Abstract
Sirtuin 2 (SIRT2), a tubulin deacetylase, is a sirtuin family protein. SIRT2 inhibitors have been shown to decrease the cell death in cellular and Drosophila models of Parkinson's disease. However, SIRT2 decreases may also compromise cellular antioxidation capacity. Our current study found that silencing of SIRT2 led to a decrease in the intracellular ATP level of PC12 cells. We also found that AGK2, a selective SIRT2 inhibitor, can exacerbate H2O2-induced decreases in the intracellular ATP level of these cells. Our study further indicated that the reduction in SIRT2 level significantly increased necrosis of PC12 cells without affecting autophagy of the cells. These results suggest that SIRT2 is a key mediator of energy metabolism and basal survival of PC12 cells.
Keywords: SIRT2, PC12 cells, necrosis, autophagy, ATP
Introduction
Sir2, a NAD+-dependent histone deacetylase, has been shown to mediate the aging process of yeast [1]. Sirtuins are the mammalian homolog of Sir2, in which there are seven members including SIRT1-SIRT7. SIRT1 has been the most extensively studied member of the sirtuin family proteins [2]. SIRT2 is a tubulin deacetylase [3], which plays a detrimental role under certain pathological conditions. For example, SIRT2 inhibitors were shown to reduce α-synuclein-induced neurotoxicity in cellular and Drosophila models of Parkinson's disease [4]. In contrast, there are also studies suggesting that SIRT2 may play beneficial roles under certain conditions. For example, decreases in the level of SIRT2 have been shown to induce cell apoptosis by affecting the levels of p53 [5].
Nevertheless, there has been no study regarding the effects of SIRT2 on intracellular ATP levels, a key factor in cellular energy metabolism. It also remains unknown if SIRT2 may affect cell necrosis. In this study we used PC12 cells as a cellular model to determine the effects of SIRT2 silencing by SIRT2 siRNA on energy metabolism and cell necrosis. We found that silencing of SIRT2 can induce necrosis as well as a decrease in the intracellular ATP level of these cells.
Materials and methods
Cell cultures
PC12 cells were purchased from the Cell Resource Center of Shanghai Institute of Biological Sciences, Chinese Academy of Sciences. The cells were plated onto 24-well cell culture plates at the initial density of 1×105cells/ml in Dul-becco's Modified Eagle Medium containing 4,500 mg/L D-glucose, 584 mg/L L-glutamine, 110 mg/L sodium pyruvate (Thermo Scientific, Waltham, MA, USA), 1% penicillin and streptomycin (Invitrogen, Carlsbad, CA, USA), and 10% fetal bovine serum (PAA, Linz, Austria).
Experimental procedures
Experiments were initiated by replacing the culture medium with medium containing various concentrations of drugs. The cells were left in an incubator with 5% CO2 at 37°C for 24 or 48 hrs.
Exctracellular lactate dehydrogenase (LDH) assay
LDH is a cytosolic enzyme that is released into cell media upon cell lysis. Extracellular LDH activity is highly correlated with a major index of cell necrosis: levels of propidium iodide-positive cells [6]. Therefore, extracellular LDH activity can be used for assessing the levels of cells undergoing primary or secondary necrosis [7]. In our study extracellular LDH activity of PC12 cells was assessed as described previously [6]. In brief, 100 μl of extracellular media of the samples was mixed with 150 μl potassium phosphate buffer (500 mM, pH 7.5) containing 1.5 mM NADH and 7.5 mM sodium pyruvate. Subsequently changes in the A340nm of the samples were monitored over 90 s by a plate reader.
SIRT2 silencing
PC12 cells were approximately 50% confluent at the time of transfection. Three small interfering RNA (siRNA) duplexes against rat SIRT2 (NM_001008368) at nucleotides 754-772 (CC UUGCUAAGGAGCUCUAUTT), 843-862 (GCUGC UACACGCAGA AUAUTT), and 1393-1411 (GGA GCAUGCCAACAUAGAUTT) were commercially synthesized (Genepharma, Shanghai, China). For controls, scrambled RNA oligonucleotides were used. Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was used for transfection according to the manufacturer's instructions. For each well, 33.3 nM of each of the three SIRT2 siRNA oligos, 2 μl lipofectamine 2000, and 600 μl serum-free media were added. After incubation for 6 hrs, the media was replaced with DMEM containing 10% fetal bovine serum.
