Abstract
Gastrodia elata (Tianma) is a traditional Chinese herb with demonstrated vasodilatory effects. This pilot study examined the effects of Tianma treatment on bladder smooth muscle contractility. Rats were treated with 2.5 g Tianma per kg body weight over 7 weeks. Contractility was measured in detrusor strips isolated in both transverse (Tr) and longitudinal (Lg) directions with the urothelium intact (+UE) or denuded (-UE). Spontaneous phasic activity was enhanced in longitudinal +UE strips. No differences between control and Tianma-treated detrusor strips were detected in contractions elicited by K+-Krebs’ solution or carbachol. Isoprenaline (IPNA)-induced relaxation remained unchanged in -UE strips after Tianma treatment. However, potency of IPNA was lower in Tianma-treated +UE strips in the longitudinal direction. These findings provided an initial assessment of how Tianma altered bladder smooth muscle function. It will be of interest in future investigations to elucidate the mechanisms via which Tianma exerts its actions on the bladder.
Keywords: Gastrodia elata, Tianma, detrusor, urothelium, contraction, relaxation, phasic activity
Introduction
Normal bladder function relies on regulated cycles of voiding and filling, which is facilitated by contraction and relaxation of the detrusor smooth muscle [1]. Bladder contractility is mainly under autonomic control. The parasympathetic neurotransmitter acetylcholine activates muscarinic receptors in the bladder to induce contraction and urine release [1]. Urine storage is the result of bladder relaxation when β-adrenoceptors are activated by the sympathetic neurotransmitter noradrenaline [1]. Spontaneous bladder contractions also play an integral part in bladder function, especially during bladder filling when detrusor wall tension and intravesical pressure fluctuate to adjust to the changing urine volume [2]. The importance of phasic activity in the bladder is implicated in various bladder conditions including overactive bladder [3]. Inhibition of excessive contractions is therefore the mainstay of overactive bladder treatment. With the unavoidable adverse effects usually associated with drugs such as muscarinic blockers and β-agonists, there is ongoing search for alternative overactive blad der drug therapies. Synthetic drugs or natural remedies with effects on bladder function, be these favourable or not, provide valuable information to patients and researchers alike. For example, drugs with other therapeutic purposes that also accentuate bladder contractions may be best avoided in overactive bladder patients.
The rhizome of Gastrodia elata, or Tianma, has the traditional Chinese medicinal effects of expelling wind from the body, pacifying the liver and relieving obstructive pain [4]. Tianma is mainly used in the treatments of convulsions and headache, but has also demonstrated effectiveness in cardiovascular conditions including hypertension [4-6]. In clinical trials, the risk of ischemic heart disease is reportedly lower with Tianma treatment, owing to its vasodilatory effects in the coronary vessels [4]. Vasodilation has also been observed in other vessels such as carotid and cerebral arteries [6]. Very little is known about the effects of Tianma on other types of smooth muscles though, except that contractility was also inhibited in the ileum [7]. In this pilot study, the effects of Tianma on bladder smooth muscle contractility were examined. In consideration of the importance of muscarinic and β-adrenergic receptors in bladder contractility, agonists at these receptors were employed to assess the actions of Tianma.
Methods
Tianma preparation
The rhizome of Gastrodia elata (Tianma) was collected from Zhaotong City, China and provided by Dr. Jun Zhou and Dr. Jiangmiao Hu (Kunming Institute of Botany, Chinese Academy of Science, Yunnan, People's Republic of China). The species was identified and chemically analyzed previously as reported in Li et al. [8]. In this study, whole dried tubers of Tianma were hammered into smaller pieces and ground to fine powder. 7.5 g of Tianma powder was mixed with 100 mL sterilized Milli-Q water and boiled for 1 h at 100 °C. The solution was centrifuged at 3000 G for 10 minutes at room temperature. The supernatant was filtered with filter paper, yielding approximately 85 mL. The Tianma solution was concentrated at 60 °C under vacuum and the final volume was reduced to 10 mL for feeding.
