Abstract
About 90% of esophageal cancers worldwide are Squamous Cell Carcinomas (SCC), mostly occurring in defined high-incidence areas of low and middle-resource countries. Historically, the highest incidences are reported in regions of Central Asia. One such region is Kashmir Valley in Northern India. In this review, we summarize a large body of epidemiological, toxicological and observational information on occurrence, dietary patterns and lifestyles to discuss factors that may be involved in the etiology of SCC in Kashmir Valley. To date, no single factor can be identified as the main cause of the excess incidence of SCC as compared to other regions of India. Three main components emerge as important factors: a societal component with poor, rural lifestyle and general deprivation, status in particular in vitamins and oligoelements; a lifestyle component with the use of copper utensil in cooking, the consumption of spicy, deep fried foodstuffs, and the drinking of hot salty tea; and an environmental component with exposure to high levels of dietary nitrosamines from diverse sources. Overall, these three components are similar to the general pattern of factors that have been involved in causing SCC in other high-incidence area in the so-called “esophageal cancer belt”, namely in central China (Cixian, Lixian) and in Northern Iran (Golestan). Further comparative studies between these regions are needed to identify the contributions of these various components.
Introduction
Esophageal cancer is the eighth most common cancer and sixth leading cause of cancer deaths in the world, with the majority of cases occurring in developing countries (1). Most of the tumours of esophagus are malignant and the diagnostic symptom, dysphagia, occurs very late. The overwhelming majority of esophageal malignancies are classified as either squamous cell carcinoma (SCC) or adenocarcinoma (ADC). Worldwide, 90% of esophageal cancers are SCC and about 5% are ADC. The remaining 5% represent rare malignancies and metastases from other organs (2;3). The aetiology of esophageal cancer is diverse and complex. There is no clear pattern of familial aggregation for esophageal cancers and the main cause appears to be combinations of environmental, dietary and lifestyle factors.
Adenocarcinoma usually develops in the lower third of the esophagus from a metaplastic lesion, Barrett’s esophagus, in which squamous epithelium of esophagus is replaced by glandular columnar epithelium (4). Barrett’s esophagus is a common condition but prospective evaluation has suggested that the incidence of adenocarcinoma in patients with Barrett’s esophagus is 40 to 125 times that expected in the general population (5;6). There is evidence that the incidence of ADC is currently increasing in several western countries, including the USA, UK, Norway and Denmark. In some instances, ADC also develops either from the very small, junctional mucosa at the eso-gastric junction, or from microscopic segments of Barrett’s esophagus. These ADC are prone to misclassification with ADC of the gastric cardia.
SCC develops according to a classical hyperplasia -dysplasia- carcinoma sequence. It occurs mostly in the upper 2/ 3 of the esophagus. There are remarkable geographic variations in SCC incidence worldwide (4). The incidence of esophageal cancer ranges from 10 to 50 /10(5)/year in Sri Lanka, India, South Africa, France and Switzerland. The disease is less common (average incidence less than 10/10(5)/year) in most areas within Japan, Europe and Canada, (7). Regions with high incidence are generally located in poor parts of the world (Table 1). High incidence areas have been identified within the Caspian littoral region of northern Iran (8), Central Asia and northern China where 60% of world’s cases occur annually and where the high incidence is clustered in sharply demarcated geographical areas. In Central Asia there is an esophageal ‘cancer belt’ extending from northern Sin-Kiang (China) through the Republics of Kazakhstan, Uzbekistan and Turkmenistan and including northern Afghanistan to northern area (Caspian littoral) of Iran. Incidences above 100/10(5)/year have been consistently reported in both males and females in some of the populations of this area (8). Kashmir Valley lies at the southern edge of this high incidence region.
Table (1).
Incidence of esophageal cancer in various regions of the world.
| Continent | Region | Area | Incidence (105/year) | |
|---|---|---|---|---|
| Men | Women | |||
| Asia | China | Linxian | 132 | 120 |
| Yang cheng | 130 | 84 | ||
| Tianjin | 16 | 8 | ||
| India | Kashmir | 43 | 27 | |
| Mumbai | 11 | 9 | ||
| Bangalore | 6 | 5 | ||
| Iran | Gonbad | 165 | 195 | |
| Ardebil | 45 | 24 | ||
| Gilan | 20 | 7 | ||
| Central Asia | Turkmenistan | 51 | 33 | |
| Kazakhstan | 47 | 26 | ||
| Uzbekistan | 28 | 13 | ||
| Europe | Sweden | 3. | 1 | |
| Poland | 5 | 1 | ||
| France | Calvados | 26 | - | |
| United Kingdom | 7 | 3 | ||
| South America | Uraguay | 40 | - | |
| Brazil | Porto Alegre | 26 | 7 | |
| North America | United states | Los Angeles | 16 | 4 |
| Washington, DC, black | 17 | 5 | ||
| Washington, DC, white | 4 | 1 | ||
| Africa | Transkei | 37 | 21 | |
Descriptive epidemiology of esophageal cancer in Kashmir
The Jammu and Kashmir state of India, situated in the extreme north of India, has three distinct geographical regions: Kashmir, Jammu and Ladakh. Kashmir Valley lies to the north of Jammu region and the south of Ladakh region at the altitude of 1800–4000 m above sea level. It is famous for its temperate climate with a severe winter and a moderate summer. The inhabitants of the three parts have different socio-religious backgrounds, life-styles and dietary habits, the features which could be helpful in aetiological/epidemiological studies of malignancies.
The region has no cancer registry, the hospital statistical data are largely unclassified and any assessment is largely based on published and unpublished hospital data available from local medical professionals. The first study on the incidence of esophageal cancer in Kashmir is by Matto and Kaul, who reported that esophageal cancer accounted for 21.5% of the patients diagnosed histopathologically for various malignancies at Shri Hari Mehtab Sing (SHMS) Hospital, Srinagar during 1962–1970 (9). In another study, covering over 5 years, 480 diagnosed cases (aged between 21 and 76 years; male-female ratio 2: 1) of esophageal cancer were reported. It was noted that the majority of the patients were using red chillies and spices in their food and consumed about 15–20 cups of hot salted tea per day (10). At the same hospital, out of 8060 pathology specimens, 2340 malignant lesions were reported over a period of 3 years time (1982–1985) (11). Gastrointestinal malignancies constituted 68.7% of all tumours, with esophageal cancer comprising 46.1% (11). More recently, a report based on hospital registration has detected incidence rates of 43.6 for men and 27 for women (age-standardized incidences/10(5) individuals/ year)(12). In a period of three years (July, 1986 to June, 1989), 1515 cases of esophageal cancer (1050 men and 465 women) were registered (12). In a limited population-based study, Gulzar and Zargar reported an incidence of esophageal cancer in the valley of 52.85/10(5) individuals (unpublished). The prevalence of precancerous conditions such as chronic esophagitis and dysplasia, similar to other high incidence areas in world, is high in the normal population of the valley. The vast majority of histologically confirmed esophageal cancers are SCC (> 95%, unpublished). Over the past 4 decades, there has been an increase in the number of detected SCC cases according to hospital data. In another local hospital, Sher-i-Kashmir Institute of Medical Sciences, from 2001 to 2003, of the 2233 cancer patients registered, 78% were esophageal cancer cases (unpublished data). In addition, a high rate of gastric cancer has also been reported in Kashmir valley (12;13). The incidence of gastro-intestinal malignancy in general and esophageal in particular is very low in the other two regions, Jammu and Ladakh.
