Abstract
Background:
Hepatitis C virus (HCV) infection can predispose to the development of insulin resistance before diabetes occurs. Such a potential link is particularly cogent in light of recent data indicate that diabetes may be associated with increased hepatic fibrosis progression in patients with chronic HCV infection. The aim of the study is to determine the prevalence of insulin resistance in non diabetic patients with chronic hepatitis C and its relation to liver fibrosis.
Methods:
Thirty eight patients with chronic liver diseases. They subdivided into 2 groups; chronic hepatitis C (CHC) with elevated liver enzymes and CHC with normal liver enzymes. Age and sex matched 12 healthy subjects as control group. All subjects were subjected to Careful history and copmlete examination with stress upon symptoms and signs of chronic liver diseases. Investigations include liver function tests; viral markers (Anti HCV antibodies & PCR for HCV). Serum fasting glucose; serum fasting insulin; homeostasis model assessment (HOMA), liver biopsy and abdominal ultrasound.
Results:
No correlation between viral load and hepatic fibrosis in HCV infected patients. Liver fibrosis is considerably higher among HCV patients with elevated serum transaminase levels. Insulin resistance is present in HCV infected cases compared with control group and it is positively correlated with liver fibrosis.
Conclusion:
The present data support the hypothesis that insulin resistance may increase the rate of fibrosis progression in non diabetic patients with chronic HCV. Follow up of hyperinsulinemia by serial measurement of HOMA test in non diabetic HCV infected patients may be a biochemical indicator for progression of liver fibrosis.
Introduction
Hepatitis C Virus (HCV) infection appears to be endemic in most parts of the world, with an estimated overall prevalence of 3% (1). Over the past decade, hepatitis C virus infection (HCV) has arisen from an obscure disease into a public health problem all around the world. It is estimated that 15% of the Egyptian population is seropositive (2) and high seroprevalence of hepatitis C infection among risk groups in Egyptian people. (3)
Chronic hepatitis C virus infection is associated with a wide spectrum of liver histological lesions, ranging from mild chronic hepatitis to cirrhosis and hepatocellular carcinoma. (4)
Chronic hepatitis C virus infection is associated with an increased risk for the development of type 2 diabetes (5). Thus, type 2 diabetes is more prevalent among patients with chronic HCV compared with those with other liver diseases and the general population, irrespective of whether cirrhosis is present. (6)
Insulin resistance plays a primary role in the development of type 2 diabetes. (7) This is supported by prospective longitudinal studies showing that insulin resistance is the best predictor for the development of diabetes, preceding the onset of diabetes by 10 to 20 years (8) and by cross-sectional studies showing that insulin resistance is a consistent finding in patients with type 2 diabetes. (9)
Insulin resistance and progressive pancreatic β-cell dysfunction have been identified as the two fundamental features in the pathogenesis of type 2 DM. As a widely validated clinical and epidemiological tool for estimating insulin resistance and β-cell function, the homeostasis model assessment (HOMA) is derived from a mathematical assessment of the balance between hepatic glucose output and insulin secretion from fasting levels of glucose and insulin. (10)
An Egyptian study showed that incidence of type 2 diabetes mellitus increased twofold in patients who had HCV infection compared with those who did not and reported that HCV infected persons with type 2 diabetes mellitus were more likely to need insulin. (11)
It is important to determine whether HCV infection can predispose to the development of insulin resistance before diabetes occurs. Such a potential link is particularly cogent in light of recent data indicate that diabetes may be associated with increased hepatic fibrosis progression in patients with chronic HCV infection. (12)
The pathogenesis of diabetes in patients with HCV is not well understood but an increase in fat or iron deposition in the liver is common in patients with HCV. (13) The profibogenic impact of high serum glucose was higher for intermediate and late fibrosis than for early stages. Thus hyperglycemia may play a role in the perpetuation and progression of fibrogenesis rather than in the initiation of the fibrotic process. (14)
The aim of the study is to assess the insulin resistance status in non diabetic patients with chronic hepatitis C and the association between insulin resistance and liver fibrosis progression.
