Summary
Cellular asymmetry is critical to metazoan development and the life cycle of many microbes. In Caulobacter, cell cycle progression and the formation of asymmetric daughter cells depend on the polarly-localized histidine kinase CckA. How CckA is regulated and why activity depends on localization are unknown. Here, we demonstrate that the unorthodox kinase DivL promotes CckA activity and that the phosphorylated regulator DivK inhibits CckA by binding to DivL. Early in the cell cycle, CckA is activated by the dephosphorylation of DivK throughout the cell. However, in later stages, when phosphorylated DivK levels are high, CckA activation relies on polar localization with a DivK phosphatase. Localization thus creates a protected zone for CckA within the cell, without the use of membrane-enclosed compartments. Our results reveal the mechanisms by which CckA is regulated in a cell-type-dependent manner. More generally, our findings reveal how cells exploit subcellular localization to orchestrate sophisticated regulatory processes.
Introduction
Asymmetric cell divisions are critical to the generation of cellular complexity in both metazoans and many microbes. However, the molecular mechanisms responsible for robustly translating asymmetry into differential cell fates remain incompletely understood. The bacterium Caulobacter crescentus represents an excellent model to dissect this process as each cell division is asymmetric (see Fig. 7). One daughter cell, the stalked cell, is sessile and commits immediately to S phase. The other daughter, the swarmer cell, is motile and locked in G1 until it differentiates into a stalked cell. Strikingly, many of the key regulatory proteins that govern cell cycle progression and cell fate asymmetry in Caulobacter are localized to specific sites within the cell (reviewed in (Curtis and Brun, 2010)). However, the role that localization plays in governing the functions and activities of these regulatory proteins is largely unknown.
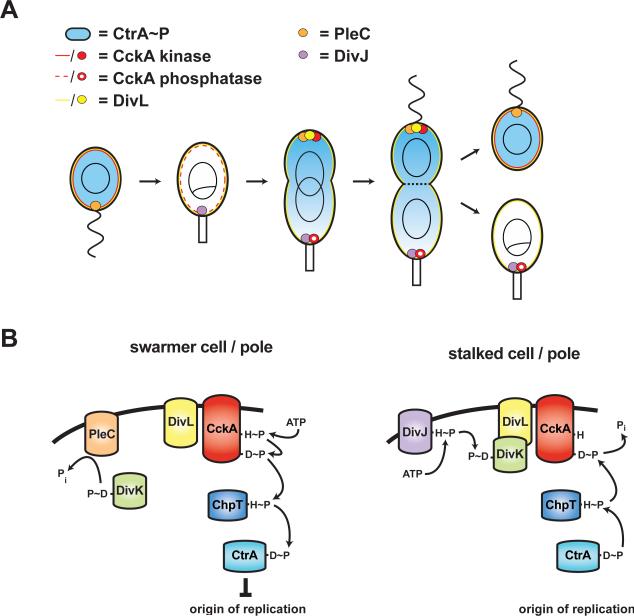
Figure 7. Model of regulatory circuitry controlling CtrA, cell cycle transitions, and cell fate asymmetry in Caulobacter crescentus.
(A) Localization of CtrA regulatory factors and CtrA activity throughout cell cycle. PleC and DivJ are localized to the swarmer and stalked poles, respectively. After DNA replication initiates in stalked cells, DivL, CckA, and PleC are recruited to the nascent swarmer pole. (B) Model of protein-protein interactions regulating CckA in swarmer and stalked cells and at the poles of predivisional cells. In swarmer cells, DivK is dephosphorylated by PleC allowing DivL to promote CckA kinase activity and, consequently, phosphorylation of CtrA. In stalked cells, DivJ phosphorylates DivK which then binds to DivL, inhibiting CckA kinase activity and ultimately driving the dephosphorylation of CtrA. In predivisional cells, CckA localizes with DivL and PleC at the swarmer pole, enabling CckA to escape downregulation by DivK~P. CckA is also frequently found at the stalked pole of stalked and predivisional cells. However, DivL is either absent from the stalked pole (not shown) or present but inhibited by phosphorylated DivK (shown); in either case, CckA remains in a phosphatase state.
Localizing regulatory proteins can serve many different functions. Cells often localize proteins that control morphogenetic processes to their primary site of action (reviewed in (Rudner and Losick, 2010)). For example, in both eukaryotes and prokaryotes, proteins regulating cell division often localize to the cytokinetic ring at mid-cell. Similarly, bacterial proteins that regulate assembly of a polar flagellum often localize, not surprisingly, to the cell pole. Localization can also facilitate the differential inheritance of proteins by daughter cells, as is the case with Ash1p in Saccharomyces cerevisiae which is preferentially retained in daughter cells to prevent mating-type switches (Sil and Herskowitz, 1996). However, the reason for subcellular localization of many proteins is not self-evident. In bacteria, regulatory proteins are frequently localized to the cell poles without having any direct function at those positions and despite regulating factors that freely diffuse.
In Caulobacter, the master histidine kinase CckA dynamically localizes to the cell poles, usually first to the nascent swarmer pole and then to both poles prior to cell division (Angelastro et al., 2009; Chen et al., 2009) (see Fig. 7). CckA is essential for cell cycle progression and the generation of daughter cells positioned at different cell cycle stages (Jacobs et al., 1999). However, why CckA must be polarly localized is mysterious as it ultimately regulates a transcription factor that is dispersed throughout the cell. Moreover, both daughter cells inherit CckA, suggesting that localization does not facilitate asymmetric inheritance.
The primary target of CckA in Caulobacter is CtrA, an essential response regulator (Quon et al., 1996) that directly controls the expression of nearly 100 genes (Laub et al., 2002). In G1 swarmer cells, phosphorylated CtrA also binds to the origin of replication to inhibit DNA replication (Quon et al., 1998). As swarmer cells differentiate into stalked cells, CtrA must be dephosphorylated or degraded to permit the initiation of DNA replication (Domian et al., 1997). Once S phase begins, new CtrA is synthesized and phosphorylated allowing it to act as a transcription factor for target genes, many of which are required for cell division.
CckA initiates two phosphorelays that control CtrA (Biondi et al., 2006) (see Fig. 7). One culminates in CtrA phosphorylation while the other leads to the phosphorylation of CpdR, which somehow inhibits CtrA proteolysis (Biondi et al., 2006; Iniesta et al., 2006). Activation of CckA as a kinase thus simultaneously drives CtrA phosphorylation and increases CtrA stability. In vivo phosphorylation assays indicate that CckA is active in swarmer cells, inactive in stalked cells, and highly active in predivisional cells (Jacobs et al., 2003). Notably, the peak in activity in predivisional cells correlates with and depends on polar localization (Angelastro et al., 2009; Chen et al., 2009; Jacobs et al., 1999).
How CckA activity is regulated remains largely undefined, although the essential, single-domain response regulator DivK may play an important role (Hecht et al., 1995). Conspicuously, a divK loss-of-function mutant arrests in G1 suggesting that without DivK, CckA may remain active, leading to a maintenance of CtrA activity and a continual silencing of DNA replication (Biondi et al., 2006; Hung and Shapiro, 2002). Consistently, CckA activity is moderately elevated in this divK mutant, but it is unclear whether DivK directly inhibits CckA.
Here, we show that (i) the non-canonical histidine kinase DivL promotes CckA kinase activity and (ii) that phosphorylated DivK downregulates CckA by binding directly to DivL. These results demonstrate that transitions in the phosphorylation state of DivK drive cell cycle transitions. When swarmer cells differentiate into stalked cells, a sharp increase in DivK phosphorylation leads to the inhibition of CckA which, in turn, permits the initiation of DNA replication. Paradoxically however, DivK remains highly phosphorylated in predivisional cells when CckA is most active as a kinase. We resolve this apparent conundrum by demonstrating that in predivisional cells CckA is activated by localizing at the swarmer pole with PleC, the primary DivK phosphatase. Our data reveal a rationale for why CckA is polarly localized and how the elaborate spatial arrangement of regulatory proteins in Caulobacter enables both cell cycle progression and the establishment of asymmetric daughter cell fates.
Results
divL acts between divK and cckA in the CtrA regulatory pathway
Previous studies have implicated DivL in the CtrA regulatory pathway, but its precise role has remained unknown (Iniesta et al., 2010; Pierce et al., 2006; Reisinger et al., 2007; Wu et al., 1999). To further characterize DivL we examined cells harboring divL346, a temperature-sensitive allele of divL (Wu et al., 1999), hereafter referred to as divLts. We found that divLts cells shifted from 30°C to 37°C became extremely filamentous and accumulated multiple chromosomes, phenotypes shared by ctrAts and cckAts mutants that result from continued growth and DNA replication in the absence of cell division (Fig. 1A). Using DNA microarrays, we also found that CtrA-dependent gene expression was affected in the divLts mutant in a manner similar to ctrAts and cckAts (Fig. S1). These data confirm that DivL positively regulates CtrA and that divL346 is a loss-of-function allele at 37°C.
