Abstract
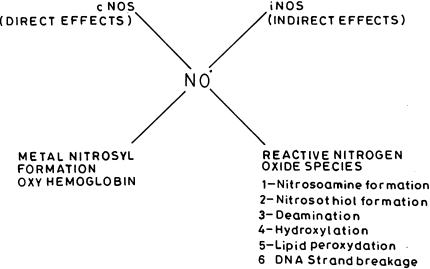
Nitric oxide (NO) a free radical having both cytoprotective as well as tumor promoting agent is formed from l-arginine by converting it to l-citrulline via nitric oxide synthase enzymes. The reaction product of nitric oxide with superoxide generates potent oxidizing agent, peroxynitrite which is the main mediator of tissue and cellular injury. Peroxynitrite is reactive towards many biomolecules which includes amino acids, nucleic acid bases; metal containing compounds, etc. NO metabolites may play a key role in mediating many of the genotoxic/carcinogenic effects as DNA damage, protein or lipid modification, etc. The basic reactions of nitric oxide can be divided as direct effect of the radical where it alone plays a role in either damaging or protecting the cell milieu and an indirect effect in which the byproducts of nitric oxide formed by convergence of two independent radical generating pathways play the role in biological reactions which mainly involve oxidative and nitrosative stress. Nitric oxide is also capable of directly interacting with mitochondria through inhibition of respiration or by permeability transition. Reaction of nitric oxide with metal ions include its direct interaction with the metals or with oxo complexes thereby reducing them to lower valent state. Excessive production of nitric oxide can be studied by inhibiting the synthetic pathway of nitric oxide using both selective or specific nitric oxide synthase inhibitor or non-selective nitric oxide synthase inhibitor with respect to isoforms of nitric oxide.
Keywords: Nitric oxide, Nitric oxide synthase, Nitric oxide inhibitors, Generation of nitric oxide, Cancer, Systemic lupus erythematosus, Direct and indirect effect of nitric oxide, Effect of nitric oxide on mitochondria
Introduction
The initial studies of the molecule (nitric oxide, NO) dates back to 1772, when Joseph Priestly called it “nitrous air,” nitric oxide was first discovered as a colorless, toxic gas. Unfortunately, the tag of toxic gas and air pollutant continued until 1987, when it was shown to actually be produced naturally in the body. By 1987, nitric oxide’s role in regulating blood pressure and relieving various heart ailments became well-established. Two years later, research revealed that nitric oxide is used by macrophages to kill tumor cells and bacteria. In 1992, nitric oxide was voted “Molecule of the Year”. The importance of the molecule became front page news in 1998 when Louis J. Ignerro, Robert F. Furchgott and Ferid Murad were awarded the Nobel Prize for Medicine and Physiology for identifying nitric oxide as a signaling molecule. The discovery has opened up newer ways of treatment for millions of patients.
Nitric oxide (NO) plays an important role in the protection against the onset and progression of cardiovascular diseases. The cardioprotective roles of NO include regulation of blood pressure and vascular tone, inhibition of platelet aggregation and leukocyte adhesion, and prevention of smooth muscle cell proliferation. Reduced bioavailability of NO is thought to be one of the central factors common to cardiovascular disease, although it is unclear whether this is a cause of, or result of, endothelial dysfunction. Any disturbance in the bioavailability of NO leads to a loss of cardio protective actions and in some cases may even increase disease progression [1].
NO is composed of an atom each of nitrogen and oxygen such that seven electrons from nitrogen and eight electrons from oxygen are involved to form an uncharged molecule (N≡O). The high reactivity of NO is not due to the fact that it contains an unpaired electron having a half life of 2–30 s. If this were the case, how would tissues survive in presence of molecular oxygen with two unpaired electrons at a concentration of 20–200 μM [2]. Nitric oxide only reacts with those biological molecules that have unpaired orbital electrons e.g., other free radicals or transition metal ions. Since most of the biological molecules have completely filled orbitals, it renders nitric oxide unreactive towards them [3]. The reactivity of NO depends upon its physical properties, such as its small size, high diffusion rate, and lipophilicity. Moreover, the reaction products of nitric oxide, i.e. the related species, also react with biological molecules and may have toxic effect as well [4]. At low levels, NO can protect cells; however, at higher levels, it is a known cytotoxin, having been implicated in tumor angiogenesis and progression [5].
The biological reactions of nitric oxide can be divided into three main pathways [6].
Diffusion
Nitric oxide trespasses the cell membrane by simple diffusion and reacts with cellular components. Once inside the cell, it may react with non-heme iron or quench tyrosyl radical of ribonucleotide reductase which may lead to inhibition of DNA synthesis [7, 8].
Auto-oxidation to form N2O3 (nitrous anhydride)
Nitric oxide combines with nitrogen dioxide to form nitrous anhydride as given under:
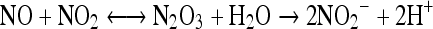 |
1 |
Reaction with superoxide to form peroxynitrite
Reaction of nitric oxide with superoxide in biological media yields peroxynitrite which is not a free radical as the new bond formed involves the free electrons on NO and O•−2. The peroxynitrite formed is a potent oxidant thus reacting with almost all biological molecules [9].
The peroxynitrite anion combine with carbon dioxide to form nitroso peroxycarbonate adduct, a known fastest reaction [10]. On decomposition it forms NO3− and CO2.
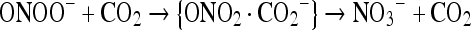 |
2 |
Nitric oxide also reacts with molecular oxygen. The reaction may take place in aqueous or in gas phase. Although the product of both phases is same but its stability differs. The rate of the reaction is second order with respect to NO and first order with respect to O2 (Eq. 3) [11]. In gaseous phase, NO2 is a stable product of NO oxidation. But in aqueous solutions, NO2 give rise to NO, NO3− [4]. NO reacts with molecular oxygen, which is present in much higher concentration than nitric oxide, to form peroxynitrite [12].
| (3) |
The nitroxyl anion (NO−) is also said to be endothelium derived relaxing factor, which is reactive but a short lived species [13]. It reacts with two nitric oxides to form nitrite and nitrous acid [14, 15]. The reaction intermediate (ONNO−) is quite similar to peroxynitrite. The only difference being that ONNO− will be one electron oxidant whereas peroxynitrite can be both one and two electron oxidant [2].
Direct Effect of NO
The kinetically fast reactions occurring physiologically are considered relevant [16]. NO does not react rapidly with amines or thiols but its reaction with metal complexes can be considered as relevant [16]. It reacts with metal complexes to form metal nitrosyls, e.g. Fe–NO complex which is quite stable. The radical is also capable of reacting with metallooxo as well as metal oxo complexes [16]. NO can also directly interact with hypervalent complex formed by agents such as H2O2 [17] and can reduce it to lower valency state.
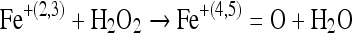 |
4 |
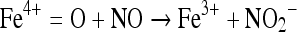 |
5 |
The presence of NO results in scavenging superoxide which besides preventing enzyme inactivation also converts any ferrous oxyadducts to active ferric state. It is also reported that at low concentration of NO, direct effects will predominate, while at higher concentration indirect effects mediated by NO/O•−2 [16].
NO protects tissue from peroxide mediated damage by scavenging metal oxo species [18]. It has been shown to inhibit lipid oxygenase activity by reacting with non-heme iron at the active site [19]. A heme protein, cyclooxygenase, involved in the conversion of arachidonic acid to prostaglandin, and other related enzymes is also influenced by NO radical reactions and metal –NO interaction [19]. A possible mechanism accounted for cyclooxygenase inhibition by superoxide involves the reduction of ferric form to the inactive ferrous state. It has also been reported that NO generation results in nitrosative reactions at nucleophilic centers resulting in the formation of S-nitrosothiols [20]. Excess production of NO has been shown to mediate glutamate induced neuronal toxicity in cortical and striatal neurons culture [21]. NO mediated apoptosis has also been reported in murine peritoneal macrophages [22]. NO also react with oxyhemoglobin and results in the formation of met-Hb and NO3−.
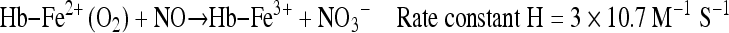 |
6 |
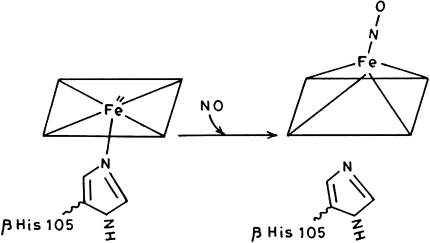
NO also interacts with deoxy-Hb and met-Hb by binding to heme ion center. The binding is hindered by a water molecule coordinated to heme Fe3+ atom is case of met-Hb and it has been reported that the association rate constant is 100 fold less for ferrous deoxy-Hb [23]. Besides these reactions, NO plays important roles in tackling diseases of cardiovascular system by improving blood supply to heart muscle [24]. The anti-impotence drug Viagra exploites the signalling role of NO and increases blood flow into the corpus cavernosum of penis. NO acts as a signaling molecule in smooth muscle cell and neurons. The effect is due to activation of soluble guanyl cyclase (SGC). The formation of guanosine 3′-5′ monophosphate (cGMP) from guanosine 5′-triphosphate is catalyzed by SGC which act as an intracellular messenger that connects the NO signal to the cellular response by activating specific protein kinases, phosphodiesterases and ion channels [9]. It has also been reported that binding of NO to heme moiety of soluble guanyl cyclase results in a pentacoordinate complex and the bond to the proximal histidine is lost [23] (Fig. 1).
Fig. 1.
Formation of pentacoordinate complex in heme moiety of guanyl cyclase
The production of nitric oxide in brain is very well established and it is quite different from other neurotransmitters like acetylcholine. The later, after the release from synapses, lasts for few milliseconds whereas NO persist for seconds, coupled with its rapid diffusion, enables it to encompass several million synapses [24].
The toxic effects of NO generally involve its oxidation products whereas NO alone is not capable of damaging DNA or ribosylate glyceraldehyde-3-phosphate dehydrogenase [25]. The radical can reversibly inhibit enzymes containing transition metal ions or free radical intermediate in their catalytic state [2]. NO in the micromolecular range can also reversibly inhibit cytochrome c oxidase [26, 27] which may result is leakage of superoxide from electron transport chain.