Western blot analysis
PC12 cells were harvested and lysed in RIPA buffer (Millipore, Temecula, CA, USA) containing Complete Protease Inhibitor Cocktail (Roche Diagnostics, Mannheim, Germany) plus 2 mM PMSF. For the Western blot of microtubule-associated protein-1 light chain 3 (LC3), an autophagic marker [8], 0.1% SDS was also included in the RIPA buffer. The cell lysates were centrifuged at 12,000 g for 20 min at 4°C. After quantifications of the protein samples using BCA Protein Assay Kit (Pierce Biotechonology, Rockford, IL, USA), 30 μg of total protein was electrophoresed through a 10% (for SIRT2 Western blot) or 14% (for LC3 Western blot) SDS-polyacrylamide gel, and then transferred to 0.45 μm nitrocellulose membranes (for SIRT2 Western blot) or 0.2 μm PVDF membranes (for LC3 Western blot) (Millipore, Billerica, MA, USA) on a semi-dry electro transferring unit (Bio-Rad Laboratories, Hercules, CA, USA). The blots were incubated overnight at 4°C with a rabbit polyclonal anti-SIRT2 antibody (1:500 dilution) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or a rabbit polyclonal anti-LC3 antibody (1:1000 dilution), then incubated with HRP-conjugated secondary antibody (EPITOMICS, Hangzhou, Zhejiang Province, China). Protein signals were detected using the ECL detection system (Pierce Biotechonology, Rockford, IL, USA). An anti-β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used to normalize sample loading and transfer. The intensities of the bands were quantified by densitometry using Gel-Pro Analyzer.
Monodansylcadaverine (MDC) staining
Cells were stained with MDC as described previously [9]. Briefly, cells were incubated with 50 μM MDC in DMEM for 30 min at 37°C, then washed with PBS three times and fixed in 4% Paraformaldehyde (PFA) for 30 minutes at room temperature. After PFA was removed the cells were washed once with PBS. Fluorescence of incorporated intracellular MDC (excitation wavelength 390 nm, emission wavelength 527 nm) was detected under a Leica fluorescence microscope.
ATP assay
ATP levels were quantified using the Roche ATP Bioluminescence Assay Kit (HS II) following the standard protocol provided by the vendor. In brief, cells were washed once with PBS and lysed with the Cell Lysis Reagent. Then 50 μl of the lysates was mixed with 150 μl of the Luciferase Reagent, and the luminescence was detected using a plate reader (Biotek Synergy 2). The protein concentrations of the samples were determined using the BCA assay. The ATP concentrations of the sample were calculated using an ATP standard, and normalized against the protein of the samples.
Statistical Analyses
All data are presented as mean + SE. Data were assessed by one-way ANOVA, followed by Student-Newman-Keuls post hoc test. P values less than 0.05 were considered statistically significant.
