Abstract
Reduced nicotinamide adenine dinucleotide (NADH) plays key roles in energy metabolism and mitochondrial functions. However, there has been little information regarding the effect of NADH on cell survival. In this study we determined the effect of NADH treatment on the survival of glioma cells. We found that treatment of C6 glioma cells with as low as 1 μM NADH for 24 hrs significantly decreased the survival of these cells, and that treatment of the cells with 1000 μM NADH for 4 days decreased the survival of the cells by nearly 90%. This effect of NADH on glioma cells appears to be mediated by oxidative stress, as indicated by our findings that NADH treatment induced an increase in intracellular reactive oxygen species, and that two antioxidants, N-acetyl cysteine and Trolox, significantly attenuated the effect of NADH. We also found that NADH treatment induced an increase in poly(ADP-ribose) polymerase (PARP) activity, and that PARP inhibitors decreased the effect of NADH on the survival of glioma cells. These observations suggest that NADH reduces the cell survival at least partially by activating PARP. Collectively, our studies demonstrated a novel biological property of NADH — NADH decreases glioma cell survival by increasing oxidative stress and PARP activation. These results also suggest that NADH may have therapeutic potential for treating gliomas.
Keywords: NADH, glioma cells, cell death, oxidative stress, poly(ADP-ribose) polymerase
Introduction
NADH plays critical roles in multiple biological processes, including energy metabolism, mitochondrial functions and gene expression [1-3]. It has also been shown that intracellular NADH is involved in modulating inositol trisphosphate (IP3)-gated Ca2+ channels [4, 5]. Excessive increases in intracellular NADH levels can produce ‘reductive stress', which results in oxidative stress in cells by mechanisms such as mobilizing iron from ferritin [6]. It has also been reported that NADH can be transported across the plasma membranes of murine astrocytes by a P2X7 receptor-dependent pathway [7]. However, there has been little information regarding the effects of NADH on cell survival.
A large number of studies have indicated that oxidized nicotinamide adenine dinucleotide (NAD+) plays important roles in energy metabolism, calcium homeostasis, gene expression, DNA repair and immunological functions [1, 8]. It has also been found that NAD+ can induce apoptosis of certain population of T cells by activating P2X7 receptors [9, 10]. Our recent study has found that treatment of tumor cells including C6 glioma cells with NAD+ significantly decreases the cell survival through oxidative stress [11], suggesting a novel property of NAD+.
Because NADH is the reduced form of NAD+, and the structure of NADH is highly similar to that of NAD+, we hypothesized that NADH treatment may also affect tumor cell survival through oxidative stress. Many studies have indicated that oxidative stress induces cell death by excessively activating poly(ADP-ribose) poly-merases (PARP), which leads to cell death by depleting intracellular NAD+ [12-15]. Based on these pieces of information, in this study we conducted experiments to test the hypothesis that NADH treatment can induce the death of glioma cells, and to determine the potential roles of oxidative stress and PARP activation in the effect of NADH on the cell survival. Our studies indicate that NADH treatment can significantly decrease glioma cell survival through oxidative stress- and PARP-dependent pathways.
Materials and methods
Cell cultures
C6 glioma cells were purchased from the Cell Resource Center of Shanghai Institute of Biological Sciences, Chinese Academy of Sciences. The cells were plated onto 24-well cell culture plates at the initial density of 1×105 cells/ml in Dulbecco's Modified Eagle Medium (containing 4,500 mg/L D-glucose, 584 mg/L L-glutamine, 110 mg/L sodium pyruvate) (Thermo Scientific, Waltham, MA, USA) that contains 1% penicillin and streptomycin (Invitrogen, Carlsbad, CA, USA) and 10% fetal bovine serum (PAA, Linz, Austria). The cells were used when the density of the cultures reached 60-80%.
Experimental procedures
Experiments were initiated by replacing the culture medium with those containing various concentrations of drugs. The cells were left in an incubator with 5% CO2 at 37 °C for 24 or 96 hrs.
Lactate dehydrogenase (LDH) assay
Cell survival was quantified by measuring LDH activity in cell lysates, as described previously [16]. In brief, cells were lysed for 20 min in lysing buffer containing 0.04% Triton-X100, 2 mM HEPES, 0.2 mM dithiothreitol, 0.01% bovine serum albumin, and 0.1% phenol red (pH 7.5). Fifty μl cell lysates were mixed with 150 μl potassium phosphate buffer (500 mM, pH 7.5) containing 1.5 mM NADH and 7.5 mM sodium pyruvate. Subsequently, changes of the A340nm of the samples were monitored over 90 s. Percentage cell survival was calculated by normalizing the LDH activity of a sample to that of control culture wells.