P53 is a protein involved in maintaining genome stability. Following exposure to DNA damaging agents rapid increase in p53 level occurs. Normally p53 has a short half life but DNA damage results in its accumulation in cells [28]. Following exposure to NO generating agents it has been shown that p53 is induced in both RAW 264.7 macrophages and RINm5F cells [29]. P53 accumulation proceed DNA fragmentation and hence apoptosis. NO inhibitors such as NMMA prevent both p53 accumulation and inducible NO generation thus leading to apoptosis [28]. NO mediates DNA damage by three mechanisms.
Formation of nitrosoamines.
Inhibition of DNA lesions repair systems which is also mediated by other genotoxic systems.
Modification of DNA not directly by NO but by its oxidation products [30].
It has been reported that NO production is increased in patients with Systemic lupus erythematosus (SLE) [31]. The disorder is characterized by immune activated state where Inducible nitric oxide synthase (iNOS) level is increased in tissues like macrophages, splenic and renal tissues and consequently NO production [32]. Induction of iNOS occurs is response to cytokine production which is a non-specific event occurring in a wide variety of conditions like ulcerative colitis [33], psoriasis [34], arthritis [35], etc. Increased production of NO is not specific for SLE rather it represent an activated state of immune system.
To relate the role of NO in SLE, its site of production and quantum is of relevance. Patients with SLE showed upregulation of iNOS in normal appearing vascular endothelium. These endothelium also over express the soluble vascular adhesion molecules Intracellular cell adhesion molecule (ICAM-1), E-Selectin and Vascular cell adhesion molecule (VCAM) [36] as have been reported in active SLE [37]. Therefore, it appears that endothelium plays an active role in the localization and propagation of leukocyte and antibody-mediated inflammation [38]. Histological examination of ICAM-1 deficient mice revealed a significant reduction in glomerulonephritis and vasculitis of the kidney, lung and skin [39].
Indirect Effect of NO
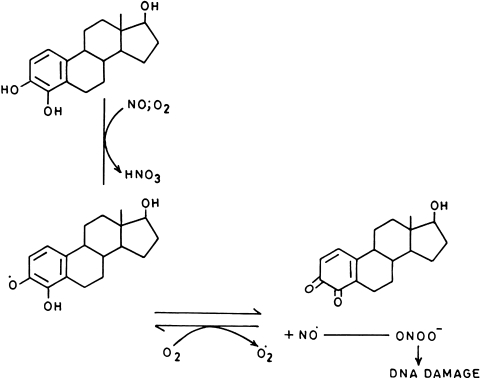
The indirect effect of NO involves the reactions between superoxide and NO which leads to the production of peroxynitrite, a powerful oxidant (rate constant—7 × 109 M−1 S−1). Formation of ONOO− is governed by the relative amount of NO and superoxide produced and also on reaction of these radicals with other biological components. In presence of excess NO or superoxide, peroxynitrite gets converted to nitrogen dioxide, an inactive entity. An intracellular source of ONOO− is mitochondria where aerobic respiration results is production of superoxide and as NO concentration is higher in lipid layers than in cytosol [16], most ONOO− formed is in hydrophobic region. Its production is controlled by manganese, SOD as well as other antioxidants. It has also been reported that catechol-estrogens adducts or complexes are oxidized to quinones which can reduce oxygen to generate O•−2 ion or in presence of NO releasing compound leads to the production of ONOO− [40]. Similarly, polyhydroxy aromatic compounds such as pyrogallol, and 1,4-hydroquinone autooxidize easily to form semiquinone radicals that react with dioxygen to generate O•−2 which in combination with the NO releasing compound like SNP, DEA-NO, SPER-NO results in production of ONOO− [41] which is responsible for DNA damage (Fig. 2). The damaged DNA has been reported to be highly immunogenic, implicating its role in the production of antibodies in diseases like SLE and Cancer [42–44].
Fig. 2.
Synergistic action of nitric oxide and catechol estrogen on DNA molecule
It has also been reported that quinone derivative of catechol-estrogen, which is produced by NO-mediated oxidation may form covalent adducts with nucleophilic groups of DNA [45]. Since human uterus and breast are site for hydroxylation of estrogens to form catechol-estrogens [46], therefore a possible mechanism of hormonal carcinogenesis associated with these organs can be related with increased production of 4-hydroxyestradiol [47–49].
The peroxynitrite can also influence protein and enzyme function, this occurs by nitration of tyrosine residues in tissues thus contributing to pathological dysfunction [9]. The formation of 3-nitrotyrosine is contributed by many nitrogen oxide species such as peroxynitrite, nitrogen dioxide, nitrous acid, nitronium ion, etc. NO alone is not capable of nitrating tyrosine [50]. In addition to this, another way to tyrosine nitration involve myeloperoxidase which is secreted by monocytes and polymorphonuclear neutrophils under inflammatory conditions. Myeloperoxidase catalyzes the formation of hypochlorous acid which is capable of reacting with NO2¯ to form NO2Cl (nitryl chloride) which is a potent nitrating agent [51].
The nitrated product 3-nitrotyrosine is found in many disease states such as chronic inflammation, atherosclerosis, acute lung injury, etc. [52, 53]. Nitration of cardiac actin impairs contractile function of heart in myocarditis. Whereas, if human neurofilament gets nitrated it interferes in the filament polymerization in amylotrophic lateral sclerosis [2]. The signal transduction cascade that depends on reversible phosphorylation of tyrosine is also disrupted by tyrosine nitration. Thus the occurrence of nitrotyrosine-containing proteins in vivo should be regarded as a general indication of tissue damage induced by reactive nitrogen species such as peroxynitrite [54].
The indirect effect of NO can be further divided as oxidation and nitrosation. Those reactions in which reactive nitrogen oxide species (RNOS) donate NO to nucleophilic groups e.g. thiols and amines, lead to formation of nitrosonium adducts known as nitrosation reactions and condition termed as nitrosative stress. Whereas, when removal of electrons or hydroxylation reactions occurs, similar to those for reactive oxygen species (ROS), leading to oxidative stress, they are termed as oxidation reactions [55]. Both these reactions have different effects on biological systems (Fig. 3).
Fig. 3.
Nitrosative and oxidative reactions of nitric oxide
Activated macrophages derived NO and its oxidative metabolite, peroxynitrite were reported to play key notes is hepatocyte injury during inflammation and cause subsequent DNA damage is surviving hepatocytes. Stimulated macrophages in vivo express iNOS which produce high amount of NO and in turn RNOS, which is capable of mediating nitrosation of amines [56, 57], a condition encountered during chronic inflammation. Thus nitrosative stress under certain in vivo conditions can result in the formation of carcinogenic nitrosoamines [58, 59].
Both mammalian and bacterial cells when exposed to NO lead to deamination of guanine, cytosine and adenine via nitrosative chemistry. This would lead to the conversion of cytosine to uracil, guanine to xanthine, adenine to hypoxanthine, methyl cytosine to thymine [60].
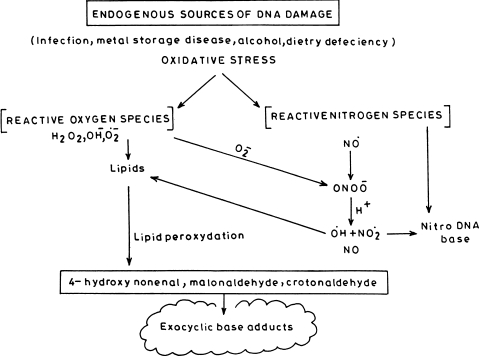
DNA damage also occurs by RNOS formed under oxidative stress. This damage is primarily caused due to formation of ONOO− [61]. It has been reported that SIN-1, a donor which is capable of generating both NO and superoxide, oxidizes guanine to OH-dG [62]. Thus, there occurs a balance between oxidative and nitrosative stress [63]. It has also been reported that NO enhances the oxidative stress caused by H2O2 [64] and also reacts with other radicals such as lipid peroxides [2]. The endogenous production of NO leading to DNA damage generates both oxidative and nitrosative stress [65] (Fig. 4).
Fig. 4.
Endogenous sources of DNA damage leading to oxidative stress
Nitric oxide can also play a protective role against oxidative damage by converting reactive species such as hydroxyl radical to less damaging and easily detoxified products [65]. Action of NO as pro- or antioxidant depends on relative production of NO and O•−2 [66].
Anti-tumor Effect of NO
Though the direct or indirect reactions mediated by NO leading to modulation/damage to biological molecules have been summarised, the better side of NO should not be neglected. It acts as an immune effector generated by iNOS in macrophages, neutrophils, etc. in large quantities which kills or inhibits the growth of many pathogens including bacteria, fungi and parasites. NO is also capable of eliminating intracellular pathogens and blocking viral replication. It has also been reported that NO derived from leukocytes showed anti-tumor effect, it also upregulates tumor suppressor p53 gene [67]. Cells exposed to NO did not show appreciable mutation in p53 gene [68]. NO donors are capable of inhibiting angiogenesis, metastasis and tumor growth. Nitric oxide also inhibits DNA damage mediated by ROS and also inhibits hydroxylation reactions. Since high risk carcinogenic sites are those exhibiting prolonged expression of iNOS during chronic inflammation, NO generated from microphages, Kupffer cells, and NK cells is capable of inhibiting replication along with anti-tumor effect in the target cell [69, 70]. Murine embryonic liver cells, BNL CL.2, are capable of expressing iNOS in response to IFN-γ thus accumulating NO.
