed by the Immulite 2000 immunoassay analyzer, DPC, USA (now Siemens) using a solid-phase, two-site, chemiluminescent enzyme immunometric assay. The low calibrator and high calibrator and control (CCCM: a bi-level nonhuman serum-based Cardiac marker control module, containing Troponin I) were supplied by the DPC company. Control values were as follows mean value 0.46 ng/ml, cv 4.1%; mean 0.97 ng/ml, cv 2.0%.
Reference ranges in the laboratory were: total CK activity in men <195 U/L, total CK women <170 U/L, CK-MB activity (both male and female) <24 U/L, and a CK-MB activity <6% of the total measured CK activity. Reference values for cTnI were <0.2 ng/ml. Cut off for diagnosis of MI ≥1.0 ng/ml.
CK isoenzyme electrophoresis was carried out according to the SEBIA Hydragel 7, IS0-CK procedure on the Sebia Automated Electrophoresis System Ref 4140, Sebia, France, supplied by Trivitron Diagnostics, India. Samples were activated by mixing 500 μl of sample with 5 μl activation solution containing 7% β-mercaptoethanol. The serum was electrophoresed and separate CK isoenzymes were visualized using a specific chromogenic substrate Phenazine Methosulphate and Tetranitro Blue Tetrazolium. The amount of resulting formazan precipitate formed is proportional to the CK enzymatic activity. Scanning of the gel was carried out according to the company protocol on an Epson Expression 1680 Pro Scanner. Densitometer scanning of the stained electrophoregrams yielded relative activities of individual isoenzyme zones. When the total CK activity was greater than 750 U/l, samples were diluted with saline to give an activity of around 750 U/L. The SEBIA Enzyme control human serum, ref 4790, was run with each gel. Gels were run one per day containing one QC. There were a total of 10 gels in the kit.
Control values for %CK activity were as follows (shown as mean and SD): CK-MM 53.0% ± 3.3, CK-MB 18.4% ± 1.5, CK-BB 28.6% ± 2.6. Nominated values: CK-MM 59.5% ± 6.0, CK-MB 17.5% ± 6.0, CK-BB 23.0% ± 6.0.
CK-MB mass activity was quantitated on the Modular Analytics E170 by electrochemiluminescence immunoassay (ECLIA) using the Roche CK-MB quantitative determination procedure Roche, Germany. Calibration was with the CK-MB CalSet provided by the manufacturer. The QC was Preci Control cardiac I Roche, mean 4.18 ng/ml, cv 6.94%.
Statistical Analysis
All measurements in groups were expressed as medians and ranges. Correlation between groups was by Anova Regression statistics with R squared values (Excel Microsoft).
Results
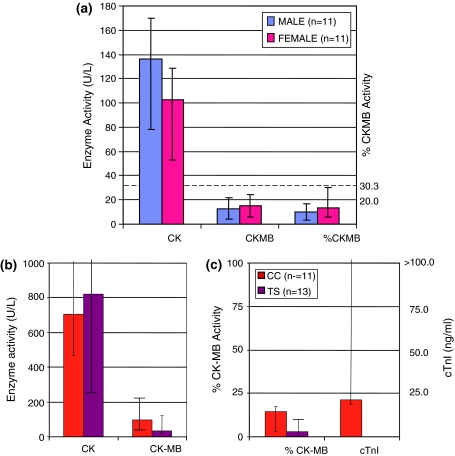
Figure 1a shows the total CK, and CK-MB activity and %CK-MB values in healthy subjects (group 1) as medians and ranges.
Fig. 1.