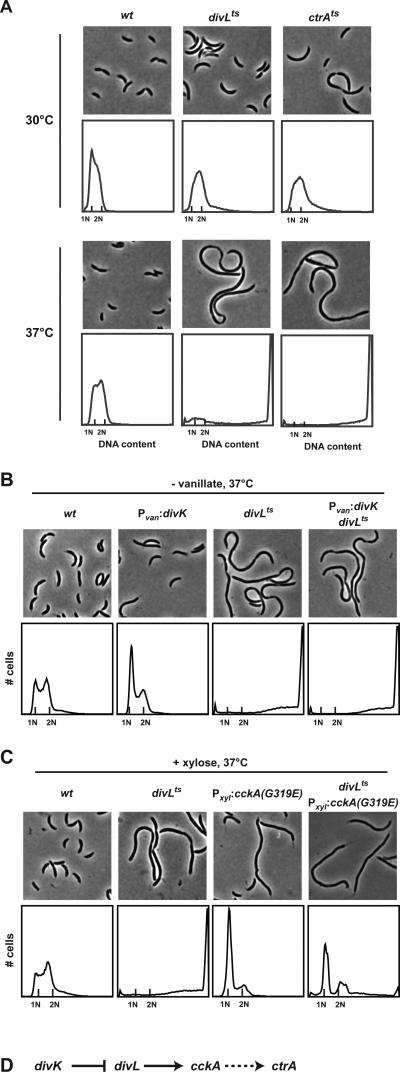
Figure 1. Epistasis analysis places divL between divK and cckA in the CtrA regulatory pathway.
(A) Phase contrast microscopy and flow cytometry analysis of wild type, divLts, and ctrAts grown at a permissive temperature (30°C) and after a shift to the restrictive temperature (37°C) for 4 hours. (B) Phase contrast images and flow cytometry analysis of wild type, divK depletion, divLts and double mutant (divK depletion and divLts) strains grown without vanillate for 4 hours to deplete divK followed by a shift to 37°C for an additional 4 hours. (C) Phase contrast images and flow cytometry analysis of wild type, divLts, cckA(G319E) overexpression, and double mutant (divLts and cckA(G319E) overexpression) strains grown with xylose for 4 hours to induce cckA(G319E) and then shifted to 37°C for an additional 4 hours. (D) Summary of genetic pathway regulating CtrA.
To map the position of divL in the regulatory circuitry controlling CtrA, we conducted epistasis experiments, using chromosome content as a readout for CtrA activity. Because CtrA silences the origin of replication, excess CtrA activity results in a G1 arrest, whereas too little CtrA activity results in a disruption of cell division and the accumulation of multiple chromosomes per cell.
First, we sought to establish the relative order of divK and divL in the CtrA regulatory pathway. DivK inhibits, either directly or indirectly, CtrA activity by decreasing both its phosphorylation (Biondi et al., 2006) and stability (Hung and Shapiro, 2002). Consequently, a loss of divK function results in increased CtrA activity and a G1 arrest. By contrast, a loss of divL function results in decreased CtrA activity and a consequent accumulation of multiple chromosomes (Fig. 1A). We engineered a strain that harbors the divLts mutation and a single copy of divK under the control of a vanillate-inducible promoter. When grown in the absence of vanillate to deplete DivK and at 37°C to inactivate DivL, this strain accumulated multiple chromosomes as with the divLts strain (Fig. 1B), suggesting that divL lies genetically downstream of divK and that DivK is a negative regulator of DivL. We corroborated this result by constructing a strain harboring the divK341 (or divKcs) mutation and in which the only copy of divL is driven by a xylose-inducible, glucose-repressible promoter (Sciochetti et al., 2005). When grown in the presence of glucose to deplete DivL and at 22°C to eliminate DivK activity, this strain accumulated multiple chromosomes, confirming that divL is genetically downstream of divK (Fig. S2).
Because divL is downstream of divK, we tested whether divL lies between divK and cckA in the CtrA regulatory pathway. Previously, we identified a mutation in CckA, G319E, that significantly increases its kinase activity and, when expressed from a high-copy plasmid, results in a G1 arrest similar to that seen with divKcs (Chen et al., 2009). To test the relationship between divL and cckA, we constructed a strain carrying a xylose-inducible copy of cckA(G319E) in a divLts background. Growth in the presence of xylose and at 37°C led to a G1 arrest indicating that CtrA activity remained high and prevented the initiation of DNA replication, despite the loss of DivL function (Fig. 1C). The overexpression of cckAG319E is thus epistatic to divL346. These data are consistent with divL lying upstream of cckA and with DivL acting as a positive regulator of CckA.
DivL regulates CtrA by promoting CckA activity
Our epistasis analyses suggest that divK and divL both lie upstream of and regulate CckA (Fig. 1D). Formally though, divK and divL could function in a pathway parallel to and independent of CckA that activates CtrA. To distinguish between these possibilities, we measured CckA activity in vivo in the divLts strain by immunoprecipitating CckA after labeling cells with [γ32P]-ATP (Fig. 2A-B). At the permissive temperature of 30°C, CckA phosphorylation levels in the divLts strain were slightly elevated relative to wild type. However, after a shift to the restrictive temperature of 37°C for 15 min, CckA phoshorylation in the divLts strain fell to ~42% that of wild type at 37°C and ~29% the level in divLts at 30°C. These data are consistent with a recent study showing that divL is necessary for full activity of a chimeric CckA-FixL reporter (Iniesta et al., 2010).
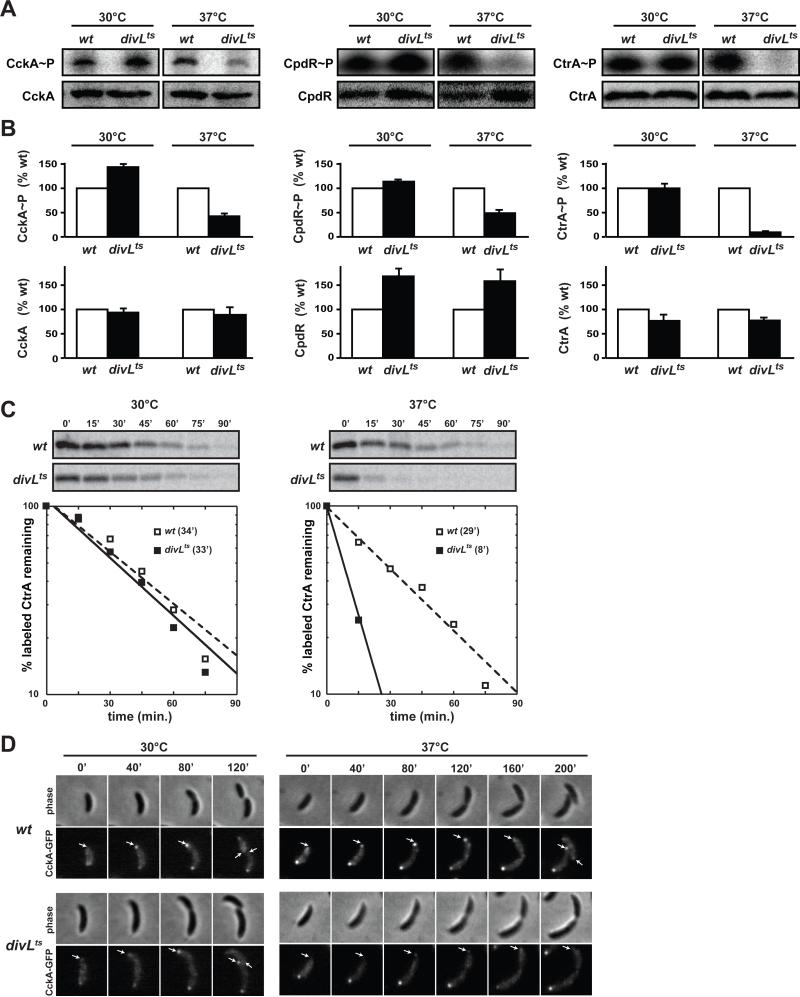
Figure 2. DivL is required to activate CckA as a kinase in vivo and to localize CckA-GFP to the swarmer pole of predivisional cells.