In humans few tumors are caused by viruses e.g. liver cancer is caused by hepatitis B virus, cervical cancer by human papilloma virus, Adult T cell leukemia by human T cell leukemia virus. Interferon γ is particularly important in limiting the spread of certain viral infections and capable of expressing iNOS. Increased concentration of NO in or near the target cell may act as tumoricidal since NO is capable of eliminating intracellular pathogens and blocking viral replication [71]. Nitric oxide is capable of protecting cell from apoptosis or mediating apoptosis depending upon the cell type e.g. NO protects rat ovarian follicles from atretic generation on one hand while on the other it induces apoptosis in tumor cells like in mastocytoma, sarcoma, melanoma [16]. NO is also reported to protect tissue form peroxide mediated damage by scavenging metallooxo species which are formed by oxidation of metal species or metal–oxygen species by H2O2 [72]. It is also been reported that animal subjects having tumorous growth acquire the ability through which their tumour tissues suppress the expression of iNOS and thus reduce the concentration of NO [73, 74]. Tumor growth is enhanced by accelerated angiogenesis by down regulating the production of vascular endothelial growth factor which is the mediator of angiogenesis [75]. Nitric oxide is also capable of suppressing metastasis by reducing intracellular stores of GSH [76, 77] or by blocking the adhesion of tumor cells to venular side of microcirculation [78]. It has also been reported that NO produced in vasculature of brain limits the spread of colon cancer to the brain [79]. Liver endothelial cells produce NO which curbs the metastases of melanoma cells to the lungs [80]. Though excessive production of NO is associated with tissue injury, it has been reported that endothelial NO production plays protective role in microvasculature [81]. NO is also reported to inhibit platelet aggregation and it reduces platelet adhesion to endothelial monolayers [82]. The defensive properties accounting for beneficial effect of NO in IL-2 induced injury include protection against tissue injury in myocardial ischemia reperfusion and adult respiratory distress syndrome [83, 84].
It is also reported that when cells were exposed to NO it resulted in DNA single strand breaks [85]. However, when purified DNA was exposed to NO at concentrations as high as 1.0 M, single stand breaks were not observed [86]. Nitric oxide is also reported to protect DNA against oxidative stress by inhibiting Fenton reaction of hydrogen peroxide which leads to single strand generation [87]. It has also been reported that NO in presence of a flavonoid having orthotrihydroxy group namely epigallocatechingallate or quercetogelin inhibits DNA damage by preventing formation of 8-nitroguanine in calf thymus DNA.
Pro-tumor Effect of NO
Carcinogenesis can be defined as a malignant transformation of a cell or group of cells. This process can be divided broadly into two stages, initiation and promotion. The initiation phase involves an irreversible modification of the genetic material of the cell caused by single exposure to any carcinogenic agent whereas promotion requires multiple exposures to the promoters to alter gene expression and produce a tumor. The promotion stage of carcinogenesis is irreversible. Cancer has multifactorial aetiology which includes genetic and environmental factors. The common environmental factors are tobacco chewing, smoking, high fat diet, contaminants, also the occupational exposures to agents like, arsenic, cadmium, chromium, etc. Other environmental factors include sunlight, radiation, pesticides, steroidal medications, etc. It is well known that most of the exogenous carcinogens act via production of reactive oxygen or reactive nitrogen species. The reactive oxygen species are generated both physiologically and pathologically in mammals and induce many kind of cellular damage [88] including DNA damage [89]. Since DNA plays a central role in information transfer, attention has focussed on oxidative damage as significant source of mutations that lead to cancer and other human pathologies [90]. The possible role of ROS modified human DNA in cancer has been indicated from our studies and it has been found that binding of circulating antibodies in cancer sera was much stronger with ROS modified DNA than native DNA. ROS modified DNA has been shown to be a better inhibitor of naturally occurring antibodies in majority of cancer patients than native human DNA [91], reiterating the enhanced recognition of ROS-DNA.
Excess production of NO has been linked to endogenous human carcinogenesis. Induction of apoptosis by NO has also been observed in culture of macrophages and pancreatic β cells. Macrophages exposed to nitric oxide exhibited typical morphology and showed DNA fragmentation indicating apoptotic cell death [28]. NO also induced cell death and showed toxic effects in two different cell lines, viz., CHO-AA8 (Chinese hamster ovary cells) and TK6 (human lymphoblastoid cells) highlighting the role of NO in the onset of mutagenesis and cell death and the involvement of these processes in cancer and inflammatory diseases [92]. Nitric oxide also showed genotoxic effects by abolishing cell growth which is a valuable parameter sensitive to different kinds of damage, e.g. membrane damage, energy depletion, organelle damage and enzyme release, etc. [93]. Peroxynitrite is short lived with t1/2 < 1 s. and is capable of oxidative damage of wide range of biological molecules e.g. nucleic acids, lipids, thiols, etc. [94]. At pH 6.8 its conjugate acid ONOOH can diffuse through membranes and cause damage at a distance from its site of synthesis [95].
It is well known that solid tumors require tumor angiogenesis for their growth i.e. the tumorous cells should be well supplied with oxygen, nutrients and growth factors. Another important feature of malignancy is enhanced vascular permeability which is regulated by endothelial cell production of vasoactive substances. The endothelial cells synthesize NO by Endothelial nitric oxide synthase (eNOS) which helps in vascular permeability and relaxation [96]. Various tumours over express NOS [97, 98]. A study between the relationship of malignancy and eNOS expression in endothelial cells of tumor vessels showed that astrocytic tumor vessels possess higher level of nitric oxide than do normal vessels and found that there was significant correlation with the proliferative potential and eNOS expression in tumor vessels [99]. Several studies have suggested that NO and its reactive derivatives e.g. ONOO− were found to be elevated in infection and inflammation and plays important role in carcinogenesis [100, 101]. The carcinogenic effect of NO may be due to its cytotoxic potential which lead to reduced cell viability [93]. As stated earlier, NO per se is not capable of reacting with biomolecules; only its reaction products lead to the production of RNOS e.g. N2O3 or ONOO− which can result in DNA lesions.
The type of mutation under oxidative and nitrosative stresses differ [102]. Oxidative stress mediate DNA damage by the formation of peroxynitrile. It has been shown that DNA strand breaks were generated when plasmid DNA was incubated with NO-donor compound and polyhydroxy aromatic compounds. On autooxidation of polyhydroxy aromatic component produce semiquinones which react with dioxygen to generate O•−2
Nitrosative stress leads to the formation of nitrosoamines e.g. RNOS generated from acidic nitrite is potentially carcinogenic in stomach [103, 104]. Nitrosamines are formed under conditions of inflammation which can lead to cancer [100]. NO also enhances tumor production by increasing the production of prostaglandin, PGE2 which increases the permeability of tumor vasculature and thus facilitate angiogenesis. Further tumor growth is supported by increased uptake of nutrients [105, 106]. Cells lacking Cu, Zn–SOD are reported to be more susceptible to NO and ONOO− [107, 108]. It has been reported that large amount of ONOO− causes necrosis where as small amounts produce apoptosis [109]. Nitric oxide also causes cellular energy deficit through DNA damage [110]. NO inhibits ribonucleotide reductase which results in impaired DNA synthesis as deoxyribonucleotides are no more available [111]. Since ONOO− causes DNA breaks this leads to activation of poly (ADP-ribose) polymerase to repair the damaged DNA [112, 113]. The activated PARP transfers about 100 ADP ribose moieties from NAD+ to nuclear proteins e.g. histones and PARP itself [112, 114]. ADP ribose polymers thus formed are degraded by glycohydrolases followed by NAD+ resynthesis which is a futile cycle and depletes ATP [114].
Reactive nitrogen oxide species have high affinity towards amino acid with thiol groups and therefore are capable of inhibiting enzymes having thiol residues required for their function [115] e.g. DNA alkyl transferases, required for repair of DNA lesions induced by alkylation, are inhibited by nitrosating its thiol residue in the active site [116, 117]. Other DNA repair enzyme inhibited by RNOS is DNA ligase. T4 DNA ligase contains lysine in partially deprotonated state which is nitrosated by NO [30]. Other DNA repair enzyme inhibited by nitrosating thiols in formamidopyrimidine glycosylase which has zinc finger motif required for its activity. Upon nitrosation, Zn is removed resulting in enzyme inactivation [118].
Effect of NO on Mitochondria
Interaction of NO with mitochondria occurs is many ways. NO synthesis may occur in organelle itself or NO produced outside may diffuse inside. NO may affect mitochondria by three main pathways.
Inactivation of mitochondrial enzymes which is irreversible
Induction of mitochondrial permeability transition
Inhibition of respiration which is reversible [110].
Therefore, effect on mitochondria mediated by NO occurs both by irreversible and reversible means. It has been shown that NO is capable of nitrosating critical thiol residues on creatinine phosphokinase which is irreversible and de-energizes mitochondria thus disrupting its ATP supply [119–121]. Nanomolar concentrations of NO are capable of reversible binding to heme as of cytochrome c oxidase [122]. This direct interaction of NO with cytochrome c is due to cNOS inhibition of mitochondrial respiration [26, 123]. Under aerobic conditions it has been shown that electron transport chain participate in forming superoxide which can then react with NO to form ONOO− [123]. In addition, ONOO− formed outside mitochondria diffuses into the matrix. The increased concentration of ONOO− inactivates Mn–SOD resulting in increase of O•−2 level and hence activating a destructive cascade of NO [124] which includes:
Irreversible damage of enzymes of citric acid cycle e.g. aconitase, iron-sulphur centers, etc. [125, 126].
Inhibition of glycolysis by inactivating glyceraldehyde-3-phosphate dehydrogenase thus impairing ATP synthesis.
Under inflammatory conditions, NADH:ubiquinone oxidoreductase (complex I) and succinate:ubiquinone oxidoreductace (complex II) are irreversibly inhibited by NO [127].
It has been found that NOS is present in mitochondria and that under normal conditions, production of NO is well regulated [2]. Induction of mitochondrial permeability transition is mainly caused by ONOO− which oxidizes thiols and NADPH of mitochondria and induces Ca2+ efflux [128, 129] along with oxidative efflux. As a consequence, Ca2+ homeostasis is disrupted [130, 131]. Mitochondrial decrease in membrane potential in induction of permeability transition leads to increase in cytoplasmic Ca2+ [131, 132]. This permeability transition is accompanied by the formation of a protein pore in mitochondrial inner membrane resulting is the leakage of its contents [131, 133].
Current evidence indicates that irreversible modification of iron binding cysteine residues releases metal and disrupts FeS structure. This is caused by ONOO− rather than by NO [126, 134]. Peroxynitrite damages membranes by hydrogen abstraction from polyunsaturated fatty acids which results is lipid peroxidation [135]. ONOO− also consumes biological antioxidants [136] enhancing the chances of mitochondrial damage.