Tianma administration
All procedures were performed in accordance with rules outlined by the Institutional Animal Care and Use Committee (Nanyang Technological University, Singapore, Project approval No.: ARF SBS/NIE-A 003). Six- to seven-week-old male Sprague-Dawley rats were obtained from the Laboratory Animals Centre (Singapore). Rats were randomly assigned to control and Tianma-treated groups. There were 10 control rats and 9 Tianma-treated rats. Each Tianma-treated rat received 0.75 g of Tianma orally per day, which equalled to a daily dose of 2.5 g Tianma per kg body weight. This dose was chosen based on efficacy demonstrated in mice (1 g per kg body weight intraperitoneal injection) and in humans (3 to 10 g per 70 kg body weight) [6]. Feeding was done with a syringe and by dispensing the Tianma solution via blunt needle drop by drop. The treatment continued on for 7 weeks before the rats were sacrificed by CO2 asphyxiation.
Tissue preparation
The whole bladder was harvested as previously described and immediately placed in carbogen (95 % O2 and 5 % CO2)-aerated ice-cold Krebs’ solution [9]. The bladder base, which made up about one third of the bladder, was discarded. Only tissues isolated from the bladder dome (detrusor) were used. The bladder dome was cut open along the lateral sides and the UE was exposed. Using a razor blade, four strips measuring 5 mm by 1 mm each were dissected from the detrusor [9]. Two of the strips were cut with the longer side parallel to the longitudinal axis of the detrusor; two others with the longer side parallel to the transverse axis of the detrusor. The longer side of the strip was in line with the direction of contractile force measurement. Both urothelium-intact (+UE) and urothelium-denuded (-UE) strips were used. In -UE strips, the UE was carefully excised with fine dissecting scissors as described elsewhere [10-12]. All strips were mounted on a tissue myograph system (Danish Myo Technology Model 800MS, Aarhus, Denmark) containing carbogen-aerated Krebs’ solution at 37 °C. Built-in clips were used to fix both ends of the tissue strip. Isometric tension was monitored in both transverse and longitudinal directions and recorded using a Powerlab interface and the LabChart software (ADInstruments, Bella Vista, Australia). The detrusor strips were allowed to equilibrate for 30 min with multiple washouts. During the equilibration and washout periods, baseline tension was consistently adjusted to 2 ± 0.1 g. Viability of the strips was tested using carbogen-aerated K+-Krebs’ solution. Experiments were commenced after an additional 30 min of continuous washout.
Contractility studies
Cumulative concentration-response curves (CRCs) of the muscarinic agonist carbachol (CCh, from 0.01 to 30 mM in half-log increments) and the nonselective β-agonist isoprenaline (IPNA, from 0.01 to 100 μM were constructed. The contraction or tension (in grams) elicited by each addition of CCh or IPNA in individual detrusor strips was used in subsequent data calculations and analysis. For the relaxation experiments, detrusor strips were first precontracted with 1 μM CCh to reach steady-state, followed by cumulative addition of IPNA. In a separate study (unpublished observations) and as reported by others [13], only minimal drop in tension occurred after prolonged CCh stimulation. Raw tension values (in g) were normalized to dry tissue weights (in mg), measured after the experiments, so that weight-insensitive data could be obtained. Maximal CCh-induced contraction in each individual strip (after normalization to weight) was considered as 100% contraction in plotting the CCh CRCs. The baseline (of 2 g) was regarded as 100 % relaxation in the IPNA experiments, where 0 % represented the 1 μM CCh-induced pre-contraction at steady-state. Maximal contractions obtained from K+-Krebs’ solution and CCh addition in Tianma-treated detrusor strips were compared with those in control strips and percent changes from control values were determined.
Spontaneous phasic activity measurements
Spontaneous phasic activity was analyzed for 2 min in unstimulated conditions. We adopted a method by Deba et al. [14] where the integral under the tension recording curve could give a simple indication of the amount of phasic activity [15]. Phasic activity was quantified this way over the 2-min period. Integral values of Tianma-treated detrusor strips were expressed as percent changes from those of control strips.
Statistical analysis
Calculations and statistical analysis were performed using the Prism 4 software (GraphPad Software Inc., La Jolla, CA, USA). All data shown in graphs were mean values ± S.E.M. CRCs were fitted by a sigmoidal regression equation of Hill slope equals unity. The equation is: response (%) = {minimal tension + [(maximal tension - minimal tension) / (1 + 10 logEC50-x)]} * 100 %, where x represents log concentration of the agonist. Replicates's tests of the data indicated no deviation from this sigmoidal equation. The potencies of CCh and IPNA between control and Tianma-treated detrusor were compared with Student's t-test. For maximal contractions induced by K+-Krebs’ solution and CCh, and for phasic activity, one-sample Student's t-test was used for determining differences from control. P values of less than 0.05 (P < 0.05) were considered to be statistically significant.