Total CK, CK-MB, %CK-MB activity and TROPONIN I (cTnI) in patient groups. a Total CK, CK-MB and %CK-MB in healthy adults. Values shown are median and range. Values from 4 males excluded: one slightly haemolysed, one after exercise and two others with the total CK elevated: Total CK 392, MB 31 (7.9%) and CK 451, MB 28 (6.2%). b Total CK and CK-MB activity control groups: group 2, cardiac control (CC) definite ACS, and group 4, thoracic surgery (TS) male patients. c %CK-MB activity and cTnI in control groups: group 2, cardiac control (CC) definite ACS, and group 4, thoracic surgery (TS) male patients. cTnI was not done in thoracic surgery patients. %CK-MB of total CK is 3.1 in TS patients compared to median 14.5 in CC patients. Upper value for total CK was 7788 and 5250 U/L in the CC and TS groups. The upper value for cTnI in the CC group was >180 ng/ml
Figure 1b and c shows the total CK, and CK-MB activity and %CK-MB values in the known ACS (CC) (group 2) and the 13 post-operative thoracic surgery (TS) patients (group 4). For ease of comparison only data from male patients was used in groups 2 and 4. Cardiac troponin I (cTnI) was requested in group 2, but not in group 4. Median total CK activity was similar in both these groups, but CK-MB median and %CK-MB was higher in the CC group. Percent CK-MB was median 14.5% in the MI group and 3.1% in the TS group. Only four patients in group 4 had a %CK-MB >6%. In the CC group values started from 4.0%.
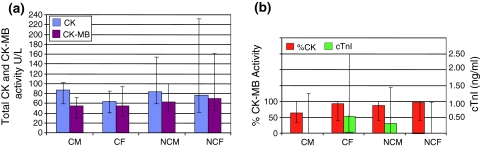
Figure 2a shows the data from the male and female patients with anomalous CK-MB values (group 3). The median total CK values were lower than for healthy laboratory personnel, but serum CK-MB activity and %CK-MB were much higher (Fig. 2b). There were no differences in values between patients with cardiac and non-cardiac involvement except in cTnI values.
Fig. 2.
Total CK, CK-MB, %CK-MB and TROPONIN I data from group 3 patients (with CK-MB anomalies). Key: CM cardiac male with anomaly, n = 6; CF cardiac female with anomaly, n = 9; NCM non-cardiac male with anomaly, n = 8; NCF non-cardiac female with anomaly, n = 12
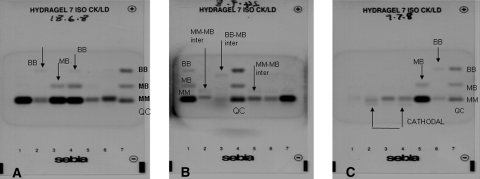
Figure 3 shows the representative examples of the electrophoretic separation of CK isoenzymes. The gels illustrate MM, MB, BB, cathodal, MM-MB intermediate and BB-MB intermediate running positions of enzyme fractions. All patient samples had a CK-MM band. The breakdown of patients with bands other than CK-MM and CK-MB is shown in Appendix 2. This appendix also contains details of patients who had more than one isoenzyme band, apart from CK-MM and CK-MB, and details of patients who had cancer.
Fig. 3.
Electrophoresis patterns in groups 2 and 3. All samples show as a minimum a CK-MM band. Gel A: lane 1 (CC), 5 (CF), 6 (NCF) patients showing CK-MM band only. Lane 2 (CM) and 4 (CC) showing CK-BB band. Lane 3 (CF control) showing CK-MB band. Lane 4 (CC) showing CK-MB and CK-BB band. Lane 7 QC. Gel B: lane 6 (NCF) and 7 (NCF control) showing CK-MM band only. Lane 2 (CF) and 5 (CF) showing MM-MB inter. Lane 3 (NCF) showing BB-MB inter. Lane 1 (CC) showing CK-MB + CK-BB bands. Lane 4 QC. Gel C: Lane 1 (NCM) and 3 (CM) showing CK-MM band only. Lane 2 (CM) and 4 (NCM) showing CK-MM and cathodal band. Lane 5 (CC) showing CK-MM and CK-MB only. Lane 6 (CM) showing CK-MM + CK-BB only. Lane 7 QC. Lane 1 and 4 is the same sample applied twice
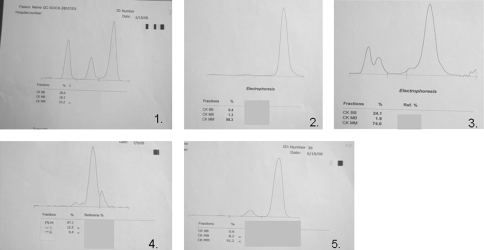
Figure 4 shows representative densitometric scans of different isoenzyme forms of CK. The BB-MB intermediate isoenzyme is shown as well as the cathodal isoenzyme. In the densitometric scan, the CK-MB activity was calculated from the total CK activity in the sample and the percent of total stain in each isoenzyme band.