The role of NO in membrane damage and necrosis was also demonstrated and the leakage of lactate dehydrogenase in TKG and CHO-AA8 cell lines both treated with NO has been observed. However, no LDH leakage was observed before 24 h in TK6 cell and 48 h in CHO-AA8 cells [137]. It has been reported that inactivation of mitochondria by ONOO− in vitro is remarkably similar to the respiratory inhibition observed in cultured tumor cells caused by activated macrophages [138]. If the internal nitric oxide concentration approaches that of mitochondrial superoxide dismutase the superoxide is reported to largely react with the nitric oxide and form peroxynitrite that would injure mitochondria irreversibly [139, 140].
Interaction with Metal Ions
Nitric oxide is capable of directly reacting with metal complexes or oxo complexes. The product of reaction is metal nitrosyls. Reaction of NO with metals may be categorized as:
Direct reaction of NO with metals
Reaction of NO with dioxygen metal complexes
Reaction of NO with oxo-complexes.
The reaction of nitric oxide to form metal nitrosyls is involved is both regulatory and cytotoxic actions [141]. Nitric oxide reacts into oxyhemoglobin. (oxy-Hb) to form met-Hb and NO3− (Eq. 7). The reaction is second order having a rate constant of 3 × 10−7 M−1 S−1.
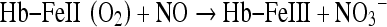 |
7 |
A variety of cellular components present inside the cell e.g. cytochrome c oxidase and cytochrome P450 is able to bind with NO which may result in inhibition of mitochondrial respiration. Since nitric oxide synthase is also present in mitochondria, it may increase the production of NO under inflammatory stressed state. NO has great affinity for iron and heme proteins due to the presence of porphyrin ligands [141]. Reactions of NO with zinc and copper containing proteins have also been reported, an important class of DNA binding protein induce zinc finger motifs [142]. Since zinc finger motif contain two to four cysteine residues and up to two histidine residues e.g. formamidopyrimidine-DNA glycosylase (Fpg protein). The protein repairs oxidative damage to guanine. NO under aerobic conditions inhibits Fpg [30]. Another example of reaction of NO with metal centers is reaction of soluble guanyl cyclase which is activated by binding of NO to ferrous heme iron resulting in generation of cGMP and transduction of NO signal [141].
Nitric oxide forms iron nitrosyl complexes by binding to iron-sulfur clusters e.g. NO is capable of inactivating aconitase due to its direct interaction with the enzymes heme iron nitrosyl complex [9]. These reactions of NO with metals generally involve covalent interactions. In addition, various metal oxygen complexes e.g. reaction of NO and oxyhemoglobin to form met-hemoglobin and nitrate (Eq. 7) also occur. This is one of the primary detoxification mechanisms of NO.
Nitric oxide also rapidly reacts with hypervalent metal complexes i.e. metallooxo species which are formed by the oxidation of metals or metal oxygen complexes by e.g. H2O2 [30]. Nitric oxide reacts with these hypervalent species and results in conversion of hypervalent complex to lower valent state thereby scavenging the metallooxo species and protecting cells from peroxide mediated damage [18]. Formation and scavenging the metallooxo species can be presented as under:
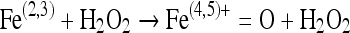 |
8 |
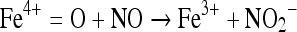 |
9 |
The high reactivity of NO with diverse chemical groups and with transition metal ions is used as a guide line for designing drugs used as NO scavengers [143]. Many coordination complexes that are Ruthenium complexes are proposed as efficient NO scavengers e.g. JM-1226 a Ruthenium coordination complex has been shown in the recovery of rat from sepsis, another NO scavenger used is 2 Phenyl-4,4,5,5 tetramethylimidazolin-1-oxyl-3 oxide (PTIO).
NOS Enzymes
Biosynthesis of nitric oxide takes place by the conversion of l-arginine to l-citrulline catalyzed by nitric oxide synthases. The enzyme has three isoforms. NOS1 or neuronal nitric oxide synthase (nNOS). NOS2 or inducible nitric oxide synthase (iNOS) and NOS3 endothelial nitric oxide synthase (eNOS). Among these, the first isoform has been purified and cloned [30]. Each isoform is a product of distinct gene [144]. Broadly speaking isoforms of nitric oxide synthase may be categorised as constitutive (cNOS) and inducible nitric oxide synthase (iNOS). Constitutive NOS is calcium dependent and continuously present whereas iNOS is Ca2+ independent and is expressed only after cytokine exposure [145]. Based on this category nNOS and eNOS are constitutively expressed and require elevated levels of Ca2+ along with activation of calmodulin to produce NO for brief period of time [146]. iNOS is induced by cytokines in almost every cell and generates locally high level of NO for prolonged periods of time [30]. It is mainly expressed in macrophages, neutrophils and epithelial cells. It has also been reported that in mice, estrogen and progesterone-induced iNOS expression is different in different cell population i.e. estrogen induces it in myometrial mast cell whereas progesterone induces it in epithelial cells.
It is also reported that cNOS are employed in physiological regulation within cardiovascular and nervous system where they produce low concentration of NO—1.0 μM in cardiovascular and 5 μM in neurological systems whereas iNOS produce NO achieving concentrations equal to 10 μM [141].
Inducible NOS
This is type 2 NOS and is induced by inflammatory stimuli e.g. cytokines or lipopolysaccharides. It is mainly expressed is macrophages and possess tightly bound calmodulin [141]. It is also reported that iNOS is not only present is activated macrophages but its synthesis can be induced in glial cells, liver and cardiac muscle.
Endothelial NOS
It is constitutively expressed in endothelial lining of blood vessels and depends on Ca2+. The NO produced by eNOS diffuses into smooth muscle cells of blood vessel and elicits cGMP dependent smooth muscle relaxation and thus increasing blood flow.
Neuronal NOS
Neuronal nitric oxide synthase is constitutively expressed in post synaptic terminals of neurons and is Ca2+ dependent. It is activated by Ca2+ influxes caused by binding of neurotransmitter glutamate, to receptor in cell membrane. nNOS is also activated by membrane depolarization through opening of voltage gated Ca2+ channels [9].
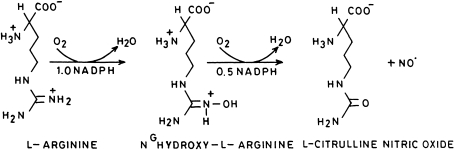
The synthesis of NO by nitric oxide synthases takes place by conversion of l-arginine to l-citrulline via the formation of NG-hydroxy-l-arginine (Fig. 5). Therefore, NOS utilize l-arginine, NADPH and oxygen to produce nitric oxide. Nitric oxide synthase enzyme is an heme iron dependent tetrahydrobiopterin enzyme where tetrahydrobiopterin binds far away and on the wrong side of porphyrin to act as hydroxylating cofactor at distal side of heme–iron center [9]. Other enzymes that use tetrahydrobiopterin are e.g. phenyl monooxygenase, tyrosine-3-monooxygenase, tryptophan-5-monooxygenase, etc. In these cases the coenzyme is directly involved in hydroxylation of substrate and which then gets oxidized to dihydrobiopterin. The dihydrobiopterin is recycled to tetrahydrobiopterin by dihydropteridine reductase.
Fig. 5.
Synthesis of nitric oxide from l-arginine
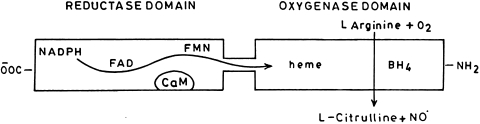
Nitric oxide synthase enzyme in its active form is a homodimer where each subunit is composed of C-terminal reductase and N-terminal oxygenase domain. It is also reported that the isolated oxygenase domain remain homodimeric whereas isolated reductase domain is monomeric indicating that the two subunits are joined by their oxygenase domains. The oxygenase domain contains a heme group and one binding site for tetrahydrobiopterin and l-arginine. Whereas, the reductase domain of nitric oxide synthase has binding sites for either of the one molecule of Flavin mononucleotide (FMN), Flavin adenine dinucleotide (FAD) and NADPH [144]. Between the reductase and oxygenase domain there is a binding site for calmodulin. In case of nNOS and eNOS only Ca2+-calmodulin complex can activate the enzyme whereas is case of iNOS, it is already bound to calmodulin and is fully active. Calmodulin is reported to improve electron flow from NADPH to flavins and also facilitates electron transfer from FMN to heme [8]. The flow of electrons in nitric oxide synthase domains are represented in Fig. 6.
Fig. 6.
Flow of electrons in nitric oxide synthase domain from FMN to heme
Inhibitors of NO
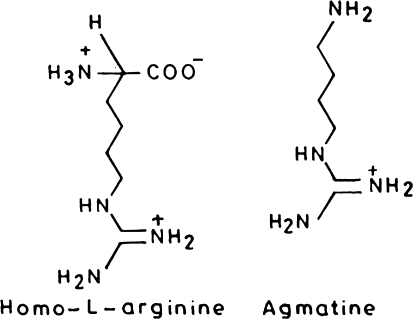
In order to study the over production of NO, inhibitors of NOS have been synthesized and investigated for their probable role in controlling the over production of NO under disease condition. Since l-arginine is a naturally occurring substrate for NO, analogs of l-arginine have been used as competitive NOS inhibitors. These inhibitors were non-selective with reference to isoforms of nitric oxide synthase e.g. N-monomethyl-l-arginine and nitro-l-arginine methyl ester [143]. Among the synthetic analogs of l-arginine only homo l-arginine and agmatine are active (Fig. 7).
Fig. 7.
Competitive inhibitors of nitric oxide synthase enzyme
It is reported that removal of α-aminopentanoic acid part of NG methyl-l-arginine (l-NMA), NG amino-l-arginine (l-NAA) and NG nitro-l-arginine (l-NNA), also the analogs of l-arginine, results in the formation of guanidine which looses binding affinity [9]. Some of the analogs of l-arginine also exhibit irreversible inactivation of NOS in presence of oxygen and NADPH e.g. l-NMA, Nε iminoethyl-l-Lysine (l-NIL), N8 iminoethyl-l-ornithine (l-NIO) i.e. inhibition is mechanism based.