Drugs and chemicals
The composition of Krebs’ solution was as follows [in mM]: NaCl [119], MgCl2 [1.2], NaH2PO4 [1.2], NaHCO3 [15], KCl [4.6], CaCl2 [1.5], D-glucose [11]. For K+-Krebs’ solution, no NaCl was added but 124 mM KCl was used instead. All constituents remained the same otherwise. All chemicals and drugs, except Tianma, used in this study were purchased from Sigma-Aldrich Co. (Singapore, Singapore). All drugs were dissolved in Ca2+-free Krebs’ solution.
Results
Tianma treatment increased spontaneous phasic activity in +UE detrusor strips
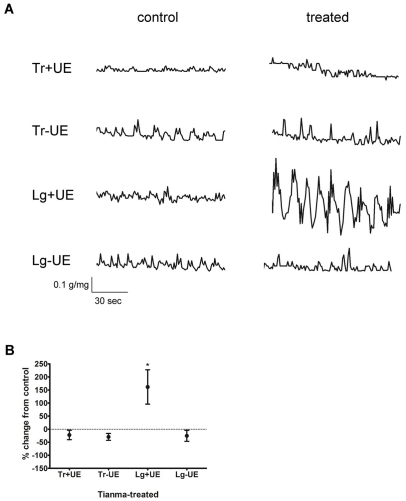
Spontaneous phasic activity was monitored in urothelium-intact (+UE) and urothelium-denuded (-UE) detrusor strips from control (i.e. untreated) and Tianma-treated rats. In this and subsequent series of experiments, strips dissected in both transverse (Tr) and longitudinal (Lg) directions were examined. Sample raw tracings of tension fluctuations are shown in Figure 1A. Both +UE and -UE strips from control rats showed comparable phasic activity, whereas that in longitudinal +UE (Lg+UE) strips from Tianma-treated rats was augmented. When expressed in terms of percent change from control values, phasic activity of all but Tianma-treated Lg+UE detrusor strips was not altered. In Lg+UE strips, phasic activity was significantly increased (Figure 1B).
Figure 1.
Spontaneous phasic activity in detrusor strips of control and Tianma-treated rats. A. Sample raw tracings showing tension fluctuations in urothelium-intact (+UE) and urothelium-denuded (-UE) detrusor strips isolated in the transverse (Tr) and longitudinal (Lg) directions. B. Percent change of spontaneous phasic activity in Tianma-treated detrusor strips from control values. Spontaneous phasic activity was greater in Tianma-treated Lg+UE strips compared with controls. * denotes P < 0.05 vs. control level which is indicated by the dotted horizontal line crossing at 0 %.
Tianma treatment had no effect on stimulated detrusor contractions
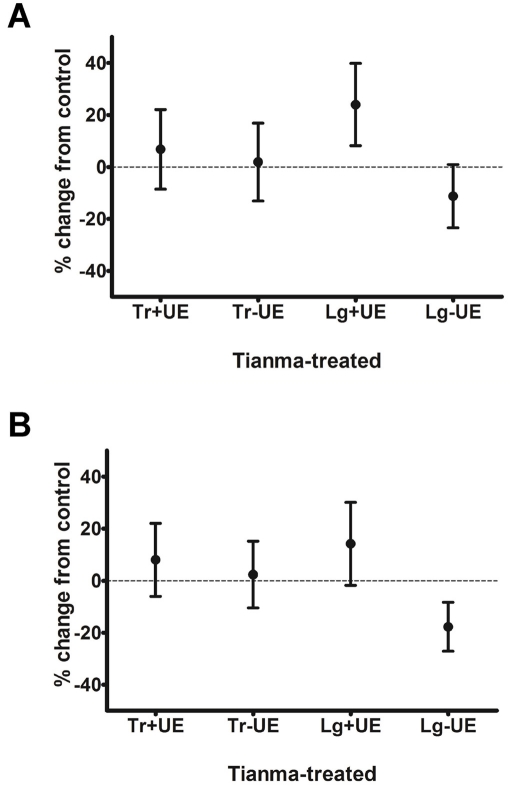
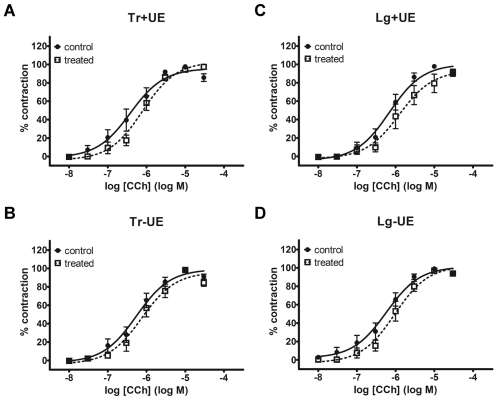
