Abstract
Purpose
To estimate the incidence of age-related macular degeneration (AMD) and the association of smoking and alcohol in a population of older women.
Design
Prospective cohort study.
Methods
Subjects were women who attended the Study of Osteoporotic Fractures year 10 and year 15 follow-up clinic visits and had fundus photographs taken at both visits (n=1958; 245 Blacks and 1713 Whites. Mean age at year 10 visit=78.2 years). Forty-five degree stereoscopic fundus photographs were graded for AMD. Logistic regression was used to test whether risk factors were associated with incident AMD.
Results
The overall 5-year AMD inci dence was 24.1% (95% confidence interval [CI]: 21.7–26.6) for early and 5.7% (95% CI: 4.6–6.8) for late. Early AMD incidence in Whites ranged from 21.9% in those 74–79 years to 33.2% in those 80–84 years, but was observed at the slightly lower rate of 29.0% in subjects ≥85 years (trend p<0.0001). After confounder adjustment, alcohol consumption was significantly associated with an elevated risk of incident early AMD (odds ratio [OR] = 1.57; 95% CI: 1.18–2.11). There was an increased risk of early AMD among subjects aged 80 years or older who were smoking compared to those younger than 80 years who were not smoking (OR=5.49; 95% CI: 1.57–19.20; p for interaction=0.026).
Conclusions
The magnitude of the greater-than-additive effect of smoking on the age-adjusted risk of AMD reinforces recommendations to quit smoking even for older individuals.
Keywords: older women, age-related macular degeneration, incidence, smoking and alcohol consumption
INTRODUCTION
Age-related macular degeneration (AMD) is the leading cause of blindness in persons 65 years of age and older in the developed world.1 Age is the strongest risk factor for AMD; however, most of the populations providing incidence and prevalence data have included relatively few subjects over the age of 75 years. For example, all of the population-based studies conducted in the United States, including the Beaver Dam Eye Study and the Baltimore Eye Survey, have included fewer than 1000 subjects aged 75 years or older.2,3 Furthermore, although previous research suggests that AMD is less common in Blacks than people of European descent,4,5 there remains a paucity of studies that provide data on incidence of AMD in Blacks.
Second to age, smoking is the most consistently identified risk factor for AMD.6–7 Although multiple studies have examined the relationship of AMD to smoking and other factors,8–10 we are not aware of any previous study that has assessed interactions between smoking and age in relation to AMD.
Findings regarding the association of alcohol consumption with AMD have been inconsistent. Alcohol has been hypothesized to have both positive and negative effects on the development of AMD, with some studies showing increased AMD risk, particularly associated with heavy drinking11 or beer consumption,12,13 whereas others showed a lower risk associated with wine consumption.14
Utilizing data from the Study of Osteoporotic Fractures, the goal of the present study was to estimate the incidence of early and late AMD in older White and Black subjects, as well as to determine the association of alcohol use and smoking status with the incidence of AMD. In addition, this study sought to determine whether the effects of alcohol use or smoking may be modified by age.
METHODS
Subjects
In 1986–1988 a cohort of 9,704 White women aged 65 years or older were enrolled in the Study of Osteoporotic Fractures (SOF), a multi-center prospective cohort study originally designed to identify risk factors for osteoporotic fractures. Details of the study design have been previously published.15 Subjects were recruited from four geographic areas in the United States using community-based listings such as health plan membership lists, Department of Motor Vehicles tapes, and voter registration lists. 15 The SOF cohort was originally focused on detecting health effects in a cohort of White women, but at the year 10 visit in 1997–1998, the cohort was enhanced by the enrollment of 662 Black women at the four centers using the same recruitment methods and inclusion/exclusion criteria as for the original cohort.16 The study complied with the tenets of the Declaration of Helsinki related to the treatment of human subjects. Subjects were eligible for the present study if they attended the year 10 and year 15 follow-up clinic visits in 1997–1998 and 2002–2004, respectively, and had fundus photographs in both eyes at both visits.
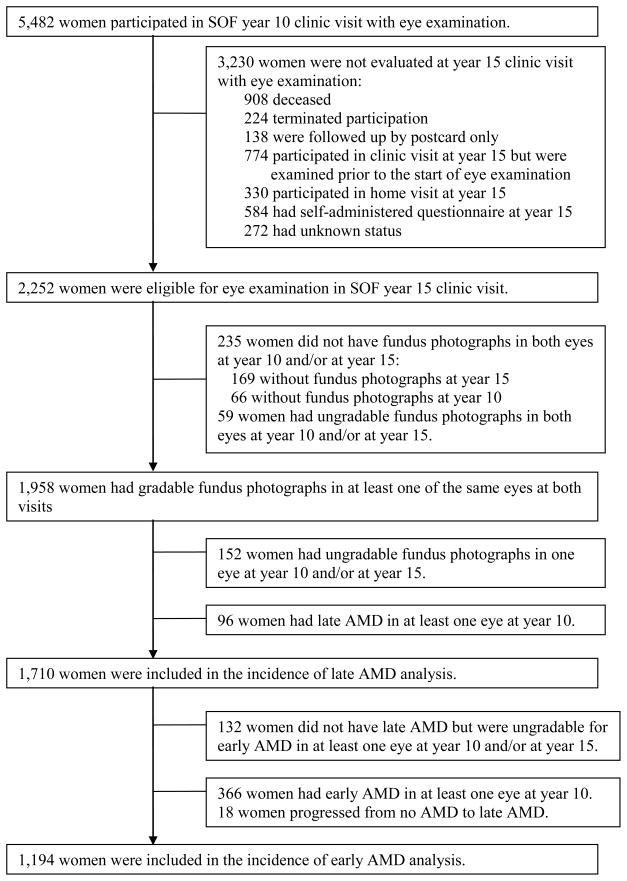
Subject selection and reasons for non-eligibility for the present study can be seen in Figure 1. Of the 5482 women attending the year 10 clinic visit, 3230 (58.9%) were not evaluated for the year 15 clinic visit with an eye examination. Of the remaining 2252 who were eligible for the present study, 294 (13%) did not have fundus photographs or had ungradable fundus photographs in both eyes at year 10 and/or year 15 visit. A comparison of baseline characteristics between participants and eligible non-participants can be found in Table 1. Participants were younger (p=.0002), more likely to walk for exercise (p=0.005), and more likely to have consumed alcohol in the previous 30 days (p=0.010) than non-participants.
