Abstract
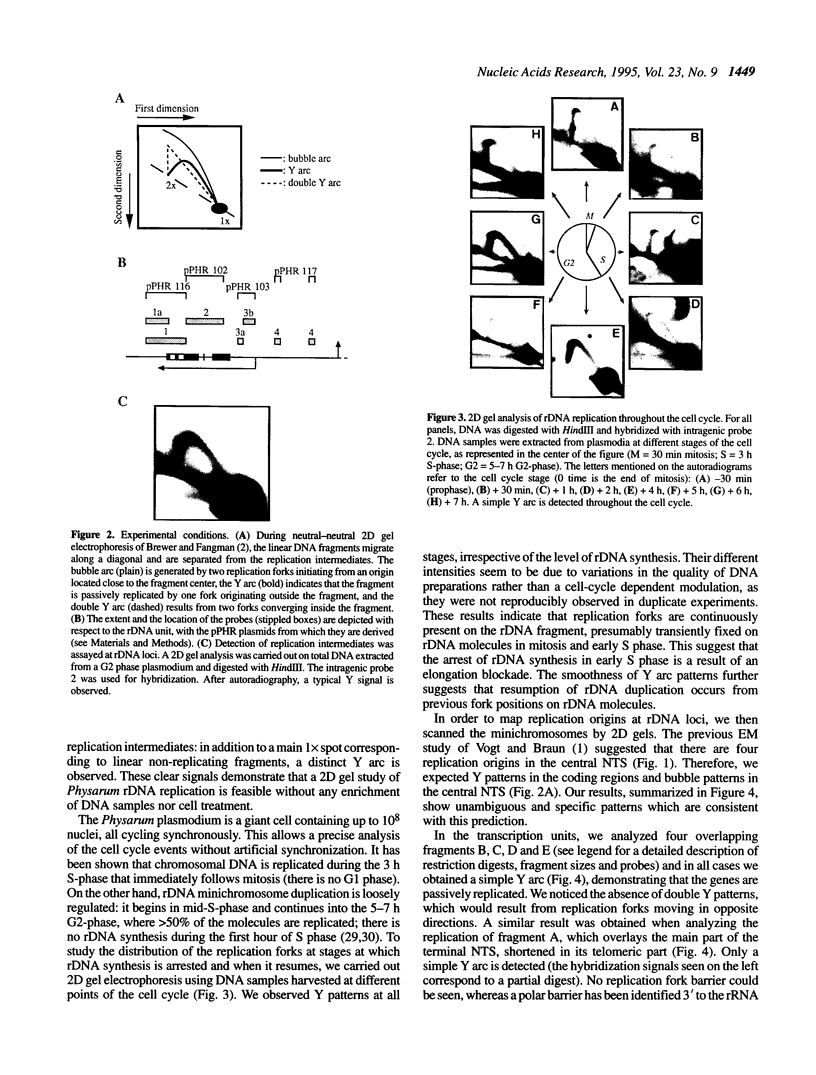
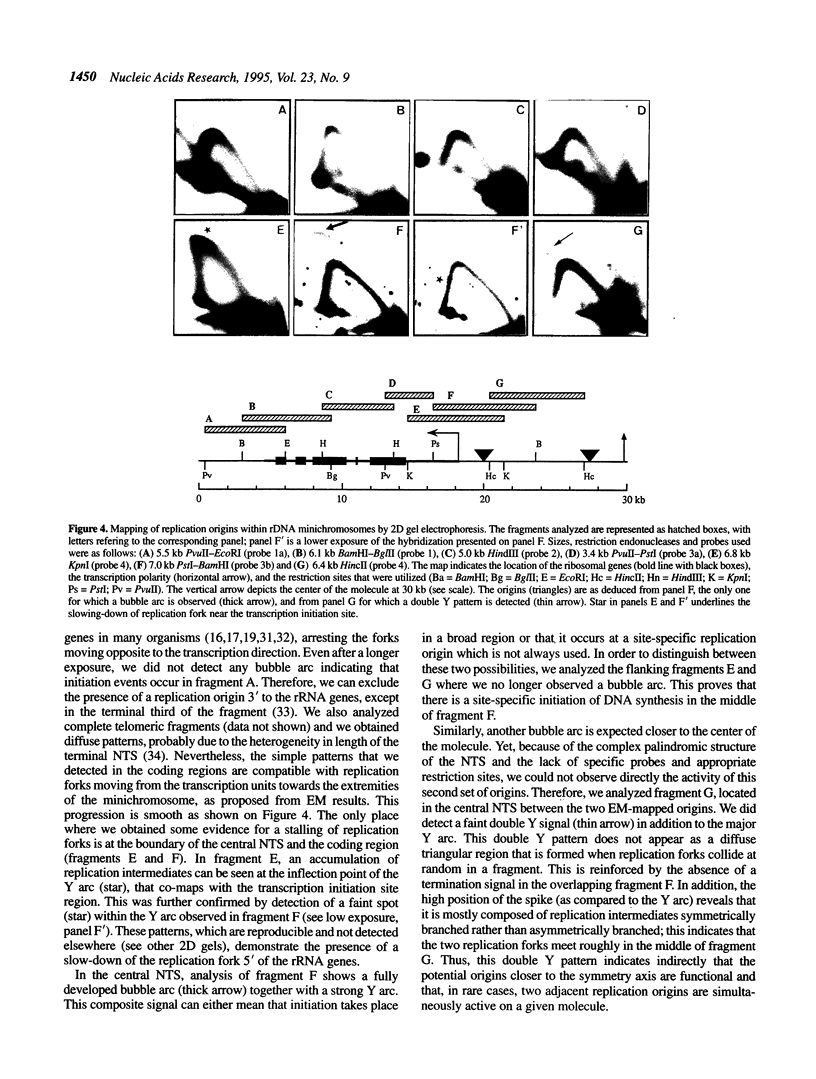
Physarum polycephalum rRNA genes are found on extrachromosomal 60 kb linear palindromic DNA molecules. Previous work using electron microscope visualization suggested that these molecules are duplicated from one of four potential replication origins located in the 24 kb central non-transcribed spacer [Vogt and Braun (1977) Eur. J. Biochem., 80, 557-566]. Considering the controversy on the nature of the replication origins in eukaryotic cells, where both site-specific or delocalized initiations have been described, we study here Physarum rDNA replication by two dimensional agarose gel electrophoresis and compare the results to those obtained by electron microscopy. Without the need of cell treatment or enrichment in replication intermediates, we detect hybridization signals corresponding to replicating rDNA fragments throughout the cell cycle, confirming that the synthesis of rDNA molecules is not under the control of S-phase. The patterns of replication intermediates along rDNA minichromosomes are consistent with the existence of four site-specific replication origins, whose localization in the central non-transcribed spacer is in agreement with the electron microscope mapping. It is also shown that, on a few molecules, at least two origins are active simultaneously.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botchan P. M., Dayton A. I. A specific replication origin in the chromosomal rDNA of Lytechinus variegatus. Nature. 1982 Sep 30;299(5882):453–456. doi: 10.1038/299453a0. [DOI] [PubMed] [Google Scholar]
- Bozzoni I., Baldari C. T., Amaldi F., Buongiorno-Nardelli M. Replication of ribosomal DNA in Xenopus laevis. Eur J Biochem. 1981 Sep 1;118(3):585–590. doi: 10.1111/j.1432-1033.1981.tb05559.x. [DOI] [PubMed] [Google Scholar]
- Brewer B. J., Fangman W. L. A replication fork barrier at the 3' end of yeast ribosomal RNA genes. Cell. 1988 Nov 18;55(4):637–643. doi: 10.1016/0092-8674(88)90222-x. [DOI] [PubMed] [Google Scholar]
- Brewer B. J., Fangman W. L. Initiation at closely spaced replication origins in a yeast chromosome. Science. 1993 Dec 10;262(5140):1728–1731. doi: 10.1126/science.8259517. [DOI] [PubMed] [Google Scholar]
- Brewer B. J., Fangman W. L. Initiation preference at a yeast origin of replication. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3418–3422. doi: 10.1073/pnas.91.8.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer B. J., Fangman W. L. The localization of replication origins on ARS plasmids in S. cerevisiae. Cell. 1987 Nov 6;51(3):463–471. doi: 10.1016/0092-8674(87)90642-8. [DOI] [PubMed] [Google Scholar]
- Brewer B. J., Lockshon D., Fangman W. L. The arrest of replication forks in the rDNA of yeast occurs independently of transcription. Cell. 1992 Oct 16;71(2):267–276. doi: 10.1016/0092-8674(92)90355-g. [DOI] [PubMed] [Google Scholar]
- Brewer B. J. When polymerases collide: replication and the transcriptional organization of the E. coli chromosome. Cell. 1988 Jun 3;53(5):679–686. doi: 10.1016/0092-8674(88)90086-4. [DOI] [PubMed] [Google Scholar]