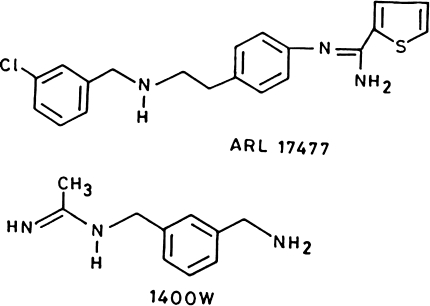
Along with the non-specific inhibitors of NOS, many isoform specific inhibitors are also available. e.g. 7-nitroindazole and aminoguanidine are inhibitors of nNOS and iNOS, respectively [143]. The importance of these isoenzyme specific inhibitors is that they inhibit selective NO biosynthesis rather than suppressing whole NO biosynthesis (Table 1). l-NIL is an exception with 30 folds more specificity towards iNOS [147]. Among these inhibitors Galaxo Wellcome compound 1400 w (N-(3-aminomethyl) benzyl) acetamide) is most selective iNOS inhibitor and Astra Arcus compound ARL 17477 is most selective for nNOS (Fig. 8).
Table 1.
Characteristics of NOS inhibitors
| Inhibitor | Specificity | Clinical importance |
|---|---|---|
| l-NIL | iNOS | Effective in arthritis and leishmaniasis |
| S-methylisothiourea, 4-amino-tetrahydrobiopterin | iNOS | NOS is most sensitive towards inhibition during de novo protein synthesis |
| Galaxo-Wellcome compound 1400 W | iNOS | Rapid reversibility and low potency Ki ≈ 2–50 μM |
| 1-Amino-S-methylisothiourea | iNOS | – |
| 7-Nitroindazole | nNOS | Experimental stroke |
| Astra Arcus Compound ARL 17477 | Infracts caused by temporary occlusion of middle cerebral artery in rats |
Fig. 8.
Specific inhibitors of neuronal nitric oxide synthase
In higher vertebrates nitric oxide has key roles in maintaining homeostasis and in vascular smooth muscle, neurons and the GI tract. It has a definite role in regulating all aspects of our lives from walking, digestion, sexual function, pain perception and pleasure, memory recall and sleeping. Finally, the way it continues to function in our bodies will influence how we degenerate with age. It has a likely role in our deaths through cardiovascular disease, stroke, diabetes and cancer. Our ability to control NO signaling and to use NO effectively in therapy must therefore have a major bearing on the future quality and duration of human life [148].
References
- 1.Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Asp Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Beckman JS, Koppenol WH. Nitric oxide, superoxide and peroxynitrite: the good the bad and the ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 3.Padmaja S, Huie RE. The reactions of nitric oxide with organic peroxyl radical. Biochem Biophys Res Commun. 1993;195:539–544. doi: 10.1006/bbrc.1993.2079. [DOI] [PubMed] [Google Scholar]
- 4.Hughes MN. Chemistry of nitric oxide and related species. Methods Enzymol. 2008;436:3–19. doi: 10.1016/S0076-6879(08)36001-7. [DOI] [PubMed] [Google Scholar]
- 5.Paradise WA, Vesper BJ, Goel A, Waltonen JD, Altman KW, Haines GK, Radosevich JA. Nitric oxide: perspectives and emerging studies of a well known cytotoxin. Int J Mol Sci. 2010;11:2715–2745. doi: 10.3390/ijms11072715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamir S, Burney S, Tannenbaum SR. DNA damage by nitric oxide. Chem Res Toxicol. 1996;9:821–827. doi: 10.1021/tx9600311. [DOI] [PubMed] [Google Scholar]
- 7.Kwon NS, Stuehr DJ, Nathan CF. Inhibitor of tumor cell ribonucleotide reductase by macrophage-derived nitric oxide. J Exp Med. 1991;174:761–767. doi: 10.1084/jem.174.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy B, Lepoivre M, Henry Y, Fontecave M. Inhibition of ribonucleotide reductase by nitric oxide derived from thionitrites: reversible modifications of both subunits. Biochemistry. 1995;34:5411–5418. doi: 10.1021/bi00016a012. [DOI] [PubMed] [Google Scholar]
- 9.Pfeiffer S, Mayer B, Hemmens B. Nitric oxide: chemical puzzles posed by a biological messenger. Angew Chem Int Ed. 1999;38:1714–1731. doi: 10.1002/(SICI)1521-3773(19990614)38:12<1714::AID-ANIE1714>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Uppu RM, Squadrito RM, Pryor WA. Acceleration of peroxynitrite oxidation by carbon dioxide. Arch Biochem Biophys. 1996;327:335–343. doi: 10.1006/abbi.1996.0131. [DOI] [PubMed] [Google Scholar]
- 11.Olbregts J. Tetramolecular reaction of nitrogen monooxide and oxygen: a still unsolved problem. Int J Chem Kinet. 1985;17:835–848. [Google Scholar]
- 12.Hughes MN, Nicklin HG, Sackrule WAC. The chemistry of peroxonitrities. Part III. The reaction of peroxynitrite with nucleophiles in alkali and other nitrite producing reactions. J Chem Soc A. 1971;23:3722–3725. [Google Scholar]
- 13.Fukuto JM, Chiang K, Hszieh R, Wong P, Chaudhri G.The pharmacological activity of nitroxyl: a potent vasodilator reversing hypoxic pulmonary vasoconstriction Circulation 1991832038–2047.2040056 [Google Scholar]
- 14.Seddon WA, Fletcher JW, Sopchyshyn FC. Pulse radiolysis of nitric oxide in aqueous solution. Can J Chem. 1973;51:1123–1130. [Google Scholar]
- 15.Seddon WA, Young MJ. Pulse radiolysis of nitric oxide in aqueous solution. Can J Chem. 1970;48:393–394. [Google Scholar]
- 16.Wink DA, Mitchel JB. Chemical biology of nitric oxide: insights into regulatory cytotoxic and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med. 1998;25:434–456. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 17.Puppo A, Halliwell B. Formation of hydroxyl radicals from hydrogen peroxide in presence of iron: is hemoglobin a biological Fenton reagent? Biochem J. 1998;249:185–190. doi: 10.1042/bj2490185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanner J, Harel S, Granit R. Nitric oxide as an antioxidant. Arch Biochem Biophys. 1991;289:130–136. doi: 10.1016/0003-9861(91)90452-o. [DOI] [PubMed] [Google Scholar]
- 19.Kanner J, Harels S, Ganit R. Nitric oxide an inhibitor of lipid oxidation by lipoxygenase, cyclooxygenase and hemoglobin. Lipids. 1992;27:46–49. doi: 10.1007/BF02537058. [DOI] [PubMed] [Google Scholar]
- 20.Mohr S, Stamler JS, Brune B. Mechanism of covalent modification of glyceraldehyde-3-phosphate dehydrogenase at its active site thiol by nitric oxide, peroxynitrite and related nitrosating agents. FEBS Lett. 1994;348:223–227. doi: 10.1016/0014-5793(94)00596-6. [DOI] [PubMed] [Google Scholar]
- 21.Dawson VL, Dawson JM, London ED, Bredt DS, Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci USA. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albina JE, Cui S, Mateo B, Reichner JS. Nitric oxide mediated apoptosis in murine peritoneal macrophages. J Immunol. 1993;150:5080–5085. [PubMed] [Google Scholar]
- 23.Stone JR, Sands RH, Dunham WR, Marletta MA. Electron paramagnetic resonance optical evidence for the formation of penta coordinate nitrosyl complex on soluble guanylate cyclase. Biochem Biophys Res Commun. 1995;207:572–577. doi: 10.1006/bbrc.1995.1226. [DOI] [PubMed] [Google Scholar]
- 24.Yoshie Y, Oshima H. Synergistic action of DNA strand breakage caused by nitric oxide together with catecholamines: implications for neurodegenerative diseases. Chem Res Toxicol. 1997;10:1015–1022. doi: 10.1021/tx970025k. [DOI] [PubMed] [Google Scholar]
- 25.Dimmeler S, Lottspeich F, Brune B. Nitric oxide causes ADP ribosylation and inhibition of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1992;267:16771–16774. [PubMed] [Google Scholar]
- 26.Cleeter MWJ, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AHV. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain by nitric oxide. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 27.Gow JP, Spruell C, Chen J, Gunn C, Ischiropoulos H, Tsai M, Smith CD, Radi R, Koppenol WH, Beckman JS. On the pH dependent yield of hydroxyl radical products from peroxynitrite. Free Radic Biol Med. 1994;16:331–338. doi: 10.1016/0891-5849(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 28.Nicotera P, Bonfoco E, Brune B. Mechanisms for nitric oxide induced cell death: involvement of apoptosis. Adv Neuroimmunol. 1995;5:411–420. doi: 10.1016/0960-5428(95)00025-9. [DOI] [PubMed] [Google Scholar]
- 29.Me Bmer UK, Lapetina EG, Brune B. Nitric oxide induced apoptosis in RAW 264.7 macrophages is antagonized by protein kinase C and protein kinase A activating compounds. Mol Pharmacol. 1995;47:757–765. [PubMed] [Google Scholar]
- 30.Wink DA, Vodovotz Y, Laval J, Laval F, Dewhirst MW, Mitchell JB. The multifaceted roles of nitric oxide in cancer. Carcinogenesis. 1998;19:711–721. doi: 10.1093/carcin/19.5.711. [DOI] [PubMed] [Google Scholar]
- 31.Belmont HM, Levartovsky D, Goel A, Amin A, Giorno R, Rediske J, Skovron ML, Abramson SB. Increased nitric oxide production accompanied by the up regulation of inducible nitric oxide synthase in vascular endothelium from patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:1810–1816. doi: 10.1002/art.1780401013. [DOI] [PubMed] [Google Scholar]
- 32.Weinberg JW, Granger DL, Pisetsky DS, Seldin MF, Misukonis MA, Mason SN, Pippen AM, Ruiz P, Wood ER, Gilkeson GS. The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-Lpr mice and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-l-arginine. J Exp Med. 1994;179:651–660. doi: 10.1084/jem.179.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Middleton SJ, Shorthouse M, Hunter JO. Increased nitric oxide synthesis in ulcerative colitis. Lancet. 1993;341:465–466. doi: 10.1016/0140-6736(93)90211-x. [DOI] [PubMed] [Google Scholar]