Figure 1.
Flow chart of participants in the study of incidence of age-related macular degeneration (IAMD) of the Study of Osteoporotic Fractures (SOF).
Table 1.
Comparison of Baseline Characteristics Between Participants and Eligible Non-Participants in the Study of Incidence of Age-Related Macular Degeneration (IAMD) of the Study of Osteoporotic Fractures (SOF).
| Characteristics | Participants (N=1958) | Non-participants† (N=294) | P-value‡ |
|---|---|---|---|
| Study Site | |||
| Baltimore, MD | 322 (16%) | 53 (18%) | 0.18 |
| Minneapolis, MN | 661 (34%) | 94 (32%) | |
| Pittsburgh, PA | 508 (26%) | 90 (31%) | |
| Portland, OR | 467 (24%) | 57 (19%) | |
| Age (years) | |||
| Mean±SD | 78.2±3.7 | 79.3±4.0 | |
| Median | 78.0 | 78.0 | 0.0002 |
| Race | |||
| White | 1713 (87%) | 262 (89%) | 0.43 |
| Black | 245 (13%) | 32 (11%) | |
| Education (years) | |||
| Mean±SD | 12.9±2.7 | 12.8±2.6 | |
| Median | 12.0 | 12.0 | 0.72 |
| <12 years | 338 (17%) | 52 (18%) | 0.86 |
| >=12 years (High School graduate or above) | 1619 (83%) | 242 (82%) | |
| Walks for exercise | |||
| Yes | 895 (46%) | 109 (37%) | 0.005 |
| No | 1056 (54%) | 184 (63%) | |
| Current smoker | |||
| Yes | 75 (4%) | 10 (3%) | 0.72 |
| No | 1881 (96%) | 284 (97%) | |
| Any alcohol in last 30 days | |||
| Yes | 942 (48%) | 118 (40%) | 0.010 |
| No | 1015 (52%) | 176 (60%) | |
| Self-rated health status | |||
| Good/Excellent | 1668 (85%) | 244 (83%) | 0.33 |
| Fair/Poor | 290 (15%) | 50 (17%) | |
| History of diabetes | |||
| Yes | 105 (5%) | 8 (3%) | 0.054 |
| No | 1853 (95%) | 285 (97%) | |
| History of hypertension | |||
| Yes | 706 (36%) | 106 (36%) | 1.00 |
| No | 1252 (64%) | 188 (64%) |
SD=Standard deviation
Subjects had gradable fundus photographs in at least one of the same eyes at both year 10 and year 15 clinic visits.
Eligible subjects did not have fundus photographs or had ungradable fundus photographs in both eyes at year 10 and/or at year 15 clinic visits.
P-values are from Wilcoxon rank-sum tests for continuous variables and chi-square tests for categorical variables.
Of the 1958 women with gradable fundus photographs, 96 were excluded from the present analyses due to the presence of late AMD in at least one eye at the year 10 visit, and 152 were excluded due to ungradable fundus photographs in one eye at either visit. Thus, 1710 subjects were included in the present analysis for incidence of late AMD. Relevant to analyzing incidence of early AMD, there were 366 with known early AMD in at least one eye at year 10, 18 progressed from no AMD to late AMD, and 132 were not gradable for early AMD in at least one eye at either year 10 or year 15. Thus, 1194 were included in the analysis for early AMD.
Definition of incident AMD
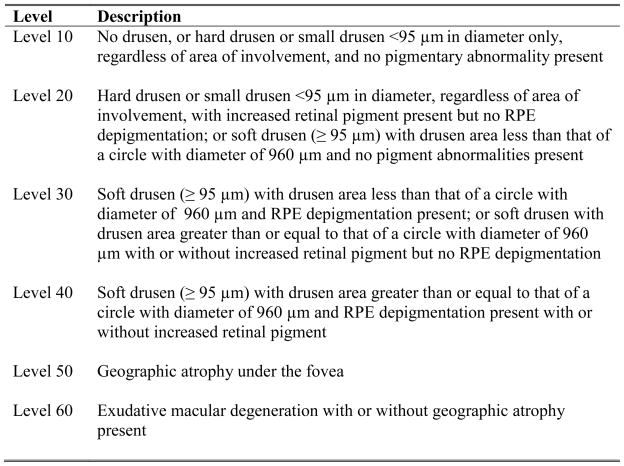
Two forty-five degree stereoscopic fundus photographs per eye were taken for each subject, and they were graded for AMD using a modification of the Wisconsin Age-Related Maculopathy Grading System (WARMGS) used in NHANES III.17 Photographs from the year 10 and 15 visits were double-graded by two independent graders in a masked fashion. AMD characteristics and severity were graded on a 6-level severity scale used in the Beaver Dam Eye Study18 and modified for use with 45-degree stereoscopic photographs (Figure 2). Incident late AMD was defined as the presence of sub-foveal geographic atrophy or choroidal neovascularization (level 50 or 60) in at least one eye at year 15 when late AMD was not present in either eye at year 10. Incident early AMD was defined as the presence of soft drusen (≥95 microns (μm) in diameter) and 1) drusen area < that of a circle with a diameter of 960 μm and retinal pigment epithelial depigmentation present; or 2) drusen area ≥ that of a circle with diameter 960 μm with or without pigmentary abnormalities (i.e. level 30 or 40) in at least one eye and without late AMD in either eye at year 15 in subjects with no AMD (level 10 or 20) in either eye at year 10. In cases of a discrepancy in the categorization of an eye as no AMD, early AMD, or late AMD, or in cases where photographs were deemed not gradable by at least one grader, photographs were evaluated by a retina specialist whose grading was taken as the final grade.
Figure 2.
Age-related macular degeneration (AMD) 6-level severity scale used in the study of incidence of age-related macular degeneration (IAMD) of the Study of Osteoporotic Fractures (SOF).
