Abstract
Resolution of Type-2 diabetes mellitus (DM) after weight loss surgery is well documented, but the mechanism is elusive. We evaluated the glucose-insulin metabolism of patients undergoing a Roux-en-Y gastric bypass (RYGB) using the intravenous glucose tolerance test (IVGTT) and compared it with patients who underwent laparoscopic adjustable gastric band (AB) placement. Thirty-one female patients (age range, 20 to 50 years; body mass index, 47.2 kg/m2) underwent RYGB. Nine female patients underwent AB placement and served as control subjects. All patients underwent IVGTT at baseline and 1 month and 6 months after surgery. Thirteen patients undergoing RYGB and one patient undergoing AB exhibited impaired glucose tolerance defined by the American Diabetes Association. By 6 months post surgery, diabetes was resolved in all but one patient undergoing RYGB and none of the patients undergoing AB. Patients with diabetes undergoing demonstrated increased insulin secretion and β-cell responsiveness 1 month after surgery and continued this trend up to 6 months, whereas none of the patients undergoing AB had changes in β-cell function. Both patients undergoing RYGB and those undergoing AB demonstrated significant weight loss (34.6 and 35.0 kg/m2, respectively) and improved insulin sensitivity at 6 months. RYGB ameliorates DM resolution in two phases: 1) early augmentation of beta cell function at 1 month; and 2) attenuation of peripheral insulin resistance at 6 months. Patients undergoing AB only exhibited reduction in peripheral insulin resistance at 6 months but no changes in insulin secretion.
Resolution of type-2 diabetes mellitus (T2DM) after weight loss surgery has been well documented with gastric bypass operations,1–3 but there is evidence that the restrictive adjustable band procedure also promotes T2DM resolution.4 The mechanisms of diabetes resolution are presumably different between a malabsorptive operation such as the Roux-en-Y gastric bypass (RYGB) and the restrictive adjustable gastric band (AB). The drastically distinct surgical approaches to weight loss surgery aiming to achieve the same benchmark of T2DM resolution beg the question of which operation is most appropriate for a morbidly obese patient with dysfunction of insulin-glucose metabolism. If both procedures are eventually equal in efficacy, the mere cost and surgical risk reduction of the simpler procedure would clearly be the best choice, and yet there are data that the mere diversion of food from the proximal gastrointestinal tract (eg, biliopancreatic diversion, RYGB) offers profound and almost immediate relief (less than 1 month) from T2DM.5–7
In this study, we used a standardized intravenous glucose tolerance test (IVGTT) to determine the baseline characteristics and subsequent response of insulin-glucose metabolism in morbidly obese patients with T2DM undergoing either RYGB or the AB. We hypothesize that RYGB induces alterations in pancreatic β-cell function, which is not observed with the AB procedure. Knowing this information may help patients and surgeons determine which procedures are most appropriate for them based on their baseline phenotype.
Methods
Patients and Inclusion Criteria
Women between the ages of 18 and 50 years were recruited into the study with ongoing treatment for T2DM and HgA1C greater than 6 per cent. The choice of surgery was a decision between the patients and their surgeons, but patients generally presented after having decided on their preferred procedure based on completely psychosocial rationale (eg, risk fear, acquaintance with the same surgery, permanent foreign body, complexity of procedure). Exclusion criteria were male, age younger than 18 or older than 50 years, body mass index (BMI) less than 35 kg/m2, and current smoking history.
Study Protocol
The study has had 6 years of continuous approval by the Institutional Review Board of Emory University, and metabolic evaluations were all conducted in the Atlanta Clinical and Translational Studies Institute (CTSI, formerly the General Clinical Research Center of Emory University). Patients admitted to the CTSI had the following measurements: IVGTT, anthropometry, and adipose tissue distribution. All measurements were obtained at baseline (0 months) and 1, 6, and 24 months after surgery. However, for the purposes of this study, the acute changes of interest were only within the first 6 months after surgery because very little metabolic change was anticipated at 24 months. Subjects were weight-stable (±1 kg) for 1 week before each measurement time point, with the exception of 1 month in which significant weight loss occurs (approximately 3 kg/week). Medications were withheld on the morning of the glucose tolerance testing.