Flow cytometry-based propidium iodide (PI) staining
C6 glioma cells were digested with 0.1% trypsin and resuspended in 1 ml phosphate-buffered saline (PBS). After wash twice with PBS, the cells were incubated with propidium iodide (20 μg/ml) at 37°C for 30 min. Subsequently, the number of PI-negative and PI-positive cells was assessed by a flow cytometer (BD FACSAriaII).
Dihydroethidium (DHE) assay
Cells were incubated with 5 μM DHE for 30 min at 37°C. Subsequently, the cells were washed with PBS, and the fluorescence signals of the cells were observed under a Leica fluorescence microscope at excitation wavelength of 545 nm and emission wavelength of 605 nm.
Poly(ADP-ribose) (PAR) immunostaining
PAR immnostaining was conducted as described previously [13]. In brief, cell cultures were fixed in 10% ice-cold TCA for 15 min, followed by washes with ice-cold 70%, 90% or 100% ethanol, respectively. The cells were incubated with monoclonal anti-PAR Ab (Trevigen, 4335-AMC-050) diluted at 1:300 in 0.1 M PBS containing 5% goat serum and 0.05% Tween-20 overnight at 4°C. After three washes with PBS containing 0.1% Tween-20, the cells were incubated with Alexa Fluor 488 goat anti-mouse secondary Ab diluted at 1:500 in blocking buffer (0.1 M PBS containing 10% goat serum and 0.1% Tween-20) for 1 hr at RT. After the cells were washed three times with PBS containing 0.1% Tween-20, the cells were counter-stained with PI (0.27 μg/ml) for 5 min at RT. Following washes with PBS, the fluorescence images of the cultures were photographed under a Leica fluorescence microscope.
Statistical analyses
All data are presented as mean + SE. Data were assessed by one-way ANOVA, followed by Student-Newman-Keuls post hoc test. P values less than 0.05 were considered statistically significant.
Results
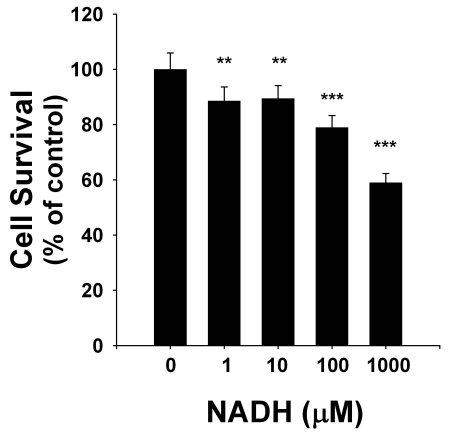
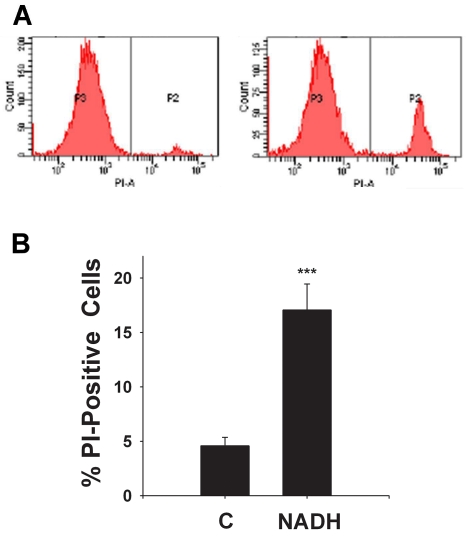
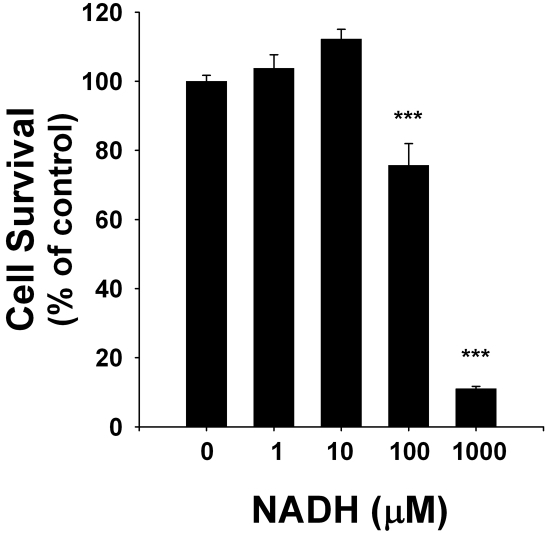
To determine the effects of NADH treatment on the survival of C6 glioma cells, we applied both LDH assay and flow cytometry-based PI assay. LDH assay showed that NADH treatment dose-dependently decreased the number of surviving cells at 24 hrs after the NADH treatment (Figure 1): NADH at concentrations of 1 - 1000 μM decreased by 10-40% the survival of the glioma cells. Similar results were obtained by flow cytometry-based PI assay (data not shown). The flow cytometry-based PI assay further showed that NADH treatment induced a significant increase in the number of PI-positive (necrotic) cells (Figures 2A & 2B). We also determined the effect of long-term treatment of C6 glioma cells with various concentrations (1, 10, 100 and 1000 μM of NADH. Treatment of the cells with 1000 μM NADH for 4 days decreased the sur vival of the cells by nearly 90% (Figure 3).