RPE=Retinal pigment epithelium
No AMD = level 10 or 20 in both eyes
Early AMD = level 30 or 40 in at least one eye, but neither levels 50 or 60 in either eye
Late AMD = level 50 or 60 in at least one eye
From July 2003 to January 2004, fundus photographs of 871 subjects were graded independently by both graders. Of those, a stratified quality control sample of 100 subjects was selected (30 subjects from the late AMD, early AMD, and non-AMD groups, respectively, and 10 subjects whose photographs were not gradable or gradable in only one eye). To measure internal reliability of the photograph grading, fundus photographs from this sample were mixed with the fundus photographs for regular grading and sent to both graders three additional times. All discrepancies were adjudicated by the retina specialist. The kappas for the status of AMD in subjects increased from 0.81 (baseline grading vs. third grading) to 0.91 (second grading vs. third grading), where baseline refers to the original grade assigned prior to inclusion in the stratified sample. To measure external reliability, photographs from the same quality control sample were sent for grading by the Wisconsin Reading Center. The kappas were relatively stable and ranged from 0.65 (Wisconsin vs. second grading) to 0.75 (Wisconsin vs. baseline grading). These kappas are indicative of good to very good agreement, and are consistent with other population based studies of AMD.
Assessment of risk factors
Alcohol consumption was ascertained with the year 10 self-administered questionnaire and was defined as consumption of any alcoholic beverage in the last 30 days (yes/no). Smoking status was ascertained with the year 10 self-administered questionnaire by asking subjects, “Do you currently smoke cigarettes” (yes/no).
Assessment of potential confounders
Potential confounders measured included age at the year 10 clinic visit, clinical site of enrollment, ethnicity, history of hypertension, self-reported diabetes, years of education, self-reported health status, and walking for exercise. Ethnicity was self-reported as either White or Black. History of hypertension, self-reported health status, and walking for exercise were ascertained with the year 10 self-administered questionnaire. For history of hypertension, subjects were asked if they were ever told by a doctor that they had hypertension. For self-reported health status, subjects were asked to rate their overall health, compared to others their own age, as very excellent, excellent, good, fair, poor, or very poor. For the purposes of the present analysis, this variable was dichotomized as excellent/good vs. fair/poor. History of diabetes was ascertained with the year 10 self-administered questionnaire by asking subjects, “Has a doctor ever told you that you have diabetes?” Regarding walking for exercise, subjects were asked if they walk for exercise (yes/no), defined as walking one block or more without stopping. Years of education was ascertained with the baseline self-administered questionnaire for White subjects and the year 10 questionnaire for Blacks.
Statistical Analysis
All statistical analyses were performed using SAS version 9.1 statistical software (SAS institute, Cary, NC). Baseline characteristics and unadjusted incidence rates were compared using Chi-square tests, T tests or Wilcoxon Rank-Sum tests when appropriate. Age-adjusted, age and race-adjusted, and multivariable-adjusted logistic regression models were used to test whether smoking or alcohol consumption were associated with incident early or late AMD, respectively. Variables that were significantly associated with each outcome in bivariate analyses at the significance level of 0.25, respectively, and/or those associated with AMD in the literature, were entered into the full multivariable model. Clinically relevant variables were retained in the model a priori, and any remaining variables were then removed after assessment of confounding to obtain the final model. The final model included alcohol consumption, current smoking, age, ethnicity, history of hypertension, study recruitment site, and walking for exercise. Interactions between current smoking and alcohol consumption, age and smoking, and age and alcohol consumption were tested by entering cross product terms in the logistic regression model and examining their effects.
To address concerns of selection bias primarily due to loss to follow-up, we performed analyses using attrition weights among Year 10 participants19 to assess the potential impact of attrition bias on the observed results. This method is based on the assumption that subjects with certain profiles of characteristics are more likely to be included in the analysis than others. A logistic regression model is developed to predict the likelihood of a subject being included in the analysis conditional on a set of observed covariates, and attrition weights for each subject are calculated by taking the inverse of the predicted probability. The attrition weights are then applied as sampling weights for each subject who was included in the analyses. From the target population of those attending the year 10 clinic visit, we calculated attrition weights from the predicted likelihood of having gradable photos at both year 10 and year 15, conditional on covariates including age at year 10, study recruitment site, education, self-rated health status, alcohol consumption, smoking, body mass index, walking for exercise, walking speed, average grip strength, use arms to stand up, hours spent with feet up, functional disability, number of chronic diseases at year 10, presence of breast cancer, history of falls, history of any fractures, depression, cognitive status, and current use of certain medications, including anticonvulsant drugs, long-acting benzodiazepines, hypotensive and/or vasodilating agents, beta and alpha blockers, anxiolytics, diuretics-thiazide. The attrition weights were calculated separately for Whites and Blacks.
RESULTS
Both unweighted and attrition weight- adjusted analyses were performed, and the results were similar from both analyses. Except for the descriptive tables, in which both results are presented, only weighted results are presented. Of 1194 subjects with no AMD at year 10 who were gradable for early AMD in both eyes at year 15, there were 286 (24.0%) with incident early AMD. The 5-year incidence of early AMD was 24.1% (95% confidence interval [CI]: 21.7–26.6). Of 1710 subjects with no AMD or early AMD at year 10 who were gradable for late AMD in both eyes at year 15, there were 94 (5.5%) who developed incident late AMD. The 5-year incidence of late AMD was 5.7% (95% CI: 4.6–6.8). Incidence of early AMD and late AMD by age and ethnicity is found in Tables 2 and 3. Overall, incidence of early AMD was higher for Whites compared to Blacks (25.1% vs. 16.7%, p=0.022), as was incidence of late AMD (6.2% vs. 0.9%, p=0.002). Only two of the 217 Black women at risk had incident late AMD; these women were 71 and 73 years old, respectively. White participants (median age = 78 years; range: 74–92 years) as a group were older than Black participants (median age = 73 years; range 65–94 years). Given that the youngest White participant was 74 years old, age-specific incidence was compared between Whites and Blacks for subjects aged 74 years and older by expanding the 75–79 year age category in Blacks to include subjects aged 74 years. Incidence of early AMD in Blacks age 74–79 was 20.0%. Age-specific incidence was higher for Whites than Blacks for both outcomes, with the exception of early AMD in subjects aged 85 and older; however, there were just two Blacks at risk in this age category, preventing reliable inferences.