Methods
This cross-sectional descriptive study was approved by the local ethical committee from the Mansoura University Hospital. Informed consent was obtained from all subjects before the beginning of the study. Thirty eight patients with chronic hepatitis C infection were selected from CHC patients attending the outpatient clinics of tropical Medicine and Internal Medicine Departments from January to Augast 2005, Mansoura University Hospital according to inclusion criteria. In addition to 12 healthy subjects of matched age and sex as a reference group. The selected patients were classified into 2 groups: Group 1 included 23 patients with chronic hepatitis C (CHC) with elevated liver enzymes: 14 males and 9 females with age ranged from 19–49 years (mean: 33.1±9.2 ys). Group 2 included 15 patients chronic hepatitis C (CHC) with normal liver enzymes: 12 males and 3 females, with age ranged from 22–41 years (mean: 30.6±7.1 ys).
Inclusion criteria were included patients with positive HCV RNA reverse transcription polymerase chain reaction (RT-PCR) for at least 6 months. Exclusion criteria were included patients with positive hepatitis B surface antigen, concomitant infection. Patients with history of Schistosomasis (rectal snip), prior anti-HCV treatment, recent use of steatosis-including agents: hepatic decompensation (ascites, jaundice, variceal bleeding, or hepatic encephalopathy) (15) or history of alcohol intake were also excluded.
Patients with fasting blood glucose level >6.2mmol/l, under antidiabetic or immuosuppressive regimens, undergoing dialysis, with clinically overt hypo- or hyperthyroidism, with clinically evident autoimmune diseases were excluded from the study.
Chronic hepatitis C patients with normal enzymes were defined as ALT levels within the normal range on three separate occasions two months apart within a 6-month period after presentation as defined by Martinot-Peignoux et al. (16)
A liver biopsy to stage CHC and availability of a fasting plasma sample collected within one week of liver biopsy.
All studied patients were subjected to full History and Clinical examination with stress on features of chronic liver disease.
The following investigations were performed to all subjects: Complete blood picture; Liver function tests; Serum Creatinine; HBsAg; Hepatitis C antibodies; PCR for HCV; Serum fasting glucose and Serum fasting insulin levels.
The HOMA index of insulin sensitivity is calculated as: HOMA-IR = (Gbx Ib)/k where Gb and Ib are basal glucose and insulin concentrations and k is a constant to scale HOMA-IR. HOMA derives from a mathematical model of the glucose–insulin homeostatic system. (17) HOMA-IR strongly predicts the development of type 2 diabetes, independent of obesity, body fat distribution, and glucose tolerance status. (18)
Insulin resistance is measured using calculated HOMO test (10) as follow: HOMAIR = (fasting insulin x fasting glucose in mmol/L) / 22.5. Where FIRI is fasting plasma insulin level (μU/ml) and FPG is fasting plasma glucose level (mmol/l).
Serum insulin was measured using an immunoradiometric assay kit (Insulin Riabead II kit; Dainabot, Tokyo, Japan). The intra- and interassay coefficients of variation of the assay were 2.0% and 2.1%, respectively.
Percutanous liver biopsy is necessary to accurately evaluate the extent of liver damage. Liver biopsy analyzed with connective tissue stains has long been considered the ‘gold standard’ for assessing liver histology, disease activity and liver fibrosis. (19) The cases with non alcoholic fatty liver diseases (NAFLD) were excluded histologically. Current clinical trials in which fibrosis are assessed in liver biopsies typically use Metavir scoring systems. The Metavir score is comprised of five progressive stages: F0, normal; F1, portal fibrosis; F2, few fibrotic septae; F3, numerous septae; and F4, cirrhosis. (20) The Metavir scoring system is well validated and reproducible, and the use of only four stages leads to greater concordance among pathologists (19). Liver biobsy reading by two pathologists.
Abdominal ultrasound, Sigmoidoscopy and Rectal mucosal biopsy (rectal snip transparency) were done to all studied patients.
Statistical analysis was done by using SPSS (Statistical package for social science) program version 10 1999. The data were parametric by using Kolomgrov Smirnov Test. The quantitative data were presented in the form of mean, standard deviation and range. Student t test was used for comparison of two groups. The qualitative data were presented in the form of number and percentage. Chi-square test was used for qualitative data. The variables (liver enzymes, degree of inflammation and stage of fibrosis) analyze by using simple and multiple linear regression methods. Significance was considered when P value less than 0.05; insignificance was considered when P value was more than 0.05.
Results
The age, sex and body mass index of the HCV patients were matched with the control (Table-1) and showed a non significant difference between HCV patients compared with control group (P=0.071, 0.068 and 0.1 respectively). However; the quantatitive PCR for HCV is significantly higher in patients with elevated liver enzymes (p =0.001).