Risk factors
Tobacco and alcohol
Smoking or chewing of tobacco, as well drinking of alcoholic drinks, are common and synergistic risk factors for SCC in many parts of the world (14;15). In Kashmir, the situation appears different. The region is almost devoid of alcohol consumption and tobacco or betel nut chewing. However smoking in form of water pipe or cigarette is widespread in the general male population. Other variants of tobacco such as snuff (“Naass”- lime based tobacco powder) are frequently used by the locals especially the rural male population. Snuff has also been reported to be a common form of tobacco consumption in other high-incidence areas, as for example in northern Iran. However, studies so far have not provided enough data to determine the exact excess of ESCC risk in relation with snuff taking.
Dietary factors
Lifestyle in Kashmir valley is characterised by specific dietary habits. The ingredients used in food and beverages as well as the method of preparation are entirely different from those found in neighbouring areas or in rest of the country. One of the most peculiar habits that needs to be considered with respect to aetiology of SCC is the use of large amounts of salt and spices with deep frying in oil and then boiling for longer time in copper utensils. Here we will discuss the food practices into two groups according to daily/frequent or occasional/ seasonal consumption.
Most common or daily dietary practices
Salted tea (‘Noon Chai’): Salted tea is the most popular beverage and freely consumed, most commonly twice a day (morning and afternoon) by almost everyone, irrespective of gender or age. The method of preparation is typical and exclusively practised in the region. Green tea leaves are brewed in the presence of sodium bicarbonate until a thick red-brown coloured extract is obtained. The extract is then diluted with water according to personal preferences, and salt and milk are added. The tea, which has a pink colour on addition of milk and tastes strong and salty, is repeatedly boiled or kept hot in ‘Samawar’ (a local version of copper kettle operated on burning charcoal) before it is served. The per capita daily consumption ranges from 200 ml to 2500 ml. Most of the population like to take it at high temperature particularly during chilly winters.
Brassica olerecea (‘Hakh’):
A cruciferous leafy vegetable of the Brassica genus, locally known as ‘Hakh’ forms an important component of the staple diet of native population. It is available throughout the year as some of its variants are resistant to subzero temperatures.
Other components of staple diet:
There is a large consumption of boiled rice brought from other parts of India or grown locally. Each person irrespective of socioeconomical status eats rice at lunch and dinner. Locally grown, sun dried red chillies are frequently consumed either in the form of Wur or mixed with vegetables during the cooking. In addition lotus stem and yoghurt are also frequently consumed. The flowers of Celosia argentea, locally known as ‘Mawal’ and saffron are used as colourants in various food preparations
Other exposures associated with nutrition:
Increasing exposure of local population, particularly rural inhabitants and fruit growers, to diverse types of fungicides and insecticides indiscriminately used in apple orchards, is a source of concern. The animals and aquatic life are also exposed to these hazardous chemicals, which can lead to biological magnification. Although studies on detailed health effects are lacking, there is concern that such exposures might synergize with other factor and impact on the incidence of various malignancies.
Seasonal/ occasional food items
Owing to long severe winter, there is no agricultural activity for about half of the year. As a result there is short growing season. Thus locally grown fresh vegetables are available for only a few months and are preserved and stored for consumption during the rest of the year. Plant-based foods are either sun-dried or pickled, whilst fish is preserved by sun drying or by smoking on grass. Preserved foodstuffs may be stored for several years because of uncertainty of harvest and their availability prior to being consumed.
Dried and pickled vegetables (‘Hokh Seun and Anchar’): Locally grown fresh vegetables, which include aubergine, gourd, tomato, cabbage, cauliflower and turnip are sliced and preserved by sun drying in the open. Dried spinach leaves are also frequently consumed. Several vegetables are also pickled by addition of salt, mixed spices, food colourants from flowers and mustard oil prior to their curing in the sun. Dried and pickled vegetables are generally stored for periods extending up to several months.
Sun-dried and smoked fish:
Fish constitutes an important component of the Kashmiri diet. Besides the fresh fish, sun-dried (‘Hu Gaard’) and smoked fish (‘Phari’) are consumed quite commonly. The process of sun drying is simple and similar to that practiced in many other places. However the method of preparation of smoked fish appears to be unique to Kashmir. The fishes are not cleaned or gutted prior to smoking, which is carried out on slow burning green grass.
Mixed Spice cake:
A mixture of commonly used dried spices with food colourants, mashed onions, garlic and red chillies which is held together by addition of mustard oil and compressed into flat circular cakes, is dried and stored. The dried spice cake (‘Wur’) is a ready source of spices and is used as a base for most Kashmiri food dishes. There is concern that these foodstuffs may frequently be contaminated by mycotoxins. Awareness of this, as well as better availability of fresh vegetable from the rest of the country, has resulted in a decrease in the consumption of sun-dried foodstuffs.
Spiced green tea (‘Kehwa’):
The ‘Kehwa’ is prepared by boiling green tea leaves in water in the presence of cardamon, cinnamon and saffron and then sugar is added. ‘Kehwa’ is taken with out milk and has a mild taste with spicy flavour. Its consumption is moderate and occasional.
Trace elements copper and zinc
In several areas of high incidence of SCC, a general deprivation in vitamins A, E, C, in beta-Carotene and in trace elements such as zinc or selenium, has been reported (16). To date, the status of most of these elements has not been assessed in the Kashmiri population. One of the main sources of vitamins and trace elements is the consumption of fresh fruits. Although Kashmir is a fruit growing areas, fruit production is very seasonal and there is adequate intake of locally grown seasonal fruits for only a few months per year. In a recent study (17), we have observed significantly elevated levels of plasma copper in patients as compared to controls (p<0.0001) with a mean concentration of 169 μg/dl and 149 μg/dl for patients and controls, respectively. The average level of zinc in patients was significantly lower than in controls (p<0.0001) with a mean concentration of 86.8 ± 9.9 μg/dl and 96.1 ± 13.9 μg/dl in patients and controls respectively. This imbalance between pro- (copper) and anti- (zinc) oxidant trace elements creates a situation wherein cells may undergo excess DNA damage. The cause of this imbalance is unknown but may be linked with the widespread use of copper utensils by general population. Water and milk samples stored in copper vessels and salted tea prepared in copper vessels showed markedly high levels of copper, which may be responsible for the high plasma copper levels (18). Zinc deficiency may be due to low meat and high whole grains consumption (19;20). Our observations are in agreement with earlier studies on zinc deficiency in cancer patients in populations at high-risk for SCC (21–23). Low plasma levels of zinc have been reported in the Indian subcontinent (24).