- Burhans W. C., Vassilev L. T., Caddle M. S., Heintz N. H., DePamphilis M. L. Identification of an origin of bidirectional DNA replication in mammalian chromosomes. Cell. 1990 Sep 7;62(5):955–965. doi: 10.1016/0092-8674(90)90270-o. [DOI] [PubMed] [Google Scholar]
- Bénard M., Pierron G. Mapping of a Physarum chromosomal origin of replication tightly linked to a developmentally-regulated profilin gene. Nucleic Acids Res. 1992 Jul 11;20(13):3309–3315. doi: 10.1093/nar/20.13.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G. R., Littau V. C., Melera P. W., Allfrey V. G., Johnson E. M. Unique sequence arrangement of ribosomal genes in the palindromic rDNA molecule of Physarum polycephalum. Nucleic Acids Res. 1979 Apr;6(4):1433–1447. doi: 10.1093/nar/6.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Brehm S. L. Replication of the extrachromosomal ribosomal RNA genes of Tetrahymena thermophilia. Nucleic Acids Res. 1981 Jul 24;9(14):3531–3543. doi: 10.1093/nar/9.14.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn A. F., Taylor W. C., Firtel R. A. Dictyostelium rDNA consists of non-chromosomal palindromic dimers containing 5S and 36S coding regions. Chromosoma. 1978 Dec 21;70(1):19–29. doi: 10.1007/BF00292212. [DOI] [PubMed] [Google Scholar]
- Collins I., Newlon C. S. Chromosomal DNA replication initiates at the same origins in meiosis and mitosis. Mol Cell Biol. 1994 May;14(5):3524–3534. doi: 10.1128/mcb.14.5.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney C. A., Eykholt R. L., Bradbury E. M. Methylation is co-ordinated on the putative replication origins of Physarum ribosomal DNA. J Mol Biol. 1988 Dec 20;204(4):889–901. doi: 10.1016/0022-2836(88)90049-6. [DOI] [PubMed] [Google Scholar]
- Daniel D. C., Johnson E. M. Selective initiation of replication at origin sequences of the rDNA molecule of Physarum polycephalum using synchronous plasmodial extracts. Nucleic Acids Res. 1989 Oct 25;17(20):8343–8362. doi: 10.1093/nar/17.20.8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diller J. D., Sauer H. W. Two early replicated, developmentally controlled genes of Physarum display different patterns of DNA replication by two-dimensional agarose gel electrophoresis. Chromosoma. 1993 Sep;102(8):563–574. doi: 10.1007/BF00368349. [DOI] [PubMed] [Google Scholar]
- Dubey D. D., Zhu J., Carlson D. L., Sharma K., Huberman J. A. Three ARS elements contribute to the ura4 replication origin region in the fission yeast, Schizosaccharomyces pombe. EMBO J. 1994 Aug 1;13(15):3638–3647. doi: 10.1002/j.1460-2075.1994.tb06671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangman W. L., Brewer B. J. Activation of replication origins within yeast chromosomes. Annu Rev Cell Biol. 1991;7:375–402. doi: 10.1146/annurev.cb.07.110191.002111. [DOI] [PubMed] [Google Scholar]
- Ferris P. J. Nucleotide sequence of the central non-transcribed spacer region of Physarum polycephalum rDNA. Gene. 1985;39(2-3):203–211. doi: 10.1016/0378-1119(85)90314-2. [DOI] [PubMed] [Google Scholar]