Fig. 4.
Representative densitometric scans from patients in group 2 and 3. 1 QC Sebia Enzyme control human serum; expected 59.5 ± 6.0; 17.5 ± 6.0; 23.0 ± 6.0; estimate from densitometer CK-MM 53.2%, CK-MB 18.2%, CK-BB 28.6%. 2 NCF control with CK-MM peak only; 70 years old female with chronic liver disease. Total CK 479 U/L, CK-MB 67 U/L, cTnI 0.742 ng/ml. 3 NCF patient with intermediate BB-MB band. 37 years old female with scrub typhus; total CK 69 U/L; CK-MB 81 U/L; cTnI 0.97 ng/ml. 4 NCM patient with cathodal CK band, 52 years old male with hairy cell leukaemia. Total CK 67 U/L, CK-MB 60 U/L; cTnI not detectable (ND). 5 CC patient with CK-MB band, 66 years old male with MI. Total CK 785 U/L, CK-MB 86 U/L, cTnI 19.5 ng/ml
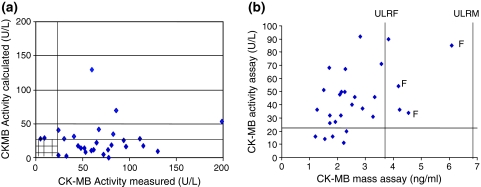
Figure 5a shows the plot of CK-MB measured versus calculated, in the 31 group 3 patients, who had a calculated value. This graph shows 19 incidences where the measured activity is abnormal, but the calculated value, from the densitometric scans is not. In 10 patients both values were elevated. There was no correlation between the measured and calculated CK-MB activity (Pearson correlation 0.006).
Fig. 5.
a Comparison of measured CK-MB activity with calculated CK-MB activity from densitometer scan in 31 patients, from group 3, with anomalous CK-MB values. The upper limit of the reference range for each CK-MB activity assay is 24 U/L. Pearson correlation R2 = 0.006, indicating no significant correlation between the two estimates. Two points, from male patients are not shown on the graphical scale: (measured, calculated CK-MB activity); (243,308) and (475,120). b Relationship CK-MB activity assay and CK-MB mass assay in 30 other patients with anomalous CK-MB values. Pearson correlation R2 = 0.004 indicating no significant correlation between the two estimates. CK-MB mass assay: Female upper limit of reference range (ULRF) 3.8 pg/ml, males 6.7 pg/ml. CK MB activity ULRF 24 U/L. Values from 2 male patients are not shown on the graph (CK-MB mass, CK-MB activity); (3.88, 915) and (31.6, 81)
Figure 5b shows the plot of the mass value for CK-MB compared to the measured activity of CK-MB in another 30 patients with anomalous CK-MB values. There was no correlation between CK-MB mass and CK-MB activity (Pearson correlation 0.004).
Nineteen samples had an elevation in CK-MB activity but not in CK-MB mass. In a later comparison of these two assays, 26 samples which had been sent for MI investigation over a 5 day period and which had no CK-MB anomalies were analysed. Here the Pearson correlation coefficient was 0.970, indicating a good correlation.
