Abstract
Objective:
To assess the difference of surface hardness of glass ionomer cement (GIC) set by conventional setting method and under ultrasonically energized method.
Method:
20 cylindrical samples measuring 2.5mm (diameter) and 5mm (length) were prepared with type IX GIC. Ten of these samples were allowed to set by conventional setting method and other ten were set under ultrasonic excitation energy. After finishing and polishing of the samples, three indentations were made on each sample using Vicker’s hardness machine with a load of 300 gm for 15 seconds. The surface microhardness of the indents was calculated by Vicker’s hardness formula.
Results:
Surface microhardness of samples set by ultrasound setting method was significantly higher than samples set by conventional method.
Conclusion:
This can be beneficial for the dental patients as when used as a restorative material, it will have a long lasting effect and can also be used in posterior load bearing areas.
Keywords: Sluggish setting of GIC, conventional setting, setting of GIC under ultrasonic waves
Introduction
Conventional GIC was introduced by Wilson and Kent in early 70’s.1 The ability of GIC to leach fluoride and adhere to the hard dental tissues made it suitable for its use in dentistry.2 Their low coefficient of thermal expansion identical to calcified tooth tissues made them a good restorative material3, but they have a poor wear resistance and are prone to fracture and moisture contamination during setting.4 They may lose water of crystallization if used in dry environment which further deteriorates their mechanical properties.5
Many of these negative effects of GIC are due to the sluggish setting of conventional GIC.6 The GIC has to bear heavy occlusal loads and increased wear during the first few days while the material is in the process of becoming fully matured.7
One solution to this slow setting is to enhance the setting rate of the cement which may be obtained by the addition of energy during the setting of the cement.8 One method of achieving this additional energy to the system is the use of ultrasonic waves.9
Surface hardness tests have been performed in the past to study the setting behavior of GIC.10 Various microhardness tests are performed for dental materials which include Brinell, Rockwell, Shore, Vickers and Knoop.3,11
Vickers hardness tester is a handy apparatus and very useful in research laboratories.11 As many studies have been done using Vicker’s hardness machine to assess surface hardness of GIC and other dental materials12, Vicker’s hardness testing has been performed in this study.
Materials and Methods
20 cylindrical samples, 5 mm in length and 2.5 mm in diameter, were prepared using Type IX GIC (Fuji Company). Ten samples were prepared by conventional setting method and designated as group “A”. The other ten samples were prepared by ultrasound setting method and designated as group “B”.
Two scoops of powder and two drops of liquid were mixed according to manufacturer’s instructions. After mixing, the cement was inserted into the PTF-e mould and filled to excess. A polyester strip was used to cover both sides of the mould for 7 minutes to allow the cement to set hard. Slight pressure was applied and the bulk of extruded excess cement was removed.
For setting of the samples of group “B” an ultrasonic scaler, Cavitron Jet Plus (DENTSPLY Professional, USA) tip was placed on top of polyester strip for 45 seconds immediately after placing the cement into the PTF-e mould covered on both sides by polyester strip.8 The rationale behind applying ultrasonic tip was to supply energy to the system to have an intimate mixing of the cement.13
Finishing
All the samples were removed from the PTF-e mould after 7 minutes. They were finished with sequential number of grit paper mounted on a Struers Knuth Rotor- 3 finishing machine14 (LEL Diamond Tools International, Inc). Initially coarser grit paper # 1000 was used followed by finer paper # 2400 and finally # 4000. The samples were finished longitudinally on both sides.
Polishing
After finishing of the samples, polishing was done on Kent-3 Automatic Lapping and Polishing unit15 (Kemet International Ltd) using diamond polishing paste.16 The polished surfaces were examined under a microscope to see if the surfaces had a smooth finish.