(A) In vivo phosphorylation assays of wild type and divLts strains grown at the permissive temperature (30°C) or shifted to the restrictive temperature (37°C) for 15 min. Equal optical densities of cells were pulsed with radiolabeled ATP, lysed, and CckA, CtrA, or CpdR immunoprecipitated. Samples from each immunoprecipitation were examined by SDS-PAGE and phosphorimaging (gel images labeled CckA~P, CpdR~P, and CtrA~P). Western blot analysis was performed on samples that were not pulsed (gel images labeled CckA, CpdR, and CtrA). (B) Quantification of bands from panel A. Error bars represent standard deviations from two independent replicates. (C) Pulse-chase analysis of CtrA. Wild type and divLts strains were pulsed with radiolabeled L-methionine for 5 min, and then chased with excess unlabeled L-methionine and casamino acids. Cultures were examined at the permissive temperature (30°C) or immediately after a shift to the restrictive temperature (37°C). Each experiment was repeated twice with representative gels and quantifications shown. The half-lives calculated for CtrA are included within each graph. (D) CckA-GFP localization through the cell cycle in wild type and divLts at the permissive (30°C) and restrictive (37°C) temperatures. Swarmer cells were isolated, placed on agarose pads and followed by time-lapse fluorescence microscopy with minutes post-synchrony indicated above the images. White arrows indicate the new pole which, in predivisional cells, becomes the swarmer pole.
If DivL regulates CckA, then DivL should also affect CtrA degradation in vivo as CckA controls the phosphorylation of CpdR through ChpT (Biondi et al., 2006). However, a previous study saw no major changes in CtrA stability in a divL510 mutant, a different ts-allele of divL, after 4 hours at the restrictive temperature (Reisinger et al., 2007). We measured the levels of phosphorylated CpdR and CtrA in our divLts strain after a 15 minute shift to the restrictive temperature and found that both were significantly decreased (Fig. 2A-B). In addition, using pulse-chase analyses, we found that CtrA stability was significantly decreased in divLts (half-life of 8 minutes) relative to wild type (half-life of 29 minutes) at the restrictive temperature (Fig. 2C). At the permissive temperature, the half-life of CtrA was nearly identical in wild type and divLts (34 and 33 minutes, respectively). These data support the notion that a loss of divL function leads to a drop in the phosphorylation of both CpdR and CtrA, further indicating that DivL promotes CtrA activity through CckA.
DivL is required to localize CckA at the nascent swarmer pole
Notably, although CckA usually localizes to both poles of a predivisional cell (Angelastro et al., 2009; Chen et al., 2009), DivL typically localizes only to the nascent swarmer pole (Sciochetti et al., 2005). These observations suggest that CckA is normally most active at the swarmer pole and that DivL may help localize CckA to that pole. We therefore examined the subcellular localization of CckA-GFP in synchronized divLts cells as they progressed through the cell cycle. For cells incubated at the restrictive temperature of 37°C, CckA-GFP localized only to the stalked pole of the predivisional cell; cells did not accumulate a swarmer pole focus of CckA nor did they divide (Fig. 2D). By contrast, divLts cells grown at 30°C localized CckA to both poles of predivisional cells and divided, as seen with wild-type cells (Fig. 2D). A similar result was obtained on mixed populations, with divLts cells shifted to 37°C for 4 hours rarely showing swarmer pole foci of CckA-GFP (Fig. S3), consistent with similar findings in a recent study (Iniesta et al., 2010). To ensure that the lack of swarmer pole localization was not due simply to a loss of CtrA activity or cell filamentation, we examined CckA-GFP localization in a ctrAts strain at 37°C. Unlike divLts, these cells accumulated CckA-GFP foci at both poles and sometimes at intervals throughout the cell (Fig. S3). Together, these data demonstrate that DivL is required for CckA to localize to the swarmer pole and that a failure to localize likely prevents the activation of CtrA and, consequently, cell division.
These observations do not, however, reveal why localization is necessary for CckA activity. There are two general possibilities: (i) DivL recruits CckA to the pole where another factor activates it, or (ii) localization of DivL and CckA to the swarmer pole sequesters them away from a negative regulator. We favored the latter, given our genetic studies indicating that DivK is an upstream, negative regulator of DivL. We therefore turned our focus to DivK.
DivK inhibits the activation of CckA as a kinase
To confirm that DivK, like DivL, affects CckA kinase activity in vivo, we measured CckA phosphorylation in the divKcs mutant strain (Fig. 3A). CckA phosphorylation was previously measured in a mixed population of divKcs cells, revealing a modest increase in CckA~P levels per cell, but not per protein (Biondi et al., 2006). However, DivK's essential function occurs during a narrow window of time immediately before DNA replication (Hung and Shapiro, 2002). We therefore measured CckA~P levels in synchronized stalked cells from the wild type and divKcs strains. CckA protein was present at similar levels in stalked cells from the two strains, but CckA~P levels were significantly higher in divKcs cells than in wild-type cells (Fig. 3B), on both a per protein and per cell level. These data demonstrate that DivK is normally required to downregulate CckA kinase activity in vivo and that the failure to do so in a divKcs strain results in a failure to downregulate CtrA and thus to initiate DNA replication (Fig. 1B).
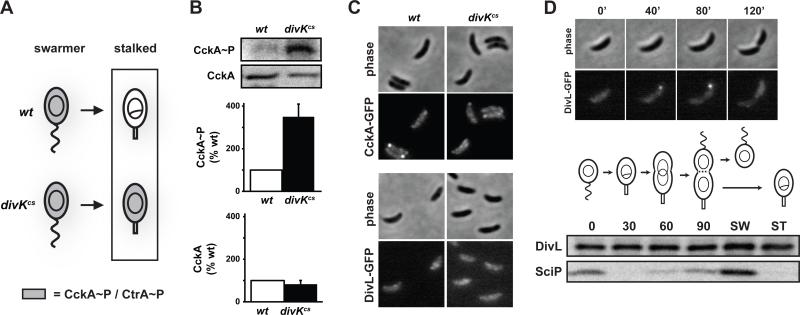
Figure 3. DivK inhibits CckA kinase activity at the G1-S transition.
(A) Diagram of CckA and CtrA activity during the swarmer-to-stalked cell transition in wild type and divKcs. (B) In vivo phosphorylation measurements of CckA in synchronized stalked cells harboring either divK or divKcs at the native chromosomal locus. Assays were performed as in Fig. 2A, except that stalked cells were isolated by allowing synchronized swarmer cells to differentiate for 50 minutes at 20°C, the restrictive temperature for divKcs. Error bars represent standard deviations from three independent replicates. (C) Fluorescence microscopy of wild type and divKcs stalked cells expressing CckA-EGFP. Strains were grown and stalked cells isolated exactly as in panel B. (D) Cell cycle localization pattern of DivL-EGFP. Swarmer cells expressing divL-gfp were isolated, placed on agarose pads containing M2G+ and followed by time-lapse fluorescence microscopy (top). Cell cycle Western blot analysis of DivL and SciP (bottom). Swarmer cells were isolated, released into rich media with samples taken for Western blot analysis every 30 minutes. Samples were also taken from swarmer (SW) and stalked (ST) cells collected immediately after cell division.
We also examined CckA-GFP localization in wild-type and divKcs cells grown in the same conditions used to measure CckA phosphorylation. For both strains, CckA-GFP was either dispersed throughout the cell or formed a focus at the stalked pole, with localization to the pole opposite the stalk seen in fewer than 2% of cells (Fig. 3C). Hence, in a divK mutant, CckA is either active at the stalked pole or the delocalized pool of CckA is active. To help distinguish between these possibilities, we examined DivL-GFP localization in the divKcs mutant and found that it was consistently delocalized (Fig. 3C). Recall that the G1-arrest phenotype of a divK depletion strain, and thus CckA activity in these cells, depends on DivL activity (Fig. 1B). Taken together, our results indicate that localization of CckA to the swarmer pole is not an obligatory step in its activation. Instead, it appears that the inactivation of DivK is sufficient to activate CckA, regardless of its cellular location, provided that DivL is functional.
If DivK does downregulate CckA kinase activity via DivL to drive the initiation of DNA replication, then stalked cells should harbor DivL. Although DivL is present in stalked cells that result from the differentiation of swarmer cells (Sciochetti et al., 2005), DivL-GFP localizes mainly to the swarmer pole of predivisional cells leaving open the question of whether stalked cells resulting from cell division harbor DivL (Fig. 3D). To address this question, we synchronized wild-type cells, allowed them to proceed once through the cell cycle, and then harvested daughter swarmer and stalked cells immediately after cell division. Western blotting revealed that DivL is present at nearly equal levels in the two daughter cells (Fig. 3D). As a control, we confirmed that SciP, a swarmer cell-specific factor, was present only in daughter swarmer cells (Gora et al., 2010).