Intravenous Glucose Tolerance Test, β-Cell Function, Insulin Sensitivity
Insulin action was assessed using the frequently sampled IVGTT. Patients were admitted to the CTSI the night before testing and fasted overnight (12 hours). Intravenous access was established for blood sampling, and the study began as previously described.8 A standard approach using minimal modeling analysis9 (MinMod Millennium, Los Angeles, CA) of glucose and insulin levels was used to quantify insulin sensitivity (Si, a measure of peripheral resistance), acute insulin response to glucose (AIRg, a measure of β-cell function and insulin secretion), and disposition index (DI = Si × AIRg), a quantitative measurement that describes the dynamic relationship between β-cell responsiveness and insulin sensitivity. In metabolically normal individuals, changes in insulin sensitivity are accompanied by compensatory alterations in the β- cell’s sensitivity to glucose. Patients with T2DM typically have lower DI than normal individuals. Patient hemoglobin A1C (HgA1C) was also measured for the purposes of this study.
Surgical Procedures
RYGB were performed laparoscopically in all patients. In brief, a 20- to 30-mL gastric pouch is formed in a longitudinal fashion. The Roux limb is typically 100 to 150 cm in length. Patients undergoing B patients had the Lap-Band system (Allergan Inc., Irvine, CA) with the first band fill 4 to 6 weeks after surgery with the aid of fluoroscopy and contrast esophagram to rapidly achieve optimal restriction.
Statistical Analysis
Analysis was performed using STATISTICA (StatSoft, Tulsa, OK). Differences between time points were analyzed using paired Student t test. Significance is P < 0.05, and results are expressed as mean ± SEM.
Results
Patient Characteristics
Patients who had RYGB had greater BMIs than those who underwent AB (47.2 vs 44.2 kg/m2, respectively) at baseline. At 6 months postsurgery, RYGB subjects and AB subjects exhibited the same trend in weight loss, but the patients undergoing RYGB had more precipitous weight reduction in the same time period than patients undergoing AB. By 6 months, both patients undergoing RYGB and those undergoing AB had similar BMIs (34.6 vs 35.0 kg/m2) (Fig. 1).
Fig. 1.
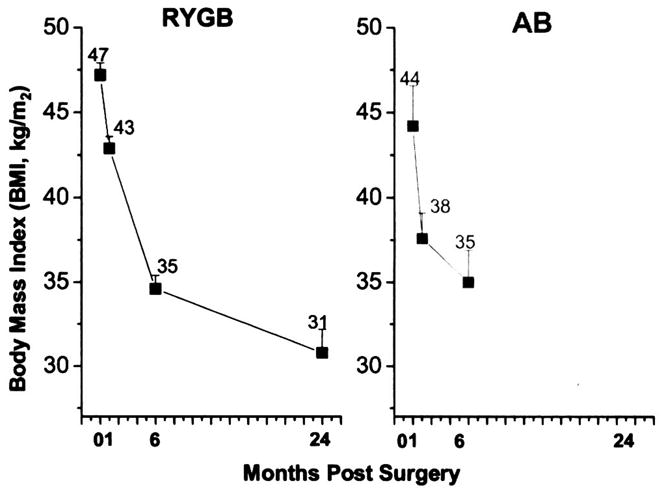
Changes in body mass index (BMI) at 6 months after Roux-en-Y gastric bypass (RYGB) and the adjustable gastric band (AB). Weight changes are no different between the two surgical groups, but significantly different at each time point for the individual surgery (P < 0.05).
Diabetes Resolution
Thirty-one patients had RYGB and nine had AB. Of these patients, 13 patients undergoing RYGB and one undergoing AB had impaired glucose tolerance or frank diabetes as defined by the American Diabetes Association. By 6 months postsurgery, diabetes was resolved in all but one patient who had undergone RYGB and none of the patients who had undergone AB.
