Abstract
Interferon (IFN)-γ is a cytokine known for its immunomodulatory and anti-proliferative action. In the liver, IFN-γ can induce hepatocyte apoptosis or inhibit hepatocyte cell cycle progression. This article reviews recent mechanistic reports that describe how IFN-γ may direct the fate of hepatocytes either towards apoptosis or a cell cycle arrest. This review also describes a probable role for IFN-γ in modulating hepatocyte fate during liver regeneration, transplantation, hepatitis, fibrosis and hepatocellular carcinoma, and highlights promising areas of research that may lead to the development of IFN-γ as a therapy to enhance recovery from liver disease.
Keywords: IFN-γ, hepatocyte, liver, proliferation, apoptosis
1. Introduction
Interferon (IFN)-γ is an inflammatory cytokine recognized for its antiviral and immunomodulatory properties. Also known as type II interferon, IFN-γ is secreted primarily by activated T cells and natural killer (NK) cells [1]. In the liver, the action of IFN-γ extends beyond immune modulation to include regulation of hepatocyte apoptosis and cell cycle progression during liver disease [2, 3]. In light of the growing understanding of IFN-γ as a contributing factor to liver disease, it is intriguing to consider emerging therapeutic approaches that modulate IFN-γ signaling. This review aims to illuminate how IFN-γ regulates hepatocyte apoptosis and cell cycle progression and explore the multifaceted role of IFN-γ during liver disease.
2. Hepatic sources and targets of IFN-γ
The liver parenchyma is comprised of hepatocytes, which are fully differentiated, metabolically active cells. Under normal conditions, hepatocytes are mitotically quiescent, yet they can be induced to replicate following injury due to toxicant exposure, viral infection, or following surgical resection of a substantial portion of the liver [4]. Hepatocytes themselves are not substantial producers of IFN-γ. Instead, IFN-γ production is attributed to activated lymphocytes, such as NK cells, T lymphocytes, and NKT cells, which either reside in the liver or are recruited to the liver in response to inflammation and injury [5]. During liver injury and inflammation, hepatocytes increase expression of the transmembrane IFN-γ receptor [6], which presumably increases their sensitivity to IFN-γ stimulation [7]. In the liver, IFN-γ also activates the IFN-γ receptor expressed on nonparenchymal cells, which include resident macrophages called Kupffer cells, the activation of which is important for mediating both innate and adaptive immune responses [8]. IFN-γ is a potent stimulus for macrophage activation, and activated macrophages produce an abundance of cytokines, such as TNF-α, which may also modulate hepatocyte function [9]. While such indirect effects of IFN-γ undoubtedly impact hepatocyte function under a variety of inflammatory conditions, this review will focus on the direct consequences of IFN-γ on hepatocyte fate, namely apoptosis and proliferation.
3. IFN-γ signaling pathways
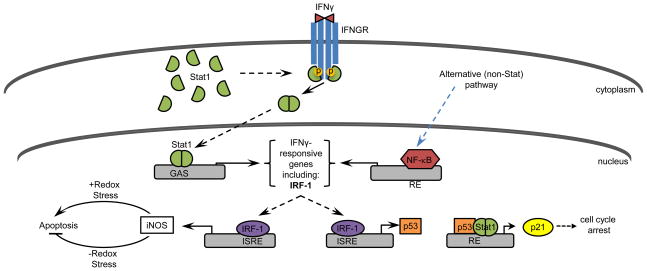
The IFN-γ receptor is a transmembrane, heterodimeric protein comprised of a constitutively expressed alpha chain (IFNGR1), which contains a ligand-binding domain and an inducible, non-ligand-binding beta chain (IFNGR2) [10]. The biologically active IFN-γ dimer interacts with IFNGR1, which initiates the association of IFNGR1 with IFNGR2 and subsequent phosphorylation of JAK1 and JAK2 kinases (Figure 1). Activated JAK kinases phosphorylate the cytoplasmic tails of IFNGR1 subunits, providing a docking site for the Src homology-2 domain of Stat1, a member of the signal transducer and activator of transcription (Stat) family of transcription factors. Stat1 becomes phosphorylated at Tyr701 [11], with maximal transcriptional activity requiring additional phosphorylation at Ser 727 [12]. Upon phosphorylation, activated Stat1 dissociates from IFNGR1, forms homo- or heterodimers, and translocates to the nucleus to modulate transcription of IFN-γ-responsive genes [13].