Figure 1.
NADH treatment dose-dependently decreased the number of surviving C6 glioma cells. The cells were treated with 1, 10, 100 and 1000 μM NADH for 24 hrs, and subsequently the number of surviving cells was assessed by LDH assay. N = 25. Data were pooled from 6 independent experiments. **, p < 0.01; ***, p < 0.001.
Figure 2.
(A) The graphs from flow cytometry-based PI assays showed that NADH treatment induced an increase in the number of PI-positive C6 glioma cells. The P2 fraction and the P3 fraction indicate the number of PI-positive cells and PI-negative cells, respectively. The number of PI-positive cells in the samples treated with 1000 μM NADH is greater than that in the controls. (B) Quantifications of the results from the flow cytometry-based PI assays showed that NADH treatment induced a significant increase in the number of PI-positive C6 glioma cells. The cells were treated with 1000 μM NADH for 24 hrs, and subsequently the number of PI-positive cells was assessed by flow cytometry-based PI assay. N = 9. Data were pooled from 3 independent experiments. ***, p < 0.001.
Figure 3.
NADH treatment for 4 days profoundly decreased the number of surviving C6 glioma cells. The cells were treated with 1, 10, 100 and 1000 μM NADH for 4 days, and subsequently the number of surviving cells was assessed by LDH assay. N = 24, Data were pooled from 3 independent experiments. ***, p < 0.001.
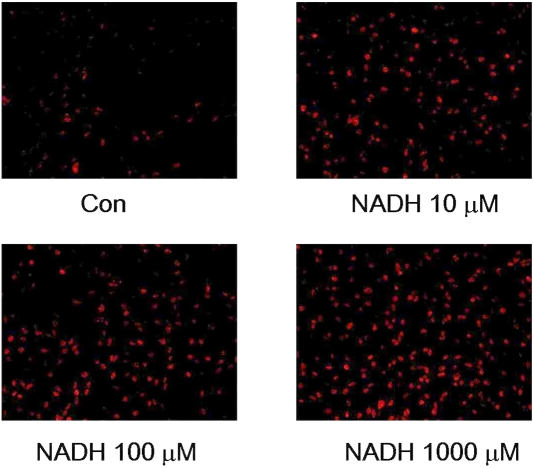
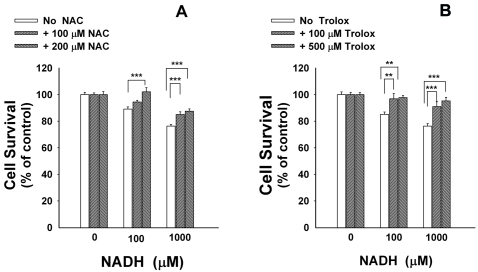
Since oxidative stress is one of the key inducers of cell death under many conditions [17, 18], we tested the hypothesis that NADH induces glioma cell death by generating reactive oxygen species (ROS). By applying DHE assay that detects superoxide levels in cells, we found that treatment of the cells with 10, 100 or 1000 μM NADH for 6 hrs induced increases in the red fluorescence signals (Figure 4), indicating increased generation of superoxide in the cells. Trolox, a water-soluble derivative of vitamin E [19], and N-acetylcysteine (NAC) are two widely used antioxidants. NAC can produce antioxidation effects due to its capacity of increasing in-tracellular glutathione levels via enhancing in-tracellular cysteine [20]. We found that treatment of the cells with NAC (Figure 5A) or Trolox (Figure 5B) significantly attenuated the effects of NADH on the cell survival.