Table 2.
Incidence of Early and Late Age-Related Macular Degeneration (AMD) in White Women in the Study of Osteoporotic Fractures.
| Age Group | Number of Women at Risk | Incidence N (%) | 95% CI of Incidence | Weighted Incidence* (95% CI) |
|---|---|---|---|---|
| Early AMD | ||||
| Age (years) | ||||
| 74–79† | 724 | 158 (21.8%) | 18.9–25.0 | 21.9 (18.8–25.0) |
| 80–84 | 271 | 90 (33.2%) | 27.6–39.2 | 33.2 (27.6–38.7) |
| 85+ | 38 | 11 (28.9%) | 15.4–45.9 | 29.0 (14.8–43.1) |
| Total | 1033 | 259 (25.1%) | 22.5–27.8 | 25.3 (22.6–28.0) |
| Trend p-value‡ | 0.0007 | 0.0009 | ||
| Late AMD | ||||
| Age (years) | ||||
| 74–79† | 991 | 41 (4.1%) | 3.0–5.6 | 4.3 (3.0–5.6) |
| 80–84 | 426 | 40 (9.4%) | 6.8–12.6 | 9.4 (6.7–12.2) |
| 85+ | 76 | 11 (14.5%) | 7.5–24.4 | 14.5 (6.7–22.3) |
| Total | 1493 | 92 (6.2%) | 5.0–7.5 | 6.4 (5.1–7.6) |
| Trend p-value‡ | <0.0001 | <0.0001 |
AMD=Age-related macular degeneration; CI=Confidence interval.
Estimates were weighted by attrition weights.
Includes 14 subjects at risk for incident early AMD who were 74 years old and four (28.6%) of them had incident early AMD, and 22 subjects at risk for incident late AMD who were 74 years old and one (4.5%) of them had incident late AMD.
Cochran-Armitage trend test.
Table 3.
Incidence of Early Age-Related Macular Degeneration (AMD) in Black Women in the Study of Osteoporotic Fractures.*
| Age Group | Number of Women at Risk | Incidence N (%) | 95% CI of Incidence | Weighted Incidence† (95% CI) |
|---|---|---|---|---|
| Early AMD | ||||
| Age (years) | ||||
| 65–69 | 15 | 3 (20.0%) | 4.3–48.1 | 17.9 (0.0–38.8) |
| 70–74 | 90 | 15 (16.7%) | 9.6–26.0 | 17.4 (9.5–25.4) |
| 75–79 | 38 | 6 (15.8%) | 6.0–31.3 | 15.7 (4.1–27.3) |
| 80–84 | 16 | 1 (6.3%) | 0.2–30.2 | 6.4 (0.0–18.3) |
| 85+ | 2 | 2 (100%) | 47.8–100 | 100 |
| Total | 161 | 27 (16.7%) | 11.4–23.5 | 17.0 (11.1–22.9) |
| Trend p-value‡ | 0.85 | 0.79 |
AMD=Age-related macular degeneration; CI=Confidence interval.
Only two (0.9%) of the 217 Blacks at risk had incident late AMD, and both subjects were 71 and 73 years old, respectively. Estimates for incident late AMD and age-stratified incident early AMD might be imprecise and should be interpreted with caution due to the small sample size of Blacks.
Estimates were weighted by attrition weights. Confidence interval was not available for the age group of 85+ years when attrition weights were used.
Cochran-Armitage trend test.
In Whites, incidence of early AMD ranged from 21.9% (95% CI: 18.8–25.0%) in those 74–79 years to 33.2% (95 % CI: 27.6–38.7%) in those 80–84 years with a lower incidence rate of 29.0% (95% CI: 14.8–43.1%) in subjects 85 years of age and older (trend p<0.0001). In contrast, incidence of early AMD in Blacks was estimated to be 17.9% (95% CI: 0.0–38.8%) in those 65–69 years of age to 6.4% (95% CI: 0.0–18.3%) in those 80–84 years of age (trend p=0.79); given the relatively few Blacks at risk in the 65–69 and 80–84 age groups, results in these groups should be interpreted with caution. Incidence of late AMD in Whites ranged from 4.3% (95% CI: 3.0–5.6%) in subjects aged 74–79 years to 14.5% (95% CI: 6.7–22.3%) in those aged 85 years and older (trend p<0.0001).
Early and late AMD incidence by baseline characteristics are shown in Table 4. Increasing age and White race were associated with incidence of both early AMD (p<0.0001 and p=0.022, respectively) and late AMD (p<0.0001 and p=0.002, respectively). In addition, any alcohol consumption in the previous 30 days (p=0.0002) was associated with incidence of early AMD (Table 4).
Table 4.
Comparison of Baseline Characteristics by the Status of Incident Age-Related Macular Degeneration (AMD) in the Study of Osteoporotic Fractures, Univariate Analyses.