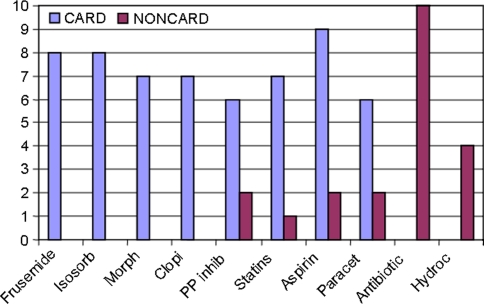
Figure 6 shows medication details in the patients with known MI and those with anomalous CK-MB values. The median number of drugs in the cardiac patients was 4 (range 0–16). The median number of drugs in the not-known cardiac involvement patients was 5 (range 0–16). Ten patients among the NCM and NCF group were receiving antibiotics, and cardiac patients were receiving more diuretics and anti-clotting agents. Both groups were receiving aspirin and paracetamol.
Fig. 6.
Medication details of patients in group 2 and 3 (n = 46). Group 2 (definite ACS) acute coronary syndrome and Group 3 (CK-MB anomalies) a those with definite cardiac involvement, termed as CARD (n = 26, 17 M, 9 F), drug chart available in 19/26 patients. Group 3 (CK-MB anomalies) b patients with no cardiac involvement, termed as NONCARD (n = 20, 8 M, 2 F) drug chart available in 17/20 patients. C median number of drugs 4 (range 0–16). NC median number of drugs 5 (range 0–16). Histogram shows Frusemide, PP Inhibitors (Proton pump inhibitors), Morphine, statins, Aspirin, Paracetamol, Clopidogrel (Anti-platelet) (ISD) Isosorbide Dinitrate, Antibiotic, various Antibiotics, hydrocortisone
Discussion
The strategy adopted for the use of cardiac biomarkers in the diagnosis of acute coronary syndromes in our institution is to request total CK, CK-MB activity and cTnI on admission and 6–9 h later. At the time of the study we received around 300 requests for cardiac biomarkers per week of which approximately 1–2% would give discrepant values for CK-MB activity.
Our justification for use of total CK in conjunction with CK-MB comes from the current guidelines on markers of cardiac damage [3, 4]. Although the use of total CK for diagnosis of MI is not recommended, the guidelines do state that a rise or a rise and fall of total CK provides additional supporting evidence of acute MI. Furthermore, total CK activity can also assist in improving myocardial tissue specificity when the ratio of CK-MB to CK is greater than previously established reference limits.
Justification for the use of %CK-MB comes from the CK-MB kit insert and Clinical Chemistry text book guidelines [5]. These state that total CK in acute MI does not usually increase beyond 5000 U/L. CK-MB constitutes about 1–3% of total CK activity in skeletal muscle and about 20% of the total CK activity in heart muscle. The three different cytosolic CK isoenzymes CK-MM, CK-MB and CK-BB are co-expressed in a tissue specific fashion together with the mitochondrial isoform. Electrophoresis shows this form will run to the cathode (macro-CK type 2) [5]. There is also an intermediate form (macro-CK type 1) running between CK-MM and CK-MB [5].
An important observation made in our study was that many %CK-MB values in healthy Indian persons (group 1) are much higher than 6%, which is the Western cutoff used to indicate a cardiac source of the CK-MB. This suggests the lack of useful discriminatory value in calculating %CK-MB to differentiate healthy individuals from those with possible acute coronary syndromes.
Generally, when the total CK activity was elevated >the upper limit of the male and female reference ranges, then anomalous CK-MB values were infrequently observed. In our definite ACS patients, group 2, %CK-MB values were from 4.0 to 17.5. The serum CK-MB activity values, as expected from the literature, were less in the “thoracic surgery” group 4, who were assumed not to have any cardiac involvement but probably sustained surgical damage to their voluntary muscles. The %CK-MB range in this group was lower than any other group (0.8–10.0 range) but overlapped with values in group 2. Since many values in both these groups exceeded 6%, this suggests that the cut off value for a cardiac source of CK-MB of >6% may not be valid in Indian subjects and should not be used.