- Ferris P. J., Vogt V. M. Structure of the central spacer region of extrachromosomal ribosomal DNA in Physarum polycephalum. J Mol Biol. 1982 Aug 15;159(3):359–381. doi: 10.1016/0022-2836(82)90289-3. [DOI] [PubMed] [Google Scholar]
- Funderud S., Andreassen R., Haugli F. Size distribution and maturation of newly replicated DNA through the S and G2 phases of Physarum polycephalum. Cell. 1978 Dec;15(4):1519–1526. doi: 10.1016/0092-8674(78)90074-0. [DOI] [PubMed] [Google Scholar]
- Gall J. G. Free ribosomal RNA genes in the macronucleus of Tetrahymena. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3078–3081. doi: 10.1073/pnas.71.8.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall L., Braun R. The organisation of genes for transfer RNA and ribosomal RNA in amoebae and plasmodia of Physarum polycephalum. Eur J Biochem. 1977 Jun 1;76(1):165–174. doi: 10.1111/j.1432-1033.1977.tb11582.x. [DOI] [PubMed] [Google Scholar]
- Hernández P., Martín-Parras L., Martínez-Robles M. L., Schvartzman J. B. Conserved features in the mode of replication of eukaryotic ribosomal RNA genes. EMBO J. 1993 Apr;12(4):1475–1485. doi: 10.1002/j.1460-2075.1993.tb05791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A., Spotila L. D., Nawotka K. A., el-Assouli S. M., Davis L. R. The in vivo replication origin of the yeast 2 microns plasmid. Cell. 1987 Nov 6;51(3):473–481. doi: 10.1016/0092-8674(87)90643-x. [DOI] [PubMed] [Google Scholar]
- Hyrien O., Méchali M. Chromosomal replication initiates and terminates at random sequences but at regular intervals in the ribosomal DNA of Xenopus early embryos. EMBO J. 1993 Dec;12(12):4511–4520. doi: 10.1002/j.1460-2075.1993.tb06140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. M. A family of inverted repeat sequences and specific single-strand gaps at the termini of the Physarum rDNA palindrome. Cell. 1980 Dec;22(3):875–886. doi: 10.1016/0092-8674(80)90564-4. [DOI] [PubMed] [Google Scholar]
- Larson D. D., Blackburn E. H., Yaeger P. C., Orias E. Control of rDNA replication in Tetrahymena involves a cis-acting upstream repeat of a promoter element. Cell. 1986 Oct 24;47(2):229–240. doi: 10.1016/0092-8674(86)90445-9. [DOI] [PubMed] [Google Scholar]
- Linskens M. H., Huberman J. A. Ambiguities in results obtained with 2D gel replicon mapping techniques. Nucleic Acids Res. 1990 Feb 11;18(3):647–652. doi: 10.1093/nar/18.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linskens M. H., Huberman J. A. Organization of replication of ribosomal DNA in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Nov;8(11):4927–4935. doi: 10.1128/mcb.8.11.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little R. D., Platt T. H., Schildkraut C. L. Initiation and termination of DNA replication in human rRNA genes. Mol Cell Biol. 1993 Oct;13(10):6600–6613. doi: 10.1128/mcb.13.10.6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Bustin M., Miller O. L., Jr Electron microscopic analysis of chromosome metabolism in the Drosophila melanogaster embryo. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):741–754. doi: 10.1101/sqb.1978.042.01.075. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Miller O. L., Jr Electron microscopic analysis of chromatin replication in the cellular blastoderm Drosophila melanogaster embryo. Cell. 1977 Nov;12(3):795–804. doi: 10.1016/0092-8674(77)90278-1. [DOI] [PubMed] [Google Scholar]