Detrusor strips from control and Tianma-treated rats were challenged with either K+-Krebs’ solution or carbachol (CCh, 30 μM). Maximal contractions in Tianma-treated detrusor strips were expressed as percent changes from those of control strips (Figure 2). In both Figures 2A and 2B, all values were not significantly deviated from zero. Thus there was no difference in contractile amplitudes between control and Tianma -treated detrusor strips under either stimulus, i.e. the depolarizating agent K+-Krebs’ solution (Figure 2A) or the muscarinic agonist CCh (Figure 2B). Concentration-dependent effects of CCh stimulation were further examined. Both control and Tianma-treated rat detrusor strips responded similarly to varying CCh concentrations. The cumulative CCh concentration-response curves (CRCs) were largely overlapping (Figure 3), and log EC50 values were not significantly different (Table 1). Collectively shown, CCh-induced contractions in control and Tianma-treated detrusor did not differ in either efficacy or potency.
Figure 2.
Comparison of maximal contractions induced by A. K+-Krebs’ solution and B. 30 μM carbachol in Tianma-treated and control detrusor strips. Responses from Tianma-treated detrusor strips are expressed as percent changes from control. Tianma treatment did not affect maximal contractile amplitude in both transverse (Tr) and longitudinal (Lg) strips with and without urothelium (UE).
Figure 3.
Concentration-response curves (CRCs) of carbachol (CCh)-induced contractions. Control (solid symbol, solid line) and Tianma-treated (open symbol, dotted line) CRCs are not different from each other. No effects of Tianma treatment were seen in urothelium-intact (+UE) or urothelium-denuded (-UE) detrusor strips of either transverse (Tr) or longitudinal (Lg) direction.
Table 1.
Potency of carbachol (CCh) and isoprenaline (IPNA) in transverse (Tr) and longitudinal (Lg) detrusor strips from control and Tianma-treated rats. CCh-induced contractions and IPNA-induced relaxations of urothelium-intact (+UE) and urothelium-denuded (−UE) strips were examined. Potency is expressed as the logarithms of the CCh or IPNA concentration achieving 50 % maximal efficacy. * denotes P < 0.05 vs. control.