Results
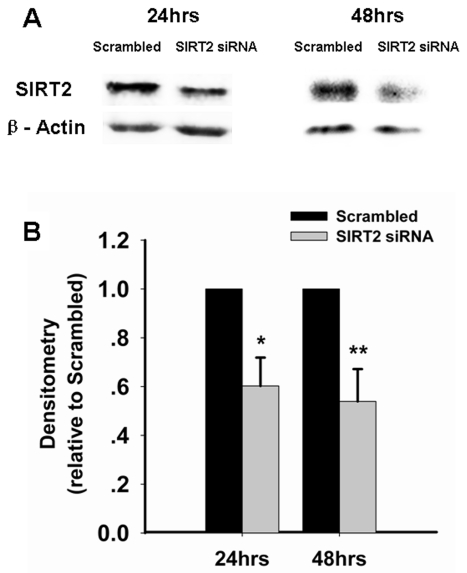
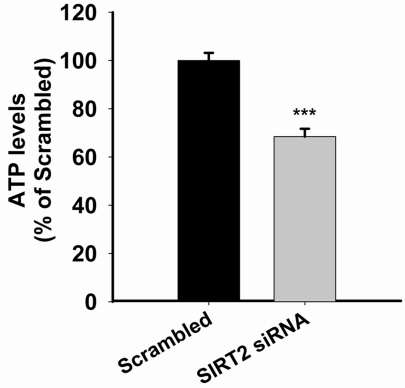
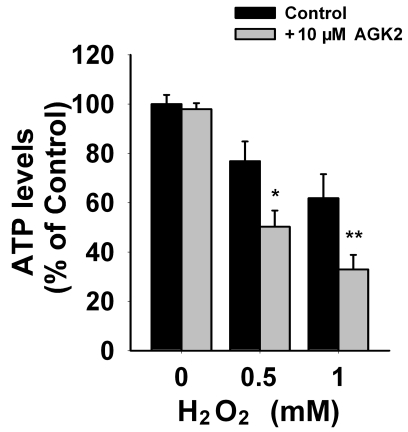
We applied SIRT2 siRNA to decrease the levels of SIRT2 in PC12 cells. At 24 or 48 hrs after the treatment of SIRT2 siRNA, Western blot assays showed decreases in SIRT2 levels in PC12 cells treated with SIRT2 siRNA compared to those treated with scrambled siRNA (Figure 1A). Quantifications of the Western blots showed that treatment with SIRT2 siRNA led to significant decreases in SIRT2 levels (Figure 1B). Because ATP is a key factor mediating cell survival [10], we assessed the effects of SIRT2 reduction on the intracellular ATP level of PC12 cells. We found that silencing of SIRT2 led to a significant decrease in the intracellular ATP levels at 24 hrs after the siRNA treatment (Figure 2). We further assessed the effects of SIRT2 inhibition on the level of intracellular ATP in the PC12 cells that had been exposed to oxidative stress. We found that treatment of the cells with 10 μM AGK2 alone, a selective SIRT2 inhibitor, for 6 hrs did not affect the ATP level (Figure 3). However, treatment with AGK2 significantly exacerbated the H2O2-induced decrease in the level of intracellular ATP (Figure 3).
Figure 1.
Treatment with SIRT2 siRNA led to significant decreases in the SIRT2 level of PC12 cells. PC12 cells were treated with SIRT2 siRNA for 6 hrs. Subsequently the cells were incubated in regular cell culture media for additional 18 hrs or 42 hrs. The levels of SIRT2 were then determined by Western blot, showing decreases in SIRT2 levels of PC12 cells (A). Quantifications of the Western blots showed that SIRT2 siRNA led to significant decreases in SIRT2 levels of PC12 cells (B). Data were collected from three to six independent experiments. *, p < 0.05; **, p < 0.01.
Figure 2.
SIRT2 reductions by SIRT2 siRNA led to significant decreases in intracellular ATP levels. PC12 cells were treated with SIRT2 siRNA for 6 hrs. Subsequently the cells were incubated in regular cell culture media for 18 hrs. Intracellular ATP levels were then assessed by luciferin/luciferase-based ATP assay. N= 11. Data were collected from three independent experiments. ***, p < 0.001.
Figure 3.
SIRT2 inhibition by AGK2 exacerbated the H2O2-induced decrease in the intracellular ATP of PC12 cells. The cells were treated with H2O2 for 1 hr. After H2O2 washout, the cells were treated with AGK2, a selective SIRT2 inhibitor, for 6 hrs, and subsequently the intracellular ATP levels were determined by luciferin/luciferase-based ATP assay. N= 12. Data were collected from three independent experiments. *, p < 0.05; **, p < 0.01.