- 34.Kolb-Bachofen V, Fehsel K, Miche G, Ruzicka T. Epidermal keratinocyte expression of inducible nitric oxide synthase in skin lesions of psoriasis vulgaris. Lancet. 1994;344:139–142. doi: 10.1016/s0140-6736(94)91328-5. [DOI] [PubMed] [Google Scholar]
- 35.Gorbunov N, Esposito E. Nitric oxide as a mediator of inflammation. Int J Immunopathol Pharmacol. 1993;6:67–75. [Google Scholar]
- 36.Belmont HM, Buyon J, Giorno R, Abramson S. Up regulation of endothelial cell adhesion molecules characterizes disease activity in systemic lupus erythematosus: the Shwartzman phenomenon revisited. Arthritis Rheum. 1994;37:376–383. doi: 10.1002/art.1780370311. [DOI] [PubMed] [Google Scholar]
- 37.Spronk PE, Bootsma H, Huitema MG, et al. Levels of soluble VCAM-1, soluble ICAM-1 and soluble E-selectin during disease exacerbations in patients with systemic lupus erythematosus (SLE): a long term prospective study. Clin Exp Immunol. 1994;97:439–444. doi: 10.1111/j.1365-2249.1994.tb06107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMurray RW. Adhesion molecules in autoimmune disease. Semin Arthritis Rheum. 1996;25:215–233. doi: 10.1016/s0049-0172(96)80034-5. [DOI] [PubMed] [Google Scholar]
- 39.Bullard DC, King PD, Hicks MJ, Dupont B, Beaudet AL, Elkon KB. Intracellular adhesion molecule-1 deficiency protects MRL/MpJ-Fas Lpr mice from early lethality. J Immunol. 1997;715:2058–2067. [PubMed] [Google Scholar]
- 40.Yumiko Y, Hiroshi O. Synergistic induction of DNA strand breakage by catechol estrogens and NO: implications for hormonal carcinogenesis. Free Radic Biol Med. 1998;24:341–348. doi: 10.1016/s0891-5849(97)00269-4. [DOI] [PubMed] [Google Scholar]
- 41.Yumiko Y, Hiroshi O. Nitric oxide synergistically enhances DNA strand breakage induced by polyhydroxy compounds but inhibits that induced by Fenton reaction. Arch Biochem Biophys. 1997;342:13–21. doi: 10.1006/abbi.1997.0100. [DOI] [PubMed] [Google Scholar]
- 42.Habib S, Moinuddin, Ali R. Acquired antigenicity of DNA after modification with peroxynitrite. Int J Biol Macromol. 2005;35:221–225. doi: 10.1016/j.ijbiomac.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Habib S, Moinuddin, Ali R. Peroxynitrite modified DNA: a better antigen for systemic lupus erythematosus anti-DNA autoantibodies. Biotechnol Appl Biochem. 2006;43:65–70. doi: 10.1042/BA20050156. [DOI] [PubMed] [Google Scholar]
- 44.Habib S, Moinuddin, Ali A, Ali R. Preferential recognition of peroxynitrite modified human DNA by circulating autoantibodies in cancer patients. Cell Immunol. 2009;254:117–123. doi: 10.1016/j.cellimm.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Dwivedy I, Devanesan P, Gemonesi P, Rogan E, Cavalieri E. Synthesis and characterization of estrogen 2,3 and 3,4 quinones. Comparison of DNA adducts formed by quinones vs. horse reddish peroxidase activated catechol estrogens. Chem Res Toxicol. 1992;5:828–833. doi: 10.1021/tx00030a016. [DOI] [PubMed] [Google Scholar]
- 46.Yager JD, Liehr JG. Molecular mechanisms of estrogen carcinogenesis. Annu Rev Pharmacol Toxicol. 1996;36:203–232. doi: 10.1146/annurev.pa.36.040196.001223. [DOI] [PubMed] [Google Scholar]
- 47.Liehr JG, Ricci MJ. 4-Hydroxylation of estrogens as marker of human mammary tumor. Proc Natl Acad Sci USA. 1996;93:3294–3296. doi: 10.1073/pnas.93.8.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liehr JG, Ricci MJ, Jefcoate CR, Hannigan EV, Hokanson JA, Zhu BT. 4-Hydroxylation of estradiol by human uterine myometrium and myoma microsomes: implications for the mechanism of uterine tumorigenesis. Proc Natl Acad Sci USA. 1995;92:9220–9224. doi: 10.1073/pnas.92.20.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan WA, Alam K, Moinuddin Catechol-estrogen modified DNA: a better antigen for cancer autoantibody. Arch Biochem Biophys. 2007;465:293–300. doi: 10.1016/j.abb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Beckman JS. Oxidative damage and tyrosine nitration by peroxynitrite. Chem Res Toxicol. 1996;9:836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- 51.Eiserich JP, Hristova M, Goss CE, Jones AD, Freeman BA, Halliwell B, Vander Vliet A. Formation of nitric oxide derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 52.Kaur H, Halliwell B. Evidence for nitric oxide mediated oxidative damage in chronic inflammation, nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS Lett. 1994;350:9–12. doi: 10.1016/0014-5793(94)00722-5. [DOI] [PubMed] [Google Scholar]
- 53.Khan F, Siddiqui AA. Prevalence of anti-3-nitrotyrosine antibodies in the joint synovial fluid of patients with rheumatoid arthritis, osteoarthritis and systemic lupus erythematosus. Clin Chim Acta. 2006;370:100–107. doi: 10.1016/j.cca.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 54.Sampson JB, Ye Y, Rosen H, Beckman JS. Myeloperoxidase and horseradish peroxidase catalyze tyrosine nitration in proteins from nitric oxide and hydrogen peroxide. Arch Biochem Biophys. 1998;356:207–213. doi: 10.1006/abbi.1998.0772. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe N, Miura S, Zeki S, Ishii H. Hepatocellular oxidative DNA injury induced by macrophage-derived nitric oxide. Free Radic Biol Med. 2001;30:1019–1028. doi: 10.1016/s0891-5849(01)00498-1. [DOI] [PubMed] [Google Scholar]
- 56.Marletta MA. Mammalian synthesis of nitrite, nitrate, nitric oxide and N-nitrosating agents. Chem Res Toxicol. 1988;1:249–257. doi: 10.1021/tx00005a001. [DOI] [PubMed] [Google Scholar]
- 57.Miles AM, Gibson M, Krishna M, Cook JC, Pacelli R, Wink DA, Grisham MB. Effects of superoxide on nitric oxide dependent N-nitrosation reactions. Free Radic Res. 1995;233:379–390. doi: 10.3109/10715769509065259. [DOI] [PubMed] [Google Scholar]
- 58.Liu RH, Baldwin B, Tennant BC, Hotchkiss JH. Elevated formation of nitrate and N-nitrosodimethylamine is wood chucks (Marmota monax) associated with chronic woodchuck hepatitis virus infection. Cancer Res. 1991;51:3925–3929. [PubMed] [Google Scholar]
- 59.Liu RH, Jacob JR, Tennant BD, Hotchkiss JH. Nitrite and nitrosoamine synthesis by hepatocytes isolated from normal woodchucks, (Marmota Monax) and woodchucks chronically infected with woodchuck hepatitis virus. Cancer Res. 1992;52:3925–3929. [PubMed] [Google Scholar]
- 60.Merchant K, Chen H, Gonzalez TC, Keefer LK, Shaw BR. Deamination of single stranded DNA cytosine residues in aerobic nitric oxide solution at micromolar total NO exposures. Chem Res Toxicol. 1996;9:891–896. doi: 10.1021/tx950102g. [DOI] [PubMed] [Google Scholar]
- 61.Wink DA, Look JA, Kim S, Vodovotz Y, Pacelli R, Krishna MC, Russo A, Mitchel JB, Jourdheuil D, Miles AM, Grisham MB. Superoxide modulates the oxidation and nitrosation of thiols by nitric oxide derived reactive intermediates. J Biol Chem. 1997;272:11147–11151. doi: 10.1074/jbc.272.17.11147. [DOI] [PubMed] [Google Scholar]
- 62.Inous S, Kawanishi S. Oxidative DNA damage induced by simultaneous generation of nitric oxide and superoxide. FEBS Lett. 1995;371:86–88. doi: 10.1016/0014-5793(95)00873-8. [DOI] [PubMed] [Google Scholar]
- 63.Billar TR. The delicate balance of nitric oxide and superoxide in liver pathology. Gastroenterology. 1995;108:603–605. doi: 10.1016/0016-5085(95)90093-4. [DOI] [PubMed] [Google Scholar]
- 64.Ioannidis I, Groot H. Cytotoxicity of nitric oxide in Fu5 hepatoma cells: evidence for co-operative action with hydrogen peroxide. Biochem J. 1993;296:341–345. doi: 10.1042/bj2960341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wink DA, Ford PC. Nitric oxide reactions important to biological systems, a survey of some kinetics investigations. Methods. 1995;7:14–20. [Google Scholar]
- 66.Darley-Usmar V, Wiseman H, Halliwell B. Nitric oxide and oxygen radicals: a question of balance. FEBS Lett. 1995;369:131–135. doi: 10.1016/0014-5793(95)00764-z. [DOI] [PubMed] [Google Scholar]
- 67.Lim S, Hung AC, Porter AG. Focused PCR screen reveals p53 dependence of nitric oxide-induced apoptosis and up-regulation of maspin and plasminogen activator inhibitor-1 in tumor cells. Mol Cancer Res. 2009;7:55–66. doi: 10.1158/1541-7786.MCR-08-0331. [DOI] [PubMed] [Google Scholar]
- 68.Forrester K, Ambs S, Upold SE, Kapust RB, Spillare EA, Weinberg WC, Felly-Bosco E, Wang XW, Geller DA, Tzeng E, Billar T, Harris CC. Nitric oxide induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild type p53. Proc Natl Acad Sci USA. 1993;93:2442–2447. doi: 10.1073/pnas.93.6.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kurose I, Miura S, Fukumura D, Yonei Y, Saito H, Tada S, Suematsu M, Tsuchiya M. Nitric oxide mediates kupffer cell-induced reduction of mitochondrial energization in hepatoma cells: a comparison with oxidative burst. Cancer Res. 1993;53:2676–2681. [PubMed] [Google Scholar]
- 70.Xio L, Eneroth PHE, Qureshi GA. Nitric oxide synthase pathway may mediate human natural killer cell cytotoxicity. Scand J Immunol. 1995;42:505–511. doi: 10.1111/j.1365-3083.1995.tb03687.x. [DOI] [PubMed] [Google Scholar]
- 71.Roit I, Brostoff J, Male DK, editors. Immunology. 3rd ed. St. Louis: Mosby. 1993;16 pp.