Insulin Sensitivity
In the nondiabetic subjects, peripheral insulin sensitivity was similar in both RYGB and AB subjects, but both were lower than normal-weight control subjects (1.90 ± 0.3 and 1.36 ± 0.21 vs 2.50 Si units in normal-weight control subjects, P < 0.05). Insulin sensitivity was normalized in both nondiabetic RYGB and AB groups at 6 months after surgery (3.02 ± 0.43 and 3.13 ± 0.85 Si units, respectively, P = not significant).
In RYGB subjects with prediabetes and diabetes, insulin sensitivity was also lower than normal-weight control subjects (2.04 ± 0.29 vs 2.50 Si units). Only one patient undergoing AB had diabetes with very low insulin sensitivity (0.11 Si units) at baseline. At 6 months, both surgical groups with diabetes had significant improvements in insulin sensitivity (RYGB 2.04 ± 0.29 vs 3.38 ± 0.51, AB 0.11 to 5.46 Si units).
Insulin Secretion
At baseline, nondiabetic RYGB subjects had much greater insulin secretion compared with those who were diabetic (672 ± 93 vs 30 ± 17 AIRg units, P < 0.005). Similarly, nondiabetic AB subjects had greater insulin secretion compared with the one AB subject who had diabetes (1011 ± 189 vs −33 AIRg units).
After surgery, nondiabetic patients undergoing RYGB had a slight elevation in insulin secretion, but then dropped below baseline 6 months postsurgery (400 ± 42 AIRg units, P < 0.05 compared with baseline). For both diabetic and nondiabetic patients undergoing AB, insulin secretion exhibited minimal to no change after surgery. In contrast, there was a pronounced surge in insulin secretion in patients with diabetes undergoing RYGB at 1 and 6 months (from 30 ± 25 at baseline to 110 ± 54 and 155 ± 38 AIR units at 1 month and 6 months, respectively, P < 0.05).
β-cell function measured by the DI was impaired at baseline in subjects with prediabetes and diabetes compared with those with normal glucose tolerance. Subjects with diabetes who underwent RYGB experienced immediate improvements in β-cell function (from 61 ± 5 at baseline to 431 ± 45 and 703 ± 27 at 1 month and 6 months postsurgery, respectively, P < 0.05) (Table 1).
Table 1.
Changes in Insulin Sensitivity (Si), Insulin Secretion (AIRg), and β-cell Function (DI) at Baseline, I Month, and 6 Months after Weight Loss Surgery
| Before Surgery |
||||
|---|---|---|---|---|
| GBP | GBP | AB | AB | |
| Nondiabetes (n = 18) | Prediabetes and Diabetes (n = 13) | Nondiabetes (n = 8) | Prediabetes and Diabetes (n = 1) | |
| Si units | 1.90 ± 0.30 | 2.04 ± 0.29 | 1.36 ± 0.21 | 0.11 |
| AIRg units | 672 ± 93 | 30 ± 17* | 1,011 ± 189 | −33 (0) |
| β-cell function (DI units) | 1,276 ± 28 | 61 ± 5* | 1,375 ± 40 | 0 |
| 1 Month Postsurgery |
||||
| GBP | GBP | AB | AB | |
| Nondiabetes (n = 15) | Prediabetes and Diabetes (n = 11) | Nondiabetes (n = 6) | Prediabetes and Diabetes (n = 1) | |
| Si units | 1.70 ± 0.32 | 2.76 ± 0.89 | 1.32 ± 0.20 | Not determined |
| AIRg units | 793 ± 194 | 156 ± 51† | 911 ± 178 | Not determined |
| β-cell Function (DI units) | 1,348 ± 62 | 431 ± 45† | 1,203 ± 36 | Not determined |
| 6 Months Postsurgery |
||||
| GBP | GBP | AB | AB | |
| Nondiabetes (n = 18) | Prediabetes and diabetes (n = 10) | Nondiabetes (n = 5) | Prediabetes and diabetes (n = 1) | |
| Si units | 3.02 ± 0.43 | 3.38 ± 0.51 | 3.13 ± 0.85 | 5.46 |
| AIRg units | 400 ± 42 | 208 ± 53† | 861 ± 207 | 2 |
| β-cell Function (DI units) | 1,200 ± 18 | 703 ± 27† | 2,695 ± 176 | 11 |
Significantly different from nondiabetes, P < 0.05.