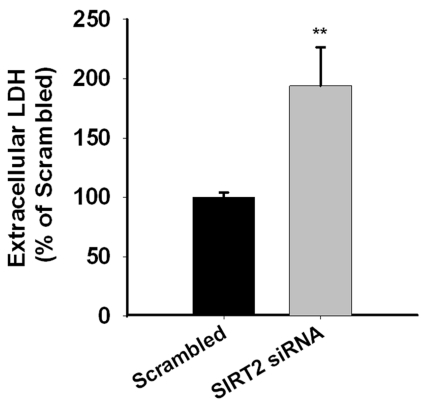
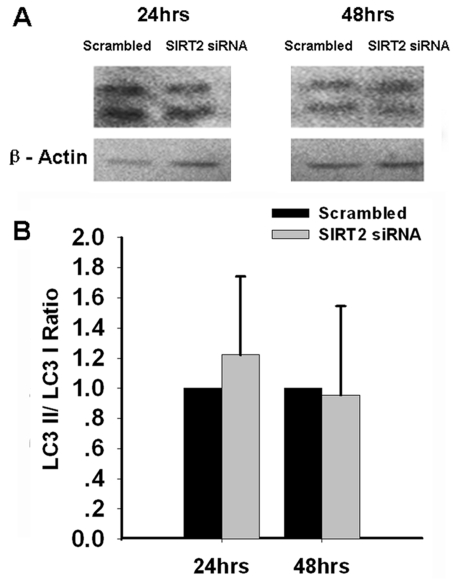
At 48 hrs after the treatment of SIRT2 siRNA, necrosis of PC12 cells was assessed by extracellular LDH assay. We found significant increases in the extracellular LDH levels of the PC12 cells treated with SIRT2 siRNA (Figure 4), suggesting that decreasing SIRT2 expression can lead to cell necrosis. We also assessed if silencing of SIRT2 may induce autophagy by determining LC3II/LC3I ratios, an index of autophagic process [8], in the cells treated with SIRT2 siRNA. Our study did not show decreases in the ratios (Figure 5), thus arguing against the possibility that SIRT2 siRNA can induce autophagy of PC12 cells. To further determine the effect of SIRT2 silencing on autophagy of PC12 cells, we applied MDC staining, another widely used approach for assessing autophagy. Similarly, MDC staining did not show observable differences between the MDC fluorescence of the cells treated with scrambled RNA and those treated with SIRT2 siRNA (data not shown).
Figure 4.
SIRT2 reductions by SIRT2 siRNA led to significant increases in necrosis of PC12 cells. PC12 cells were treated with SIRT2 siRNA for 6 hrs. Subsequently the cells were incubated in regular cell culture media for additional 42 hrs. Necrosis levels of the cells were determined by assessing the levels of extracellular LDH activity. N= 20. Data were collected from five independent experiments. **, p < 0.01; ***, p < 0.001.
Figure 5.
SIRT2 reductions by SIRT2 siRNA did not affect cell autophagy. PC12 cells were treated with SIRT2 siRNA for 6 hrs. At 24 or 48 hrs after the treatment, Western blot assays were conducted to determine the LC3II/LC3I ratios in the cells (A). Quantifications of the of the Western blots did not showed significant differences in the ratios between the cells treated with scrambled RNA and the cells treated with SIRT2 siRNA (B). Data are representative of two to four independent experiments.
Discussion
There are three major findings from this study: First, silencing of SIRT2 can lead to a significant decrease in the level of intracellular ATP in PC12 cells; second, silencing of SIRT2 can exacerbate oxidative stress-induced decreases in the level of intracellular ATP; and third, silencing of SIRT2 can significantly increase necrosis of PC12 cells without affecting autophagy of the cells. Collectively, these findings suggest that SIRT2 plays an important role in mediating energy metabolism and cell survival of PC12 cells.
Multiple biological functions of SIRT2, including affecting cell mitosis, cell motility and oligodendrocyte differentiation, have been reported [11-14]. There are also studies suggesting that SIRT2 may play either detrimental or beneficial roles on cell survival. Several studies have suggested that SIRT2 activation can reduce cell survival under certain conditions. For example, SIRT2 activation induces increased expression of Bid thus leading to apoptosis [15]; and SIRT2 inhibition reduces α-synuclein-induced neurotoxicity in cellular and Drosophila models of Parkinson's disease [4]. However, SIRT2 may also play beneficial roles under certain conditions: The effect of SIRT2 on FOXO3a could produce cytoprotective effects by inducing increased expression of antioxidantion enzymes such as Mn-SOD [15]; and SIRT2 reductions have been shown to induce cell apoptosis by affecting the levels of p53 [5]. SIRT2 has also been shown to be severely reduced in a number of human brain tumor cell lines, which implicates that the absence of SIRT2 may mediate cellular transformation and development of cellular malignancy [16].