In our study, (17) we also observed that patients with poorly differentiated tumours had higher plasma copper levels than patients with moderately or well differentiated tumour (p<0.0001). On the other hand, we found a positive correlation between zinc deficiency and presence of a TP53 mutation in the tumour (the median zinc level was 81.5 and 88 μg/dl for patients with and with out p53 mutations, respectively, p=0.03). Thus, alteration in plasma levels of trace elements may have an impact on the aggressiveness of cancer. Experimentally, zinc deficiency has been shown to enhance esophageal cell proliferation by disrupting cell cycle checkpoints (25) and/or by weakening apoptosis (26), facilitating the formation of esophageal SCC in rats exposed to nitrosamines. Accumulation of copper may also enhance tumour angiogenesis (27).
Potential Sources of Carcinogen Exposures
In high-incidence areas, the prevalence of non-cancerous esophageal lesions such as esophagitis is high in the general population [28]. While it is unclear whether mild or moderate forms of esophagitis carry an increased risk of SCC, this observation is compatible with the hypothesis that these populations are chronically exposed to pro-inflammatory agents that cause injury in the esophageal mucosa. Occurrence of a premalignant lesion and progression towards malignancy is believed to be further enhanced by factors such as coarse or abrasive food, hot beverages and irritant dietary components (29;30). In the following sections we will discuss possible exposures to a number of toxic and mutagenic chemicals present in the foodstuffs and lifestyle risk factors of Kashmiries as described above.
Salted tea
A potential source of irritants is the high consumption of hot tea prepared using sodium bicarbonate at the time of boiling tea leaves and containing added common salt (NaCl). NaCl is a well known epithelial irritant that has been considered as risk factor for gastric cancer (31). This salted tea exhibits a high methylating activity (equivalent to 3 p.p.m N-methylnitrosourea (NMNU)), upon in vitro nitrosation of caffeidine [1-methyl-4-(methylamino)-5-(N-ylcarbamoyl)imidazole] and caffeidine acid [N-[4-(5-carboxy-1-methylimidazolyl)]-N,N′-dimethylurea], the hydrolysis products of caffeine under alkaline conditions (32). Nitrosition of caffeidine in acidic conditions produces mononitrosocaffeidine (MNC) [1-methyl-4-(N-methyl-N-nitrosoamino)-5-(N-thylcarbamoyl) imidazole], an asymmetric nitrosamine and dinitrosocaffeidine (DNC) [1-methyl-4-(N-methyl-N-nitrosoamino)-5-(N-methyl-N-nitrosocarbamoyl) imidazole], a N-nitrosamide. Nitrosation of caffeidine acid under acidic conditions produces N,N′-dimethylparabanic acid (DMPA, N,N′-dimethylimidazolidinetrione) as a major product, with low amounts of mononitrosocaffeidine and N,N′-dimethyl-N-nitrosourea. In experimental animals, DNC is highly mutagenic both with and without metabolic activation, whereas MNC, like several other aromatic asymmetric nitrosamines, does not exhibit genotoxic or mutagenic properties (33;34). The range of tumour histologies detected in rats include neuroeptithelioma of the olfactory bulb and squamous cell carcinomas of the nasal cavity and of the forestomach (33). In view of the well-known structure-activity relationships of these N-nitroso compounds, their possible endogenous formation due to high consumption of salted tea may be a critical risk factor for the high occurrence of esophageal and gastric cancers in Kashmir.
‘Salted tea’ prepared according to local method also formed considerable amounts of N-nitrosoproline (NPRO) (360 micrograms/kg) and N-nitrosopipecolic acid (NPIC) (5870 micrograms/kg) along with 3 yet unidentified non-volatile N-nitroso compounds besides the presence of preformed N-nitrosodimethylamine (NDMA), on nitrosation of tea extracts under conditions simulating the fasting human stomach (35;36). Tannins isolated from salted tea have been found to give a positive result in ribosomal degranulation tests and extract showed genotoxicity to rat hepatocytes in alkaline elucidative assays (35).
‘Salted tea’ is usually consumed at high temperature and may cause thermal injury to the esophageal mucosa. This hyperthermic effect may contribute to the general inflammatory state of the epithelium, favouring the production of potentially damaging oxygen and nitrogen species and reasonably contribute to enhanced mutagenesis (37).
Sun-dried and smoked foodstuffs
Sun drying of spices and vegetables is a widely used strategy for preservation and storage of foodstuffs in Kashmir. Presence of trace levels of several volatile and non-volatile N-nitroso compounds has been reported in a variety of stored foodstuffs from Kashmir (35), including the presence of NDMA and N-nitrosopyrrolidine (NPYR) in dried and smoked fish, dried and pickled vegetables, red chilies and spice cake. NDMA was also detected in Mawal. Studies have shown that considerable human exposure to N-nitroso compounds may result from the consumption of staple dietary items in Kashmir (Table 2). The practice of drying raw foodstuffs in open sun or shade under humid conditions, and storage procedures that may be subject to bacterial or fungal growth, appear to be the main reason for the presence of preformed N-nitroso compounds in preserved foods.
Table (2).
Average daily consumption of various chemical through food items/ beverages.
| Chemical | Consumption (μg /day) | Source |
|---|---|---|
| Nitrate | 237 | Hakh |
| Methylamine | 1200 | Noon chai |
| Ethylamine | 14320 | Noon chai |
| Dimethylamine | 150 280 |
Noon chai Red chillies |
| Diethylamine | 400 | Noon chai |
| Pyrrolidine | 517 | Red chillies |
| Methylbenzylamine | 40 | Red chillies |
The presence of N-introso compounds was analysed in food stuffs frequently consumed in Kashmir, including dried, pickled and smoked foodstuffs. Nine out of 11 food items were found to contain low concentrations of NDMA, NPYR, N-nitrososarcosine (NSAR), NPRO and N-nitrosothaizolidine-4-carboxylic acid (NTCA). This survey showed a widespread contamination of raw foodstuffs from Kashmir by N-nitroso compounds (35). Analysis of levels of volatile N-nitrosamines revealed relatively high levels of N-NDMA (20 μg/kg), N-nitrosopiperidine (NPIP) and NPYR in smoked fish, sun-dried spinach (5.8 μg NDMA/kg; 23.8 μg NPIP /kg), sun-dried pumpkin (24.6 μg NPYR /kg) and dried, mixed vegetables (10 μg NDMA /kg) (Table 3) (38). Hakh (Brassica olerecea) was analysed for nitrosatable aliphatic amines, N-nitrosamines (prior to and after nitrosation) and alkylating activity due to N-nitrosamides following nitrosation.
Table (3).