Table (1).
Age; sex distribution and body mass index (BMI) of the studied groups.
| Groups | Age | Male | Female | BMI | Quantitative PCR |
|---|---|---|---|---|---|
| Group-1 (23) CHC with elevated enzymes |
33.13±9.15 | 14/60.9 % | 9/39.1% | 28.13±2.44 | 177±159 c |
| Group -2 (15) CHC with normal enzymes |
30.61±7.10 | 12/80 % | 3/20 % | 27.81±3.34 | 42.9±36.3 |
| Group -3 Control |
34.08±8.51 | 6/50 % | 6/50 % | 25.58±1.72 | |
| Signifcant test | One Way Anova F test F=2.35 P=0.071 |
Chi-square test X2=8.70 P=0.068 |
One Way Anova F test F=2.22 P=0.101 |
t= 7.93 P 0.001** |
|
Comparison between the different groups by using ANOVA test for numerical data and Chi square test for percentages.
P: mildly statistically significant (P<0.05).
P: highly statistically significant (P<0.01).
Group1 versus Group3.
Group2 versus Group3.
Group1 versus Group2. %: Percentage. CHC: chronic hepatitis C virus. BMI: Body mass index. PCR: polymeraze chain reaction.
In Table (2), the HCV patients with elevated liver enzymes showed significant high fasting insulin, HOMA-IR and liver fibrosis grading than those with normal liver enzymes or healthy control (17.85±4.5, 3.98±1.41, 21/2 versus 12.68±2.5, 2.69±0.76, 13/2 or 9.1±3.6, 1.92±0.74 respectively).
Table (2).
Serum fasting glucose; fasting insulin (pmol/l) and HOMA-IR of the studied groups (CHC with elevated enzymes; CHC witn normal enzymes versus normal healthy control).
| FBG (mmol/l) | FI (μU/l) | HOMA-IR | Fibrosis | ||
|---|---|---|---|---|---|
| +ve | −ve | ||||
| Group -1 CHC with elevated enzymes |
4.9±0.75 a | 17.85±4.5ac | 3.98±1.41ac | 21 | 2 |
| Group -2 CHC with normal enzymes |
4.6±0.51 | 12.68±2.5b | 2.69±0.76 | 13 | 2 |
| Group -3 Healthy Control |
4.1±0.42 | 9.1±3.6 | 1.92±0.74 | ||
| Group -1 versus Group -3 | P=0.05* | P<0.001** | P<0.001** | ||
| Group -2 versus Group -3 | P=0.112 | P<0.001** | P=0.071 | ||
| Group -1 versus Group -2 | P=0.071 | P=0.009** | P=0.09** | P=0.023* | |
Comparison between the different groups by using ANOVA test for numerical data and Chi square test for percentages.
P: mildly statistically significant (P<0.05).
P: highly statistically significant (P<0.01). F=6.21 = p < 0.001**.
Group1 versus Group3.
Group2 versus Group3.
Group1 versus Group2. %: Percentage. NS: non-significant. CHC: chronic hepatitis C virus. FBG: Fasting blood glucose. FI: Fasting insulin. HOMA-IR: Homeostasis model assessment-Insulin resistance.
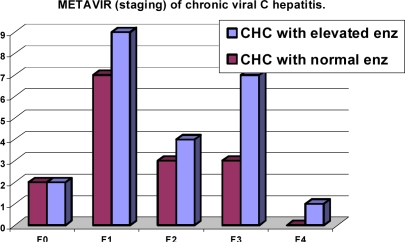
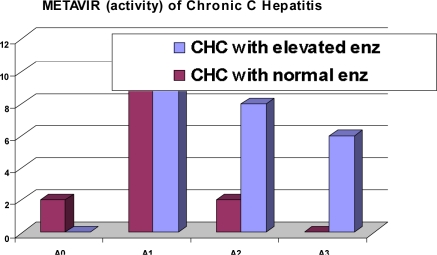
There was no significant difference of METAVIR fibrosis Staging between CHC patients with elevated liver enzymes versus those without elevated liver enzymes (P value = 0.51) table-3 andfigure-1. However in table-4 and figure-2; METAVIR activity score is significantly higher in CHC patients with elevated liver enzymes than those without elevated liver enzymes (P value = 0.021)
Table (3).