Many studies have shown that there is a high false-positive rate in assays measuring CK-MB activity. Alpert and Thygesen [6] discuss a noticeable group of patients diagnosed as having had an MI by elevated CK-MB measurements in the face of normal troponin values. Others, like Lee et al. [7] quote a prevalence of 0.43% for macro-CK type 1 (MM-MB inter form) and 1.2% for macro-CK type 2 (mitochondrial, cathodal type) in 136/8322 patients. This means a possible rate of anomalous CK-MB activity values of 1.6%. In this regard, our study has highlighted some of the well known limitations of the CK-MB immunoinhibition assay, specifically aspects pertaining to specificity of the assay. As early as 1981 Laing [8] conveyed that “the prerequisite for the CK-MB inhibition method is the absence of CK-BB in the sample and a high CK-MB value >30% of the total CK is an indication to check the sample for CK-BB isoenzymes.” Our study has further confirmed that in patients where the %CK-MB is >33.0, in 23/35 anomalous cases, another isoenzyme (CK-BB) and/or an atypical isoform of CK was being included in the total.
The cause of the discrepant CK-MB values was revealed in most of our anomalous cases by CK isoenzyme electrophoresis. We noted that in our study, an important cause of false elevation of CK-MB activity could be attributed to the presence of CK-BB, macro-CK type 1, macro-CK type 2, or a form that has not previously been reported in the literature, running between CK-MB and CK-BB bands (CK-MB-BB inter). Further, it appears that elevation of CK-BB isoenzyme in Indian subjects is much more common than reports from Western studies. Laing [8] reported the frequency of elevation of CK-BB isoenzyme in serum to be 1:1000. Direct damage to CNS and brain ischaemia can release CK-BB from the brain, but it can only reach the circulation from the CNS if the blood/brain barrier is damaged. Reference values for CK-BB include 0.5–0.9 U/L in healthy subjects; 200–300 U/L in CNS damage; 3–76 U/L in GI tract disease and 10–17 U/L in muscular damage. Tsai et al. [9] report a case of prostatic carcinoma with spurious elevation of CK-MB activity >the total CK activity of 964 U/L. Lee et al. [7] state that macro-CK type 2 (cathodal, mitochondrial) may account for 25% of measured total CK activity and type 1 (MM-MB inter) 10% of the total activity. Tsai et al. [9] observed that macro-CK type 1 (MM-MB inter) was associated with myositis and autoimmune process, while the mitochondrial, cathodal form was more likely to be malignant cell proliferation. They also mention many reports of prostatic or other cancers with elevated CK-BB or the presence of macro-CK type 1 or 2. Macro-CK is found transiently in up to 6% of patients [9]. Type 1 is usually a complex of CK-BB and IgG or CK-MM and IgA. Type 2 is oligomeric mitochondrial CK with a reported prevalence of 0.5–2.6% [5]. Four of our patients had a concurrent diagnosis of cancer, all had other CK isoforms but only one had the cathodal band said to be associated with cancer.
In a recent study by Hoshino et al. [10], sacromeric and ubiquitous mitochondrial CK (MtCK) isoforms of CK were shown to make up nearly 80% of measured CK-MB activity in normal subjects. Similar to our study, they carried out isoenzyme electrophoresis of patient samples, assessed CK-MB enzymatic activity by densitometry and demonstrated an MtCK dimer band running concurrently with the CK-MM band and a MtCK octamer that ran cathodally. Thus, the appearance of macro-CK type 1 and mtCK in the serum renders the specificity of the immunoinhibition method low in the CK-MB activity assay. Schmid et al. [11] reported macroenzyme creatine kinase Type 2 accumulation in sera of HIV infected patients and a significant association with Tenofovir disoproxil fumarate (TDF) treatment. Our study corroborates these earlier reports that the CK-MB immunoinhibition assay is not specific for CK-MB and the assay would be better described as measuring “non-CK-MM activity.”