| CCh |
IPNA |
||||
|---|---|---|---|---|---|
| Control | Tianma-treated | Control | Tianma-treated | ||
| Tr | +UE | -6.5 ± 0.2 | -6.1 ± 0.1 | -6.3 ± 0.2 | -6.2 ± 0.1 |
| -UE | -6.3 ± 0.2 | -6.1 ± 0.1 | -6.4 ± 0.1 | -6.3 ± 0.2 | |
| Lg | +UE | -6.2 ± 0.1 | -6.0 ± 0.2 | -6.3 ± 0.1 | -5.8 ± 0.1* |
| -UE | -6.3 ± 0.2 | -6.1 ± 0.1 | -6.37 ± 0.09 | -6.2 ± 0.1 | |
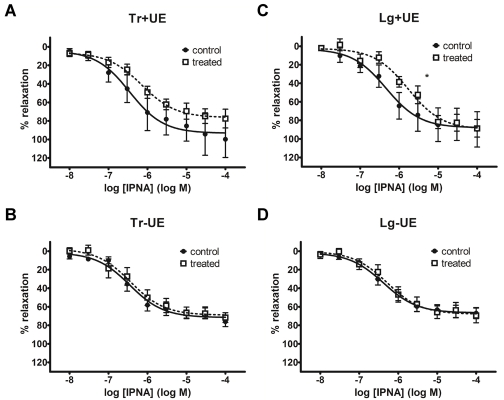
Potency of isoprenaline (IPNA)-induced relaxations in +UE detrusor strips was decreased after Tianma treatment
Since chemically stimulated contractile function in the detrusor was not affected by Tianma treatment, relaxant responses were examined next. Cumulative CRCs of the nonselective β-agonist isoprenaline (IPNA) against 1 μM CCh pre-contraction are shown in Figure 4. Maximal relaxations were not different between control and Tianma-treated detrusor strips. In -UE strips, the control and Tianma-treated CRCs were also largely indistinguishable (Figures 4B and 4D). The IPNA CRC in Tianma-treated Tr+UE strips appeared to be shifted to the right (Figure 4A), but the resultant log EC50 was not different from control (Table 1). The rightward shift of CRC was more apparent in Lg+UE strips (Figure 4C), in which IPNA potency was significantly lower in Tianma-treated detrusor (-5.8 ± 0.1) than control (-6.3 ± 0.1) (Table 1). The data here suggested that Tianma may elicit an inhibitory action on the urothelium (UE), resulting in less potent IPNA responses. Direction-specific responses may differ in their sensitivity to Tianma, as demonstrated by the greater effect on IPNA potency in only longitudinal relaxations.
Figure 4.
Concentration-response curves (CRCs) of isoprenaline (IPNA)-induced relaxations. The CRCs are shown as: control in solid symbol and solid line; Tianma-treated in open symbol and dotted line. No effects of Tianma treatment were seen in -UE strips of either transverse (Tr) or longitudinal (Lg) direction (panels C and D). In Tr+UE strips, a small inhibitory effect was observed (panel A). The rightward shift of the CRC was more prominent in Lg+UE strips (panel B). * denotes P < 0.05 of IPNA potency vs. control.
Discussion
According to the practice of traditional Chinese medicine, Tianma can be used for its sedative, anticonvulsant and vasodilatory effects [4,6]. In carotid, cerebral and coronary vessels, Tianma induces relaxation and increases blood flow [6]. These vasodilatory effects suggest a possible role of Tianma in mediating the contractility of other types of smooth muscle as well. The only other published report on non-vascular smooth muscle function was one by Hayashi et al. [7], where Tianma showed an overall inhibitory effect on ileum smooth muscle contraction. However, no information on the effects of Tianma on bladder smooth muscle contractility is available. In the present study, we compared various aspects of smooth muscle contractility between detrusor strips from control and Tianma-treated rats. Various effects of Tianma on spontaneous phasic contractile activity and on agonist-stimulated relaxations were noted.
For its cardiovascular effects, Tianma has been tested in patients with hypertension and myocardial ischaemia and with promising results [6]. The prevalence of these cardiovascular disorders increases with age, to take hypertension as an example [5]. According to Burt et al. [5], less than 10 % of young adults (i.e. those below 40 years old) are hypertensive compared with half of the older adults (i.e. those above 40 years old) suffering from high blood pressure. Based on the correlation between age and cardiovascular disorders, Tianma is more likely prescribed to the middle-aged and aged population. It is also in this age group that the vasodilatory effects of Tianma are more clinically applicable. For a similar reason, bladders from mature adult rats instead of younger ones were used in the current pilot study.
Unlike in vascular and ileum smooth muscles [6,7], Tianma treatment did not promote smooth muscle relaxation in the bladder in this study. A rather opposite phenomenon was observed instead. Detrusor strips from Tianma-treated rats showed greater spontaneous phasic activity and were more resistant to IPNA-induced relaxation. The latter finding was evidenced by lower IPNA potency in Tianma-treated detrusor strips. The contrasting effects on bladder and vascular or ileum smooth muscles could not be well explained presently due to the paucity of information on the mechanistic actions of Tianma.