- Newlon C. S., Sonenshein G. E., Holt C. E. Time of synthesis for ribosomal ribonucleic acid in Physarum. Biochemistry. 1973 Jun 5;12(12):2338–2345. doi: 10.1021/bi00736a024. [DOI] [PubMed] [Google Scholar]
- Puvion-Dutilleul F., Pierron G. Localization by high resolution in situ hybridization of the ribosomal minichromosomes during the nucleolar cycle of Physarum polycephalum. Exp Cell Res. 1992 Dec;203(2):354–364. doi: 10.1016/0014-4827(92)90009-w. [DOI] [PubMed] [Google Scholar]
- Saffer L. D., Miller O. L., Jr Electron microscopic study of Saccharomyces cerevisiae rDNA chromatin replication. Mol Cell Biol. 1986 Apr;6(4):1148–1157. doi: 10.1128/mcb.6.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev L. T., Burhans W. C., DePamphilis M. L. Mapping an origin of DNA replication at a single-copy locus in exponentially proliferating mammalian cells. Mol Cell Biol. 1990 Sep;10(9):4685–4689. doi: 10.1128/mcb.10.9.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev L. T., DePamphilis M. L. Guide to identification of origins of DNA replication in eukaryotic cell chromosomes. Crit Rev Biochem Mol Biol. 1992;27(6):445–472. doi: 10.3109/10409239209082569. [DOI] [PubMed] [Google Scholar]
- Vaughn J. P., Dijkwel P. A., Hamlin J. L. Replication initiates in a broad zone in the amplified CHO dihydrofolate reductase domain. Cell. 1990 Jun 15;61(6):1075–1087. doi: 10.1016/0092-8674(90)90071-l. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Braun R. Structure of ribosomal DNA in Physarum polycephalum. J Mol Biol. 1976 Sep 25;106(3):567–587. doi: 10.1016/0022-2836(76)90252-7. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Braun R. The replication of ribosomal DNA in Physarum polycephalum. Eur J Biochem. 1977 Nov 1;80(2):557–566. doi: 10.1111/j.1432-1033.1977.tb11912.x. [DOI] [PubMed] [Google Scholar]
- Yu G. L., Blackburn E. H. Amplification of tandemly repeated origin control sequences confers a replication advantage on rDNA replicons in Tetrahymena thermophila. Mol Cell Biol. 1990 May;10(5):2070–2080. doi: 10.1128/mcb.10.5.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger A., Ryser U., Braun R. Ribosomal genes of Physarum: their isolation and replication in the mitotic cycle. J Mol Biol. 1972 Mar 14;64(3):681–691. doi: 10.1016/0022-2836(72)90091-5. [DOI] [PubMed] [Google Scholar]
- Zhu J., Newlon C. S., Huberman J. A. Localization of a DNA replication origin and termination zone on chromosome III of Saccharomyces cerevisiae. Mol Cell Biol. 1992 Oct;12(10):4733–4741. doi: 10.1128/mcb.12.10.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]