Another possibility is that the very high % of CK-MB found in some of our patients could indicate the inability of the anti-M antibody to effectively inhibit the M subunit. This was described by Laing in 1981 [8] as being caused by butyrophenon compounds and droperidol. Many of our patients with anomalies in the study were on multiple drug therapies including 8 on statins. Could ineffectiveness of CK-M subunit inhibition be a factor in their high CK-MB values?
Studies have shown that elevation of total CK and CK-MB activity could be caused by drug induced damage to the myocytes and rhabdomyolysis secondary to use of statins, compromised hepatic and renal function, hypothyroidism, diabetes and concomitant medication [12, 13]. We have also observed anomalous values of CK-MB activity in patients on statin therapy.
Technicians must always be aware that even slight haemolysis can elevate CK-MB values. A further point is that in CK-MB values in the reference range produce only very low absorbance changes per minute producing large intra-assay variations in %cv.
There is also a situation of “MB leak” in patients who develop an elevated CK-MB as measured by a mass assay, but in whom total CK activity is normal [14, 15]. This MB leak pattern seems to represent microscopic myocardial infarction or reversible ischemic injury just severe enough to compromise the myocardial sarcolemma and allow leakage of intracellular contents. An elevated MB conferred a higher adverse event rate that did a normal MB whether or not total CK is elevated [15].
Conclusions
This paper has outlined evidence suggesting that the high rate of spurious CK-MB activity values in Indian patients are due to the following causes: presence of other forms of CK in the sample namely CK-BB, macro-CK Type 1 (MM-MB inter), mitochondria CK (cathodal), and another isoform running between CK-MB and CK-BB (MB-BB inter), and possible drug related effects on CK-MB activity analysis. Macro-CK type 1 was seen in cardiac control and anomalous patients, as well as non-cardiac patients. However, the cathodal and MB-BB isoform forms occurred predominantly in patients who did not have cardiac involvement. Our future strategy for screening cardiac biomarkers is to replace the CK-MB activity assay by the CK-MB mass assay. This will also enable us to stop analyzing the total CK activity and possibly eliminate false positives in these samples. We are currently assessing the performance of the CK-MB mass assay.
Limitations of the Study
The possibility of artifactual bands in the specimen electrophoresis could not be completely ruled out. The consistency of sample application and of staining could cause some variation, although electrophoresis controls were run on all gels. On sample which was run twice (Fig. 3, gel c, lane 1 and 4) had a cathodal band in the second run, not seen in the much fainter first run. However, repeat samples from three other patients did show consistent patterns.
The clinical records for the group after thoracic surgery (group 4) were not examined. Some of them may have had cardiac involvement.
Only male patients were included in comparison groups 2 and 4, ideally these groups should have included an equal number of females to draw conclusions about cut off values for %CK-MB in cardiac and non-cardiac patients.
Acknowledgments
Conflicts of interest None.
Appendix 1
Clinical Details of Patients
Group 2 (Definite ACS, CC): Individual diagnoses were as follows: anterior wall myocardial infarction AWMI (3), inferior wall myocardial infarction (IWMI) (1), non ST elevation myocardial infarction (NSTEMI) (2), ACS (1), myocardial infarction (MI) (1), coronary artery CABG (1), unstable angina (1), left ventricular (LV) dysfunction (1).
Group 3 (CK-MB anomalies) (a) those with definite cardiac involvement (n = 15) consisting of 6 male patients (CM). They had individual diagnoses of: acute coronary syndrome (ACS) (2), pre-ACS (1), pericardial effusion with query TB (1), ischaemic heart disease (1). Angina (1). Nine female patients (CF) with diagnoses of: NSTEMI (1), NSTEMI plus scrub typhus (1), ACS (2), chronic cardiac failure (1), IWMI (1), scrub typhus and viral myocarditis (1), acute myocardial infarction (AMI) plus cancer of the caecum (1), atrial fibrillation (AF) (1).