METAVIR fibrosis staging in patients with chronic hepatitis C infection.
| Groups | METAVIR (Staging)(F) | ||||
|---|---|---|---|---|---|
| F0 | F1 | F2 | F3 | F4 | |
| CHC with elevated enz. (N/%) | 2/8.7% | 9/39.1% | 4/17.4% | 7/30.4% | 1/4.3% |
| CHC with normal enz. (N/%) | 2/13.3% | 7/46.7% | 3/30% | 3/20% | -/- |
| Comparison between two groups | NS (P =0.51) | ||||
Comparison between the different groups by using Chi square test for percentages.
P: mildly statistically significant (P<0.05).
P: highly statistically significant (P<0.01). X2= 9.71 = P= 0.64.
N: number. %: Percentage. NS: non-significant. CHC: chronic hepatitis C virus.
Figure (1).
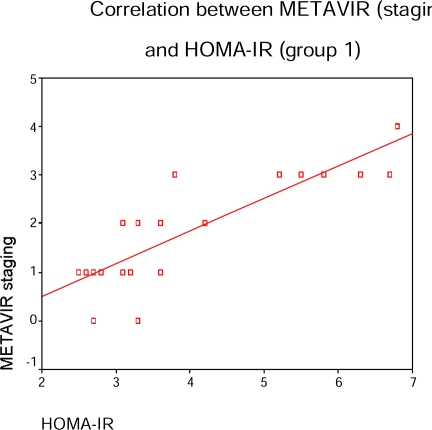
In CHC patients with elevated liver enzymes; FBG, HOMA-IR and prothrombin time were correlated with METAVIR activity and liver fibrosis scores in table (5) & figure (3) (p=0.01, 0.02, p=0.02, 0.01 and p=0.01, 0.01 respectively) while viral load (PCR) was correlated METAVIR activity score only (p=0.031).
Table (4).
METAVIR activity score in patients with chronic hepatitis C infection.
| Groups | METAVIR (Activity)(A) | |||
|---|---|---|---|---|
| A0 (No) | A1 (Mild) | A2 (Moderate) | A3 (Severe) | |
| CHC with elevated enz. (N/%) | -/- | 9/39.1 | 8/34.8 | 6/26.1 |
| CHC with normal enz. (N/%) | 2/13.3 | 11/73.3 | 2/13.3 | -/- |
| Comparison between two groups | P=0.021* | |||
Comparison between the different groups by using Chi square test for percentages.
P: mildly statistically significant (P<0.05).
P: highly statistically significant (P<0.01). X2= 20.1 (P= 0.012)* X2= 6.65 (p= 0.063).
N: number. %: Percentage. NS: non-significant. CHC: chronic hepatitis C virus
Figure (2).
Discussion
The recent data indicate that insulin resistance and diabetes may be associated with increased hepatic fibrosis progression in patients with chronic HCV infection. (10)
This study revealed that mild hepatitis was the most common histological finding; 73.3 % in CHC patients with normal liver enzymes and 39.1% in patients with elevated liver enzymes table (4). METAVIR Staging showed that F1 was the most common in CHC patients with normal enzymes (46.7%) and 39.1% in CHC patients with elevated enzymes (table-3). Puoti et al (21) found that subjects with normal liver enzymes had moderate to severe degree of hepatitis. The discordance between serum aminotransferase levels and histologically assessed liver damage has been well documented in the group of HCV infected patients with persistently normal ALT levels who can show substantial liver inflammation and fibrosis on liver biopsy. (22) The number of apoptotic hepatocytes correlates with necroinflammation but not with serum aminotransferase levels. This could be explained by the hepatocytes die without membrane injury leading to a reduced release of aminotransferases. Therefore, a different extent of apoptosis could explain the discordance between histological activity and serum aminotransferase levels in patients with CHC. (23)
In the present study, ALT were significantly correlated with fibrosis in CHC patients with elevated enzymes Table (5), while it was not correlated with fibrosis in CHCpatients with normal enzymes Table (6). There is conflicting evidence on the association between the degree of ALT abnormality and extent of histological fibrosis. This is consistent with Marthurin et al. (24) who demonstrated that the risk of high degree of fibrosis and disease progression is considerably higher among people with abnormal serum transaminase levels compared with those with consistently normal levels; On other hand, several studies have shown either no correlation (25) or very weak correlation between ALT and hepatic fibrosis. (26)
Table (5).