One of the key biological changes in cell necrosis is ATP depletion [10]. Our study found that, at 24 hrs after treatment with SIRT2 siRNA, there was a nearly 40% decrease in the intracellular ATP levels in PC12 cells. To our knowledge, this is the first report showing that SIRT2 reductions are sufficient to decrease intracellular ATP levels, suggesting a key influence of SIRT2 on energy metabolism. We further found that AGK2, a selective SIRT2 inhibitor, can exacerbate the H2O2-induced decrease in the level of intracellular ATP. These findings have collectively suggested that SIRT2 plays a key role in the energy metabolism of PC12 cells under both basal and oxidative stress conditions. Because ATP depletion is a key factor mediating cell necrosis, our study suggests that SIRT2 reductions may induce cell necrosis by decreasing intracellular ATP levels.
Our current study provides evidence suggesting that reductions in the level of SIRT2 are sufficient to induce cell necrosis of PC12 cells. Previous studies have suggested that SIRT2 reductions can induce apoptosis of certain types of cells by inducing accumulation of p53 [5]. However, our current study further suggests that SIRT2 reductions can induce necrosis of PC12 cells. Our observations, together with the studies suggesting the capacity of SIRT2 reductions to induce cell apoptosis, indicate a critical role of SIRT2 in maintaining basal survival of certain types of cells. Previous study has suggested that dissociation of FoxO1 from SIRT2 can lead to binding of the acetylated FoxO1 to Atg7 thus affecting autophagic process [17]. Our study did not show that SIRT2 reductions can induce autophagy of PC12 cells, suggesting that SIRT2 may play significant roles in autophagy only in certain types of cells.
Previous studies have suggested that SIRT2 inhibition produces neuroprotective effects by decreasing sterol biosynthesis [18]. Our study provided evidence suggesting that SIRT2 plays a key role in mediating intracellular ATP levels. It has also been indicated that SIRT1 can modulate such factors in energy metabolism as glucose levels [19]; and SIRT3 has been shown to regulate mitochondrial fatty-acid oxidation [20]. Our findings, together with the observations suggesting key roles of SIRT1 and SIRT3 on energy metabolism, have indicated crucial roles of sirtuns in energy metabolism. NAD+ and NADH are regulators of sirtuins, which have been shown to play significant roles in various biological functions and multiple major diseases [21-24]. Future studies are warranted to further investigate the roles of SIRT2 in energy metabolism of other types of cells both in vitro and in vivo, and to investigate the mechanisms underlying the relationships among SIRT2, NAD+/ NADH, energy metabolism and cell survival.
Acknowledgments
This study was supported by a National Key Basic Research ‘973 Program Grant’ #2010CB834306 (to W. Y. and W. Xia), a Pujiang Scholar Program Award 09PJ1405900 (to W. Y.), and a Key Research Grant of Shanghai Municipal Educational Committee #09ZZ21 (to W. Y.).
References
- 1.Guarente L. Sir2 links chromatin silencing, metabolism, and aging. Genes Dev. 2000;14:1021–1026. [PubMed] [Google Scholar]
- 2.Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J. Sirtuins: the ‘magnificent seven', function, metabolism and longevity. Ann Med. 2007;39:335–345. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- 3.Milne JC, Denu JM. The Sirtuin family: therapeutic targets to treat diseases of aging. Curr Opin Chem Biol. 2008;12:11–17. doi: 10.1016/j.cbpa.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 4.Outeiro TF KE, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson's disease. Science. 2007;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Matsumori H, Nakayama Y, Osaki M, Kojima H, Kurimasa A, Ito H, Mori S, Katoh M, Oshimura M, Inoue T. SIRT2 down-regulation in HeLa can induce p53 accumulation via p38 MAPK activation-dependent p300 decrease, eventually leading to apoptosis. Genes Cells. 2011;16:34–45. doi: 10.1111/j.1365-2443.2010.01460.x. [DOI] [PubMed] [Google Scholar]