Group 3(b) those with no demonstrated cardiac involvement (n = 20). This group consisted of 8 males (NCM) with the following diagnoses: chronic obstructive pulmonary disease(COPD) (1), COPD plus small cell carcinoma (1), acute pulmonary oedema with chronic kidney disease CKD (1), alcohol related chronic liver disease CLD (1), CLD plus lung disease (1), cerebral palsy (1), hairy cell leukaemia (1), bronchopneumonia and acute respiratory distress syndrome (ARDS) (1),
Twelve non-cardiac female patients (NCF patients) were diagnosed with : Tuberculosis TB (1), bronchial asthma (1), scrub typhus (1), diabetic ketoacidosis (DKA) with pneumonia (1), osteoarthritis (1), acute gastritis (1), CLD (1), deep vein thrombosis (DVT) (1), acute myeloid leukaemia (AML) with sepsis (1), disseminated vascular coagulation (1), acute leukaemia (1), hand trauma (1). Many patients in the non-cardiac (NC) subgroup also presented with symptoms of breathlessness.
In addition there were three other control specimens i.e. non-anomalous, consisting of samples from a 13 year old female with rheumatic heart disease, 70 year old female with chronic liver disease and a 34 year old male with alcohol induced liver damage.
Appendix 2
Total CK and CK-MB Activity Data from Group 3 Patients with cTnI > 1.0 ng/ml
1 male cTnI 1.25 ng/ml (CK, CK-MB: 99/33 U/L), diagnosis: IWMI.
3 patients cTnI 1.22 ng/ml, (CK, CK-MB: 84/34 U/L), diagnosis: STEMI; cTnI 2.38 ng/ml (CK, CK-MB: 60/52 U/L), diagnosis: IWMI; cTnI 2.50 ng/ml (CK, CK-MB: 64/77 U/L), diagnosis: AMI.
2 patients cTnI 1.0 ng/ml (CK/CK-MB: 70/59 U/L) MB band and MB-BB band; diagnosis CLD alcohol; cTnI 1.44 ng/ml (CK/CK-MB: 62/45 U/L) MM band only; diagnosis bronchopneumonia (ARDS).
none.
Appendix 3
Frequency of Occurrence of CK Isoenzyme Bands in Definite ACS (CC) Group and in Group 3. The Listing is by Bands
CC-5, CM-4, CF-1, NCM-3, NCF-2, total 15.
CC-2, CM-3, CF-0, NCM-3, NCF-2, total 10.
CC-3, CM-2, CF-4, NCM-0, NCF-5, total 14.
CC-0, CM-1, CF-0, NCM-3, NCF-3, total 7.
CC-0, CM-0 CF-0, NCM-1, NCF-2, total 3.
Occurrence of Multiple CK Isoenzyme Bands in the Same Patient
In the NM group, 1 patient with IHD had MB + BB + cathodal band.
NCM group, 1 patient with alcoholic CLD and breathlessness had BB + MB-BB inter band.
NCF group 2 patients. One with TB had MB + BB + MB-BB inter + cathodal band, the second NCF patient with scrub typhus had MB + BB + MB-BB inter + cathodal band.
Occurrence of CK Isoenzyme Bands in Patients with Cancer
Four patients had a diagnosis of cancer,
CF group, 1 patient, with carcinoma of the caecum, had an MM-MB inter band.
NCM group, 2 patients, one had small cell carcinoma of the lung with MB and BB band, the second hairy cell leukaemia with cathodal band (see Fig. 3, gel C, slot 1 and 4).
NCF group 1 patient with acute leukaemia had the MM-MB inter band.