Relationship between METAVIR activity & fibrosis scores and laboratory data in patients CHC with elevated enzymes.
| Laboratory Data | METAVIR (activity) (A) | METAVIR (staging) (F) | ||
|---|---|---|---|---|
| r | p | r | p | |
| FBG | 0.80 | 0.01** | 0.43 | 0.02* |
| FI | 0.38 | 0.1* | 0.60 | 0.01** |
| HOMA-IR | 0.49 | 0.02* | 0.66 | 0.01** |
| PCR | 0.41 | 0.031* | 0.32 | 0.22 |
| ALT | 0.42 | 0.04* | 0.40 | 0.04* |
| PT | 0.87 | 0.01** | 0.49 | 0.01** |
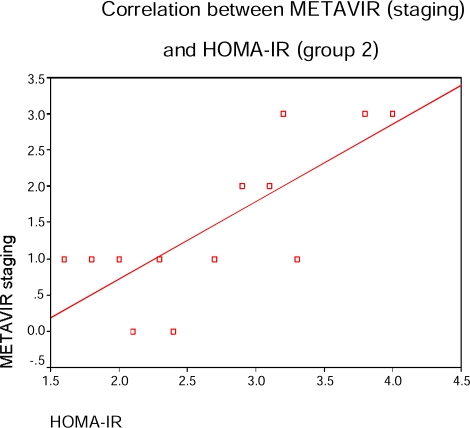
In CHC patients with normal liver enzymes; FBG & HOMA-IR were correlated with METAVIR activity and liver fibrosis scores in table (6) & figure (4) (p=0.04, 0.01 and p=0.01, 0.02 respectively) while viral load (PCR) was correlated with METAVIR activity score only (p=0.031) and prothrombin time was correlated with METAVIR fibrosis score (p=0.02). There is a correlation between serum fasting insulin and liver fibrosis progression in CHC patients with elevated enzymes (p=0.01) table (5) and in those with normal enzymes (p=0.02) (table-6).
Table (6).
Relationship between METAVIR activity & fibrosis scores and laboratory data in Patients with CHC with normal enzymes.
| Laboratory Data | METAVIR (activity) (A) | METAVIR (staging) (F) | ||
|---|---|---|---|---|
| r | p | r | p | |
| FBG | 0.53 | 0.04* | 0.77 | <0.01** |
| FI | 0.76 | 0.01** | 0.61 | 0.02* |
| HOMA-IR | 0.76 | 0.01** | 0.73 | 0.02** |
| PCR | 0.66 | 0.034* | 0.07 | 0.92 |
| ALT | 0.48 | 0.04* | 0.21 | 0.43 |
| PT | 0.08 | 0.91 | 0.50 | 0.02* |
P: mildly statistically significant (P<0.05).
P: highly statistically significant (P<0.01). FBG: Fasting blood glucose. FI: Fasting insulin. HOMA-IR: Homeostasis model assessment-Insulin resistance. CHC: chronic hepatitis C virus. PCR: polymeraze chain reaction. ALT: Alanine transferase. PT: Prothrombin time
In the present study, there is a correlation between prothrombin time and liver fibrosis in CHC patients with elevated enzymes (table-5) and in those with normal enzymes (Table-6). This agrees with Croquet et al (27) who demonstrated that prothrombin time is well correlated with the pathological degree of liver fibrosis and with area of fibrosis even at initial stages of hepatic fibrosis in chronic liver disease. Therefore, in patients with chronic liver disease, the prothrombin time is an indirect marker of liver fibrosis even in the initial stages of fibrosis before the decrease in hepatic function.
In this work, there is no correlation between quantitative PCR (viral load) and hepatic fibrosis in CHC with elevated enzymes (table-5) and in CHC with normal enzymes (Table-6). This is consistent with Marcellin (4) who reported that there is no correlation between HCV RNA levels and severity of liver fibrosis. On other hand, Zeuzem et al (28) who noted that there is correlation between serum levels of HCV RNA to the degree of liver injury and liver fibrosis.