Vicker’s Hardness Indentation
Immediately after polishing, one end of each sample was marked with red marker to identify it from the other uncolored end. Three micro-hardness indentations were made on each polished surface of the samples. First indentation was made at the colored end and noted as “Reading 1.” Second reading (Reading 2) was taken at the centre of the sample and the third (Reading 3) at the uncolored end of the samples. The indentations were performed under a load of 300 gm for 15 seconds.17
Optical microscopy
After the indentations, these samples were observed under Olympus BX-60 optical microscope (Olympus America, Inc.) using 20 x magnifications.
Taking image of the microindents
Image of the indents were taken by a camera connected to the Olympus BX-60 optical microscope (Olympus America, Inc.) and were viewed on the computer screen also connected to the microscope. The images were viewed and saved using a computer software “Pro-image.”
Vickers hardness test
This test was performed by an indenter which was pyramidal in shape having a pointed tip and a square base. The test piece was pushed against the indenter at a specific applied load (P) of 300 gm for 15 seconds. The average diagonal length (L) of the resultant indentation on the work piece was measured and the Vickers hardness number was calculated by the Equation given below
n the equation, value of K is 1.854.
Results
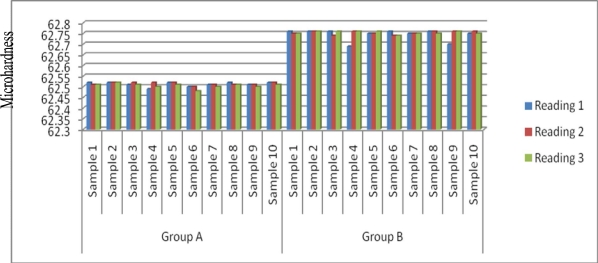
Microhardness values are plotted in figure 1 and clearly depict the difference between the samples in group “A” and group “B”. Group “B” samples (ultrasonically cured) have microhardness values higher than group “A” samples (conventionally cured).
Figure 1.
Comparison of microhardness values between Group A and Group B
Table 1 show the mean microhardness values of group “A” and group “B”. The values are statistically significant (P<0.05).
Table 1.
Mean Micro Hardness Values of Group “A” and Group “B”
| Number | Reading # 1 | Reading # 2 | Reading # 3 | |
|---|---|---|---|---|
| GROUP A | 10 | 65.512 | 62.514 | 62.505 |
| GROUP B | 10 | 62.744 | 62.753 | 62.754 |
| P-value* | < 0.05 | < 0.05 | < 0.05 | |
P-value reflects the statistical significance of the difference between two groups
Discussion
In this study Vickers hardness test was performed to measure the microhardness of the conventionally cured and ultrasonically cured samples as plotted in Figure 1 respectively to compare surface hardness of the samples set by the two methods. The ultrasonically cured material has microhardness higher than the chemically cured material. In another study ultrasonically cured samples showed higher microhardness value than conventionally set samples.13
The result of this study also matches the results of another study that reveals that the cement when set by ultrasonically energized method show decreased wear17.
In one study it was mentioned that closer mixing of polyacid and glass powder occurs with ultrasonic waves and therefore more contact occurs between glass and the acid. Also it was mentioned that ultrasonic waves diminish the mean particle size of the glass phase which causes more surface area for reaction with the acid and hence more compaction occurs between the resulting solid because of better packing arrangement of the residual glass particles.18
Hence from this study it is indicated that surface hardness of glass ionomer cement set under ultrasonic waves is more than chemically set. This can be beneficial for the dental patients as when used as a restorative material, it will have a longer lasting effect and can also be used in posterior load bearing areas.