| Characteristics | No AMD at both visits† | Incident Early AMD | P-value* | No or Early AMD at both visits | Incident Late AMD | P-value* |
|---|---|---|---|---|---|---|
| Total | 908 | 286 | 1616 | 94 | ||
| Study Site | ||||||
| Baltimore, MD | 156 (78%) | 43 (22%) | 0.13 | 259 (94%) | 16 (6%) | 0.45 |
| Minneapolis, MN | 307 (73%) | 116 (27%) | 555 (96%) | 25 (4%) | ||
| Pittsburgh, PA | 239 (80%) | 61 (20%) | 419 (94%) | 26 (6%) | ||
| Portland, OR | 206 (76%) | 66 (24%) | 383 (93%) | 27 (7%) | ||
| Age (years) | ||||||
| Mean±SD | 77.4±3.4 | 78.6±3.4 | 77.9±3.6 | 80.0±3.5 | ||
| Median | 77.0 | 78.0 | <0.0001 | 77.0 | 80 | <0.0001 |
| Race | ||||||
| White | 774 (75%) | 259 (25%) | 0.022 | 1401 (94%) | 92 (6%) | 0.002 |
| Black | 134 (83%) | 27 (17%) | 215 (99%) | 2 (1%) | ||
| Education (years) | ||||||
| <12 years | 155 (78%) | 43 (22%) | 0.43 | 279 (94%) | 17 (6%) | 0.84 |
| >=12 years (High School graduate or above) | 753 (76%) | 242 (24%) | 1336 (95%) | 77 (5%) | ||
| Walks for exercise | ||||||
| Yes | 399 (74%) | 142 (26%) | 0.09 | 734 (95%) | 40 (5%) | 0.57 |
| No | 505 (78%) | 143 (22%) | 876 (94%) | 54 (6%) | ||
| Current smoker | ||||||
| Yes | 32 (76%) | 10 (24%) | 0.98 | 62 (95%) | 3 (5%) | 0.75 |
| No | 874 (76%) | 276 (24%) | 1552 (94%) | 91 (6%) | ||
| Any alcohol in last 30 days | ||||||
| Yes | 422 (72%) | 168 (28%) | 0.0002 | 787 (95%) | 42 (5%) | 0.45 |
| No | 486 (81%) | 117 (19%) | 828 (94%) | 52 (6%) | ||
| Self-rated health status | ||||||
| Good/Excellent | 769 (76%) | 239 (24%) | 0.65 | 1380 (94%) | 83 (6%) | 0.44 |
| Fair/Poor | 139 (75%) | 47 (25%) | 236 (96%) | 11 (4%) | ||
| History of diabetes | ||||||
| Yes | 59 (84%) | 11 (16%) | 0.10 | 88 (96%) | 4 (4%) | 0.62 |
| No | 849 (76%) | 275 (24%) | 1528 (94%) | 90 (6%) | ||
| History of hypertension | ||||||
| Yes | 309 (74%) | 111 (26%) | 0.14 | 584 (95%) | 33 (5%) | 0.84 |
| No | 599 (77%) | 175 (23%) | 1032 (94%) | 61 (6%) |
AMD=Age-related macular degeneration; SD=Standard deviation.
P-values were obtained from T tests for age and chi-square tests for all other variables.
Excludes 18 subjects with late AMD at year 10 visit.
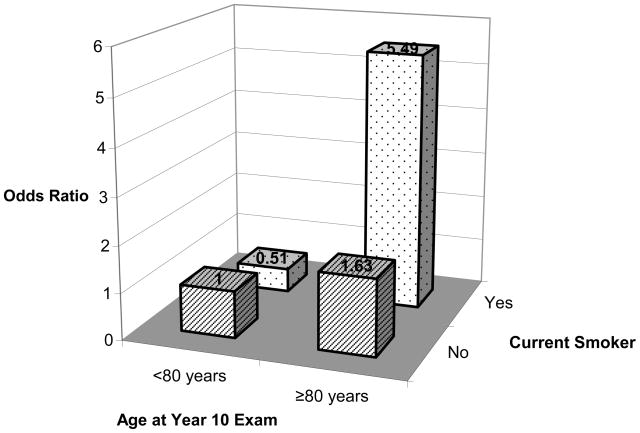
The age-adjusted, age and race-adjusted, and multivariable-adjusted associations of alcohol consumption and smoking with early AMD are shown in Table 5. Alcohol consumption was significantly associated with evevated risk of early AMD in all models tested. The multivariable model showed that subjects with any alcohol consumption in the previous 30 days were approximately 60% more likely to develop early AMD than those without (odds ratio [OR] = 1.57; 95% CI: 1.18–2.11). No association of current smoking to early or late AMD was seen when smoking was examined alone. However, there was a significant interaction between age at year 10 and smoking (Figure 3 and Table 6), suggesting substantially increased risk of early AMD among subjects aged ≥80 years who were smoking compared to those aged <80 years who were not smoking (OR=5.49; 95% CI: 1.57–19.20; p for interaction=0.026) as opposed to a moderately greater risk of early AMD among non-smoking subjects 80 years compared to those aged <80 who were not smoking (OR=1.63.; 95% CI: 1.22–2.18; p =.001) (Table 6). None of the other interactions tested was significant (p=0.35 for interaction between age and alcohol, and p=0.46 for interaction between smoking and alcohol).
Table 5.
Risk factors for incident Age-Related Macular Degeneration (AMD) in the Study of Osteoporotic Fractures Eye Study.*
| Risk Factor | Unadjusted | Age-adjusted | Age and Race-adjusted | Multivariable-adjusted† |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Early AMD | ||||
| Any alcohol in last 30 days: Yes vs. No | 1.67 (1.27–2.19) | 1.58 (1.20–2.09) | 1.58 (1.20–2.08) | 1.57 (1.18–2.11) |
| Current smoker: Yes vs. No | 0.99 (0.48–2.04) | 1.13 (0.54–2.35) | 1.13 (0.55–2.36) | 1.11 (0.53–2.35) |
| Late AMD | ||||
| Any alcohol in last 30 days: Yes vs. No | 0.86 (0.57–1.31) | 0.79 (0.52–1.21) | 0.75 (0.49–1.15) | 0.79 (0.51–1.23) |
| Current smoker: Yes vs. No | 0.79 (0.24–2.58) | 0.99 (0.30–3.25) | 1.01 (0.31–3.33) | 1.04 (0.31–3.47) |
AMD=Age-related macular degeneration; OR=Odds ratio; CI = Confidence interval.
Estimates were weighted by attrition weights.
Model includes alcohol consumption, current smoking, age, race, hypertension, walks for exercise, and study sites.
Figure 3.
Effects of interaction between age at year 10 and smoking history on risk for incident early age-related macular degeneration (AMD) in the study of incidence of age-related macular degeneration (IAMD) of the Study of Osteoporotic Fractures (SOF). (P-value for interaction=0.026).
Table 6.