- 6.Ying W, Han SK, Miller JW, Swanson RA. Acidosis potentiates oxidative neuronal death by multiple mechanisms. J Neurochem. 1999;73:1549–1556. doi: 10.1046/j.1471-4159.1999.0731549.x. [DOI] [PubMed] [Google Scholar]
- 7.Roy MK, Takenaka M, Kobori M, Nakahara K, Isobe S, Tsushida T. Apoptosis, necrosis and cell proliferation-inhibition by cyclosporine A in U937 cells (a human monocytic cell line) Pharmacol Res. 2006;53:293–302. doi: 10.1016/j.phrs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Tanida I, Ueno T, Kominami E. LC3 and Autophagy. Methods Mol Biol. 2008;445:77–88. doi: 10.1007/978-1-59745-157-4_4. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Qin Z, Liang Z. The prosurvival role of autophagy in Resveratrol-induced cytotoxicity in human U251 glioma cells. BMC Cancer. 2009;9:215. doi: 10.1186/1471-2407-9-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang F, Vemuri MC, Schneider JS. Modulation of ATP levels alters the mode of hydrogen peroxide-induced cell death in primary cortical cultures: effects of putative neuroprotective agents. Brain Res. 2004;997:79–88. doi: 10.1016/j.brainres.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 11.Harting K, Knoll B. SIRT2-mediated protein deacetylation: An emerging key regulator in brain physiology and pathology. Eur J Cell Biol. 2010;89:262–269. doi: 10.1016/j.ejcb.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Tang BL, Chua CE. SIRT2, tubulin deacetylation, and oligodendroglia differentiation. Cell Motil Cytoskeleton. 2008;65:179–182. doi: 10.1002/cm.20253. [DOI] [PubMed] [Google Scholar]
- 13.Pandithage R, Lilischkis R, Harting K, Wolf A, Jedamzik B, Luscher-Firzlaff J, Vervoorts J, Lasonder E, Kremmer E, Knoll B, Luscher B. The regulation of SIRT2 function by cyclin-dependent kinases affects cell motility. J Cell Biol. 2008;180:915–929. doi: 10.1083/jcb.200707126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Zhang B, Tang J, Cao Q, Wu Y, Wu C, Guo J, Ling EA, Liang F. Sirtuin 2, a mammalian homolog of yeast silent information regulator-2 longevity regulator, is an oligodendroglial protein that decelerates cell differentiation through deacetylating alpha-tubulin. J Neurosci. 2007;27:2606–2616. doi: 10.1523/JNEUROSCI.4181-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang F, Nguyen M, Qin FX, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell. 2007;6:505–514. doi: 10.1111/j.1474-9726.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- 16.Voelter-Mahlknecht S, Ho AD, Mahlknecht U. FISH-mapping and genomic organization of the NAD-dependent histone deacetylase gene, Sirtuin 2 (Sirt2) Int J Oncol. 2005;27:1187–1196. [PubMed] [Google Scholar]
- 17.Zhao Y, Yang J, Liao W, Liu X, Zhang H, Wang S, Wang D, Feng J, Yu L, Zhu WG. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat Cell Biol. 12:665–675. doi: 10.1038/ncb2069. [DOI] [PubMed] [Google Scholar]
- 18.Luthi-Carter R, Taylor DM, Pallos J, Lambert E, Amore A, Parker A, Moffitt H, Smith DL, Runne H, Gokce O, Kuhn A, Xiang Z, Maxwell MM, Reeves SA, Bates GP, Neri C, Thompson LM, Marsh JL, Kazantsev AG. SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proc Natl Acad Sci U S A. 107:7927–7932. doi: 10.1073/pnas.1002924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elliott PJ, Jirousek M. Sirtuins: novel targets for metabolic disease. Curr Opin Investig Drugs. 2008;9:371–378. [PubMed] [Google Scholar]
- 20.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV, Jr, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao C, Hong Y, Han J, Ma Y, Chen H, Xia W, Ying W. NAD+ treatment decreases tumor cell survival by inducing oxidative stress. Front Bio-sci (Elite Ed) 2011;3:434–441. doi: 10.2741/e258. [DOI] [PubMed] [Google Scholar]
- 22.Ma Y, Chen H, Xia W, Ying W. Oxidative stress and PARP activation mediate the NADH-induced decrease in glioma cell survival. IJPPP. 2011;3:21–28. [PMC free article] [PubMed] [Google Scholar]
- 23.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 24.Ying W, Xiong ZG. Oxidative stress and NAD+ in ischemic brain injury: current advances and future perspectives. Curr Med Chem. 2009;17:2152–2158. doi: 10.2174/092986710791299911. [DOI] [PMC free article] [PubMed] [Google Scholar]