Phosphorylated DivK directly binds DivL
DivK was previously found to bind DivL in a yeast two-hybrid system (Ohta and Newton, 2003). To test whether DivK binds directly to DivL in vitro we used Förster resonance energy transfer (FRET). We purified C-terminal fusions of DivK and DivL to CFP and YFP, respectively. For DivL, we used a construct lacking only the putative N-terminal transmembrane domain; for FRET studies we refer to this construct simply as DivL. The FRET ratio measured after mixing DivK-CFP and DivL-YFP was not significantly different from that of free CFP and YFP (Fig. S4A), indicating that no significant FRET occurs between DivL and unphosphorylated DivK in our conditions. However, as a response regulator, DivK activity likely depends on phosphorylation. To test the effect of phosphorylation on binding, we added substoichiometric amounts of untagged DivJ, the cognate kinase for DivK (Ohta et al., 1992), and ATP to a reaction containing DivK-CFP and DivL-YFP. We then observed a rapid and significant increase in FRET efficiency (Fig. 4A). A construct containing only the DHp and CA domains of DivL fused to YFP also strongly interacted with DivK-CFP upon addition of DivJ and ATP, with a FRET efficiency ~85% that seen with the longer version of DivL (Fig. S4B). These experiments demonstrate that the phosphorylation of DivK strongly increases its affinity for DivL.
Figure 4. Mutations in DivL that affect DivK binding in vitro affect CckA activity in vivo.
(A) In vitro FRET analysis of the DivL-DivK interaction. DivK-CFP and DivL-YFP (each at 2.5 μM) were mixed together with 5mM MgCl2 and 500μM ATP. At t=0, 100 nM DivJ was added and the ratio of the 527 nm to 475 nm emissions (FRET ratio) was measured while exciting the samples at 433 nm. A mixture of free CFP and YFP (denoted with minus signs) each at 2.5 μM was included as a control. DivK-CFP was tested for binding to DivL-YFP and the mutants indicated. (B) Phase contrast microscopy and flow cytometry analysis of cells expressing either divL or divL(A601L) under the control of a xylose-inducible promoter on a high-copy plasmid. Cells were grown in the presence of glucose; leaky expression from the high-copy plasmid leads to moderate, constitutive levels of expression. (C) In vivo phosphorylation measurements of CckA in synchronized stalked cells expressing either divL or divL(A601L) as in panel B. Assays were performed as in Fig. 2A, except that stalked cells were obtained by allowing synchronized swarmer cells to differentiate for 35 minutes. Error bars represent standard deviations from three independent replicates. (D) Fluorescence microscopy of CckA-EGFP in stalked cells expressing divL or divL(A601L). Strains were grown and stalked cells exactly as in panel C. (E) Phase contrast microscopy and flow cytometry analysis of strains harboring the pleC::Tn5 disruption with the chromosomal copy of divL deleted and expressing either divL or divL(Y550F) from the native divL promoter on a low-copy plasmid grown at 30°C or 37°C for 4 hours.
Mutations in DivL that affect DivK binding in vitro affect CckA kinase activity in vivo
To bolster the notion that DivK~P binding to DivL is relevant in vivo, we tested whether mutations in divL and divK that perturb CtrA activity in vivo also affect their interaction in vitro. A transposon insertion in divL causing a truncation after amino acid 657 was previously identified in a screen for suppressors of pleC (Reisinger et al., 2007). As the loss of pleC decreases CtrA activity, suppression requires a compensatory mutation that increases CtrA activity. We hypothesized that the divL657 mutation may achieve such an increase by disrupting the ability of DivK~P to inhibit DivL and thereby downregulate CckA as a kinase. To test this hypothesis, we purified a construct, DivL°CA-YFP, that lacks the putative transmembrane domain and the last 112 amino acids of DivL. This construct did not show a significant FRET signal with DivK, either with or without DivJ (Fig. 4A), suggesting that it indeed no longer had the ability to strongly bind DivK.
Next, we wanted to examine a point mutation in DivL that disrupts binding to DivK, as point mutants are less likely to affect folding or tertiary structure. We created a series of DivL point mutants at sites predicted to interface with DivK based on comparison to a co-crystal structure of a histidine kinase-response regulator complex from T. maritima (Casino et al., 2009). One mutation, A601L, completely eliminated binding of DivL-YFP to phosphorylated DivK-CFP in vitro (Fig. 4A). To test whether this mutation also disrupted binding in vivo, we expressed divL(A601L) from a xylose-inducible promoter on a plasmid in wild-type cells. Growth in the presence of glucose led to leaky, constitutive expression of divL(A601L). Using flow cytometry we found that most cells expressing divL(A601L) contained a single chromosome (Fig. 4B), similar to the G1 arrest seen with the divKcs strain. We then synchronized swarmer cells expressing either divL(A601L) or divL, released them into media at 30°C, and allowed them to develop into stalked cells for 35 minutes. We measured CckA phosphorylation in each population of cells and found that CckA~P levels were more than five times higher in the cells expressing divL(A601L) (Fig. 4C). CckA-GFP was also not localized to the swarmer pole in these cells (Fig. 4D), again indicating that CckA activation does not require swarmer pole localization if DivK cannot bind and inhibit it via DivL. Collectively, these findings suggest that DivK does not bind DivL(A601L) in vitro or in vivo, thereby preventing the normal downregulation of CckA and CtrA, and so yielding a G1 arrest (Fig. 4B). We infer that DivL(A601L) is not simply misfolded as it can still activate CckA; this mutant appears specifically disrupted for binding DivK~P. Importantly, these results also indicate that DivL is the primary target of DivK in regulating CckA and CtrA, as the divL(A601L) strain retains wild-type DivK but cannot properly down-regulate CckA or CtrA.
We also tested the effect of mutating tyrosine-550 in DivL to phenylalanine. DivL shares extensive homology to histidine kinases but contains a tyrosine in place of the usual phosphorylatable histidine (Wu et al., 1999). DivL(Y550F) does not affect CckA localization (Iniesta et al., 2010), but could affect DivK binding and hence CckA activity. We thus purified DivL(Y550F)-YFP and tested binding to DivK-CFP by measuring FRET. Compared to the wild-type construct, DivL(Y550F) produced a higher FRET signal when mixed with DivK-CFP and substochiometric amounts of DivJ and ATP (Fig. 4A). If DivL(Y550F) binds DivK more tightly than wild-type DivL in vivo, introducing this mutation should negatively affect the activity of CckA and CtrA. To test this possibility, we constructed strains in which either divL or divL(Y550F) is carried on a low-copy plasmid as the only copy of divL. At 30°C both strains had relatively normal morphology and chromosomal content (Fig. S5). However, at 37°C, cells expressing divL(Y550F) became filamentous and showed a modest accumulation of chromosomes per cell, reflecting a loss of CtrA activity (Fig. S5). These phenotypes were significantly exacerbated by introducing a pleC::Tn5 mutation which, as noted above, sensitizes cells to other mutations that downregulate CtrA (Fig. 4E). We conclude that the Y550F mutation renders DivL better at binding DivK~P in vitro and, consistently, disrupts CtrA activation in vivo.
Mutations in DivK that affect DivL binding in vitro affect CckA activity in vivo
Next, we tested the ability of DivL to bind mutants of DivK. First, we tested DivK(D90G), the mutant encoded by divKcs that prevents downregulation of CckA and CtrA in vivo. DivK(D90G) is phosphorylated in vivo to a similar extent as wild-type DivK suggesting its defect may be an inability to bind and inhibit DivL (Hung and Shapiro, 2002). Indeed, purified DivK(D90G)-CFP produced a significantly weaker FRET ratio when incubated with YFP-DivL along with DivJ and ATP (Fig. 5A).
Figure 5. Mutations in DivK that affect DivL binding in vitro affect CckA activity in vivo.