Effect of Age and Smoking on Incidence of Early Age-Related Macular Degeneration (AMD).
| Age | Current Smoker | OR | 95% CI | P-value |
|---|---|---|---|---|
| <80 years | No | 1.0 (Reference) | ||
| <80 years | Yes | 0.51 | 0.17 – 1.50 | 0.22 |
| ≥80 years | No | 1.63 | 1.22 – 2.18 | 0.001 |
| ≥80 years | Yes | 5.49 | 1.57 – 19.20 | 0.008 |
Neither alcohol consumption nor smoking was significantly associated with incidence of late AMD in any of the models tested (Table 5). None of the interactions tested in the late AMD models was significant (p=0.22 for interaction between age and alcohol, p=0.98 for interaction between age and smoking, and p=0.98 for interaction between smoking and alcohol).
DISCUSSION
Our finding of a greater-than-additive risk of early AMD associated with current smoking among subjects ≥ 80 years leads us to conclude that even older individuals stand to benefit from quitting smoking. Prior studies on the incidence of AMD and smoking were not able to address this greater risk in the oldest old because of the smaller numbers of individuals in this age group in these studies.20–28 Although the number of smokers in the study was not large, the magnitude of the difference in early AMD incidence (4 out of 42 or 12.5% among smokers <80 versus 6 out of 10 or 60% among smokers ≥ 80) was sufficient both to imply statistical significance and to be a source of great concern. Cigarette smoking has been hypothesized to increase the risk for AMD by reducing serum antioxidant levels, 29 altering choroidal blood flow,27 and decreasing retinal luteal pigments.30 Women in this study represent a survivor cohort, which might be one reason why smoking was not associated with late AMD. The incidence rate for smokers under age 80 gave rise to an estimated odds ratio less than 1.0 that was not significant with reference to non-smokers under age 80; while this finding could be written off as a small-sample phenomenon, it also might be interpreted as suggesting that the underlying causal mechanisms are not simple. Still, our findings overall are consistent with recommendations to quit smoking even for older individuals.
Our results are also consistent with several studies that have found an increased risk of AMD associated with alcohol consumption.31 The Beaver Dam Eye Study found beer consumption in the past year to be associated with an increased odds of retinal pigment degeneration (OR=1.13; 95% CI: 1.02–1.25) and exudative AMD (OR=1.41; 95% CI: 1.05–1.88) cross-sectionally.12 In the 5-year follow-up study, beer consumption in men was also associated with incidence of soft indistinct drusen (OR per 100g/wk consumed=1.18; 95% CI: 1.00–1.39) and confluence of soft drusen (OR 1.33; 95% CI: 1.04–1.70), and former heavy drinking was associated with increased drusen area (OR=1.44; 95% CI: 1.09–1.89) in men and women combined.13 In contrast, the 10-year follow-up study showed no associations with early AMD lesions, but instead showed that current or past heavy drinkers (≥4 servings per day) had an increased risk of developing exudative AMD (OR=6.94; 95% CI: 1.85–26.1).11 More recently, the Reykjavik study found that those who consumed at least 1 drink per month were at increase risk of developing early AMD,24 and the Los Angeles Latino Eye Study (LALES) study also reported significant associations of heavy alcohol consumption with prevalence of early and late AMD.25 Furthermore, the Copenhagen City Eye study found that those drinking >250g/week (more than approximately 3 drinks per day) were at increased risk of developing any AMD over a 14-year follow-up period (OR=4.61; 95% CI: 1.1–19.2).26 Interestingly, among studies that examined type of alcohol consumed, several reported higher risk associated with beer,12,13 yet lower risk associated with wine.13,14,24 We did not examine alcohol consumption by type. It is possible that the type of alcohol consumed is important in assessing AMD risk, and thus requires further investigation. Although the Beaver Dam Eye Study, Blue Mountains Eye Study, and Rotterdam Study used similar classification criteria for AMD, there were some differences in their findings.20–22
The greater incidence of early AMD in the White cohort compared to the Black cohort is intriguing. Other eye diseases show different prevalence rates across ethnic groups,32–33 suggesting that the differences observed here are real and not an artifact of the cohorts starting at different times. We are not aware of a biological mechanism that might explain the difference, but we believe that investigation of potential mechanisms would be a worthwhile direction for future research.
Our cohort was considerably older than those previously studied. When comparing to age groups used in other studies, our results were fairly similar to those observed for women in the Blue Mountains Study and for both genders combined in the Rotterdam study (the Rotterdam Study did not provide gender-specific data, although rates between males and females were reported to be similar and not statistically significantly different). AMD incidence among women aged 75 and older was lower in SOF than in Beaver Dam; however, the classification criteria for early AMD in Beaver Dam was more liberal, including any type of drusen associated with retinal pigmentary abnormalities. To our knowledge, the Barbados Eye Study is the only study to provide AMD incidence data for people of African origin.23 Comparisons to the Barbados Eye Study are made difficult by the older age distribution of SOF as well as differences in the AMD classification criteria used, particularly for early AMD. Incidence in the Barbados Eye Study was reported in 10-year age strata from ages 40 to 69, and for all subjects aged 70 and older combined. Thus, of the 13 women in the Barbados Eye Study that were 70 years of age and older, there were no incident cases of early macular changes, compared to an incidence of 16% in SOF. In terms of late AMD, our estimates were similar to those observed in Beaver Dam, but somewhat higher than those observed in Blue Mountains or Rotterdam (Table 7). Although AMD incidence is strongly associated with increasing age, AMD may not be an inevitable part of the aging process because the incidence of early AMD leveled off at around age 85, similar to the pattern observed for Alzheimer’s disease.34 Although the explanation of this pattern may relate to our evaluations being based on a survivor cohort, the same pattern was observed both in unweighted analyses and in analyses that incorporated attrition weights.
Table 7.
Comparison of Early and Late AMD Incidence in Whites among Different Population-Based Studies.*
| SOF | Blue Mountains21† | Rotterdam22 | Beaver Dam20† | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Early (%) | Late (%) | Age | Early (%) | Late (%) | Age | Early (%) | Late (%) | Age | Early (%) | Late (%) |
| 74–79 | 21.9 | 4.3 | 70–79 | 19.1 | 2.4 | 75–79 | 14.7 | 1.9 | 75+ | 28.8 | 7.6 |
| 80+ | 32.6 | 10.2 | 80+ | 17.7 | 8.8 | 80+ | 22.5 | 3.4 | |||
AMD=Age-related macular degeneration; SOF= Study of Osteoporotic Fractures.