Figure 4.
NADH treatment increases the levels of ROS in C6 glioma cells. The cells were treated with 10, 100 or 1000 μM NADH for 6 hrs, and subsequently the ROS levels in the cells were assessed by dihydroethidium (DHE) assay. The photos are representatives of the photos taken from three independent experiments.
Figure 5.
Antioxidants attenuated the effects of NADH treatment on the survival of C6 glioma cells. The cells were pre-treated with N-acetyl cysteine (NAC) (A) or Trolox (B) for 30 min, followed by co-treatment with 100 or 1000 μM NADH for 24 hrs. The percentage of cell survival was assessed by LDH assay. N = 16. Data were pooled from 4 independent experiments. *, p < 0.05, **, p < 0.01, ***, p < 0.001.
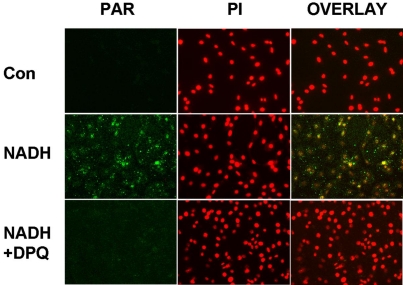
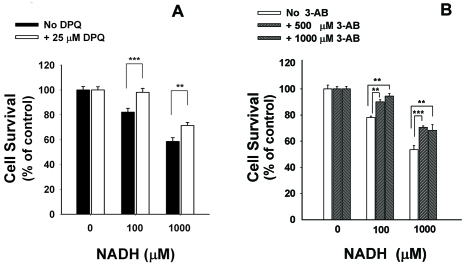
A number of in vitro and in vivo studies have indicated that PARP is a key enzyme in oxidative stress-induced cell death [1, 15]. We tested if PARP activation contributes to the effect of NADH on the cell survival. 4-dihydro-5-[4-(1- piperidinyl)butoxy]-1(2H-isoquinolinone)(DPQ) and 3-aminobenzaimde (3-AB) are two widely used PARP inhibitors [12, 21]. We found that treatment of C6 glioma cells with 1000 μM NADH for 2 hrs induced increased PAR formation in the cells, which is an index of PARP activity [22] (Figure 6). The PAR formation was prevented by the PARP inhibitor DPQ (Figure 6), indicating that NADH induces PARP activation in the cells. We further determined if PARP inhibitors can decrease the effect of NADH on the cell survival: The NADH-induced decrease in C6 glioma cell survival was significantly attenuated by treatment of the cells with DPQ (Figure 7A) or 3-AB (Figure 7B), suggesting a significant role of PARP in this effect of NADH.
Figure 6.
NADH induced increased poly(ADP-ribose) (PAR) formation in C6 glioma cells. C6 glioma cells were pre-treated with 25 μM DPQ for 30 min, followed by co-treatment with 1000 μM NADH. Immunostainingfor PAR formation in the cultures was conducted 2 hours after the NADH exposures. Nuclei were counterstained with propidium iodide (PI). Overlaid images show that NADH induced PAR formation in the nuclei (yellow), which was attenuated by DPQ pre-treatment. Results are representative of three independent experiments.
Figure 7.
PARP contributes to the NADH-induced decreases in C6 glioma cell survival. The cells were pre-treated with DPQ (A) or 3-aminobenzaimde (3-AB) (B) for 30 min, followed by co-treatment with 100 or 1000 μM NADH for 24 hrs. Subsequently, the cell survival was assessed by LDH assay. N= 14-20. Data were pooled from 3 independent experiments. **, p < 0.01, ***, p < 0.001.
Discussion
The key findings of this study include: (1) treatment of C6 glioma cells with NADH can significantly decrease the survival of the cells; (2) oxidative stress mediates the effects of NADH on tumor cell survival, and (3) PARP activation also contributes to the effect of NADH on tumor cell survival.
The finding that as low as 1 μM NADH can induce a significant decrease in the number of surviving C6 glioma cells indicates a novel biological property of NADH: NADH treatment can induce decreases in glioma cell survival. Our study also indicated that NADH can significantly increase cell necrosis, as assessed by the flow cytometry-based PI assay.
Our studies suggest that oxidative stress mediates the effect of NADH on the cell survival. This was supported by the findings that (1) NADH can induce marked increases in ROS in the cells at 6 hours after NADH treatment; and (2) two structurally different antioxidants, Trolox and N-acetyl cysteine, can significantly attenuate the effects of NADH. Because oxidative stress is one of the major factors in cell death under a number of physiological and pathological conditions [23-25], it is not surprising that ROS also plays a significant role in the NADH-induced tumor cell death.