Figure 1.
Pathways by which IFN-γ elicits apoptosis and cell cycle arrest. IFN-γ activates Stat or non-Stat-mediated pathways, leading to the induction of IRF-1. IRF-1 induces iNOS and p53, which regulate apoptosis and cell cycle progression, respectively. RE = response element.
Stat1 exists in two forms: the 91-kDa Stat1α or the 84-kDa Stat1β, the latter of which is a splice variant that lacks a transcriptional activation domain. In response to IFN-γ, Stat1 isoforms may combine with each other or with Stat3 to produce two distinct transcription factors: a Stat1α homodimer and a Stat1(α/β)/Stat3 heterodimer [14]. Upon translocation to the nucleus, these transcription factors bind to gamma-interferon activation site (GAS) elements for the rapid induction of gene transcription. IFN-γ-responsive genes with identified GAS elements include genes involved in regeneration, antiviral defense, cell cycle progression and apoptosis [15]. Stat1 also binds to GAS elements to induce expression of interferon regulatory factor (IRF)-1. IRF-1 is a transcription factor that binds to interferon-sensitive response elements (ISREs) to drive the expression of an additional set of IFN-γ-responsive genes, including those involved in apoptosis and cell cycle regulation [16].
While the contribution of the JAK-Stat pathway to IFN-γ signal transduction has been well characterized, accumulating evidence demonstrates that regulation of IFN-γ-responsive genes can and does occur independently of this pathway [17]. For example, IFN-γ induces the expression of the immediate-early gene c-myc in Stat1-deficient tumor cells and immortalized fibroblasts [18]. Likewise, IFN-γ treatment modulates the expression of numerous genes in bone marrow-derived macrophages from Stat1-deficient mice [19]. One possible explanation for these findings is that, in the absence of Stat1, IFN-γ treatment stimulates IRF-1 expression via a putative NF-κB site in the IRF-1 promoter [20]. Furthermore, IFN-γ has been shown to increase degradation of the NF-κB inhibitor, IκBβ, thereby enhancing NF-κB activity [20]. While some of these findings are cell type-specific, collectively they illustrate the complexity associated with IFN-γ-mediated gene regulation.
4. IFN-γ induces hepatocyte apoptosis
IFN-γ elicits apoptosis in numerous cell types, including hepatocytes, through mechanisms that are poorly understood and likely to involve multiple pathways [2, 21]. In primary mouse hepatocytes, IFN-γ treatment induces apoptosis through a p53-independent, IRF-1-dependent mechanism that requires de novo protein synthesis [2, 22]. As a well-established tumor suppressor, IRF-1 is implicated in cell growth control, oncogene transformation and apoptosis [23]. IRF-1 is primarily regulated at the transcriptional level [24], and IRF-1 mRNA levels are elevated in IFN-γ-treated hepatocyte cultures [22]. While IRF-1-regulated genes vary based on the cell type and stimulus, IRF-1 target genes related to apoptosis include caspase-1, caspase-7 and FasL [25–27]. Nevertheless, IFN-γ induces apoptosis in primary mouse hepatocytes through a mechanism that does not appear to involve caspase-1 or caspase-3 [22]. Furthermore, although IFN-γ treatment slightly increases mRNA levels of Fas and caspase-11, this effect was not observed in IRF1−/− hepatocytes, suggesting that these pathways are negligible for IFN-γ-mediated apoptosis in mouse hepatocytes [22].