It is interesting to note that despite spontaneous phasic activity and IPNA potency were altered after Tianma treatment, these effects were not observed universally in detrusor strips isolated in both transverse and longitudinal directions. Only in the longitudinal strips was where Tianma treatment elicited changes in the contractile characteristics. Similar observations of direction-specific differences in contractility have been reported elsewhere. For example, transverse detrusor strips from young adult rats were more sensitive to voltage-gated K+ channel blockade [9]. On the other hand, longitudinal strips were more prone to ATP-sensitive K+ channel blockade [16]. Also in young adult rat detrusor, longitudinal strips were more responsive than transverse strips to increasing concentrations of IPNA [15]. This directional difference was not seen in this study, possibly attributed to the older rat bladders used here. A study by Pagala et al. [17] showed data suggesting that contractile differences between transverse and longitudinal detrusor strips were effectively abolished in older rats. A comparison of young and adult rat detrusor strips, presumably in the longitudinal direction, did not reveal age-related differences under IPNA stimulation [13]. However, selective β3-agonists were less efficacious in inducing relaxations in older rat bladders [13]. It remains to be investigated whether such age-related differences also exist in transverse detrusor strips. Spontaneous phasic activity also varies with age, being highly prevalent in both neonatal and old bladders [18]. It is possible that the Tianma-associated increase in spontaneous phasic activity (as observed in this study) may be accentuated in bladders from older rats. Clinically, this may caution against the prescription of Tianma to older individuals who are also suffering from overactive bladders symptoms. Nevertheless much is yet to be elucidated regarding the mechanisms underlying age-related changes in bladder function. More notably, it will be of interest to conduct a comparative study across bladders from all ages on the effects of Tianma treatment.
As with age-related contractile differences in the detrusor, much remains unknown about the urothelial regulatory mechanisms of bladder function. Although it has been widely accepted that urothelial secretions exert an inhibitory control on detrusor contractions, identifying the secretory substances has been less successful due to species and methodological variations in different reports. Several potential candidates, including purine neurotransmitters, noradena-line, nitric oxide and cyclooxygenase products, have been ruled out as urothelial-derived inhibitory factor in human and pig detrusor strips in the longitudinal direction [10,11]. Possibly transverse strips from humans and pigs might show different results, as in the case of transverse rat detrusor strips where a role of nitric oxide synthase has been demonstrated in enhancing contractions [19]. Species differences may also be a contributing factor since longitudinal rat detrusor strips contracted more when cyclooxygenase activity was inhibited [19,20] but the same drug treatment did not alter human or pig detrusor contractility [10,11]. In this study, we presented evidence of an urothelium-dependent increase in spontaneous phasic activity and a decrease in potency of IPNA-induced relaxation in longitudinal rat detrusor strips. The findings here add to the growing list of directional specificity of contractile regulation in the bladder. The functional implication of urothelial function has also been established with Tianma treatment, warranting more rigorous mechanistic investigations of bladder function in the future.
In summary, this study describes for the first time how Tianma treatment mediated bladder smooth muscle contractility, in spite of demonstrated effects on vascular and ileum smooth muscles reported elsewhere. Directional contractile differences were revealed as only Tianma-treated detrusor strips in the longitudinal direction showed greater spontaneous phasic activity and lower IPNA potency. A key finding in this study was the dependence on urothelium in the Tianma-associated changes. The results here may serve as a step toward understanding the mechanisms via which Tianma elicits its actions on the urothelium in regulating bladder function.
Acknowledgments
The authors thanked Professor Jun Zhou and Dr. Jiangmiao Hu (Kunming Institute of Botany, Chinese Academy of Science, Yunnan, People's Republic of China) for providing Tianma. This study was supported by grants from the Singapore Ministry of Education (RG83/07) and from the Institute of Advanced Studies, Nanyang Technological University.