Also; there is a significant correlation between quantitative PCR (viral load) and necroinflammatory activity in CHC patients with elevated enzymes (Table-5) and in CHC patients with normal enzymes (Table-6). This is in agreement with Adinolfi et al (29) who noted that a serum level of HCV RNA is correlated with necroinflammatory activity. This association is more in patients with higher serum ALT levels. Thus, it indicates that viral load is an important factor in determining liver necroinflammatory activity and consequently influence the progression of the stage of liver disease. (29) The HCV contributes to liver damage with a direct cytopathic effect. Alternatively, the host immune response may play a role in the pathogenesis of liver injury and which may reflect the viral load. This hypothesis, is also possible that both a direct viral effect and a host immune-mediated response play a role in the pathogenesis of chronic liver injury. (30)
In the present study, there is a significant correlation between serum fasting blood glucose and liver fibrosis in CHC patients with either elevated enzymes or normal enzymes (Table-5,6). This is consistent with Ratziu et al, (14) who demonstrated that high serum glucose is associated with liver fibrosis and higher fibrosis progression rate in chronic hepatitis C, this may be due to that hyperglycemia results in enhanced formation and deposition of advanced glycation end products. (31) Specific receptors for these advanced glycation end products have been detected in the liver where they are restricted to hepatic stellate cells, the main cellular source of liver collagen. (32)
In this study, there is a correlation between serum fasting insulin and liver fibrosis progression in CHC patients with elevated enzymes and in those with normal enzymes. This is in agreement with Fukui et al, (33) who reporting that fasting serum insulin levels were significantly elevated in patients with chronic HCV infection compared with control subjects.
In this work, there is significant higher HOMA-IR in CHC with elevated enzymes and those with normal enzymes compared with control group (Table 2). This is in agreement with Maeno et al, (34) who founded that insulin resistance was elevated among HCV infected patients compared with control group, this may be due to decreased liver carbohydrate metabolism and hypersecretion of insulin-resistant cytokines, such as interleukin-6 and tumor necrosis factor, (35) which have been shown to be elevated in patients with chronic HCV infection. The HCV induced TNF alpha that played an important role in the pathogenesis of insulin resistance and may provide the pathogenic link between chronic HCV infection and insulin resistance. (36) This also was demonstrated in Egyptian patients with HCV infection had a significantly higher level of TNF alpha and ferritin which may explain their insulin resistance. (37)
We found a significant correlation between both serum fasting glucose and HOMA-IR with degree of inflammation (METAVIR activity) and liver fibrosis score, respectively, regardless of the liver enzymes. This is agree with Hui et al, (36) who approved that there is a correlation between insulin resistance and liver fibrosis in patients with chronic hepatitis C. The insulin plays a significant role in the development of fibrosis via a mechanism involving steatosis. In this regard, steatosis promotes cellular insulin resistance which, in turn, induces compensatory hyperinsulinaemia. (38) Hyperinsulinaemia has been shown to directly stimulate hepatic stellate cell proliferation and increase expression of connective tissue growth factor, a key factor in the progression of fibrosis. (39) This was also reported by D Souza et al., (40) who found that insulin resistance contributes to liver fibrosis in chronic HCV infection; this relationship is not genotypic specific.
In conclusion, The present data support the hypothesis that insulin resistance may increase the rate of fibrosis progression in non diabetic patients with chronic HCV. Follow up of hyperinsulinemia in non diabetic HCV infected patients may be a biochemical indicator for progression of liver fibrosis. The use insulin resistance to predict progression of fibrosis is interesting. We need a longitudinal study to address this issue as recommendation from our study.
Figure (3).
Figure (4).