Significantly different from nondiabetes, P < 0.05.
Discussion
It is widely accepted that T2DM is a combination of dysfunctional insulin secretion by β-cells and insulin sensitivity (peripheral resistance) that results in impaired glucose homeostasis. Indeed, it is possible that a patient with T2DM possesses very robust β-cell function to compensate for impaired peripheral resistance or has combined attenuations in β-cell function and insulin sensitivity. Unfortunately, most patients with T2DM presenting for weight loss surgery have no information on the phenotype they possess.
In this study, we observed that patients with T2DM undergoing RYGB exhibit and early restoration of pancreatic β-cell function measured by AIRg at 1 month. There is a second phase of improved insulin-glucose metabolism as exhibited by profound improvements in peripheral Si at 6 months, which also led to an improved DI. However, although the amount of weight loss after AB was similar to RYGB, there is never an improvement observed in AIRg in patients undergoing AB, but only a modest improvement in insulin sensitivity at 6 months, which is presumably from weight loss.
Data for normoglycemic patients undergoing both weight loss procedures were also presented to give context to the data observed in patients with diabetes. Although it is beyond the scope of this discussion, it is readily evident that morbidly obese patients without diabetes probably maintain normoglycemia by high-output insulin secretion, and once peripheral insulin sensitivity improves after weight loss, insulin secretion is reduced below baseline levels. Although there was only one patient with diabetes who had AB, the data found in normoglycemic patients undergoing AB demonstrate that this procedure has virtually no effect on β-cell function. These data are also novel in that there are no reports to our knowledge using IVGTT to follow patients undergoing AB for weight loss.
It is not clear how the RYGB augments β-cell function so soon after surgery, but several plausible explanations have been proposed. Several experimental studies have shown that ghrelin inhibits insulin release in mice, rats, and humans.10 We and others have shown early decreases in the “hunger” hormone ghrelin after gastric bypass.11 Blockade of ghrelin signaling markedly increase glucose-induced insulin release in vitro. In high-fat diet-induced mildly obese mice, ghrelin deficiency enhances insulin release and prevents impaired glucose tolerance.12 If these observations are validated, it is potentially feasible to manipulate ghrelin signaling as a treatment for T2DM.
Another plausible and popular explanation is that the intestinal rearrangement sending nutrients rapidly to the distal small bowel induces a significant rise in GLP-1.13 Other investigators administering GLP-1 intravenously demonstrated a dose-dependent rise in insulin secretion as well.14 Insulin secretion by the β-cell undoubtedly occurs by multiple hormone stimuli and the mechanisms are incompletely understood.
There is also molecular explanation for the reversal of peripheral insulin resistance after weight loss.15 Bikman and colleagues demonstrated how muscles become desensitized to insulin transport by inappropriate phosphorylation of the subcellular insulin-receptor substrate-1 (by the inhibitor of kappa B kinase β, which commonly accumulates with high adipose volumes.
In summary, this study showed that weight loss between the patients undergoing AB and those undergoing RYGB follow the same early trends, but RYGB clearly induces an acute insulin response from β-cells early postoperatively, whereas AB does not. With this information, it is possible that patients with T2DM with intact or robust β-cell function should be offered a restrictive operation such as the sleeve gastrectomy or an adjustable gastric band as a way to increase insulin sensitivity and thereby increase the DI. In patients with attenuated β-cell function, gastric bypass may offer the more expeditious route to T2DM reversal. In essence, the decision to choose a restrictive procedure or a combined restrictive-malabsorptive procedure can potentially be made based on a patient’s insulin secretion and peripheral resistance profile. Although such a concept is ideal, there are other complementary biomarkers that predict resolution of T2DM such as inflammatory mediators16 and adiponectin.17 Therefore, the ability to predict a patient’s response from weight loss surgery and choosing the ideal treatment will most likely rely on an aggregate of related physiological profiles rather than independent parameters.