Concentration of different chemicals present in local food stuffs under different conditions.
| Food/Item Beverage | Active Ingredients | Concentration (μg/kg) | Nature/Condition | |
|---|---|---|---|---|
| Smoked fish | NDMA | 20 | Uncooked | |
| NPIP | - | Uncooked | ||
| NPYP | - | Uncooked | ||
| NTCA | 3294 | Nitrosation under simulated gastric conditions with realistic nitrite concentration | ||
| Sun dried fish | NDMA | 20 | Nitrosation under simulated gastric conditions with realistic nitrite concentration | |
| Hakh | Fresh | NDMA | 11 | Cooked |
| Sun dried | NDMA | 69.9 | Nitrosation under simulated gastric conditions with realistic nitrite concentration | |
| NPYR | 21 | Cooked | ||
| NMNU | 1200 | Nitrosation under chemical condition | ||
| Sun dried mixed vegetables | NDMA | 10 | Uncooked | |
| NDMA | 35.6 | Nitrosation under simulated gastric conditions with realistic nitrite concentration | ||
| Spinach | NDMA | 5.8 | Uncooked | |
| NPIP | 23.8 | Uncooked | ||
| Pumpkin | NPYR | 24.6 | Uncooked | |
| Red chilies & wur | NPIC | 43115 | Nitrosation under simulated gastric conditions with realistic nitrite concentration | |
| Pickled vegetables | NDMA | 7.7 | Nitrosation under simulated gastric conditions with realistic nitrite concentration | |
| Noon chai | NPRO | 360 | Nitrosation of tea extract under conditions simulating the fasting human stomach | |
| NPIC | 5870 | Nitrosation of tea extract under conditions simulating the fasting human stomach | ||
| 3 non-volatile unidentified nitrosamines | - | Nitrosation of tea extract under conditions simulating the fasting human stomach | ||
| Methylating activity | 3ppm of NMNU | In vitro nitrosation | ||
| Kehwa | NDMA | 9.2 | Under simulated gastric conditions with realistic nitrite concentration | |
The cooked vegetable contained 11 μg /kg nitrosodimethylamine and 21 μg /kg nitrosopyrrolidine. Nitrosation under chemical conditions yielded 1200 μg /kg of NMNU (39).
Several foodstuffs from Kashmir were studied under simulated gastric conditions with a realistic nitrite concentration for the formation of N-nitroso compounds. NDMA, NPRO, NTCA and NPIC were the main products in different foodstuffs. Significant amounts of NDMA were formed from dried fish (20 μg /kg), dried and pickled vegetables (35.6 μg /kg and 7.3 μg /kg), Hakh leaves (69.9 micrograms/kg), and the traditional tea ‘Kehwa’ (9.2 μg /kg). The highest level of NTCA was formed in smoked fish (3294 μg /kg). High values of 4315 μg /kg NPIC were also obtained following nitrosation of red chilies and mixed spice cake (‘Wur’) under simulated gastric conditions (Table 3). These results suggest an appreciable endogenous formation of N-nitroso compounds from local foods in Kashmir (36).
Dietary amines, nitrite and nitrate
The population is also exposed to dietary amines, nitrite and nitrate, (40–42) (Table-3). In different age groups, the mean levels of salivary nitrate/nitrite ranged between 21–36 ppm and 12–17 ppm, respectively (Table 4) (41). Exposure estimates for the adult population show that high consumption of boiled Hakh leads to a high nitrate intake of 237 mg/day. The frequent consumption of hot salted tea results in exceptionally high exposure to methylamine (1200 μg/day), ethylamine (14,320 μg /day), dimethylamine (150 μg /day) and diethylamine (400 μg /day). The excess use of red chilies leads to exposure to dimethylamine (280 μg /day), pyrrolidine (517 μg /day) and methylbenzylamine (40 μg /day) (41) (Table 2). These studies provide evidence of a chronic exposure to methylbenzylamine of Kashmiri population at high risk of esophageal cancer (42).
Table (4).
Concentration of different chemicals in saliva and tobacco.
| Medium | Constituents | Concentrations |
|---|---|---|
| Saliva | Nitrate | 21–36 ppm |
| Nitrite | 12–17 ppm | |
| Different brands of Indians tobacco | NNN | 68–730 ng/c* (PF) 11–156ng/c (MSS) |
| NNK | 19–174 ng/c (PF) 7–173 ng/c (MSS) |
|
| NAB/NAT | 98–519 ng/c (PF) 12–146ng/c (MSS) |
Tobacco products
Tobacco is consumed either as snuff or in the form of “Hukka” or cigarette smoking in Kashmir. Heavy smokers smoke 20–30 cigarettes or up to 375 g tobacco through ‘Hukka’ smoking a day. Different brands of Indian cigarettes have been analysed, by gas chromatography-thermal energy analysis, for the presence of carcinogenic tobacco-specific N-nitrosamines (TSNA) in both tobacco and mainstream smoke (43). Preformed TSNA in cigarette tobacco ranged between 68 and 730 ng N-nitrosonornicotine (NNN)/cigarette, between 19 and 174 ng 4-(N-nitrosomethylamino)-1-(3-pyridyl)-1-butanone (NNK)/cigarette and between 98 and 519 ng N-nitrosoanabasine (NAB) together with N-nitrosoanatabine (NAT)/cigarette. The amounts of NNN, NNK and NAB/NAT in mainstream smoke were 11–156, 7–73 and 17–146 ng/cigarette, respectively (43) (Table 4).
Genetic alterations and cancer progression
Alterations in the genetic make-up or rearrangement of the cell genome can produce profound changes in cell functions, their differentiation and in the control of their proliferation and lifespan. Genetic changes common in esophageal cancer include mutations in TP53 (overall, in over 50% of advanced cancers), alterations in P16/CDKN2a (promoter methylation, deletions, mutations and/or loss of expression, in 40 to 75%), amplification of CyclinD1 (15–20%) or MYC (10%), HST (10%). In ADC, over expression of Cyclin E and of Cyclooxygenase-2 (Cox-2) is common and is often detectable in Barrett’s mucosa. In SCC, over expression of Cox-2 occurs at variable rates, depending upon the general, pro-inflammatory context in which the tumour occurs. Amplification of TP63, a homologue of TP53 involved in the regulation of squamous differentiation, is a specific alteration in SCC, not detected in ADC. Other, common genetic changes include loss of alleles (LOH) at 5q, 13q, as well as over expression of EGFR. Interestingly, mutation in RAS genes, a common occurrence in many cancers including in particular adenocarcinoma of the lung, breast, colon and pancreas, is extremely rare in esophageal cancers. In Barrett’s mucosa, genomic instability often precedes the appearance of histologic abnormalities (44;45).
Studies on TP53 mutations have shown that the distribution along the coding sequence and type of mutations vary in esophageal cancers, depending upon the geographic origin or the patient. These variations have been interpreted as the consequence of different mutagenic mechanisms, reflecting the heterogeneity of the factors involved in the aetiology of esophageal cancers. In Kashmir valley, mutations in the TP53 gene (exons 5 to 8, containing all common mutation hotspots) have been analysed by us in SCC of 55 patients from different districts of the valley (46). This study revealed the presence of mutations in 36.36% (20/55) tumours. The 20 mutations included 17 single-base substitutions (11 transitions + 6 transversions, of which 12 missense mutations, 2 non-sense mutations and 3 sequence variations in intron 6) and 3 deletions. Mutations were more common in women than in men (p = 0.016; OR = 4.13; 95% CI = 1.26–13.46). Overall, the mutation frequency in Kashmir was comparable with countries such as Thailand (41%) (47), Southern Brazil (34.8%) (37) and a previous report from India (39.0%) (48) but was much lower than in other well-documented areas of high risk for ESCC in Western Europe (Normandy 60–70%, Northern Italy 50–70%) (49;50), Iran (50–65%) (51;52) and China (40–77%) (53).