PARP-1 is a major member of PARP family proteins [22]. This enzyme is rapidly activated by single-strand DNA damage, which leads to poly (ADP-ribosyl)ation of target proteins such as histones and PARP-1 itself by consuming NAD+ [22]. PARP-1 plays important role in various biological functions, including DNA repair, gene expression, genomic stability, cell cycle, and long-term memory [1, 22]. A number of studies have indicated that excessive PARP activation is a key factor in cell death induced by oxidative stress both in vitro and in vivo [1, 22]. Increased PARP activation leads to cell death by depleting intracellular NAD+, increasing mitochondrial permeability transition and inducing nuclear translocation of apoptosis-inducing factors [13, 14, 26, 27]. Multiple studies, applying either pharmacological or genetic approaches to inhibit PARP-1, have also indicated that PARP-1 inhibition can produce protective effects both in vitro and in vivo [12]. Our study indicated that PARP activation plays a significant role in the effects of NADH on cell survival. Together with our finding that oxidative stress mediates the NADH-induced decease in cell survival, our study suggests that NADH produces its effects on the cell survival by inducing oxidative stress and subsequent PARP activation.
Malignant glioma is a major type of brain cancer, and most of the patients of malignant glioma have poor prognosis [28]. It is of great theoretical and clinical importance to find novel approaches to kill glioma cells. Our study has suggested that NADH is a novel agent that can profoundly decrease the survival of C6 glioma cells, suggesting that NADH might have therapeutic potential for treating gliomas.
In summary, our studies demonstrate a new biological property of NADH. That is, NADH treatment can significantly decrease glioma cell survival by inducing increases in oxidative stress and PARP activation. Our studies also suggest that NADH may be used for treating gliomas. Future studies are warranted to investigate the effect of NADH on tumor survival in animal models, and to further investigate the mechanisms underlying the effect of NADH treatment on tumor cell survival.
Acknowledgments
The authors would like to acknowledge the technical support of Mrs. Jin Xu. This study was supported by Shanghai Engineering Center Grant of Equipment and Technology of Physical Therapy for Major Diseases #08DZ2211200 (to W. Y.), a Key Research Grant of Shanghai Municipal Scientific Committee #08JC1415400 (to W. Y.), a National Key Basic Research ‘973 Program Grant’ #2010CB834306 (to W. Y. and W. Xia), a Pujiang Scholar Program Award 09PJ1405900 (to W. Y.), a Project Grant for Promoting Cooperation of Scientific Research and Education of High-Level Talents between China and USA& Canada (to W. Y.), a Key Research Grant of Shanghai Municipal Educational Committee #09ZZ21 (to W. Y.), and a Morning Star Program Award (to W. Xia).
References
- 1.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Q, Piston DW, Goodman RH. Regulation of corepressor function by nuclear NADH. Science. 2002;295:1895–1897. doi: 10.1126/science.1069300. [DOI] [PubMed] [Google Scholar]
- 3.Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293:510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 4.Kaplin AI, Snyder SH, Linden DJ. Reduced nicotinamide adenine dinucleotide-selective stimulation of inositol 1,4,5-trisphosphate receptors mediates hypoxic mobilization of calcium. J Neurosci. 1996;16:2002–2011. doi: 10.1523/JNEUROSCI.16-06-02002.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patterson RL, van Rossum DB, Kaplin AI, Barrow RK, Snyder SH. Inositol 1,4,5-trisphosphate receptor/GAPDH complex augments Ca2+ release via locally derived NADH. Proc Natl Acad Sci U S A. 2005;102:1357–1359. doi: 10.1073/pnas.0409657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaeschke H, Kleinwaechter C, Wendel A. NADH-dependent reductive stress and ferritin-bound iron in allyl alcohol-induced lipid peroxida-tion in vivo: the protective effect of vitamin E. Chem Biol Interact. 1992;81:57–68. doi: 10.1016/0009-2797(92)90026-h. [DOI] [PubMed] [Google Scholar]