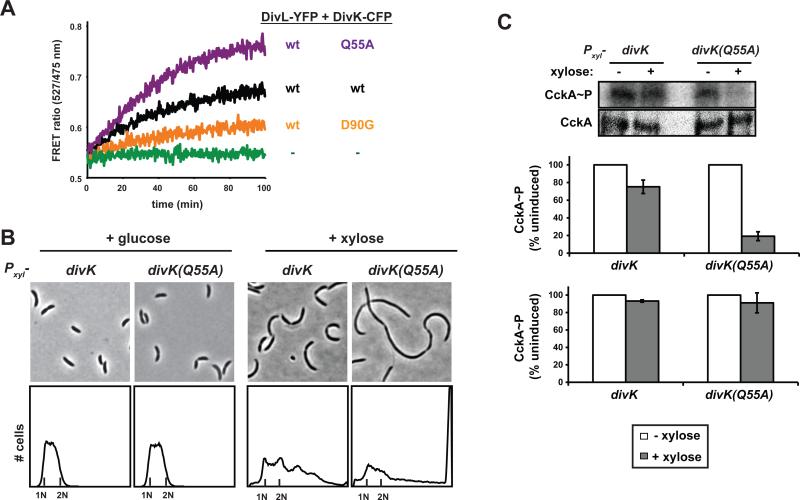
(A) In vitro FRET analysis of DivL-YFP binding to wild type DivK-CFP and the mutants indicated. Assays were performed as in Fig. 5A. Wild-type and free CFP/YFP traces are the same experiments as in Fig. 5A and are duplicated to facilitate comparison. (B) Phase contrast microscopy and flow cytometry analysis of strains expressing either divK or divK(Q55A) from a low-copy plasmid under the control of a xylose-inducible promoter. Cells were grown in glucose or in the presence of xylose for 6 hours. (C) In vivo phosphorylation measurements of CckA in a mixed population of cells expressing divK or divK(Q55A). Assays were performed as in Fig. 2A, except strains were induced with xylose for 2 hours and compared to identically treated, but uninduced cultures. Error bars represent standard deviations from three independent replicates.
We also examined a mutation in DivK that increases binding. In a screen for point mutants of DivK that affect its interaction with DivL, we found that the substitution Q55A significantly increased binding in our FRET assay (Fig. 5A). We predicted that this mutant would hyperactivate DivK in vivo and, consequently, downregulate the CtrA regulatory pathway. To test this possibility, we engineered strains expressing either wild-type divK or divK(Q55A) under the control of a xylose-inducible promoter on a low-copy plasmid. In the presence of glucose, neither strain exhibited major defects in cellular morphology or chromosomal content. However, when grown in xylose for 6 hours, cells expressing divK(Q55A) became extremely filamentous and accumulated multiple chromosomes, similar to divL, cckA, and ctrA mutants (Fig. 5B). The phenotypes for divK(Q55A) were more severe than for cells overexpressing wild-type divK. Using in vivo phosphorylation assays, we verified that overproducing DivK(Q55A) for 2 hours led to a significant decrease in CckA phosphorylation levels, similar to the decrease seen in divLts cells (Fig. 5C). These data lend further support to a model in which phosphorylated DivK antagonizes CckA by binding directly to DivL. Mutations that increased or decreased DivK-DivL binding in vitro led to a corresponding decrease or increase, respectively, of CckA kinase activity in vivo.
Localization to the swarmer pole activates CckA by localizing it with a DivK phosphatase
In sum, our findings support a model in which (i) DivK inhibits CckA by binding to DivL and (ii) cell cycle transitions are ultimately driven by changes in the phosphorylation state of DivK. Such a model is consistent with the reciprocal changes in DivK~P and CckA~P early in the cell cycle (Jacobs et al., 2003; Lam et al., 2003). In G1 swarmer cells, DivK is predominantly unphosphorylated while CckA retains activity and is phosphorylated. In stalked cells, DivK phosphorylation increases while CckA phosphorylation drops to its lowest level during the cell cycle. However, in predivisional cells, DivK remains phosphorylated and yet CckA is highly active, in apparent conflict with the model. Conspicuously though, the DivK phosphatase PleC (Ohta et al., 1992) is located at the swarmer pole of predivisional cells (Wheeler and Shapiro, 1999), along with DivL and CckA. Thus, we hypothesized that PleC phosphatase activity may protect DivL and CckA from DivK~P at the nascent swarmer pole in predivisional cells, thereby allowing the accumulation of high levels of phosphorylated CtrA in this cell type.
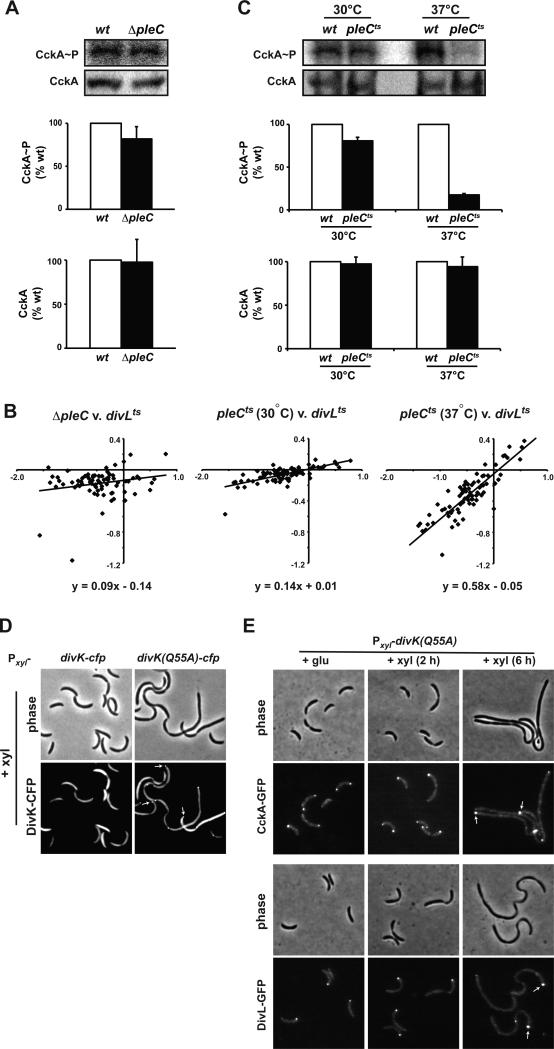
If this hypothesis is correct, the phosphorylation levels of CckA and CtrA should decrease in a pleC mutant. In a ΔpleC mutant we found that CckA~P levels in vivo dropped to ~82% of wild-type levels (Fig. 6A), while in a pleC::Tn5 mutant CtrA~P drops to ~10% of wild type levels (Biondi et al., 2006). Consistently, pleC mutants are highly sensitive to other mutations that decrease CtrA activity, often with synthetic, nearly lethal phenotypes (Chen et al., 2009). Conversely, pleC null mutants are suppressed by mutations in genes that promote CtrA activity (Sommer and Newton, 1991). Nevertheless, for cells harboring only a pleC null mutation, the consequent decrease in CckA~P and CtrA~P does not lead to a severe cell cycle phenotype or major changes in CtrA-dependent gene expression (Fig. 6B), as with divL and cckA mutants. Either another DivK phosphatase exists or cells compensate for the loss of pleC; the latter possibility is suggested by previous observations that pleC null strains exhibit alternative patterns of localization for many key regulatory proteins (Reisinger et al., 2007; Wheeler and Shapiro, 1999).
Figure 6. CckA and DivL both localize at the swarmer pole with PleC to avoid downregulation by DivK.
(A) In vivo phosphorylation measurements of CckA in wild type and ΔpleC. (B) CtrA-dependent gene expression in pleC mutants. Oligonucleotide microarrays were used to measure global gene expression patterns in ΔpleC and pleCts relative to wild type at 30°C and in pleCts relative to wild type at 37°C for 1 hr. The log ratio for each CtrA-regulated gene was compared to the log ratio of expression in divLts relative to wild type, each grown at 37°C for 4 hours (see Fig. S1). The best fit line and equation are shown on each plot. (C) In vivo phosphorylation measurements of CckA in wild type and pleCts at the permissive temperature (30°C) and after shift to the restrictive temperature (37°C) for 15 minutes. Error bars represent standard deviations from three independent replicates. (D) divK and divK(Q55A) were each fused to cfp and expressed from a low-copy plasmid under the control of a xylose-inducible promoter. Subcellular localization was examined by epi-fluorescence microscopy after growth in xylose for 6 hrs. For cells expressing divK(Q55A)-cfp, white arrows indicate swarmer pole foci, where swarmer poles were identified as those opposite stalked poles. (E) CckA-EGFP and DivL-EGFP localization in cells harboring Pxyl-divK(Q55A) on a low-copy plasmid and grown in the presence of gluocse or in xylose for 2 or 6 hours. At the 6 hour time point, white arrows indicate foci of CckA-GFP or DivL-GFP at the putative swarmer pole, identified as the pole opposite the stalked pole.
To better address the consequence of losing PleC phosphatase activity, we measured CckA~P levels in a pleCts strain 15 minutes after shifting to the restrictive temperature. In this case, we found that CckA~P levels dropped to ~18% of wild-type, similar to the decrease measured indivLts cells, and with virtually no change in CckA protein level (Fig. 6C). Moreover, DNA microarray analysis revealed that in pleCts cells grown at 37°C for 1 hour, CtrA regulated genes were down-regulated much more significantly than in the ΔpleC, and comparable to that seen in divLts (Fig. 6B). These data demonstrate that pleC is, in fact, critical to maintaining the activity of CckA in predivisional cells.