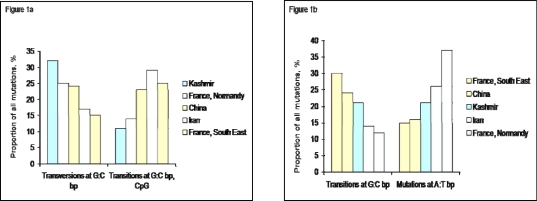
In Kashmir, 25% of the mutations (n=5) were transitions or transversion at A:T base pairs, a type of mutation very common in tumours from western Europe. This type of mutation is similar to the one experimentally induced by metabolites of ethanol such as acetaldehyde in vitro. In Europe, they have been interpreted as evidence for a role of alcohol as a mutagen in esophageal cancer. However, many other carcinogens may generate mutations at A:T base pairs and epidemiological observations do not support the notion that these mutations might be caused by alcohol in patients from Kashmir valley. Of the 12 other single nucleotide mutations, 6 were transversions at G bases (30%; 3 G:C>C:G transversions and 3 G:C>T:A transversions) and 6 were G:C to A:T transitions (30%), two of them occurring at CpG dinucleotide. Transversions at G:C base pairs are often caused by the formation of bulky DNA adducts on guanine. Polymerase bypass of the adduct results in misincorporation of the wrong nucleotide and mutation. This type of mutation is formed, for example, as a result of adduct formation by the mycotoxin aflatoxin (in liver cancers), or by metabolites or polycyclic aromatic hydrocarbons (in lung cancers of smokers). Of note, the 3 G:C to T:A transversions detected in Kashmiri patients were all found in male smokers. G:C to T:A transversions are also a common mutation induced by oxidative DNA damage leading to the formation of 8-oxodeoxiguanosine (8-oxodG). G:C to A:T transitions are, overall, the most common type of TP53 mutations in human cancer, irrespective of histology and site. Mutations at CpG dinucleotide occur spontaneously at a high rate, enhanced by exposure to reactive nitrogen species generated during inflammation. Mutations occurring at non-CpG sites may have very diverse molecular causes. For instance, they may result form the mutagenic action of nitrosamines. Presence of alkyl nitrosamine in food stuffs, leading to O6-alkyl guanine adducts and base mispairing during replication, resulting in G>A (or C>T on the other strand of the DNA) transition (54) has been considered to be a major risk factor in China (55). In a previous report from Delhi (India), a complete absence of mutations at CpG sites was reported. Interestingly, in Kashmir, there was a higher prevalence of G:C>A:T mutation in females (27.27%) than in males (11.11%).
Figure (1) compares the proportion of selected mutation types in tumors from Kashmir and from other parts of the world including areas of high (China, Iran, France-Normandy) and low (France-South East) incidence. This comparison illustrate the inverse relationship between the relative proportion of different types of mutations, which is consistent across different regions. Figure 1a shows the inverse relationship between mutations that may be due to bulky, environmental carcinogens (transversions at G:C base pairs) and G:C transitions at CpG sites, mainly due to endogenous mutation mechanisms. Kashmir tumors show the highest rate of G:C transversions and the lowest rate of CpG transitions, supporting the hypothesis of a high impact of exogenous carcinogens. Figure 1b compares two mutation types that have been proposed to be associated with smoking and with the consumption of alcoholic beverages. None of these mutations are particularly prevalent in Kashmir. In contrast, they strongly distinguish between the two sets of tumors from France. Overall, these results also concur with the notion that etiologic risk factors in Kashmir are different than in Western Europe, where tobacco and alcohol are considered as main causes.
Figure (1).
Comparison between main TP53 mutation types in Esophageal cancer from Kashmir and from selected other regions of the world.
1a: Proportion of transversions at G:C base pairs (often resulting from DNA damage by carcinogens forming bulky adducts or from the formation of 8-oxo-deoxyguanosine) and of transitions at C:C base pairs occurring in a CpG context (mostly resulting from an endogenous mutagenic process).
1b: Proportion of transitions at G:C base pairs at non-CpG sites (that can be induced by many types of carcinogens including alkylating agents) and of mutations (transitions and transversions) at A:T bp (often resulting from mutagenesis by metabolites of alcohol such as acetaldehyde) Data from IARC TP53 mutation database [60].
The same specimens as those screened for TP53 mutation were also analysed for BRAF, beta-catenin, EGFR or HER2 mutations, as well as for the presence of Human Papilloma viruses (HPV16, 18, 33). No mutation was found in BRAF, beta-catenin or HER2. However, 3 of 53 SCC were found to contain mutations in EGFR (1 in exon 18 and 2 in exon 19) (56), identical to those that have been shown to constitutively activate the receptor kinase in lung cancers of never-smokers. Regarding HPV, none of the lesions were found to contain any of the HPV types tested. Another study, published in 2005, has reported results on 33 tumours collected more than a decade ago (29 SCC and 4 ADC). Only 3 TP53 mutations (9%) were found, but 8/33 tumours (24%) were found to contain oncogenic HPV types (HPV 16: 6 cases, HPV 18: 2 cases) (57). Thus, studies conducted on the two series of specimens analysed so far have generated divergent results. It is unclear whether these differences are due to technical factors in assessing biomarkers or to differences in patient’s recruitment or sampling. In any case, further studies are required to provide a more detailed picture of the pattern of genetic alterations in esophageal cancers in Kashmi.
With the exception of a very rare familial syndrome, Tylosis, there is no evidence of strong familial aggregation of esophageal cancers. Familial clusters have been reported in high-incidence areas in China, but it is unclear whether these clusters result from common, widespread exposure rather than from the Mendelian transmission of predisposing gene(s). On the other hand, a number of genetic polymorphisms in genes of the xenobiotics metabolism, detoxification, ethanol metabolism and DNA repair, have been suggested to carry a low, increased risk of esophageal SCC. Although none of these polymorphic genes would result into a familial inheritance pattern, their combination in an individual may result into high susceptibility in the context of particular exposure patterns. This aspect has not been studied so far in patients of the Kashmir valley.