- 72.Gorbunov NV, Osipov AN, Day BW, Zayas RB, Kagan VE, Elsayed NM. Reduction of ferryl myoglobulin and ferryl hemoglobulin by nitric oxide: a protective mechanism against ferryl hemoprotein induced oxidations. Biochemistry. 1995;34:6689–6699. doi: 10.1021/bi00020a014. [DOI] [PubMed] [Google Scholar]
- 73.Gardner TE, Naoma H, Daly JM. Peritoneal and splenic macrophage function in tumor bearing host. J Surg Res. 1995;59:305–310. doi: 10.1006/jsre.1995.1169. [DOI] [PubMed] [Google Scholar]
- 74.Lejeune P, Lagade CP, Orier N, Pinard D, Oshima H, Jeannin JF. Nitric oxide involvement in tumour induced immunosuppression. J Immunol. 1994;152:5077–5083. [PubMed] [Google Scholar]
- 75.Tsurumi Y, Murohara T, Krasinski K, Chen D, Witzenbchler B, Kearney M, Couffinhal T, Isner JM. Reciprocal relation VEGF and NO in the regulation of endothelial integrity. Nat Med. 1997;3:879–886. doi: 10.1038/nm0897-879. [DOI] [PubMed] [Google Scholar]
- 76.Luperchio S, Tamir S, Tannenbaum SR. Nitric oxide induced oxidative stress, glutathione metabolism in rodent and human cells. J Exp Med. 1996;181:1333–1343. doi: 10.1016/0891-5849(96)00219-5. [DOI] [PubMed] [Google Scholar]
- 77.Petit JF, Nicaise M, Lopoivre M, Guissani A, Lemaire G. Protection by glutathione against the antiproliferative effects of nitric oxide: dependence on kinetics of nitric oxide release. Biochem Pharmacol. 1996;52:205–212. doi: 10.1016/0006-2952(96)00177-3. [DOI] [PubMed] [Google Scholar]
- 78.Kong L, Dunn GD, Keefer LK, Korthuis RJ. Nitric oxide reduces tumor cell adhesion to isolated rat post capillary vennules. Clin Exp Metastasis. 1996;14:335–343. doi: 10.1007/BF00123392. [DOI] [PubMed] [Google Scholar]
- 79.Murata J, Ricciardi-Castagnoli P, Dessous LE, Mange P, Martin F, Juitlert-Jeanneret L. Microglial cells induce cytotoxic effects towards colon carcinoma cells: measurement of tumor cytotoxicity with a gamma glutamyl transpeptidase assay. Int J Cancer. 1997;70:169–174. doi: 10.1002/(sici)1097-0215(19970117)70:2<169::aid-ijc6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 80.Hiranao S. In vitro and in vivo cytotoxic effects of nitric oxide on metastatic cells. Cancer Lett. 1997;115:56–62. doi: 10.1016/s0304-3835(97)04706-x. [DOI] [PubMed] [Google Scholar]
- 81.Claney RM, Abramson SB. Nitric oxide: a novel mediator of inflammation. Soc Exp Biol Med. 1995;210:93–101. doi: 10.3181/00379727-210-43927aa. [DOI] [PubMed] [Google Scholar]
- 82.Kubes PM, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leucocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roissaint R, Falke KJ, Lobez F, Slama K, Pison U, Zapol WM. Inhaled nitric oxide for the adult respiratory distress syndrome. N Eng J Med. 1993;328:339–405. doi: 10.1056/NEJM199302113280605. [DOI] [PubMed] [Google Scholar]
- 84.Lafer AM. Cytoprotective action of nitric oxide donors in ischemia reperfusion injury. In: Moncada S, Marletta MA, Higgs EA, editors. The biology of nitric oxide. London: Portland Press; 1992. pp. 55–58. [Google Scholar]
- 85.Nguyen J, Brunson D, Crespi CL, Penman BW, Wishnok JS, Tannenbaum SR. DNA damage and mutation in human cells exposed to nitric oxide. Proc Natl Acad Sci USA. 1992;89:3030–3034. doi: 10.1073/pnas.89.7.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Routledge MN, Wink DA, Keefer LK, Dipple A. Mutations induced by saturated aqueous nitric oxide in psp189 sup. F gene in human Ad293 and E. coli MBM 7070 cells. Carcinogenesis. 1993;14:1251–1254. doi: 10.1093/carcin/14.7.1251. [DOI] [PubMed] [Google Scholar]
- 87.Oshima H, Yoshie Y, Auriol S, Gilbert I. Anti-oxidant and pro-oxidant actions of flavonoids: effect on DNA damage induced by nitric oxide, peroxynitrite and nitroxyl anion. Free Radic Biol Med. 1998;25:1057–1065. doi: 10.1016/s0891-5849(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 88.Carreira BP, Morte MI, Inácio A, Costa G, Rosmaninho-Salgado J, Agasse F, Carmo A, Couceiro P, Brundin P, Ambrósio AF, Carvalho CM, Araújo IM. Nitric oxide stimulates the proliferation of neural stem cells bypassing the epidermal growth factor receptor. Stem Cells. 2010;28:1219–1230. doi: 10.1002/stem.444. [DOI] [PubMed] [Google Scholar]
- 89.Cerutti P, Gosh R, Oya Y, Amstad P. The role of cellular defense in oxidant carcinogenesis. Environ Health Prospect. 1994;102:123–129. doi: 10.1289/ehp.94102s10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ames BN. Endogenous oxidative DNA damage, ageing and cancer. Free Radic Res Commun. 1989;7:121–128. doi: 10.3109/10715768909087933. [DOI] [PubMed] [Google Scholar]
- 91.Abdi S, Ali A. Role of ROS modified human DNA in pathogenesis and etiology of cancer. Cancer Lett. 1999;142:1–9. doi: 10.1016/s0304-3835(99)00112-3. [DOI] [PubMed] [Google Scholar]
- 92.Burney S, Tamir S, Gal A, Tannenbaum SR. A mechanistic analysis of nitric oxide induced cellular toxicity. Nitric Oxide Biol Med. 1997;1:130–144. doi: 10.1006/niox.1996.0114. [DOI] [PubMed] [Google Scholar]
- 93.Stopper H, Moller M, Bommell HM, Schmid HHHW. Cytotoxic versus genotoxic effects of nitric oxide (NO) Toxicol Lett. 1999;106:59–67. doi: 10.1016/s0378-4274(99)00019-3. [DOI] [PubMed] [Google Scholar]
- 94.Beckman JS. Peroxynitrite versus hydroxy radical: the role of nitric oxide in superoxide dependant cerebral injury. Ann NY Acad Sci. 1994;738:69–74. doi: 10.1111/j.1749-6632.1994.tb21791.x. [DOI] [PubMed] [Google Scholar]
- 95.Marla SS, Lee J, Groves JT. Peroxynitrite rapidly permeates phospholipid bilayers. Proc Natl Acad Sci USA. 1997;94:14243–14248. doi: 10.1073/pnas.94.26.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Angard E. Nitric oxide: mediator, murderer and medicine. Lancet. 1994;343:1199–1206. doi: 10.1016/s0140-6736(94)92405-8. [DOI] [PubMed] [Google Scholar]
- 97.Thomsen LL, Lawton FG, Knowles RG, Beesley JE, Riveros-Moreno V, Moncada S. Nitric oxide synthase activity in human gynecological cancer. Cancer Res. 1994;54:1352–1354. [PubMed] [Google Scholar]
- 98.Thomsen LL, Miles DW, Happerfield L, Bobrow LG, Knowles RG, Moncada S. Nitric oxide synthase activity in human breast cancer. Br J Cancer. 1995;72:41–44. doi: 10.1038/bjc.1995.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Iwata S, Nakagawa K, Hironobou H, Oka Y, Kumon S, Sakaki S. Endothelial nitric oxide synthase expression in tumor vasculature is correlated with malignancy in human supratentorial astrocytic tumors. Neurosurgery. 1999;45:1–4. doi: 10.1097/00006123-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 100.Oshima H, Bartch H. Chronic infection and inflammatory process as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res. 1994;305:253–264. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 101.Wiseman H, Halliwell B. Damage to DNA by the reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. J Biochem. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wink DA, Hanbaurer I, Grisham MB, Laval F, Nims RW, Laval J, Cook JC, Pacelli R, Liegmann J, Krishna MC, Ford MC, Mitchell JB. The chemical biology of NO: insights into regulation, protective and toxic mechanisms of nitric oxide. Curr Top Cell Regul. 1996;34:159–187. doi: 10.1016/s0070-2137(96)80006-9. [DOI] [PubMed] [Google Scholar]
- 103.Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60. doi: 10.1016/s0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- 104.Lintas CL, Clark A, Fox J, Tannenbaum SR, Newberne PM. In vivo stability of nitrite and nitrosamine formation in the dog stomach: effect of nitrite and amine concentration and of ascorbic acid. Carcinogenesis. 1982;3:161–165. doi: 10.1093/carcin/3.2.161. [DOI] [PubMed] [Google Scholar]
- 105.Maeda H, Noguchi Y, Sato K, Akaike T. Enhanced vasculature permeability in solid tumors is mediated by nitric oxide and inhibited by both nitric oxide scavenger and nitric oxide synthase inhibitor. Jpn J Cancer Res. 1994;85:331–334. doi: 10.1111/j.1349-7006.1994.tb02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Noguchi Y, Fujii S, Beppu T, Ogawa M, Maeda H. Excessive production of nitric oxide in rat solid tumours and its implications in rapid tumour growth. Cancer. 1996;77:1598–1604. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1598::AID-CNCR27>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 107.Estevez AG, Radi R, Barbeito L, Shin JT, Thompson TA, Beckman JS. Peroxynitrite induced cytotoxicity in PC12 cells: evidence for an apoptic mechanism differentially modulated by neurotrophic factors. J Neurochem. 1995;65:1543–1550. doi: 10.1046/j.1471-4159.1995.65041543.x. [DOI] [PubMed] [Google Scholar]