Another possibility is that IFN-γ elicits apoptosis by enhancing the production of reactive oxygen species (ROS) or through ER stress. Indeed, treatment of mouse hepatocytes with IFN-γ prolonged ROS generation for 48 hr and increased expression of caspase-12, a marker of ER stress [28]. Inhibition of ER stress by glycerol treatment was shown to inhibit caspase-4 and -12 gene expression in IFN-γ-treated hepatocytes without affecting IRF-1 gene expression or ROS induction [29], suggesting that ER stress, rather than ROS, may be a crucial mediator in IFN-γ-induced apoptosis. Along these lines, IFN-γ was also shown to induce ROS production in IRF-1−/− hepatocytes, which are not susceptible to IFN-γ-mediated apoptosis [22]. Hence, ROS production alone is not sufficient to elicit apoptosis in IFN-γ-treated hepatocytes.
Intriguingly, hepatoma cell lines vary in their susceptibility to IFN-γ-mediated apoptosis, with some cell lines being altogether resistant. Such variation is not likely due to altered Stat1 protein expression among cell lines because, although basal expression levels of Stat1 are relatively low, expression is strongly upregulated in response to IFN-γ in many hepatoma cell lines [30]. Variation in susceptibility to IFN-γ-mediated apoptosis is also not due to altered expression of Fas or caspases. In fact, expression of pro- and anti-apoptotic proteins, such as caspase-1, caspase-3, caspase-8 and Bcl-2, remain largely unchanged in sensitive and resistant cell lines, as do levels of phosphorylated Stat1 [31]. An alternative explanation may stem from discrepancies in iNOS expression, which was shown to be increased in the IFN-γ-resistant HuH-7 human hepatoma cell line [31]. When transfected with siRNA to suppress iNOS expression, HuH-7 cells displayed increased sensitivity to IFN-γ-mediated apoptosis. Furthermore, IFN-γ-sensitive Hep3B cells exhibited less apoptosis when cocultured with HuH-7 cells, presumably due to the protective effect of increased NO production by the iNOS-expressing HuH-7 cells [31]. iNOS has also been shown to protect against hepatocyte apoptosis in animal models of endotoxemia [32, 33], hepatic regeneration [34], and ischemia/reperfusion injury [35]. In hepatocytes, IFN-γ synergizes with bacterial lipopolysaccharide, TNF-α and IL-1 to induce expression of the iNOS gene, which contains two ISREs and a GAS element in the upstream enhancer region [36–38]. It has been suggested that iNOS may promote or inhibit hepatocyte apoptosis depending on the redox state of the cell [39]. In the presence of redox stress, iNOS production may create oxidizing species that potentiate cell death. However, when proapoptotic signals are delivered in the absence of redox stress, iNOS production may facilitate the S-nitrosation of caspases and production of cGMP, both of which have been shown to inhibit apoptosis [40–44]. Understanding how iNOS confers resistance to IFN-γ-mediated apoptosis in hepatocytes under various physiological and pathological conditions may provide a basis for developing new strategies to treat hepatocellular carcinoma.
The consequences of IFN-γ on hepatocyte apoptosis are complicated by the presence of other cytokines and growth factors in the liver. Apoptosis is important for regulation of liver size and hepatic immune responses, and it can also mediate hepatitis [45]. In contrast to IFN-γ, the proinflammatory cytokines TNF-α, IL-6 and IL-1β are not cytotoxic to freshly isolated mouse hepatocytes [21]. However, hepatocytes treated with both IFN-γ and TNF-α exhibit increased apoptosis when compared to cells treated with IFN-γ alone, indicative of synergy between these two cytokines [21]. Understanding the mechanism by which this occurs is confounded by observations that TNF-α has been shown to enhance apoptosis, as well as suppress it, in primary mouse hepatocytes stimulated under other conditions [46, 47].
Whereas IFN-γ-mediated apoptosis is typically enhanced by TNF-α, it can be inhibited by other soluble mediators such as hepatocyte growth factor (HGF) [21]. HGF is a multifunctional cytokine that has a protective effect against liver injury and stimulates hepatocyte proliferation during liver regeneration [48, 49]. The mechanism by which HGF protects against IFN-γ-mediated apoptosis has been attributed to a growth factor-induced survival signal that requires p21 [50], although other mechanisms may exist. Understanding how growth factors and cytokines influence hepatocyte susceptibility to IFN-γ-mediated apoptosis will provide insight into mechanisms of IFN-γ cytotoxicity under both physiological and pathological conditions.