Based on these findings, we conclude that in swarmer cells, PleC maintains a low level of DivK~P allowing DivL to associate with and promote CckA activity. In stalked cells, DivJ replaces PleC at the old pole and drives a surge in DivK phosphorylation, resulting in the down-regulation of CckA. In predivisional cells, DivJ continues to phosphorylate DivK, but the localization of CckA and DivL to the swarmer pole along with PleC enables CckA to function again as a kinase and drive CtrA phosphorylation.
This model further suggests that the mutant DivK(Q55A) may downregulate CckA as a kinase by binding more tightly to DivL at the swarmer pole and thus overcoming the effects of PleC. To test this prediction, we examined the localization of a DivK(Q55A)-CFP fusion expressed from a low-copy plasmid in an otherwise wild type background. Most cells expressing DivK(Q55A)-CFP showed clear, significant polar foci as well as irregular foci within filamentous cells at pinched sites that likely represent nascent poles (Fig. 6D). In cells producing DivK(Q55A), we also found that DivL-GFP and CckA-GFP formed foci at the cell poles and at highly pinched, nascent poles within the cell, similar to the pattern seen with DivK(Q55A)-CFP (Fig. 6E). Collectively, our data indicate that DivK(Q55A), by virtue of its tighter binding to DivL, can effectively overcome the PleC phosphatase, infiltrate the swarmer pole, and downregulate CckA, without disrupting the polar localization of DivL or CckA. We speculate that this ability to bypass PleC may be due in part to competition between DivL and PleC for DivK~P binding; enhanced binding to DivL may thus protect DivK~P from PleC. Taken together with our analyses of pleC mutants, these data strongly support a model in which the joint localization of PleC, DivL, and CckA at the swarmer pole normally enables CckA to avoid downregulation by DivK~P.
Discussion
Throughout biology, developmental processes rely heavily on the subcellular localization of key regulatory proteins. For many proteins, localization enables the regulation of a morphogenetic or structural process that is itself localized, such as the cytokinetic ring, DNA replication, and flagellar assembly. For other proteins, localization may promote asymmetric inheritance following cell division, as with Ash1p in S. cerevisiae (Sil and Herskowitz, 1996) and with DivJ and PleC in Caulobacter (Wheeler and Shapiro, 1999). Localization can also directly stimulate the activity of some regulatory proteins. For instance, the polar localization of chemotaxis proteins in E. coli (Maddock and Shapiro, 1993) facilitates the assembly of a supramolecular cluster that enables signal adaptation and exquisite sensitivity, properties critical to chemotaxis (Hansen et al., 2010). Finally, localization can act to sequester regulatory proteins from their targets, as with the nucleolar localization of the phosphatase Cdc14 in S. cerevisiae (Visintin et al., 1999).
Why CckA localizes to the poles of Caulobacter predivisional cells had previously been unclear. CckA does not directly regulate a morphogenetic process nor is it asymmetrically inherited. A major clue came from our observation that in certain mutants, the activity of CckA is no longer dependent on localization to the swarmer cell pole (Fig. 3B-C, 4C-D). Conversely, in cells producing hyperactive DivK, CckA remains localized to the swarmer pole but is not active (Fig. 5C, 6E). These results highlight another reason for subcellular localization: to create a microenvironment within the cell where CckA can avoid downregulation by its inhibitor, DivK~P. In predivisional cells, bulk measurements indicate that DivK~P levels are high (Jacobs et al., 2001). Although this DivK~P can diffuse throughout the cell, our data suggest that the enforced proximity of CckA and PleC, a DivK phosphatase, at the pole promotes CckA kinase activity. Consistently, the immediate consequence of losing PleC activity is a downregulation of CckA and CtrA (Fig. 6C). It is then the transition from a delocalized to localized state which triggers CckA kinase activity and, in turn, drives the late stages of cell cycle progression.
DivK dictates cell cycle progression and cellular asymmetry by regulating CckA
Our results underscores DivK as a key regulator of the Caulobacter cell cycle and the establishment of cellular asymmetry. Although DivK was first identified almost 20 years ago (Hecht et al., 1995; Sommer and Newton, 1991), it has been unknown precisely how it regulates development and the cellular asymmetry of Caulobacter. DivK is a single-domain response regulator and hence was presumed not to directly affect transcription. Indeed, our results indicate that DivK's primary cell cycle role is the regulation of CckA through a direct, phosphorylation-dependent interaction with the essential, non-canonical kinase DivL.
Synthesis of our results with those published previously yields a molecular-level model for the regulation of Caulobacter cell cycle progression and cell fate asymmetry (Fig. 7). In swarmer cells, polarly localized PleC actively dephosphorylates DivK to permit a productive interaction between DivL and CckA and, consequently, to maintain the phosphorylation of CtrA and a G1 state. During the swarmer-to-stalked cell transition, PleC is replaced by DivJ at the stalked pole, resulting in the rise of DivK phosphorylation and, consequently, the downregulation of CckA kinase activity via DivL. The inhibition of CckA and consequent loss of CtrA binding to the origin permits DNA replication to initiate. As the stalked cell develops into a predivisional cell, CckA, DivL, and PleC are recruited to the nascent swarmer pole. PleC phosphatase activity shields CckA from DivK~P and thus drives the phosphorylation of CtrA, enabling the late stages of cell cycle progression and morphogenesis. CckA is also found at the stalked pole of predivisional cells. DivL is usually absent from this pole, but even when present, it would be inhibited by DivK~P. Like most histidine kinases, CckA is bifunctional such that when not stimulated as a kinase, it functions as a phosphatase (Chen et al., 2009). Predivisional cells thus have CckA in the kinase and phosphatase states at opposing poles, resulting in a gradient of phosphorylated CtrA across the cell (Chen et al., 2010). Following cell division, the daughter swarmer cell retains PleC and hence dephosphorylates DivK to maintain CckA and CtrA activity. The daughter stalked cell inherits DivJ, leading to DivK phosphorylation, which prevents DivL from stimulating CckA kinase activity, thereby facilitating the onset of DNA replication in this cell type.
Protein-protein interactions underlying the control of CckA activity
At the heart of our model is a dynamic protein-protein interaction system comprising DivK, DivL, and CckA. Our results indicate that a complex of DivL and CckA is active with respect to CckA autophosphorylation and phosphotransfer, and that the binding of DivK~P to DivL inhibits CckA. Toggling the phosphorylation state of DivK thus inversely toggles the phosphorylation state of CckA and, consequently, CtrA. Whether DivL and CckA directly interact is not yet clear, although both proteins localize to the swarmer pole and were suggested to co-immunoprecipitate (Iniesta et al., 2010).
Our results do, however, demonstrate that the interaction between DivK~P and DivL is direct and several lines of evidence indicate that binding is similar to canonical two-component signaling interactions, but without phosphotransfer occurring. First, binding requires only the DHp and CA domains of DivL, the same domains used in canonical HK-RR interactions. Also, the substitutions Y550F and A601L in DivL that affect binding are at sites likely to mediate canonical two-component protein interactions. In the co-crystal structure of HK853 and RR468 from Thermotoga maritima (Casino et al., 2009), the residues in HK853 corresponding to Y550 and A601 directly contact RR468. Similarly, for DivK, the substitution D90G decreases binding to DivL (Fig. 5A) and the corresponding residue in RR468 is in contact with HK853. Notably, aspartate-90 resides at the N-terminus of alpha-helix 4 in DivK (Guillet et al., 2002). For most response regulators, the α4-β5-α5 face changes conformation in a phosphorylation-dependent manner to effect an output (Gao et al., 2007), often by modulating protein-protein interactions. We propose that the phosphorylation of DivK induces a conformational change that enables tighter binding to DivL.
Although binding occurs, DivL and DivK likely do not participate in phosphotransfer reactions. DivL does not harbor significant autokinase or DivK~P phosphatase activity in vitro (CGT and MTL, unpublished) and a previous report found that the ATPase domain of DivL is not required to support viability (Reisinger et al., 2007). Nevertheless, we cannot rule out that tyrosine phosphorylation of DivL plays a regulatory role.
Finally, our data suggest that DivL is the primary output for phosphorylated DivK during cell cycle progression. DivK was suggested to independently control CpdR (Iniesta and Shapiro, 2008). However, the fact that divL(A601L) led to an increase in CckA activity and a G1 arrest indicates that DivK acts primarily through DivL to downregulate CpdR and CtrA.