There are some differences in the classification of early AMD among different population-based studies.
Estimates are for women only.
Limitations of our study include that the results are not applicable to all older adults since the participants were women and represented a relatively healthy population. In addition, there were only 114 women 85 years or older in the cohort, which resulted in relatively wide 95% confidence intervals for estimates. Data on past smoking exposure and alcohol consumption were not available, precluding us from commenting on risks associated with cumulative exposure. Another limitation is that measurements of confounding factors, including current smoking exposure and current alcohol consumption, were based on self-report.
This study does have several strengths. This was a prospective study, and therefore exposure was likely to precede disease onset. In addition, the population studied was considerably older than others to date, allowing for examination of AMD risk in the oldest old. All of the photographs in SOF were double-graded whereas in other population-based studies only a random sample of the cohort was double-graded and the rest of the cohort was graded once. The masked, double-grading of the cohort, as well as the adjudication of discrepancies by a retina specialist, should increase the study’s precision and diminish the likelihood of disease misclassification. Lastly, we addressed concerns about attrition bias by performing attrition-weight analyses.
In conclusion, this investigation adds to the evidence base pointing to AMD-related concerns associated with smoking and alcohol use, both modifiable risk factors. The greater-than-additive risk of AMD associated with smoking among those 80 years or older compared to those under age 80 is a particularly noteworthy finding that reinforces recommendations to quit smoking even for older adults. Additional research in older populations would be valuable to refine our understanding of the underlying risks related to AMD.
Acknowledgments
Funding/Support: Supported by NIH Grant EY013626-03 and Research to Prevent Blindness. The Study of Osteoporotic Fractures (SOF) is supported by Public Health Service research grants from the National Institutes of Health (AG05407, AR35582, AG05394, AR35584, AR35583, R01 AG005407, R01 AG027576-22, 2 R01 AG005394-22A1, 2 R01 AG027574-22A1). Support for Dr. Mangione was also provided by the UCLA Center for Health Improvement in Minority Elders/Resource Centers for Minority Aging Research, National Institutes of Health, National Institute of Aging (AG-02-004).
Other Acknowledgments: None.
Footnotes
Financial Disclosures: None.
Author Contribution: Design and conduct of the study (ALC, SRC, JAC, KEE, KLS, MCH, CMM); collection and management (ALC, SRC, JAC, KEE, KLS, MCH, CMM); analysis and interpretation of the data (ALC, RLS, SRC, FY, JAC, KEE, KLS, MCH, KLP, ET, CMM); preparation (ALC, RLS, FY); review and approval of the manuscript (ALC, RLS, SRC, FY, JAC, KEE, KLS, MCH, KLP, ET, CMM).
Statement about Conformity with Author Information: Institutional Review Board approvals were obtained from the University of California, Los Angeles, the University of California, San Francisco, the University of Maryland, the University of Minnesota, Kaiser Permanente Center for Health Research in Portland, Oregon, and the University of Pittsburgh prior to the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klein R, Wang Q, Klein BE, et al. The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity. Invest Ophthalmology Vis Sci. 1995;36:182–191. [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–43. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 3.Rahmani B, Tielsch JM, Katz J, et al. The cause-specific prevalence of visual impairment in an urban population. The Baltimore Eye Survey. Ophthalmology. 1996;103:1721–1726. doi: 10.1016/s0161-6420(96)30435-1. [DOI] [PubMed] [Google Scholar]
- 4.Friedman DS, Katz J, Bressler NM, et al. Racial differences in the prevalence of age-related macular degeneration: the Baltimore Eye Survey. Ophthalmology. 1999;106:1049–1055. doi: 10.1016/S0161-6420(99)90267-1. [DOI] [PubMed] [Google Scholar]
- 5.Klein R, Klein BE, Jensen SC, et al. Age-related maculopathy in a multiracial United States population: the National Health and Nutrition Examination Survey III. Ophthalmology. 1999;106:1056–1065. doi: 10.1016/S0161-6420(99)90255-5. [DOI] [PubMed] [Google Scholar]
- 6.Klein R, Klein BE, Linton KL, DeMets DL. The Beaver Dam Eye Study: the relation of age-related maculopathy to smoking. Am J Epidemiol. 1993;137:190–200. doi: 10.1093/oxfordjournals.aje.a116659. [DOI] [PubMed] [Google Scholar]
- 7.Clemons TE, Molton RC, Klein R, Seddon JM, Ferris FL., 3rd Age-Related Eye Disease Study Reseradch Group. Risk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology. 2005;112:533–539. doi: 10.1016/j.ophtha.2004.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seddon JM, Reynolds R, Maller J, et al. Prediction Model for Prevalence and Incidence of Advanced Age-Related Macular Degeneration Based on Genetic, Demographic, and Environmental Variables. Invest Ophthalmol Vis Sci. 2009;50:2044–2053. doi: 10.1167/iovs.08-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan JSL, Paul Mitchell O, Kifley A, et al. Smoking and the Long-term Incidence of Age-Related Macular Degeneration: The Blue Mountains Eye Study. Arch Ophthalmology. 2007;125:1089–1095. doi: 10.1001/archopht.125.8.1089. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt S, Haines JL, Postel EA, et al. Joint effects of smoking history and APOE genotypes in age-related macular degeneration. Mol Vis. 2005;11:941–949. [PubMed] [Google Scholar]