5. IFN-γ inhibits hepatocyte cell cycle progression
IFN-γ inhibits the proliferation of freshly isolated hepatocytes [2] and some hepatocyte-derived cell lines [51, 52]. In primary mouse hepatocytes, IFN-γ treatment inhibits DNA synthesis through a G1 cell cycle arrest that requires both Stat1 and the tumor suppressor protein, p53 [2, 53]. This G1 arrest coincides with increased expression the G1 cyclin-dependent kinase inhibitor, p21 [2]. As transcription factors, p53 and Stat1 proteins can individually activate the p21 promoter in response to IFN-γ [54, 55]. However, in the absence of p53, IFN-γ treatment only slightly increases p21 expression and fails to elicit a cell cycle arrest [2]. This suggests that activation of Stat1 alone is not sufficient to drive p21 and inhibit S-phase progression.
The requirement for Stat1 in mediating the anti-proliferative action of IFN-γ may stem from findings that Stat1 enhances the transcriptional activity of p53. For instance, in mouse embryonic fibroblasts, Stat1 directly interacts with p53 to function as a coactivator in the transcription of p53-responsive genes [56]. Furthermore, Stat1 increases p53 stability by suppressing expression of Mdm2 [56], an E3 ubiquitin ligase that targets p53 for proteosomal degradation [57]. Moreover, the Stat1-target gene interferon-induced transmembrane protein 1 (IFITM1) also stabilizes the transcriptional activity of p53, resulting in increased p21 expression and subsequent cell cycle arrest in response to IFN-γ [52]. Finally, the anti-proliferative consequences of IFN-γ also require the Stat1 target gene IRF-1, which is essential for the increased p53 expression observed in response to IFN-γ [22]. Hence, the cross talk that exists between Stat1 and p53 signaling pathways is intricate, and Stat1 likely influences p53 expression and activity in hepatocytes through multiple mechanisms. Determining how such complex transcriptional events are regulated in response to IFN-γ may provide a mechanistic explanation for how IFN-γ regulates hepatocyte cell cycle progression.
While p21 expression is central to the IFN-γ-mediated cell cycle arrest, IFN-γ has also been shown to prevent the downregulation of p27Kip1, another inhibitor of the cyclin-dependent kinases that regulate S-phase cell cycle progression. For example, in human bronchial epithelial cells, IFN-γ treatment inhibited proliferation by preventing the downregulation of p27 that typically occurs in response to HGF and serum [58]. The possibility that IFN-γ inhibits p27 degradation to elicit a cell cycle arrest is intriguing, but this mechanism has not yet been established in HGF-stimulated hepatocyte cultures treated with IFN-γ. Given the significance of HGF during liver growth and repair, understanding the opposing effects of IFN-γ and HGF on cell cycle progression is important for developing therapies to enhance recovery from inflammatory-based liver disease.
6. IFN-γ and liver disease
6.1. IFN-γ and liver regeneration
Liver regeneration is a multistep process in which normally quiescent hepatocytes proliferate to replace damaged or lost liver mass due to widespread injury or partial surgical resection [59]. During liver regeneration following 70% partial hepatectomy (PH), hepatocytes proliferate in response to cytokine and growth factor signals that are largely provided by nonparenchymal cells in the liver [60]. Understanding mechanisms of liver regeneration is complicated by the existence of interconnected and redundant signaling pathways in hepatocytes and nonparenchymal cells, as well as extrahepatic influences on regeneration, including hormones and neurotransmitters. As a result, the precise signals that initiate and terminate hepatocyte proliferation in the regenerating liver are unclear.