References:
- 1.Wislon AD, Kent BE. A new translucent cement for dentistry. The glass ionomer cement. Br Dent J. 1972;99:132–135. doi: 10.1038/sj.bdj.4802810. [DOI] [PubMed] [Google Scholar]
- 2.Sidhu SK, Watson TF. Resin- modified glass ionomer materials. Am J Dent. 1995;8:59–67. [PubMed] [Google Scholar]
- 3.Anusavice KJ. Phillip’s Science of Dental Materials. 11th edition. St.Louis: Elsevier; 2004. pp. 471–479. [Google Scholar]
- 4.McCabe JF. Applied Dental Materials. 9th Edition. New Jersey, Wiley: Blackwell; 2008. pp. 285–286. [Google Scholar]
- 5.Powers JM, Sakaguchi RL, Craig RG. Craig’s restorative dental materials. 12th Edition. Toronto: Mosby; 2006. pp. 173–176. [Google Scholar]
- 6.Kilpatrick NM, McCabe JF, Murray JJ. Factors that influence the setting characteristics of encapsulated glass ionomer cements. J Dent. 1994;22:182–187. doi: 10.1016/0300-5712(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 7.Koenraads H, Van der Kroon G, Frencken JE. Compressive strength of two newly developed glass ionomer materials for use with the Atraumatic Restorative Treatment (ART) approach in class II cavities. Dent Mater. 2009;25:51–556. doi: 10.1016/j.dental.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Kleverlaan CJ, Van Duinen RN, Feilzer AJ. Mechanical properties of glass ionomer cements affected by curing methods. Dent Mater. 2004;20(1):45–50. doi: 10.1016/s0109-5641(03)00067-8. [DOI] [PubMed] [Google Scholar]
- 9.Talal A, Tanner KE, Billington R, Pearson GJ. Effect of ultrasound on the setting characteristics of glass ionomer cements studied by Fourier Transform Infrared Spectroscopy. J Mater Sci Mater Med. 2009;20:405–11. doi: 10.1007/s10856-008-3578-z. [DOI] [PubMed] [Google Scholar]
- 10.Crisp S, Wilson AD. Reactions in glass ionomer cements. V-effect of incorporating tartaric acid in the cement liquid. J Dent Res. 1976;55:1023–31. doi: 10.1177/00220345760550060401. [DOI] [PubMed] [Google Scholar]
- 11.Buschow KHJ. Encyclopedia of material science: Science and Technology. 2001;4:3565–3570. [Google Scholar]
- 12.Khouw-Liu VHW, Anstice HM, Pearson GJ. An in vitro investigation of a poly (vinyl phosphonic acid) based cement with four conventional glass-ionomer cements: Part 2: maturation in relation to surface hardness. J Dent. 1999;27:359–365. doi: 10.1016/s0300-5712(98)00062-1. [DOI] [PubMed] [Google Scholar]
- 13.Towler MR, Bushby AJ, Billington RW, Hill RG. A preliminary comparison of the mechanical properties of chemically cured and ultrasonicallycured glass ionomer cements, using nano-indentation techniques. Biomaterials. 2001;22:1401–1406. doi: 10.1016/s0142-9612(00)00297-0. [DOI] [PubMed] [Google Scholar]
- 14.Cattell MJ, Palumbo RP, Knowles JC, Clarke RL, Samarawickrama DYD. The effect of veneering and heat treatment on the flexural strength of Empress® 2 ceramics. J Dent. 2002;30:161–169. doi: 10.1016/s0300-5712(02)00013-1. [DOI] [PubMed] [Google Scholar]
- 15.Lippert F, Parker DM, Jandt KD. In vitro demineralization/remineralization cycles at human tooth enamel surfaces investigated by AFM and nanoindentation. J Colloid Interface Sci. 2004;280:442–448. doi: 10.1016/j.jcis.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Haywood VB, Heymann HO, Kusy RP, Whitley JQ, Andreaus SB. Polishing porcelain veneers: an SEM and specular reflectance analysis. Dent Mater. 1988;4:116–121. doi: 10.1016/s0109-5641(88)80003-4. [DOI] [PubMed] [Google Scholar]
- 17.Okada K, Tosaki S, Hirota K, Hume WR. Surface hardness change of restorative filling materials stored in saliva. Dent Mater. 2001;17:34–39. doi: 10.1016/s0109-5641(00)00053-1. [DOI] [PubMed] [Google Scholar]
- 18.Towler MR, Crowley CM, Hill RG, Hill RG. Investigation into the ultrasonic setting of glass ionomer cements. Part I Postulated modalities. J Mater Sci Letters. 200 [Google Scholar]