References
- 1.Wasley A, Alter M. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin liver Dis. 2000;20(1):1–60. doi: 10.1055/s-2000-9506. [DOI] [PubMed] [Google Scholar]
- 2.EI-Sayed NM, Gomatos PJ, Rodier GR, et al. Seroprevalence survey of Egyptian tourism workers to hepatitis B virus, hepatitis C virus, human in immunodeficiency virus and Treponema pallidum infections: Association of hepatitis C virus infections with specific regions of Egypt. Am J trop Med Hyg. 1996;55:179–84. doi: 10.4269/ajtmh.1996.55.179. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Wahab M, Zakaria S, Kamel M, et al. High seroprevalence of hepatitis C infection among risk groups in Egypt. Am J Trop Med Hyg. 1994;51(5):563–7. doi: 10.4269/ajtmh.1994.51.563. [DOI] [PubMed] [Google Scholar]
- 4.Marcellin P, Hepatitis C. Clinical spectrum of the disease. J Hepatol. 1999;31:9–16. doi: 10.1016/s0168-8278(99)80368-7. [DOI] [PubMed] [Google Scholar]
- 5.Allison M, Wreghitt T, Palmer C, et al. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J hepatol. 1994;21:1135–1139. doi: 10.1016/s0168-8278(05)80631-2. [DOI] [PubMed] [Google Scholar]
- 6.Mehta SH, Brancati FI, Sulkowski MS, et al. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the Unites States. Ann Intern Med. 2000;133:592–599. doi: 10.7326/0003-4819-133-8-200010170-00009. [DOI] [PubMed] [Google Scholar]
- 7.Haffner S, Stern M, Dunn J, et al. Diminished insulin sensitivity and increased insulin response in nonobese, nondiabetic Mexican Americans. Metabolism. 1900;39:842–847. doi: 10.1016/0026-0495(90)90130-5. [DOI] [PubMed] [Google Scholar]
- 8.Warram JH, Martin BC, Krolewski AS, et al. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring diabetic parents. Ann Intern Med. 1990;113:909–915. doi: 10.7326/0003-4819-113-12-909. [DOI] [PubMed] [Google Scholar]
- 9.Cahill GF. Beta-cell deficiency, insulin resistance, or both. N Engl J Med. 1988;318:1268–1270. doi: 10.1056/NEJM198805123181909. [DOI] [PubMed] [Google Scholar]
- 10.Yiqing S, JoAnn E, Lesley T, et al. Insulin Sensitivity and Insulin Secretion Determined by Homeostasis Model Assessment (HOMA) and Risk of DM in a Multiethnic Cohort of Women. Diabetes Care. 2007 Jul;30(7):1747–1752. doi: 10.2337/dc07-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Zayadi A, Selim O, Hamdy H, et al. Association of chronic hepatitis C infection and diabetes mellitus. Trop Gastroenterol. 1998;19:141–4. [PubMed] [Google Scholar]
- 12.Monto A, Alonzo J, Watson J, et al. Steatosis in chronic hepatitis C: relative contributions of obesity, diabetes mellitus, and alcohol. Hepatology. 2002;36:729–736. doi: 10.1053/jhep.2002.35064. [DOI] [PubMed] [Google Scholar]
- 13.Czaja AJ, Carpenter HA, Santrach PJ, et al. Host and disease specific factors affecting steatosis in chronic hepatitis C. J Hepatol. 1998;29:198–206. doi: 10.1016/s0168-8278(98)80004-4. [DOI] [PubMed] [Google Scholar]
- 14.Ratziu V, Munteanu M, Charlotte F, et al. Fibogenic impact of high serum glucose in chronic hepatitis C. J Hepatol. 2003;39:1049–1055. doi: 10.1016/s0168-8278(03)00456-2. [DOI] [PubMed] [Google Scholar]
- 15.Hu KQ, Tong MJ. The long-term outcomes of patients with compensated hepatitis C virus-related cirrhosis and history of parental exposure in the united state. Hepatology. 1999;29:1311–1316. doi: 10.1002/hep.510290424. [DOI] [PubMed] [Google Scholar]
- 16.Martinot-Peignoux M, Boyer N, Cazals-Hatem D, et al. Prospective study on anti-hepatitis C viruspositive patients with persistently normal serum alanine transaminase with or without detectable serum hepatitis C virus RNA. Hepatology. 2001;34:1000–1005. doi: 10.1053/jhep.2001.28458. [DOI] [PubMed] [Google Scholar]
- 17.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 18.Hui JM, Sud A, Farreii GC. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression. Gastroenterology. 2003;125:1695–1704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 19.Friedman SL. Liver fibrosis- from bench to bedside. J Hepatol. 2003;38:S38–S53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 20.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 21.Puoti C, Magrini A, Stati T, Rigato P, et al. Clinical, histological and virological features of hepatitis C virus carriers with persistently normal or abnormal alanine transaminase levels. Hepatology. 1997;26:1393–1398. doi: 10.1053/jhep.1997.v26.pm0009397976. [DOI] [PubMed] [Google Scholar]