Non-canonical topologies and activities for two-component signaling proteins
The connectivity of the two-component signaling proteins that regulate the Caulobacter cell cycle includes both canonical and non-canonical features. The phosphorylation and dephosphorylation of DivK by DivJ and PleC, respectively, and the multistep phosphorelays initiated by CckA exemplify the two most common topologies for two-component proteins. These pathways are, however, connected in a highly unconventional manner, with the response regulator DivK~P binding the non-canonical kinase DivL to, in turn, modulate the activity of another histidine kinase, CckA. There are very few examples of other two-component proteins wired together in such unorthodox ways. In P. aeruginosa, the histidine kinase RetS directly modulates the activity of another histidine kinase, GacS (Goodman et al., 2009), although in that case, the two kinases have nearly identical DHp domains and probably heterodimerize.
Most histidine kinases mediate adaptive responses to environmental signals by binding small molecule inducers or ligands. However, CckA may not respond to anything other than DivK and DivL. Although DivL and CckA are transmembrane proteins, neither has a substantial periplasmic domain. The transmembrane domains thus may serve mainly to facilitate polar localization. Each kinase does have several intracellular PAS domains, and while these domains sometimes modulate response to environmental or metabolic signals, they are also often involved in protein-protein interactions (Lee et al., 2008). While CckA and DivL may not directly integrate environmental signals, PleC and DivJ may.
The regulation of DivL and CckA by DivK also highlights the expanding role of single-domain response regulators in bacteria. Although the majority of response regulators control transcription, single-domain regulators are relatively common and modulate a wide range of physiological processes through protein-protein interaction (Jenal and Galperin, 2009).
Molecular mechanisms for producing and maintaining cellular asymmetry
The identification of DivL as an intermediary between DivK and CckA fills a major gap in our understanding of the regulatory circuit governing the Caulobacter cell cycle. Central to this circuit is the response regulator DivK, which ultimately dictates cell cycle progression and replicative asymmetry via DivL. Our work further suggests that the subcellular localization of regulatory proteins is crucial to the development and cell cycle of Caulobacter for at least two reasons. First, as noted, the localization of factors such as DivJ and PleC likely promotes their asymmetric inheritance, helping to enforce the asymmetry of daughter cells. Second, we now find that the localization of CckA, DivL, and PleC to a single pole of the predivisional cell effectively partitions the cytoplasm but without the use of membrane-enclosed compartments or other physical barriers. Our findings reveal a remarkable mechanism through which bacterial cells can create and exploit a heterogeneous cytoplasm to activate a master kinase and to produce cell fate asymmetry.
Experimental Procedures
Growth conditions
Caulobacter crescentus strains were grown in PYE (rich medium), M2G (minimal medium), M2G+ (M2G + 1% PYE), or M5G (phosphate-deplete medium) supplemented when necessary with oxytetracycline (1 μg/mL), kanamycin (25 μg/mL), chloramphenicol (2 μg/mL), gentamycin (0.6 μg/mL), novobiocin (100 μg/mL), 0.2% glucose, or 0.3% xylose. Cultures were grown at 30°C unless otherwise noted and diluted when necessary to maintain exponential growth. Escherichia coli strains were grown at 37°C in LB supplemented when necessary with carbenicillin (100 μg/mL), oxytetracycline (12 μg/mL), kanamaycin (50 μg/mL), chloramphenicol (30 μg/mL), or gentamycin (15 μg/mL). Synchronies were performed as described previously (Jones et al., 2001).
Protein expression, purification, and antibody production
Protein expression and purifications were performed as described (Skerker et al., 2005) except with modified expression conditions. After reaching mid-exponential phase, cultures were induced with 0.5 mM IPTG for 16 hours at 18°C. Fluorescent fusion protein concentrations were determined using absorbances at 433 nm for CFP fusions (molar extinction coefficient 32,500 M-1cm-1) or 514 nm for YFP fusions (molar extinction coefficient 83,400 M-1cm-1). Non-fluorescent protein concentrations were determined by measuring absorbance at 280 nm and using extinction coefficients calculated with the Protparam tool (http://ca.expasy.org/tools/protparam.html). Purified His6-DivL, expressed from pHIS-divL and lacking only the putative N-terminal transmembrane domain, was used to generate rabbit polyclonal antiserum (Covance). Crude antisera were used at a 1:5000 dilution.
In vivo phosphorylation measurements
In vivo phosphorylation measurements were carried out as described previously (Domian et al., 1997) with the following modifications. One colony was inoculated into M5G medium and grown overnight at 30°C until the optical density at 660 nm was between 0.2 to 0.4. Cultures were normalized by optical density to the least dense culture in the batch and 1 mL of cells from each culture pulsed with 1 μM [γ32P]-ATP having a specific activity of 30 Ci/mmol (Perkin Elmer) for 5 minutes. Labeling was carried out at the temperatures indicated. Immunoprecipitations were performed using Protein A agarose beads (Roche). In synchrony experiments, swarmer cells were isolated from cultures at OD660 ~0.2 and resuspended in the original media, which was filter sterilized, to avoid replenishing phosphate in the culture. Cells were grown at the temperatures and for the times indicated to isolate synchronized stalked cells.
In vivo CtrA stability measurments
CtrA pulse-chase experiments were performed as described previously (Gora et al., 2010) with the exception that Protein A agarose beads from Roche were used.
Band quantification
Quantifications of bands on SDS-PAGE gels were done using the Gel Analyzer function in ImageJ (http://rsbweb.nih.gov/ij).
FRET
FRET was performed at 30°C, reading 70 μL reactions from 96-well polystyrene plates (Corning) using a Varioskan Flash fluorescence plate reader (ThermoFisher Scientific). Samples were excited at 433 nm and emission measured at 525 nm and 475 nm.
DNA microarrays
Gene expression profiles were obtained as described previously (Gora et al., 2010) using custom Agilent arrays. RNA was collected from divLts cells grown to mid-exponential phase in rich media at 30°C and compared to RNA from cells shifted to 37°C for two or four hours.
Flow Cytometry
DNA content per cell was determined as described previously (Chen et al., 2009) except cells were not treated with rifampicin.
Microscopy
Both live and fixed cells were mounted onto M2G+ 1.5% agarose pads (supplemented with xylose when applicable) and imaged using a Axiovert 200 microscope (Zeiss) with a 63X/1.4NA objective (Zeiss) with 1.6X Optivar and an Orca II camera (Hamatsu) controlled using software from Metamorph (Universal Imaging, PA). Fluorescent images were obtained using an EXFO X-cite 120 light source and CFP or GFP filters (Chroma). Fluorescence images were taken on live cells transferred from culture to agarose pads and kept at the temperatures indicated using an objective heater (Bioptechs) during the imaging process. Cells examined were in mid-exponential phase.