References
- 1.Michel MC, Barendrecht MM. Physiological and pathological regulation of the autonomic control of urinary bladder contractility. Pharmacol Ther. 2008;117:297–312. doi: 10.1016/j.pharmthera.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Brading AF. Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. J Physiol. 2006;570:13–22. doi: 10.1113/jphysiol.2005.097311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fry CH, Meng E, Young JS. The physiological function of lower urinary tract smooth muscle. Auton Neurosci. 2010;154:3–13. doi: 10.1016/j.autneu.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Chen JK, Chen TT. Tianma. In: Crampton L, editor. Chinese medical herbology and pharmacology. Art of Medicine Press, City of Industry; 2004. pp. 785–787. [Google Scholar]
- 5.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 6.Chang H-M, But P P-H. Chang H-M, But P P-H. Pharmacology and applicaions of Chinese Mate-ria Medica. New Jersey: World Scientific; 1986. Tianma and mihuanjun; pp. 171–174. [Google Scholar]
- 7.Hayashi J, Sekine T, Deguchi S, Lin Q, Horie S, Tsuchiya S, Yano S, Watanabe K, Ikegami F. Phenolic compounds from Gastrodia rhizome and relaxant effects of related compounds on isolated smooth muscle preparation. Phytochem. 2002;59:513–519. doi: 10.1016/s0031-9422(02)00008-0. [DOI] [PubMed] [Google Scholar]
- 8.Li N, Wang K-J, Chen J-J, Zhou J. Phenolic compounds from the rhizomes of Gastrodia elata. J Asian Nat Prod Res. 2007;9:373–377. doi: 10.1080/10286020600780979. [DOI] [PubMed] [Google Scholar]
- 9.Lo WN, Liang W. Blockade of voltage-sensitive K+ channels increases contractility more in transverse than in longitudinal rat detrusor strips. Urology. 2009;73:400–404. doi: 10.1016/j.urology.2008.08.465. [DOI] [PubMed] [Google Scholar]
- 10.Chaiyaprasithi B, Mang CF, Kilbinger H, Hohenfellner M. Inhibition of human detrusor contraction by a urothelium derived factor. J Urol. 2003;170:1897–1900. doi: 10.1097/01.ju.0000091870.51841.ae. [DOI] [PubMed] [Google Scholar]
- 11.Hawthorn MH, Chapple CR, Cock M, Chess-Williams Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br J Pharmacol. 2000;129:416–419. doi: 10.1038/sj.bjp.0703068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santoso AGH, Sonarno IAB, Arsad NAB, Liang W. The role of the urothelium and ATP in mediating detrusor smooth muscle contractility. Urology. 2010 doi: 10.1016/j.urology.2010.06.040. doi: 10.1016/j.urology.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 13.Frazier EP, Schneider T, Michel MC. Effects of gender, age and hypertension on b-adrenergic receptor function in rat urinary bladder. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:300–309. doi: 10.1007/s00210-006-0077-y. [DOI] [PubMed] [Google Scholar]
- 14.Deba A, Palea S, Rouget C, Westfall TD, Lluel P. Involvement of b3-adrenoceptors in mouse urinary bladder function: role in detrusor muscle relaxation and micturition reflex. Eur J Pharmacol. 2009;618:76–83. doi: 10.1016/j.ejphar.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Liang W, Lo WN. b-adrenoceptor-mediated differences in transverse and longitudinal strips from the rat detrusor. Int Urol Nephrol. 2010 doi: 10.1007/s11255-010-9759-y. doi: 10.1007/s11255-010-9759-y. [DOI] [PubMed] [Google Scholar]
- 16.Lo WN, Santoso AGH, Liang W. Differences in transverse and longitudinal rat detrusor contractility under K+ channel blockade. UroToday Int J. 2010 doi: 10.3834/uij.1944-5784.2010.04.07. [Google Scholar]
- 17.Pagala MK, Tetsoti L, Nagpal D, Wise GJ. Aging effects on contractility of longitudinal and circular detrusor and trigone of rat bladder. J Urol. 2001;166:721–727. [PubMed] [Google Scholar]
- 18.Szigeti GP, Somogyi GT, Csernoch L, Széll E. Age-dependence of the spontaneous activity of the rat urinary bladder. J Muscle Res Cell Motil. 2005;26:23–29. doi: 10.1007/s10974-005-9003-z. [DOI] [PubMed] [Google Scholar]
- 19.Santoso AGH, Lo WN, Liang W. Urotheliumdependent and urothelium-independent detrusor contractility mediated by nitric oxide synthase and cyclooxygenase inhibition. Neurourol Urodynam. 2010 doi: 10.1002/nau.21015. doi: 10.1002/nau.21015. [DOI] [PubMed] [Google Scholar]
- 20.Azadzoi KM, Heim VK, Tarcan T, Siroky MB. Alteration of urothelial-mediated tone in the ischemic bladder: role of eicosanoids. Neurourol Urodynam. 2004;23:258–264. doi: 10.1002/nau.20029. [DOI] [PubMed] [Google Scholar]