- 11.Klein R, Klein BE, Tomany SC, Moss SE. Ten-year incidence of age-related maculopathy and smoking and drinking: the Beaver Dam Eye Study. Am J Epidemiol. 2002;156:589–598. doi: 10.1093/aje/kwf092. [DOI] [PubMed] [Google Scholar]
- 12.Ritter LL, Klein R, Klein BE, Mares-Perlman JA, Jensen SC. Alcohol use and age-related maculopathy in the Beaver Dam Eye Study. Am J Ophthalmology. 1995;120:190–196. doi: 10.1016/s0002-9394(14)72607-8. [DOI] [PubMed] [Google Scholar]
- 13.Moss SE, Klein R, Klein BE, Jensen SC, Meuer SM. Alcohol consumption and the 5-year incidence of age-related maculopathy: the Beaver Dam eye study. Ophthalmology. 1998;105:789–794. doi: 10.1016/S0161-6420(98)95016-3. [DOI] [PubMed] [Google Scholar]
- 14.Obisesan TO, Hirsch R, Kosoko O, Carlson L, Parrott M. Moderate wine consumption is associated with decreased odds of developing age-related macular degeneration in NHANES-1. J Am Geriatr Soc. 1998;46:1–7. doi: 10.1111/j.1532-5415.1998.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 15.Cummings SR, Nevitt MC, Browner WS, et al. Study of Osteoporotic Fractures Research Group. Risk factors for hip fracture in white women. N Engl J Med. 1995;332:767–73. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 16.Cauley JA, Lui LY, Ensrud KE, Zmuda JM, Stone KL, Hochberg MC, Cummings SR. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA. 2005;293:2102–2108. doi: 10.1001/jama.293.17.2102. [DOI] [PubMed] [Google Scholar]
- 17.Klein R, Davis MD, Magli YL, et al. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98:1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 18.Klein R, Klein BE, Jensen SC, Cruickshanks KJ. The relationship of ocular factors to the incidence and progression of age-related maculopathy. Arch Ophthalmology. 1998;116:506–513. doi: 10.1001/archopht.116.4.506. [DOI] [PubMed] [Google Scholar]
- 19.Afifi AA, Kotlerman JB, Ettner SL, Cowan M. Methods for improving regression analysis for skewed continuous or counted responses. Annu Rev Public Health. 2007;28:95–111. doi: 10.1146/annurev.publhealth.28.082206.094100. [DOI] [PubMed] [Google Scholar]
- 20.Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21. doi: 10.1016/s0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell P, Wang JJ, Foran S, Smith W. Five-year incidence of age-related maculopathy lesions: the Blue Mountains Eye Study. Ophthalmology. 2002;109:1092–1097. doi: 10.1016/s0161-6420(02)01055-2. [DOI] [PubMed] [Google Scholar]
- 22.van Leeuwen R, Klaver CC, Vingerling JR, Hofman A, de Jong PT. The risk and natural course of age-related maculopathy: follow-up at 6 ½ years in the Rotterdam study. Arch Ophthalmology. 2003;121:519–26. doi: 10.1001/archopht.121.4.519. Erratum in: Arch Ophthalmology 2003;121:955. [DOI] [PubMed] [Google Scholar]
- 23.Leske MC, Wu SY, Hyman L, Hennis A, Nemesure B, Schachat AP Barbados Eye Studies Group. Four-year incidence of macular changes in the Barbados Eye Studies. Ophthalmology. 2004;111:706–11. doi: 10.1016/j.ophtha.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Arnarsson A, Sverrisson T, Stefansson E, et al. Risk factors for five-year incident age-related macular degeneration: the Reykjavik Eye Study. Am J Ophthalmology. 2006;142:419–28. doi: 10.1016/j.ajo.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Varma R, Fraser-Bell S, Tan S, Klein R, Azen SP Los Angeles Latino Eye Study Group. Prevalence of age-related macular degeneration in Latinos: the Los Angeles Latino eye study. Ophthalmology. 2004;111:1288–97. doi: 10.1016/j.ophtha.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 26.Buch H, Vinding T, la Cour M, Jensen GB, Prause JU, Nielsen NV. Risk factors for age-related maculopathy in a 14-year follow-up study: the Copenhagen City Eye Study. Acta Ophthalmology Scand. 2005;83:409–418. doi: 10.1111/j.1600-0420.2005.00492.x. [DOI] [PubMed] [Google Scholar]
- 27.Bettman JW, Fellows V, Chao P. The effect of cigarette smoking on the intraocular circulation. AMA Arch Ophthalmology. 1958;59:481–488. doi: 10.1001/archopht.1958.00940050037002. [DOI] [PubMed] [Google Scholar]
- 28.Wang JJ, Tomany SC, van Leeuwen R, Klein R, Mitchell P, Vingerling JR, Klein BEK. Risk Factors for Incident Age-Related Macular Degeneration. Pooled Findings from 3 Continents. Ophthalmology. 2004;111:1280–1287. doi: 10.1016/j.ophtha.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Alberg A. The influence of cigarette smoking on circulating concentrations of antioxidant micronutrients. Toxicology. 2002;180:121–137. doi: 10.1016/s0300-483x(02)00386-4. [DOI] [PubMed] [Google Scholar]
- 30.Hammond BR, Jr, Wooten BR, Snodderly DM. Cigarette smoking and retinal carotenoids: implications for age-related macular degeneration. Vision Res. 1996;36:3003–3009. doi: 10.1016/0042-6989(96)00008-9. [DOI] [PubMed] [Google Scholar]
- 31.Chong EWT, Kreis AJ, Wong TY, Simpson JA, Guymer RH. Alcohol Consumption and the Risk of Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis. Am J Ophthalmology. 2008;145:707–715. doi: 10.1016/j.ajo.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Rudnicka AR, Mt-Isa S, Owen CG, Cook DG, Ashby D. Variations in primary open-angle glaucoma prevalence by age, gender, and race: a Bayesian meta-analysis. Invest Ophthalmol Vis Sci. 2006;47:4254–4261. doi: 10.1167/iovs.06-0299. [DOI] [PubMed] [Google Scholar]
- 33.Leske MC, Chylack LT, Wu SY The Lens Opacities Case-Control Study Group. The Lens Opacities Case-Control Study: Risk Factors for Cataract. Arch Ophthalmology. 1991;109:244–251. doi: 10.1001/archopht.1991.01080020090051. [DOI] [PubMed] [Google Scholar]
- 34.Lautenschlager NT, Cupples LA, Rao VS, et al. Risk of dementia among relatives of Alzheimer’s disease patients in the MIRAGE study: What is in store for the oldest old? Neurology. 1996;46:641–50. doi: 10.1212/wnl.46.3.641. [DOI] [PubMed] [Google Scholar]