Supplementary Material
Acknowledgments
We thank A. Newton, N. Ohta, L. Shapiro, and K. Ryan for providing strains, C. Jacobs-Wagner for the CckA antibody, P. Chien for the CpdR antibody, and P. Viollier and S. Radhakrishnan for technical assistance with in vivo phosphorylation assays. We thank A. Yuan for helping in identifying the DivL(A601L) mutant and the Laub lab and S. Bell for comments on the manuscript. This work was supported by an NIH grant (5R01GM082899) to MTL. MTL is an Early Career Scientist of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angelastro PS, Sliusarenko O, Jacobs-Wagner C. Polar localization of the CckA histidine kinase and cell cycle periodicity of the essential master regulator CtrA in Caulobacter crescentus. J Bacteriol. 2009;192:539–552. doi: 10.1128/JB.00985-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi EG, Reisinger SJ, Skerker JM, Arif M, Perchuk BS, Ryan KR, Laub MT. Regulation of the bacterial cell cycle by an integrated genetic circuit. Nature. 2006;444:899–904. doi: 10.1038/nature05321. [DOI] [PubMed] [Google Scholar]
- Casino P, Rubio V, Marina A. Structural insight into partner specificity and phosphoryl transfer in two-component signal transduction. Cell. 2009;139:325–336. doi: 10.1016/j.cell.2009.08.032. [DOI] [PubMed] [Google Scholar]
- Chen YE, Tropini C, Jonas K, Tsokos CG, Huang KC, Laub MT. Spatial gradient of protein phosphorylation underlies replicative asymmetry in a bacterium. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1015397108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YE, Tsokos CG, Biondi EG, Perchuk BS, Laub MT. Dynamics of two Phosphorelays controlling cell cycle progression in Caulobacter crescentus. J Bacteriol. 2009;191:7417–7429. doi: 10.1128/JB.00992-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis PD, Brun YV. Getting in the loop: regulation of development in Caulobacter crescentus. Microbiol Mol Biol Rev. 2010;74:13–41. doi: 10.1128/MMBR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domian IJ, Quon KC, Shapiro L. Cell type-specific phosphorylation and proteolysis of a transcriptional regulator controls the G1-to-S transition in a bacterial cell cycle. Cell. 1997;90:415–424. doi: 10.1016/s0092-8674(00)80502-4. [DOI] [PubMed] [Google Scholar]
- Gao R, Mack TR, Stock AM. Bacterial response regulators: versatile regulatory strategies from common domains. Trends in Biochemical Sciences. 2007;32:225–234. doi: 10.1016/j.tibs.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AL, Merighi M, Hyodo M, Ventre I, Filloux A, Lory S. Direct interaction between sensor kinase proteins mediates acute and chronic disease phenotypes in a bacterial pathogen. Genes Dev. 2009;23:249–259. doi: 10.1101/gad.1739009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gora KG, Tsokos CG, Chen YE, Srinivasan BS, Perchuk BS, Laub MT. A cell-type-specific protein-protein interaction modulates transcriptional activity of a master regulator in Caulobacter crescentus. Mol Cell. 2010;39:455–467. doi: 10.1016/j.molcel.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet V, Ohta N, Cabantous S, Newton A, Samama JP. Crystallographic and biochemical studies of DivK reveal novel features of an essential response regulator in Caulobacter crescentus. J Biol Chem. 2002;277:42003–42010. doi: 10.1074/jbc.M204789200. [DOI] [PubMed] [Google Scholar]
- Hansen CH, Sourjik V, Wingreen NS. A dynamic-signaling-team model for chemotaxis receptors in Escherichia coli. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1005017107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht GB, Lane T, Ohta N, Sommer JM, Newton A. An essential single domain response regulator required for normal cell division and differentiation in Caulobacter crescentus. EMBO J. 1995;14:3915–3924. doi: 10.1002/j.1460-2075.1995.tb00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung DY, Shapiro L. A signal transduction protein cues proteolytic events critical to Caulobacter cell cycle progression. Proc Natl Acad Sci U S A. 2002;99:13160–13165. doi: 10.1073/pnas.202495099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniesta AA, Hillson NJ, Shapiro L. Cell pole-specific activation of a critical bacterial cell cycle kinase. Proc Natl Acad Sci U S A. 2010;107:7012–7017. doi: 10.1073/pnas.1001767107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniesta AA, McGrath PT, Reisenauer A, McAdams HH, Shapiro L. A phospho-signaling pathway controls the localization and activity of a protease complex critical for bacterial cell cycle progression. Proc Natl Acad Sci U S A. 2006;103:10935–10940. doi: 10.1073/pnas.0604554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniesta AA, Shapiro L. A bacterial control circuit integrates polar localization and proteolysis of key regulatory proteins with a phospho-signaling cascade. Proc Natl Acad Sci U S A. 2008;105:16602–16607. doi: 10.1073/pnas.0808807105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs C, Ausmees N, Cordwell SJ, Shapiro L, Laub MT. Functions of the CckA histidine kinase in Caulobacter cell cycle control. Mol Microbiol. 2003;47:1279–1290. doi: 10.1046/j.1365-2958.2003.03379.x. [DOI] [PubMed] [Google Scholar]
- Jacobs C, Domian IJ, Maddock JR, Shapiro L. Cell cycle-dependent polar localization of an essential bacterial histidine kinase that controls DNA replication and cell division. Cell. 1999;97:111–120. doi: 10.1016/s0092-8674(00)80719-9. [DOI] [PubMed] [Google Scholar]
- Jacobs C, Hung D, Shapiro L. Dynamic localization of a cytoplasmic signal transduction response regulator controls morphogenesis during the Caulobacter cell cycle. Proc Natl Acad Sci U S A. 2001;98:4095–4100. doi: 10.1073/pnas.051609998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenal U, Galperin MY. Single domain response regulators: molecular switches with emerging roles in cell organization and dynamics. Curr Opin Microbiol. 2009;12:152–160. doi: 10.1016/j.mib.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SE, Ferguson NL, Alley MR. New members of the ctrA regulon: the major chemotaxis operon in Caulobacter is CtrA dependent. Microbiology. 2001;147:949–958. doi: 10.1099/00221287-147-4-949. [DOI] [PubMed] [Google Scholar]
- Lam H, Matroule JY, Jacobs-Wagner C. The asymmetric spatial distribution of bacterial signal transduction proteins coordinates cell cycle events. Dev Cell. 2003;5:149–159. doi: 10.1016/s1534-5807(03)00191-6. [DOI] [PubMed] [Google Scholar]
- Laub MT, Chen SL, Shapiro L, McAdams HH. Genes directly controlled by CtrA, a master regulator of the Caulobacter cell cycle. Proc Natl Acad Sci U S A. 2002;99:4632–4637. doi: 10.1073/pnas.062065699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Tomchick DR, Brautigam CA, Machius M, Kort R, Hellingwerf KJ, Gardner KH. Changes at the KinA PAS-A dimerization interface influence histidine kinase function. Biochemistry. 2008;47:4051–4064. doi: 10.1021/bi7021156. [DOI] [PubMed] [Google Scholar]
- Maddock JR, Shapiro L. Polar location of the chemoreceptor complex in the Escherichia coli cell. Science. 1993;259:1717–1723. doi: 10.1126/science.8456299. [DOI] [PubMed] [Google Scholar]
- Ohta N, Lane T, Ninfa EG, Sommer JM, Newton A. A histidine protein kinase homologue required for regulation of bacterial cell division and differentiation. Proc Natl Acad Sci U S A. 1992;89:10297–10301. doi: 10.1073/pnas.89.21.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta N, Newton A. The core dimerization domains of histidine kinases contain recognition specificity for the cognate response regulator. J Bacteriol. 2003;185:4424–4431. doi: 10.1128/JB.185.15.4424-4431.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce DL, O'Donnol DS, Allen RC, Javens JW, Quardokus EM, Brun YV. Mutations in DivL and CckA rescue a divJ null mutant of Caulobacter crescentus by reducing the activity of CtrA. J Bacteriol. 2006;188:2473–2482. doi: 10.1128/JB.188.7.2473-2482.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quon KC, Marczynski GT, Shapiro L. Cell cycle control by an essential bacterial two-component signal transduction protein. Cell. 1996;84:83–93. doi: 10.1016/s0092-8674(00)80995-2. [DOI] [PubMed] [Google Scholar]
- Quon KC, Yang B, Domian IJ, Shapiro L, Marczynski GT. Negative control of bacterial DNA replication by a cell cycle regulatory protein that binds at the chromosome origin. Proc Natl Acad Sci U S A. 1998;95:120–125. doi: 10.1073/pnas.95.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisinger SJ, Huntwork S, Viollier PH, Ryan KR. DivL performs critical cell cycle functions in Caulobacter crescentus independent of kinase activity. J Bacteriol. 2007;189:8308–8320. doi: 10.1128/JB.00868-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner DZ, Losick R. Protein subcellular localization in bacteria. Cold Spring Harb Perspect Biol. 2010;2:a000307. doi: 10.1101/cshperspect.a000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciochetti SA, Ohta N, Newton A. The role of polar localization in the function of an essential Caulobacter crescentus tyrosine kinase. Mol Microbiol. 2005;56:1467–1480. doi: 10.1111/j.1365-2958.2005.04652.x. [DOI] [PubMed] [Google Scholar]
- Sil A, Herskowitz I. Identification of asymmetrically localized determinant, Ash1p, required for lineage-specific transcription of the yeast HO gene. Cell. 1996;84:711–722. doi: 10.1016/s0092-8674(00)81049-1. [DOI] [PubMed] [Google Scholar]
- Skerker JM, Prasol MS, Perchuk BS, Biondi EG, Laub MT. Two-component signal transduction pathways regulating growth and cell cycle progression in a bacterium: a system-level analysis. Plos Biol. 2005;3:e334. doi: 10.1371/journal.pbio.0030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer JM, Newton A. Pseudoreversion analysis indicates a direct role of cell division genes in polar morphogenesis and differentiation in Caulobacter crescentus. Genetics. 1991;129:623–630. doi: 10.1093/genetics/129.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visintin R, Hwang ES, Amon A. Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature. 1999;398:818–823. doi: 10.1038/19775. [DOI] [PubMed] [Google Scholar]
- Wheeler RT, Shapiro L. Differential localization of two histidine kinases controlling bacterial cell differentiation. Mol Cell. 1999;4:683–694. doi: 10.1016/s1097-2765(00)80379-2. [DOI] [PubMed] [Google Scholar]
- Wu J, Ohta N, Zhao JL, Newton A. A novel bacterial tyrosine kinase essential for cell division and differentiation. Proc Natl Acad Sci U S A. 1999;96:13068–13073. doi: 10.1073/pnas.96.23.13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.