Several compelling findings implicate IFN-γ as a negative regulator of hepatocyte proliferation during liver regeneration. For example, injection of IFN-γ was found to inhibit DNA synthesis in the regenerating liver 40 hr after PH [61]. Moreover, hepatocyte DNA synthesis was increased 32–40 hr after PH in IFN-γ−/− mice, as well as in IFNGR−/− mice [61]. During PH-induced liver regeneration, IFN-γ production is largely attributed to NK cells, supported by the finding that NK cells constitute 90% of all IFN-γ-positive cells in the liver 36 hr after PH [61]. When NK cells were depleted by administration of anti-asialo GM-1 prior to PH [62], hepatocyte DNA synthesis was increased in the regenerating liver [61], which suggests that NK cells likely produce the IFN-γ that attenuates regeneration. This notion is strengthened by other studies in which reduced NK cell activity correlated with enhanced liver regeneration [63–65].
There is evidence that IFN-γ may inhibit hepatocyte proliferation in the regenerating liver through the Stat1-mediated induction of IRF-1 and p21 [66, 67]. Administration of IFN-γ was found to induce the expression of IRF-1, p21 and phosphorylated Stat1 within the first 4 hr after PH [61]. A separate set of studies examined the effects of IFN-γ on liver regeneration by using the immunostimulant polyinosinic:polycytidylic acid (poly I:C). Poly I:C is a synthetic dsRNA analog that simulates viral infection by binding to toll-like receptor-3 and activating the innate immune system [68].
Administration of poly I:C inhibited PH-induced liver regeneration and augmented the production of IFN-γ by NK cells in the regenerating liver [61]. It also induced the expression of IRF-1, p21 and phosphorylated Stat1 after PH [67]. Moreover, disruption of the Stat1 gene abolished poly I:C suppression of liver regeneration and reduced the expression of IRF-1 and p21, indicating that IFN-γ may exert its anti-proliferative effects on regenerating hepatocytes through a Stat1-dependent manner. Likewise, disruption of IRF-1 and p21 gene expression also diminished poly I:C suppression of hepatocyte proliferation following PH [66]. These data implicate IFN-γ as a negative regulator of liver regeneration via a Stat1-dependent mechanism involving downstream expression of IRF-1 and p21. Thus, it can be surmised that IFN-γ, produced by activated NK cells, inhibits hepatocyte proliferation in the regenerating liver by mechanisms similar to those that have been identified in vitro.
Although NK cells are an abundant source of IFN-γ, NKT cells also secrete IFN-γ and may be an important regulator of liver regeneration under certain conditions, such as infection with hepatitis B virus (HBV). Studies show that liver regeneration is attenuated in HBV-transgenic (HBV-tg) mice, which express high levels of hepatitis B surface antigen and have detectable HBV DNA in their serum [69]. Impaired liver regeneration in HBV-tg mice coincided with an accumulation of NKT cells in the remnant livers, and these cells were in an activated state, based on increased expression of CD69 and IFN-γ production. Impaired liver regeneration in these mice was largely rectified by NKT cell depletion, but not by NK cell depletion, which implicates NKT cells and presumably NKT cell-derived IFN-γ in attenuation of regeneration. Hence, both NK and NKT cells may regulate PH-induced liver regeneration through the production of IFN-γ. The cellular source of IFN-γ may vary according to the liver environment, including status of viral infection and accompanying inflammation.
6.2. IFN-γ and liver transplantation
Suppression of IFN-γ signaling represents a promising approach for inducing tolerance during liver allograft transplantation, which has been used to ameliorate liver disease for nearly half a century. There are three types of liver transplantation methods: orthotopic liver transplantation (OLT) (replacement of a whole diseased liver with a healthy donor liver), heterotopic liver transplantation (addition of a donor liver at another site, leaving the diseased liver intact), and reduced-size liver transplantation (replacement of a whole diseased liver with a portion of a healthy liver) [70]. One problem that transplant recipients inevitably face is the possibility of graft rejection, which occurs in approximately 20–60% of transplant recipients [70]. Immune suppression is routinely used to induce graft acceptance and is typically aimed at suppressing the adaptive immune system, particularly the expression of Th1 cytokines such as IFN-γ [71, 72]. In a study investigating OLT in syngeneic and allogeneic rat models, allogeneic liver grafts exhibited greater hepatocyte apoptosis and less hepatocyte proliferation than syngeneic grafts after OLT [73]. Expression of IFN-γ mRNA, activation of Stat1, and expression of the downstream genes, IRF-1 and p21, were also elevated in the allogeneic grafts compared with the syngeneic grafts [73]. NK cells appear to be an abundant source of IFN-γ, as depletion of NK cells in allografts decreased IFN-γ expression and Stat1 activation, resulting in enhanced hepatocyte proliferation in the grafts [73]. These data suggest that, after allogeneic transplantation, NK cell-derived IFN-γ likely contributes to rejection and inhibition of hepatocyte proliferation [73]. Thus, inhibition of IFN-γ expression or activity in the allograft tissue following OLT represents a potential strategy to improve host tolerance and enhance recovery from liver disease.