Perspectives: Primary, Secondary Prevention, Therapy
The results and observations summarized here support the hypothesis that the high incidence of esophageal cancer in Kashmir valley is the consequence of a multi-factorial process. To date, no single factor can be clearly identified as the main cause of the excess incidence of esophageal cancer as compared to other regions of India. As in most other regions of central Asia with a high incidence of SCC, three main component emerge as important factors: a societal component with general, deprivation status in particular with respect to vitamin and other nutrients, a lifestyle component with specific practices such the use of copper utensil as well as the consumption of a typical, very hot tea, and an environmental component with potential exposure to high levels of dietary nitrosamines from diverse sources. In addition, one cannot exclude the possible contribution of genetic susceptibility factors, although there is so far no evidence for familial transmission of cancer risk. Overall, these three components are reminiscent of the general pattern of factors involved in other high-incidence area in the so-called “esophageal cancer belt”, namely in central China (Cixian, Lixian) and in Northern Iran (Golestan). Further comparative studies between different regions are needed to identify the respective contributions of these various components.
In theory, each of the components may be amenable to several forms of preventive intervention. However, in practice, such intervention is difficult to achieve. Surveys in different regions have shown that trends in SCC incidence show considerable variations with time in relation with global changes in lifestyles. For example, in Normandy, a region of Western France with traditionally high incidence of SCC attributed to the consumption of home made alcohols, a sharp decrease in incidence has been observed over the past 15 years. In Northern Iran, current figures suggest that the incidence of SCC may also have sharply decreased since the initial reports of very high incidence rates in the 1970’s. However, the lack of continuous cancer registration precludes analysis of these trends. In Central China, incidence rates remain elevated in both men and women despite considerable changes in lifestyle. In this area, several intervention trials based on supplementation of diet with selenium and oligoelements have shown some reduction in the overall risk of SCC.
Addressing the problem of esophageal cancer in Kashmir valley will require first proper monitoring of incidence rates and of time trends through cancer registration. This must be combined with improved histological or endoscopy-based diagnosis to better identify the exact proportion of SCC versus ADC of the gastric cardia.
Second, clinical surveys are needed to determine the prevalence of early esophageal lesions and to identify whether subjects tend to develop common, possible precursor lesions. In particular, the existence of inflammatory conditions, as well, as a better assessment of the contribution of inflammatory mechanisms to carcinogenesis, need better revaluations. Incorporating molecular markers such as mutations in TP53 and other genes, or changes in gene expression, will help to better understand the sequence of events leading to cancer in the Kashmiri population and will help to identify intermediate en-points for diagnosis or therapeutic intervention.
Third, it will be important to develop epidemiological studies aimed at better assessing the contribution of the factors discussed in this review. This may be achieved, in first instance, by developing a case-control study with careful collection of information on dietary and lifestyle patterns. Assessing the contribution of factors such as excess copper or low vitamins/nutrients will require more complex cohort studies with long-term follow-up of a large number of subjects. In the studies above, it will be needed to take molecular markers into consideration.
Finally, it is important to take advantage of the experience developed in other parts of the world in order to achieve better clinical management of esophageal cancer patients. For example, Chinese surgeons have acquired a high level of expertise in resecting and post-operative management of SCC in high-risk population, with significant and spectacular increase in patient survival. The availability of novel, endoscopy-guided techniques for local treatment may also help to reduce cancer burden. In India, a recent study on early oral lesions has demonstrated that early detection of such lesions by simple visual detection, followed by local treatment, has a significant impact on oral cancer mortality (58;59). The development of such approach, adapted to esophageal SCC, may have an immediate impact in the population of the Kashmir Valley. In the long-term, however, decreasing the incidence of esophageal SCC will require a sustained improvement in the general socio-economical and health status of the whole region.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics. 2002, CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Stoner GD, Rustigi AK. Biology of esophageal squamous cell carinoma., in Gastrointestinal cancers: biology, diagnosis and therapy. In: Rustgi AK, editor. Lippincott-Raven Publishers; Philadelphia: 1995. pp. 141–148. [Google Scholar]
- 3.Vizcaino AP, Moreno V, Lambert R, Parkin DM. Time trends incidence of both major histologic types of esophageal carcinomas in selected countries. 1973–1995. Int J Cancer. 2002;99:860–868. doi: 10.1002/ijc.10427. [DOI] [PubMed] [Google Scholar]
- 4.Fleischer DE, Haddad NG. Neoplasms of the esophagus. In: Castel DO, editor. The Esophagus. Lippincott Williams and Wilkins; Philadelphia: 1999. p. 441. [Google Scholar]
- 5.Cameron AJ, Ott BJ, Payne WS. The incidence of adenocarcinoma in columnar-lined (Barrett's) esophagus. N Engl J Med. 1985;313:857–859. doi: 10.1056/NEJM198510033131404. [DOI] [PubMed] [Google Scholar]
- 6.Hameeteman W, Tytgat GN, Houthoff HJ, van den Tweel JG. Barrett's esophagus : development of dysplasia and adenocarcinoma. Gastroenterology. 1989;96:1249–1256. doi: 10.1016/s0016-5085(89)80011-3. [DOI] [PubMed] [Google Scholar]
- 7.Blot WJ, McLaughlin JK. The changing epidemiology of esophageal cancer. Semin Oncol. 1999;26:2–8. [PubMed] [Google Scholar]