- 22.Puoti C, Guido M, Mangia A, et al. Clinical management of HCV carriers with normal aminotransferase levels. Dig Liver Dis. 2003;35:362–369. doi: 10.1016/s1590-8658(03)00185-3. [DOI] [PubMed] [Google Scholar]
- 23.Bantel H, Lugering A, Poremba C. Caspase activation correlates with the degree of inflammatory liver injury in chronic hepatitis C virus infection. Hepatology. 2001;34:758–767. doi: 10.1053/jhep.2001.28229. [DOI] [PubMed] [Google Scholar]
- 24.Marthurin P, Moussalli J, Candranel JF. Slow progression rate of fibrosis in hepatitis C virus patients with persistently normal alanine transaminase activity. Hepatology. 1998;27:868–872. doi: 10.1002/hep.510270333. [DOI] [PubMed] [Google Scholar]
- 25.Luo JC, Hwang SJ, Lai CR. Relationship between serum aminotransferase levels, liver histologies and virological status in patients with chronic hepatitis C in Taiwan. J Gastroenterol Hepatol. 1998;13:685–690. doi: 10.1111/j.1440-1746.1998.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 26.Fanning L, Kenny E, Sheehan M. Viral load and clinicopathological features of chronic hepatitis C (1b) in a homogeneous patient population. Hepatology. 1999;29:904–907. doi: 10.1002/hep.510290310. [DOI] [PubMed] [Google Scholar]
- 27.Croquet V, Vuillemin E, Ternisien C, et al. Prothrombin index is an indirect marker of severe liver Fibrosis. Eur J Gastroenterol Hepatol. 2002;14:1133–1141. doi: 10.1097/00042737-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Zeuzem S, Franke A, Lee J-H, et al. Phylogenetic analysis of hepatitis C virus isolates and their correlation to viremia, liver function tests and histology. Hepatology. 1996;24:1003–1009. doi: 10.1002/hep.510240505. [DOI] [PubMed] [Google Scholar]
- 29.Adinolfi LE, Utili R, Andreana A, et al. Serum HCV RNA Levels Correlate with Histological Liver Damage and Concur with Steatosis in Progression of Chronic Hepatitis C Dig Dis Sci. 2001468: 1677–1683. [DOI] [PubMed] [Google Scholar]
- 30.Hiroishi K, Kita H, Kojima M, et al. Cytotoxic lymphocyte response and viral load in hepatitis C infection. Hepatology. 1997;25:705–712. doi: 10.1002/hep.510250336. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt AM, Hori O, Brett J, et al. Cellular receptors for advanced glycation end products. Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler Thromb. 1994;14:1521–1528. doi: 10.1161/01.atv.14.10.1521. [DOI] [PubMed] [Google Scholar]
- 32.Fehrenbach H, Weiskirchen R, Kasper M, et al. Up-regulated expression of the receptor for advanced glycation end products in cultured rat hepatic stellate cells during transdifferentiation to myofibroblasts. Hepatology. 2001;34:943–952. doi: 10.1053/jhep.2001.28788. [DOI] [PubMed] [Google Scholar]
- 33.Fukui M, Kitagawa Y, Nakamura N, Yoshikawa T. Insulin sensitivity in patients with chronic hepatitis C virus infection. Diabetes Care. 2002;25:1900–1901. doi: 10.2337/diacare.25.10.1900. [DOI] [PubMed] [Google Scholar]
- 34.Maeno T, Okumura A, Ishikawa T, et al. Mechanisms of increased insulin resistance in non-cirrhotic patients with chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2003;18:1358–1363. doi: 10.1046/j.1440-1746.2003.03179.x. [DOI] [PubMed] [Google Scholar]
- 35.Kern PA, Ranganathan S, Li C, et al. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 36.Hui C, Belaye T, Montegrande K, et al. A comparison in the progression of liver fibrosis in chronic hepatitis C between persistently normal and elevated transaminase. J Hepatol. 2003;38:511–517. doi: 10.1016/s0168-8278(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 37.Elsammak M, Refai W, Elsawaf A, et al. Elevated serum tumor necrosis factor alpha and ferritin may contribute to the insulin resistance found in HCV positive Egyptian patients. Curr Med Res Opin. 2005;21(4):527–34. doi: 10.1185/030079905X38141. [DOI] [PubMed] [Google Scholar]
- 38.Fartoux L, Poujol-Robert A, Guéchot J, et al. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut. 2003;54:1003–1008. doi: 10.1136/gut.2004.050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paradis V, Perlemuter G, Bonvoust F. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738–44. doi: 10.1053/jhep.2001.28055. [DOI] [PubMed] [Google Scholar]
- 40.D’Souza R, Sabin CA, Foster GR. Insulin resistance plays a significant role in liver fibrosis in chronic hepatitis C and in the response to antiviral therapy. Am J Gastroenterol. 2005;100(7):1509–15. doi: 10.1111/j.1572-0241.2005.41403.x. [DOI] [PubMed] [Google Scholar]