6.3. IFN-γ and T cell-mediated hepatitis
Autoimmune hepatitis is a chronic disease of unknown etiology that can progress to cirrhosis. Liver injury is attributed to activated CD4+ T cells and NKT cells that either directly damage parenchymal cells or cause damage through the production of proinflammatory cytokines [74]. To study autoimmune hepatitis, several experimental mouse models of T cell-dependent liver injury may be used, including administration of concanavalin A (ConA), a plant lectin that is also a T cell mitogen [75]. A single intravenous injection of ConA induces evidence of hepatitis within 8–24 hours, characterized by increased transaminase activity, infiltration of the liver by neutrophils and T cells, and hepatocyte apoptosis and necrosis [76–80]. ConA-induced hepatitis is dependent on CD4+ T cells, NKT cells and macrophages [79, 81] and requires the production of pro-inflammatory cytokines TNF-α and IFN-γ [82–85], the latter of which peaks about 10 hr after ConA administration [82]. The requisite role of IFN-γ during ConA-induced hepatitis has been established using neutralizing IFN-γ antibodies, an IFN-γ receptor-immunoglobulin fusion protein, as well as transgenic mice that do not express IFN-γ, collectively demonstrating that ConA-treated mice do not develop liver injury in the absence of IFN-γ signaling [78, 84, 86, 87].
Endogenous IFN-γ likely mediates ConA-induced hepatitis through several mechanisms. Compelling evidence supports the possibility that, after ConA administration, increased levels of IFN-γ stimulate hepatocyte apoptosis through a Stat1-dependent pathway. Indeed, Stat1 activation occurs within 3 hr after ConA treatment [82]. This activation is specifically attributed to IFN-γ and is required for ConA-induced liver injury [82]. One consequence of Stat1 activation is the induction of IRF-1, which mediates the apoptotic effects of IFN-γ in cultured hepatocytes [22]. Along these lines, IRF-1 expression is detected 3 and 9 hr after ConA administration, along with concomitant increases in pro-apoptotic Bax and caspase-3 proteins [82]. Disruption of the IRF-1 gene was shown to protect against ConA-induced liver injury and mortality [88–90]. Collectively, these findings support the notion that, following ConA administration, increased levels of IFN-γ may induce hepatocyte apoptosis through a Stat1/IRF-1-dependent pathway.
Intriguingly, Stat3 activation also occurs after ConA administration, and evidence indicates that liver injury may result from an imbalance between Stat1 and Stat3 activation. For instance, Stat1−/− mice treated with ConA show increased Stat3 activation and expression of the Stat3-regulated anti-apoptotic gene Bcl-xL [82, 91]. This indicates that Stat3 may normally protect against liver injury and that ConA-induced IFN-γ production and subsequent Stat1 activation may suppress Stat3 activation. The observation that Stat1 activation and IRF-1 gene expression are increased in the absence of Stat3 signaling further supports a reciprocal relationship between Stat1 and Stat3 [82]. Furthermore, these proteins appear to regulate the activation of one another through induction of suppressor of cytokine signaling (SOCS), especially SOCS1 and SOC3, which curtail Stat activation by inhibiting Jaks [92, 93]. Hence, ConA-induced activation of Stat1 and subsequent induction of SOCS gene expression may suppress the activation of Stat3 and abolish its protective effects against hepatocyte apoptosis.