- 8.Munoz N, Day NE. Esophageal cancer, in : Cancer Epidemiology and Prevention. In: Schottenfeld D, Fraumeni JF, editors. Oxford University Press; New York: 1996. pp. 681–706. [Google Scholar]
- 9.Mattoo AR, Kaul HK. Incidence of malignant neoplasms in Kashmir. J Indian Med Assoc. 1974;62:309–311. [PubMed] [Google Scholar]
- 10.Maqbool AR, Ahad A. Carcinoma of esophagus in Kashmir. Indian J Otolaryngol. 1976;28:118. [Google Scholar]
- 11.Jan GM, Zargar SA. Role of brush cytology in GI and biliary tract Lesions. Indian J Cancer. 1988;25:22–27. [PubMed] [Google Scholar]
- 12.Khuroo MS, Zargar SA, Mahajan AR, Banday MA. High incidence of oesophageal and gastric cancer in Kashmir in a population with special personal and dietary habits. Gut. 1992;33:11–15. doi: 10.1136/gut.33.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durrani HA. The presentation of gastric cancer in Kashmir natives: a study of 850 cases. Am J Gastroentrol. 1982;77:700. [Google Scholar]
- 14.Yang CS. Research on esophageal cancer in China: a review. Cancer Res. 1980;40:2633–2644. [PubMed] [Google Scholar]
- 15.Van Rensburg SJ. Epidemiologic and dietary evidence for a specific nutritional predisposition to esophageal cancer. J Natl Cancer Inst. 1981;67:243–251. [PubMed] [Google Scholar]
- 16.Lu H, Cai L, Mu LN, Lu QY, Zhao J, Cui Y, Sul JH, Zhou XF, Ding BG, Elashoff RM, et al. Dietary mineral and trace element intake and squamous cell carcinoma of the esophagus in a Chinese population. Nutr Cancer. 2006;55:63–70. doi: 10.1207/s15327914nc5501_8. [DOI] [PubMed] [Google Scholar]
- 17.Dar NA, Mir MM, Slam I, Malik MA, Gulzar GM, Yatoo GN, Ahmad A, Shah A. Association between copper excess, zinc deficiency and TP53 mutations in esophageal squamous cell carcinoma from Kashmir valley, India -a high risk area. Nutrition and Cancer. 2008;60:585–591. doi: 10.1080/01635580802290231. [DOI] [PubMed] [Google Scholar]
- 18.Narang AP, Verma A, Kumar GR, Sanyal B. Serum copper levels in gastrointestinal tract (GIT) cancer. J Trace Elem Electrolytes Health Dis. 1989;3:147–150. [PubMed] [Google Scholar]
- 19.Gibson RS. Content and bioavailability of trace elements in vegetarian diets. Am J Clin Nutr. 1994;59:1223S–1232S. doi: 10.1093/ajcn/59.5.1223S. [DOI] [PubMed] [Google Scholar]
- 20.Lonnerdal B. Dietary factors influencing zinc absorption. J Nutr. 2000;130:1378S–1383S. doi: 10.1093/jn/130.5.1378S. [DOI] [PubMed] [Google Scholar]
- 21.Zowczak M, Iskra M, Torliski L, Cofta S. Analysis of serum copper and zinc concentrations in cancer patients. Biological Trace Element Research. 2001;82:1–8. doi: 10.1385/BTER:82:1-3:001. [DOI] [PubMed] [Google Scholar]
- 22.Azin F, Raie RM, Mahmoudi MM. Correlation between the levels of certain carcinogenic and anticarcinogenic trace elements and esophageal cancer in northern Iran. Ecotoxicol Environ Saf. 1998;39:179–184. doi: 10.1006/eesa.1997.1601. [DOI] [PubMed] [Google Scholar]
- 23.Abnet CC, Lai B, Qiao YL, Vogt S, Luo XM, Taylor PR, Dong ZW, Mark SD, Dawsey SM. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst. 2005;97:301–306. doi: 10.1093/jnci/dji042. [DOI] [PubMed] [Google Scholar]
- 24.Abdulla M, Suck C. Blood levels of copper, iron, zinc, and lead in adults in India and Pakistan and the effect of oral zinc supplementation for six weeks. Biol Trace Elem Res. 1998;6:323–331. doi: 10.1007/BF02789092. [DOI] [PubMed] [Google Scholar]
- 25.Fong LY, Mancini R, Nakagawa H, Rustgi AK, Huebner K. Combined cyclin D1 overexpression and zinc deficiency disrupts cell cycle and accelerates mouse fore-stomach carcinogenesis. Cancer Res. 2003;63:4244–4252. [PubMed] [Google Scholar]
- 26.Fong LY, Nguyen VT, Farber JL. Esophageal cancer prevention in zinc-deficient rats: rapid induction of apoptosis by replenishing zinc. J Natl Cancer Inst. 2001;93:1525–1533. doi: 10.1093/jnci/93.20.1525. [DOI] [PubMed] [Google Scholar]
- 27.Nasulewicz A, Mazur A, Opolski A. Role of copper in tumour angiogenesis-clinical implications. J Trace Elem Med Biol. 2004;18:1–8. doi: 10.1016/j.jtemb.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Crespi M, Munoz N, Grassi A, Qiong S, Jing WK, Jien LJ. Precursor lesions of oesophageal cancer in a low-risk population in China : comparison with high-risk populations. Int J Cancer. 1984;34:599–602. doi: 10.1002/ijc.2910340503. [DOI] [PubMed] [Google Scholar]
- 29.O'Neill CH, Hodges GM, Riddle PN, Jordan PW, Newman RH, Flood RJ, Toulson EC. A fine fibrous silica contaminant of flour in the high oesophageal cancer area of north-east Iran. Int J Cancer. 1980;26:617–628. doi: 10.1002/ijc.2910260514. [DOI] [PubMed] [Google Scholar]
- 30.Ghadirian P. Thermal irritation and esophageal cancer in northern Iran. Cancer. 1987;60:1909–1914. doi: 10.1002/1097-0142(19871015)60:8<1909::aid-cncr2820600840>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 31.Correa P. Modulation of gastric carcinogenesis: updated model based on itragastric nitrosation. In: Bartch HR, editor. The relevance of nitrosocompounds to human cancer : Exposure and Mechanism. IARC; Lyon: 1987. p. 485. [PubMed] [Google Scholar]
- 32.Kumar R, Mende P, Wacker CD, Spiegelhalder B, Preussmann R, Siddiqi M. Caffeine-derived N-nitroso compounds--I: Nitrosatable precursors from caffeine and their potential relevance in the etiology of oesophageal and gastric cancers in Kashmir, India. Carcinogenesis. 1992;13:2179–2182. doi: 10.1093/carcin/13.11.2179. [DOI] [PubMed] [Google Scholar]
- 33.Ivankovic S, Seibel J, Komitowski D, Spiegelhalder B, Preussmann R, Siddiqi M. Caffeine-derived N-nitroso compounds. V. Carcinogenicity of mononitrosocaffeidine and dinitrosocaffeidine in bd-ix rats. Carcinogenesis. 1998;19:933–937. doi: 10.1093/carcin/19.5.933. [DOI] [PubMed] [Google Scholar]
- 34.Razdan R, Frei E, Spiegelhalder B, Siddiqi M. Caffeine-derived N-nitroso compounds. IV: Kinetics of mononitrosocaffeidine demethylation by rat liver microsomes. Cancer Lett. 1994;79:117–122. doi: 10.1016/0304-3835(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 35.Siddiqi M, Tricker AR, Preussmann R. The occurrence of preformed N-nitroso compounds in food samples from a high risk area of esophageal cancer in Kashmir, India. Cancer Lett. 1988;39:37–43. doi: 10.1016/0304-3835(88)90038-9. [DOI] [PubMed] [Google Scholar]
- 36.Siddiqi M, Tricker AR, Preussmann R. Formation of N-nitroso compounds under simulated gastric conditions from Kashmir foodstuffs. Cancer Lett. 1988;39:259–265. doi: 10.1016/0304-3835(88)90068-7. [DOI] [PubMed] [Google Scholar]