- 108.Lin KY, Xue JT, Nomen M, Spur B, Wong PYK. Peroxynitrite induced apoptosis in HL 60 cells. J Biol Chem. 1995;270:16487–16490. doi: 10.1074/jbc.270.28.16487. [DOI] [PubMed] [Google Scholar]
- 109.Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced respectively by mild and intense insults with N-methyl-d-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci USA. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Michael PM. Nitric oxide and cell death. Biochim Biophys Acta. 1999;1411:401–414. doi: 10.1016/s0005-2728(99)00029-8. [DOI] [PubMed] [Google Scholar]
- 111.Lepoivre M, Flaman JM, Bobe P, Lemaire G, Henry Y. Quenching of the tyrosyl free radical of ribonucleotide reductase by nitric oxide. Relationship to cytostasis induced in tumour cells by cytotoxic macrophages. J Biol Chem. 1994;269:21891–21897. [PubMed] [Google Scholar]
- 112.Lautier D, Logueux J, Thibodeau J, Menard L, Poirier GG. Molecular and biochemical features of poly(ADP) ribose metabolism. Mol Cell Biochem. 1993;122:171–193. doi: 10.1007/BF01076101. [DOI] [PubMed] [Google Scholar]
- 113.Zhang J, Dawson VL, Dawson TM, Snyder SH. Nitric oxide activation of poly(ADP-ribose) synthase in neurotoxicity. Science. 1994;263:687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- 114.Szabo C, Zingreli B, Connor MO, Salzman AL. DNA strand breakage, activation of poly(ADP-ribose) synthase, and cellular energy depletion are involved in cytotoxicity in macrophages and smooth muscle cells exposed to peroxynitrite. Proc Natl Acad Sci USA. 1996;93:1753–1758. doi: 10.1073/pnas.93.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wink DA, Nims RW, Darbyshire JF, Christodoulou D, Hanbauer I, Cox GW, Laval F, Laval J, Cook JA, Krishna MC, Graff W, Mitchell JB. Reaction kinetics for nitrosation of cysteine and glutathione in aerobic nitric oxide solutions at neutral pH. Insights into the fate and physiological effects of intermediates generated in NO/O2 reaction. Chem Res Toxicol. 1994;7:519–525. doi: 10.1021/tx00040a007. [DOI] [PubMed] [Google Scholar]
- 116.Ling-Ling C, Nakamura T, Nakatsu Y, Sakumi K, Hayakawa H. Specific amino acid sequences required for O6 methyl guanine-DNA methyl transferase activity: analysis of these residues at or near the methyl residues site. Carcinogenesis. 1992;83:837–843. doi: 10.1093/carcin/13.5.837. [DOI] [PubMed] [Google Scholar]
- 117.Zak P, Kliebl K, Laval F. Repair of O6 methyl guanine and O4 methyl thymine by human and rat O6 methyl guanine-DNA-methyl transferase. J Biochem. 1994;269:730–733. [Google Scholar]
- 118.Wink DA, Laval J. The Fpg protein, a DNA repair enzyme is inhibited by the biomediator nitric oxide, in vitro and in vivo. Carcinogenesis. 1994;15:2125–2129. doi: 10.1093/carcin/15.10.2125. [DOI] [PubMed] [Google Scholar]
- 119.Wolosker H, Panizzutti R, Engeklender S. Inhibition of creatine kinase by S-nitrosoglutathione. FEBS Lett. 1996;392:274–276. doi: 10.1016/0014-5793(96)00829-0. [DOI] [PubMed] [Google Scholar]
- 120.Schweizer M, Richter C. Nitric oxide potently and reversibly deenergizes mitochondria at low oxygen tensions. Biochem Biophys Res Commun. 1994;204:169–175. doi: 10.1006/bbrc.1994.2441. [DOI] [PubMed] [Google Scholar]
- 121.Konorev EA, Kalyanaraman B. Rapid and irreversible inhibition of creatine kinase by peroxynitrile. FEBS Lett. 1998;427:171–174. doi: 10.1016/s0014-5793(98)00413-x. [DOI] [PubMed] [Google Scholar]
- 122.Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 123.Poderoso JJ, Carreras MC, Lisdero C, Riobo N, Schopfer F, Boveris A. Nitric oxide inhibits electron transfer and increase superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys. 1996;328:85–92. doi: 10.1006/abbi.1996.0146. [DOI] [PubMed] [Google Scholar]
- 124.MacMillan LA, Crog JP, Crow JD, Kerby JS, Beckman JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci USA. 1996;95:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sazabo C, Salzman AL. Endogenous peroxynitrite is involved in the inhibition of mitochondrial respiration in immuno-stimulated J774.2 macrophages. Biochem Biophys Res Commun. 1995;209:739–743. doi: 10.1006/bbrc.1995.1561. [DOI] [PubMed] [Google Scholar]
- 126.Hausladen A, Fridovich I. Superoxide and peroxynitrite inactivate aconitases, nitric oxide does not. J Biol Chem. 1994;269:29405–29408. [PubMed] [Google Scholar]
- 127.Brown GC. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett. 1995;369:136–139. doi: 10.1016/0014-5793(95)00763-y. [DOI] [PubMed] [Google Scholar]
- 128.Scarlet JL, Packer MA, Porteous CM, Murphy MP. Alteration to glutathione and nicotinamide nucleotides during the mitochondrial permeability transition induced by peroxynitrite. Biochem Pharmacol. 1996;52:1047–1055. doi: 10.1016/0006-2952(96)99426-5. [DOI] [PubMed] [Google Scholar]
- 129.Schweizer M, Richter C. Peroxynitrite stimulates the pyridine nucleotide linked Ca release from intact rat liver mitochondria. Biochemistry. 1996;35:4524–4528. doi: 10.1021/bi952708+. [DOI] [PubMed] [Google Scholar]
- 130.Nicotera P, Orrenius S. Ca2+ and cell death. Ann NY Acad Sci. 1992;648:17–27. doi: 10.1111/j.1749-6632.1992.tb24520.x. [DOI] [PubMed] [Google Scholar]
- 131.Gunter TE, Gunter KK, Sheu SS, Gavin CE. Mitochondrial Ca transport: physiology and pathological relevance. Am J Physiol. 1994;267:C313–C339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- 132.Richter C, Godvadge V, Schlapbach R, Schhweizer M, Schlegel J. Nitric oxide kills hepatocytes by mobilizing mitochondrial calcium. Biochem Biophys Res Commun. 1994;205:1143–1150. doi: 10.1006/bbrc.1994.2785. [DOI] [PubMed] [Google Scholar]
- 133.Zoratti M, Sazabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 134.Cassina A, Radi R. Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys. 1996;328:309–316. doi: 10.1006/abbi.1996.0178. [DOI] [PubMed] [Google Scholar]
- 135.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 136.Vander VA, Smith D, O’Neill CA, Kaur H, Usmar VD, Halliwell CEB. Interactions of peroxynitrite with human plasma and its constituents: oxidative damage and antioxidant depletion. Biochem J. 1994;303:295–301. doi: 10.1042/bj3030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Burney S, Tamir S, Gal A, Tannenbaum SR. A mechanistic analysis of nitric oxide induced cellular toxicity. Nitric Oxide Biol Chem. 1997;1:130–144. doi: 10.1006/niox.1996.0114. [DOI] [PubMed] [Google Scholar]
- 138.Radi R, Rodriguez M, Castro L, Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys. 1994;308:89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 139.Packer MA, Murphy MP. Peroxynitrite causes Ca efflux from mitochondria which is prevented by cyclosporin A. FEBS Lett. 1994;345:237–240. doi: 10.1016/0014-5793(94)00461-7. [DOI] [PubMed] [Google Scholar]
- 140.Richter C, Goavadze V, Laffranchi R, Schlapbach R, Schweizer M, Suter M, Walter P, Yaffee M. Oxidants in mitochondria: from physiology to diseases. Biochim Biophys Acta. 1995;1271:67–74. doi: 10.1016/0925-4439(95)00012-s. [DOI] [PubMed] [Google Scholar]
- 141.Gordge MP. How cytotoxic is nitric oxide. Exp Nephrol. 1998;6:12–16. doi: 10.1159/000020499. [DOI] [PubMed] [Google Scholar]
- 142.Rodes D, Klug A. Zinc fingers. Sci Am. 1993;268:55–56. doi: 10.1038/scientificamerican0293-56. [DOI] [PubMed] [Google Scholar]
- 143.Marcello NM, John LW. Nitric oxide. V. Therapeutic potential of nitric oxide donors and inhibitors. Am J Physiol. 1999;276:G1313–G1316. doi: 10.1152/ajpgi.1999.276.6.G1313. [DOI] [PubMed] [Google Scholar]
- 144.Nathan C, Xie Q. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994;269:13725–13728. [PubMed] [Google Scholar]
- 145.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide physiology pathophysiology and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 146.Bredt DS, Snyder SH. Nitric oxide a novel neuronal messenger. Neuron. 1992;8:3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- 147.Sikora AG, Gelbard A, Davies MA, Sano D, Ekmekcioglu S, Kwon J, Hailemichael Y, Jayaraman P, Myers JN, Grimm EA, Overwijk WW. Targeted inhibition of inducible nitric oxide synthase inhibits growth of human melanoma in vivo and synergizes with chemotherapy. Clin Cancer Res. 2010;16:1834–1844. doi: 10.1158/1078-0432.CCR-09-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hirst DG, Robson T. Nitric oxide physiology and pathology. Methods Mol Biol. 2011;704:1–13. doi: 10.1007/978-1-61737-964-2_1. [DOI] [PubMed] [Google Scholar]