Acknowledgments
This study is funded in part by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases R03 DK067167 (NGM) and National Institutes of Health/ACTSI Grant M01 RR00039.
Footnotes
Presented at the Annual Scientific Meeting and Postgraduate Course Program, Southeastern Surgical Congress, Atlanta, GA, February 7–10, 2009.
References
- 1.Torquati A, Lufti R, Abumrad N, Richards WO. Is Roux-en-Y gastric bypass surgery the most effective treatment for type 2 diabetes mellitus in morbidly obese patients? J Gastrointest Surg. 2005;9:1112–8. doi: 10.1016/j.gassur.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en-Y gastric bypass on type-2 diabetes mellitus. Ann Surg. 2003;238:467–85. doi: 10.1097/01.sla.0000089851.41115.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–85. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–23. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 5.DePaula AL, Macedo ALV, Rassi N, et al. Laparoscopic treatment of metabolic syndrome in patients with type 2 diabetes mellitus. Surg Endosc. 2008;22:2670–8. doi: 10.1007/s00464-008-9808-0. [DOI] [PubMed] [Google Scholar]
- 6.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strader AD, Calusen TR, Goodin SZ, Wendt D. Ileal interposition improves glucose tolerance in low dose streptozotocin-treated diabetic and euglycemic rats. Obes Surg. 2009;19:96–104. doi: 10.1007/s11695-008-9754-x. [DOI] [PubMed] [Google Scholar]
- 8.Miller NG, Hansen JM, Jones DP, et al. Loss of total and visceral adipose tissue mass predicts decreases in oxidative stress after weight-loss surgery. Obesity (Silver Spring) 2009;17:439–46. doi: 10.1038/oby.2008.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 10.Dezaki K, Sone H, Yada T. Ghrelin is a physiological regulator of insulin release in pancreatic islets and glucose homeostasis. Pharmacol Ther. 2008;118:239–49. doi: 10.1016/j.pharmthera.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Lin E, Gletsu N, Fugate K, et al. The effects of gastric division on systemic ghrelin concentrations in the morbidly obese. Arch Surg. 2003;124:754–61. doi: 10.1001/archsurg.139.7.780. [DOI] [PubMed] [Google Scholar]
- 12.Dezaki K, Sone H, Koizumi M, et al. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes. 2006;55:3486–93. doi: 10.2337/db06-0878. [DOI] [PubMed] [Google Scholar]
- 13.Bose M, Olivan B, Teixeira J, et al. Do incretins play a role in the remission of type 2 diabetes after gastric bypass surgery: what are the evidence. Obes Surg. 2009;19:217–29. doi: 10.1007/s11695-008-9696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kjems LL, Hoist JJ, Volund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003;52:380–6. doi: 10.2337/diabetes.52.2.380. [DOI] [PubMed] [Google Scholar]
- 15.Bikman BT, Zheng D, Pories WJ, et al. Mechanism for improved insulin sensitivity after gastric bypass surgery. J Clin Endocrinol Metab. 2008;93:4656–63. doi: 10.1210/jc.2008-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gletsu N, Lin E, Khaitan L, et al. Changes in C-reactive protein predict insulin sensitivity in severely obese individuals after weight loss surgery. J Gastrointest Surg. 2005;9:1119–28. doi: 10.1016/j.gassur.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Lin E, Phillips LS, Ziegler TR, et al. Increases in adiponectin predict improved liver, but not peripheral, insulin sensitivity in severely obese women during weight loss. Diabetes. 2007;56:735–42. doi: 10.2337/db06-1161. [DOI] [PubMed] [Google Scholar]