- 37.Putz A, Hartmann AA, Fontes PP, Alexandre CO, Silveira DA, Klug SJ, Rabes HM. TP53 mutation pattern of esophageal squamous cell carcinomas in a high risk area (Southern Brazil): role of life style factors. Int J Cancer. 2002;98:99–105. doi: 10.1002/ijc.10128. [DOI] [PubMed] [Google Scholar]
- 38.Siddiqi MA, Tricker AR, Kumar R, Fazili Z, Preussmann R. Dietary sources of N-nitrosamines in a high-risk area for oesophageal cancer--Kashmir, India. IARC Sci Publ; 1991. pp. 210–213. [PubMed] [Google Scholar]
- 39.Kumar R, Mende P, Tricker AR, Siddiqi M, Preussmann R. N-nitroso compounds and their precursors in Brassica oleracea. Cancer Lett. 1990;54:61–65. doi: 10.1016/0304-3835(90)90092-c. [DOI] [PubMed] [Google Scholar]
- 40.Kumar R, Siddiqi M, Fazili Z, Wacker CD, Spiegelhalder B, Preussmann R. Effect of dietary nitrate on endogenous nitrosation of piperazine in humans. Cancer Lett. 1992;65:139–143. doi: 10.1016/0304-3835(92)90158-r. [DOI] [PubMed] [Google Scholar]
- 41.Siddiqi M, Kumar R, Fazili Z, Spiegelhalder B, Preussmann R. Increased exposure to dietary amines and nitrate in a population at high risk of oesophageal and gastric cancer in Kashmir (India) Carcinogenesis. 1992;13:1331–1335. doi: 10.1093/carcin/13.8.1331. [DOI] [PubMed] [Google Scholar]
- 42.Siddiqi M, Kumar R, Kaul D, Spiegelhalder B, Preussmann R. Salivary nitrate and nitrite concentrations from a sample population of children and adults in high risk area for esophageal and gastric cancers in Kashmir, India. Cancer Lett. 1992;64:133–136. doi: 10.1016/0304-3835(92)90073-5. [DOI] [PubMed] [Google Scholar]
- 43.Kumar R, Siddiqi M, Tricker AR, Preussmann R. Tobacco-specific N-nitrosamines in tobacco and mainstream smoke of Indian cigarettes. Food Chem Toxicol. 1991;29:405–407. doi: 10.1016/0278-6915(91)90081-h. [DOI] [PubMed] [Google Scholar]
- 44.Blount PL, Galipeau PC, Sanchez CA, Neshat K, Levine DS, Yin J, Suzuki H, Abraham JM, Meltzer SJ, Reid BJ. 17p allelic losses in diploid cells of patients with Barrett's esophagus who develop aneuploidy. Cancer Res. 1994;54:2292–2295. [PubMed] [Google Scholar]
- 45.Barrett MT, Sanchez CA, Galipeau PC, Neshat K, Emond M, Reid BJ. Allelic loss of 9p21 and mutation of the CDKN2/p16 gene develop as early lesions during neoplastic progression in Barrett's esophagus. Oncogene. 1996;13:1867–1873. [PubMed] [Google Scholar]
- 46.Mir MM, Dar NA, Gochhait S, Zargar SA, Ahangar AG, Bamezai RN. p53 mutation profile of squamous cell carcinomas of the esophagus in Kashmir (India): a high-incidence area. Int J Cancer. 2005;116:62–68. doi: 10.1002/ijc.21002. [DOI] [PubMed] [Google Scholar]
- 47.Taniere P, Martel-Planche G, Puttawibul P, Casson A, Montesano R, Chanvitan A, Hainaut P. TP53 mutations and MDM2 gene amplification in squamous-cell carcinomas of the esophagus in south Thailand. Int J Cancer. 2000;88:223–227. doi: 10.1002/1097-0215(20001015)88:2<223::aid-ijc12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 48.Ralhan R, Arora S, Chattopadhyay TK, Shukla NK, Mathur M. Circulating p53 antibodies, p53 gene mutational profile and product accumulation in esophageal squamous-cell carcinoma in India. Int J Cancer. 2000;85:791–795. doi: 10.1002/(sici)1097-0215(20000315)85:6<791::aid-ijc9>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 49.Esteve A, Lehman T, Jiang W, Weinstein IB, Harris CC, Ruol A, Peracchia A, Montesano R, Hollstein M. Correlation of p53 mutations with epidermal growth factor receptor over expression and absence of mdm2 amplification in human esophageal carcinomas. Mol Carcinog. 1993;8:306–311. doi: 10.1002/mc.2940080414. [DOI] [PubMed] [Google Scholar]
- 50.Rugge M, Bovo D, Busatto G, Parenti AR, Fawzy S, Guido M, Ancona E, Ninfo V, Ruol A, Shiao YH. p53 alterations but no human papillomavirus infection in pre invasive and advanced squamous esophageal cancer in Italy. Cancer Epidemiol Biomarkers Prev. 1997;6:171–176. [PubMed] [Google Scholar]
- 51.Biramijamal F, Allameh A, Mirbod P, Groene HJ, Koomagi R, Hollstein M. Unusual profile and high prevalence of p53 mutations in esophageal squamous cell carcinomas from northern Iran. Cancer Res. 2001;61:3119–23. [PubMed] [Google Scholar]
- 52.Sepehr A, Taniere P, Martel-Planche G, Zia'ee AA, Rastgar-Jazii F, Yazdanbod M, Etemad-Moghadam G, Kamangar F, Saidi F, Hainaut P. Distinct pattern of TP53 mutations in squamous cell carcinoma of the esophagus in Iran. Oncogene. 2001;20:7368–7374. doi: 10.1038/sj.onc.1204912. [DOI] [PubMed] [Google Scholar]
- 53.Shi ST, Yang GY, Wang LD, Xue Z, Feng B, Ding W, Xing EP, Yang CS. Role of p53 gene mutations in human esophageal carcinogenesis: results from immunohistochemical and mutation analyses of carcinomas and nearby non-cancerous lesions. Carcinogenesis. 1999;20:591–597. doi: 10.1093/carcin/20.4.591. [DOI] [PubMed] [Google Scholar]
- 54.Lozano JC, Nakazawa H, Cros MP, Cabral R, Yamasaki H. G-->A mutations in p53 and Ha-ras genes in esophageal papillomas induced by N-nitrosomethylbenzylamine in two strains of rats. Mol Carcinog. 1994;9:33–39. doi: 10.1002/mc.2940090107. [DOI] [PubMed] [Google Scholar]
- 55.Gao H, Wang LD, Zhou Q, Hong JY, Huang TY, Yang CS. p53 tumor suppressor gene mutation in early esophageal precancerous lesions and carcinoma among high-risk populations in Henan, China. Cancer Res. 1994;54:4342–4346. [PubMed] [Google Scholar]
- 56.Dar NA, Mounawar M, Mir MM, Gulzar GM, Khan BA, Khan MA, Singh J, Martel G, Hainaut P.Epidermal Growth Factor Receptor Mutations in Esophageal Squamous cell carcinomas from Kashmir, a high incidence area Cancer Letterssubmitted.2009
- 57.Katiyar S, Hedau S, Jain N, Kar P, Khuroo MS, Mohanta J, Kumar S, Gopalkrishna V, Kumar N, Das BC. p53 gene mutation and human papillomavirus (HPV) infection in esophageal carcinoma from three different endemic geographic regions of India. Cancer Lett. 2005;218:69–79. doi: 10.1016/j.canlet.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 58.Sankaranarayanan R, Ramadas K, Thomas G, Muwonge R, Thara S, Mathew B, Rajan B. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet. 2005;365:1927–1933. doi: 10.1016/S0140-6736(05)66658-5. [DOI] [PubMed] [Google Scholar]
- 59.Sankaranarayanan R, Dinshaw K, Nene BM, Ramadas K, Esmy PO, Jayant K, Somanathan T, Shastri S. Cervical and oral cancer screening in India. J Med.Screen. 2006;13(Suppl 1):S35–38. [PubMed] [Google Scholar]