References
- 1.Hong Y. Burden of cardiovascular disease in Asia: big challenges and ample opportunities for action and making a difference. Clin Chem. 2009;55:1450–1452. doi: 10.1373/clinchem.2009.125369. [DOI] [PubMed] [Google Scholar]
- 2.Venkatesh T. Accreditation requirement of laboratory medicine in India. Indian Journal of clinical biochemistry. 2008;23(3):207–208. doi: 10.1007/s12291-008-0047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christenson RH, Apple FS, Cannon CP, Francis G, Jesse Rl, Morrow DA, Newby LK, Ravkilde J, Storrow AB, Tang W, Wu AHB. The National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem. 2007;53:552–574. doi: 10.1373/clinchem.2006.079749. [DOI] [PubMed] [Google Scholar]
- 4.Apple FS, Christenson RH, Jaffe AS, Mair J, Ordonez-Llanos J, Pagani F, Panteghini M, Tate J, Wu AHB. National Academy of Clinical Biochemistry and IFCC committee for standardization of markers of cardiac damage Laboratory medicine Practice guidelines: analytical issues for biochemical markers of acute coronary syndromes. Clin Chem. 2007;53:547–551. doi: 10.1373/clinchem.2006.084715. [DOI] [PubMed] [Google Scholar]
- 5.Panteghini M, Bais R, Solinge WW. Enzymes, chapter 21. In: Burtis CA, Ashwood ER, Bruns DE, editors. Tietz textbook of clinical chemistry and molecular diagnostics. 4. St Louis: WB Saunders, Elsevier Inc; 2006. pp. 598–601. [Google Scholar]
- 6.Alpert JS, Thygesen K. A call for universal definitions in cardiovascular disease. Circulation. 2006;14:757–758. doi: 10.1161/CIRCULATIONAHA.106.648030. [DOI] [PubMed] [Google Scholar]
- 7.Lee KN, Csako G, Bernhardt P, Elin RJ. Relevance of macro creatine kinase type 1 and 2 isoenzymes to laboratory and clinical data. Clin Chem. 1994;40:1278–1283. [PubMed] [Google Scholar]
- 8.Laing H, editor. Creatine kinase isoenzymes. Berlin: Springer-Verley; 1981. [Google Scholar]
- 9.Tsai S-J, Wang T-E, Lin S-C, Lo C-I, Lo C-I, Tseng C-Y. Extremely high CK-MB levels exceeding total CK levels in a patient with chest pain: a case report. J Intern Med Taiwan. 2003;14:243–247. [Google Scholar]
- 10.Hoshino T, Sakai Y, Yamashita K, Shirahase Y, Sakaguchi K, Asaeda A, et al. Development and performance of an enzyme immunoassay to detect creatine kinase isoenzyme MB activity using anti-mitochondrial creatine kinase monoclonal antibodies. Scand J Clin Lab Invest. 2009;69:687–695. doi: 10.3109/00365510902981171. [DOI] [PubMed] [Google Scholar]
- 11.Schmid H, Muhlbayer D, Bogner JR, Goebel FD. Macroenzyme Creatine Kinase Type 2 accumulation in sera of HIV-infected patients: significant association with Tenofovir Df (TDF) treatment. Poster 827, 12th conference on retroviruses and opportunistic infections, Boston, USA; 2005.
- 12.Yeter E, Keles T, Durmaz T, Bozkurt E. Rhabdomyolysis due to the additive effect of statin therapy and hypothyroidism: a case report. Med Case Reports. 2007;1:130. doi: 10.1186/1752-1947-1-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Midi I, Bilgin O, Koytak PK, Tanridag T. Myopathy due to concomitant use of statin and gemfibrosil in a patient with chronic renal failure: case report. Marmara Medical Journal. 2004;17:129–132. [Google Scholar]
- 14.Lloyd-Jones DM, Camargo CA, Giugliano RP, Walsh CR, O’Donnell CJ. Characteristics and prognosis of patients with suspected acute myocardial infarction and elevated MB relative index but normal total creatine kinase. Am J Cardiol. 1999;84:957–962. doi: 10.1016/S0002-9149(99)00480-4. [DOI] [PubMed] [Google Scholar]
- 15.Peacock WF, Emerman CL, McErlean ES, DeLuca SA, VanLente F, Lowrie M, Rao JS, Nissen SE. Normal CK, elevated MB predicts complications in acute coronary syndromes. J Emerg Med. 2001;20:385–390. doi: 10.1016/S0736-4679(01)00317-1. [DOI] [PubMed] [Google Scholar]