- 7.Lu H, Burns D, Garnier P, Wei G, Zhu K, Ying W. P2X7 receptors mediate NADH transport across the plasma membranes of astrocytes. Biochem Biophys Res Commun. 2007;362:946–950. doi: 10.1016/j.bbrc.2007.08.095. [DOI] [PubMed] [Google Scholar]
- 8.Ziegler M. New functions of a long-known molecule. Emerging roles of NAD in cellular signaling. Eur J Biochem. 2000;267:1550–1564. doi: 10.1046/j.1432-1327.2000.01187.x. [DOI] [PubMed] [Google Scholar]
- 9.Aswad F, Kawamura H, Dennert G. High sensitivity of CD4+CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: a role for P2X7 receptors. J Immunol. 2005;175:3075–3083. doi: 10.4049/jimmunol.175.5.3075. [DOI] [PubMed] [Google Scholar]
- 10.Haag F, Adriouch S, Brass A, Jung C, Moller S, Scheuplein F, Bannas P, Seman M, Koch-Nolte F. Extracellular NAD and ATP: Partners in immune cell modulation. Purinergic Signal. 2007;3:71–81. doi: 10.1007/s11302-006-9038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao C, Hong Y, Han J, Ma Y, Chen H, Xia W, Ying W. NAD+ treatment decreases tumor cell survival by inducing oxidative stress. Front in Biosci (In Press) doi: 10.2741/e258. [DOI] [PubMed] [Google Scholar]
- 12.Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 13.Alano CC, Ying W, Swanson RA. Poly(ADP-ribose) polymerase- 1-mediated cell death in astrocytes requires NAD+ depletion and mito-chondrial permeability transition. J Biol Chem. 2004;279:18895–18902. doi: 10.1074/jbc.M313329200. [DOI] [PubMed] [Google Scholar]
- 14.Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neuro-sci. 2010;30:2967–2978. doi: 10.1523/JNEUROSCI.5552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ying W. NAD+ and NADH in cellular functions and cell death. Front Biosci. 2006;11:3129–3148. doi: 10.2741/2038. [DOI] [PubMed] [Google Scholar]
- 16.Ying W, Swanson RA. The poly(ADP-ribose) glycohydrolase inhibitor gallotannin blocks oxidative astrocyte death. Neuroreport. 2000;11:1385–1388. doi: 10.1097/00001756-200005150-00007. [DOI] [PubMed] [Google Scholar]
- 17.Orrenius S, Gogvadze V, Zhivotovsky B. Mito-chondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 18.Ying W. Deleterious network hypothesis of apop-tosis. Med Hypotheses. 1998;50:393–398. doi: 10.1016/s0306-9877(98)90211-0. [DOI] [PubMed] [Google Scholar]
- 19.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decol-orization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 20.Martin H, Abadie C, Heyd B, Mantion G, Richert L, Berthelot A. N-acetylcysteine partially reverses oxidative stress and apoptosis exacer bated by Mg-deficiency culturing conditions in primary cultures of rat and human hepatocytes. J Am Coll Nutr. 2006;25:363–369. doi: 10.1080/07315724.2006.10719547. [DOI] [PubMed] [Google Scholar]
- 21.Czapski GA, Cakala M, Kopczuk D, Strosznajder JB. Effect of poly(ADP-ribose) polymerase inhibitors on oxidative stress evoked hydroxyl radical level and macromolecules oxidation in cell free system of rat brain cortex. Neurosci Lett. 2004;356:45–48. doi: 10.1016/j.neulet.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 22.D'Amours D, Desnoyers S, D'Silva I, Poirier GG. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J. 1999;342 (Pt 2):249–268. [PMC free article] [PubMed] [Google Scholar]
- 23.Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 24.Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in car-cinogenesis. Toxicol Pathol. 38:96–109. doi: 10.1177/0192623309356453. [DOI] [PubMed] [Google Scholar]
- 25.Harman D. Aging and oxidative stress. J Int Fed Clin Chem. 1998;10:24–27. [PubMed] [Google Scholar]
- 26.Ying W, Garnier P, Swanson RA. NAD+ repletion prevents PARP-1-induced glycolytic blockade and cell death in cultured mouse astrocytes. Biochem Biophys Res Commun. 2003;308:809–813. doi: 10.1016/s0006-291x(03)01483-9. [DOI] [PubMed] [Google Scholar]
- 27.Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of poly(ADP-ribose) polymerase- 1-dependent cell death by apoptosis-inducingfactor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 28.Robins HI, Lassman AB, Khuntia D. Therapeutic advances in malignant glioma: current status and future prospects. Neuroimaging Clin N Am. 2009;19:647–656. doi: 10.1016/j.nic.2009.08.015. [DOI] [PubMed] [Google Scholar]