Yet another mechanism by which IFN-γ may orchestrate T cell-mediated hepatitis is through the upregulation of chemokines and adhesion molecules, which facilitate the infiltration of leukocytes to the liver. After ConA administration, NKT cells and other cells produce IFN-γ, which activates the Stat1/IRF-1 pathway in hepatocytes, sinusoidal endothelial cells and Kupffer cells, modulating the expression of chemokines and adhesion molecules, such as VCAM, ICAM-1, Mig, IP-10 and I-TAC, CCL20 and ENA-78 [88]. ConA-mediated induction of these genes requires IFN-γ but is influenced to a lesser extent by Stat1 and IRF-1, suggesting that other Stats or other IRF transcription factors may contribute to the IFN-γ-mediated induction of these genes. Many of these chemokines and adhesion molecules alter neutrophil and/or T cell recruitment to the liver in other models of liver disease [94–98], which provides compelling justification for further investigating the role of these molecules during T cell-mediated hepatitis.
6.4. IFN-γ and viral hepatitis
Viral hepatitis is the leading cause of hepatocellular carcinoma and a common reason for liver transplantation. A prevalent cause of viral hepatitis is chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV), which are hepatotropic, noncytopathic viruses that are transmitted parenterally [99]. Although HBV and HCV are unrelated viruses with different strategies for replication, recovery from infection with either virus depends on a robust adaptive immune response that includes activation of cytotoxic T lymphocytes (CTL) and the production of CTL-derived IFN-γ [100, 101]. In the absence of viral clearance, HBV and HCV establish chronic infection, which occurs in about 5–10% of adults infected with HBV and in about 70–90% of HCV cases [100, 102]. Complications of chronic infection include the development of cirrhosis and hepatocellular carinoma (HCC). Disease progression is generally attributed to immune-mediated pathology that results from CTL cytotoxicity and the recruitment of antigen-nonspecific inflammatory cells to necroinflammatory lesions in the liver [99].
IFN-γ plays a crucial role in limiting HBV and HCV pathogenesis during acute infection. Whereas activated NK and NKT cells are typically abundant sources of IFN-γ during viral infection [103], these innate immune cells are not believed to play a significant role in viral clearance during HBV or HCV infection [104, 105]. Instead, production of IFN-γ is generally attributed to virus-specific CD8+ CTL [106, 107]. Although CTL can directly lyse virus-infected hepatocytes through perforin and Fas-dependent mechanisms [108], most of the antiviral action of these cells occurs noncytopathically through the production of IFN-γ [109]. IFN-γ likely contributes to viral clearance through several mechanisms, including direct inhibition of viral replication. This is supported by the observation that IFN-γ suppresses HCV replication in the HuH-7 human hepatoma cell line [110] and inhibits HBV replication in transgenic mice [111, 112], the latter of which occurs through a NO-dependent pathway that may involve IRF-1 [113, 114]. IFN-γ can also exert antiviral action by increasing antigen processing, transport and MHC expression in virus-infected cells to facilitate viral clearance. Along these lines, IFN-γ has been shown to induce MHC class I and class II proteins through mechanisms that involve IRF-1 and CIITA, respectively [115, 116], and enhance antigen processing by modulating expression of enzymatic subunits of the proteosome [117]. Finally, IFN-γ induces the expression of numerous chemokines that bind to the chemokine receptors CXCR3 and CCR5, which are selectively expressed on the Th1 subset of CD4+ T cells [118]. The recruitment of virus-specific CD4+ T helper cells to the liver most likely contributes to viral clearance by enhancing CTL activation [99].
CTL-derived IFN-γ may also exacerbate liver damage during viral hepatitis. For instance, by inducing the hepatic expression of IFN-γ-inducible genes, such as chemokines CXCL9 and CXCL10, IFN-γ enhances the recruitment of antigen-nonspecific mononuclear and polymorphonuclear cells to the liver where they accumulate in necroinflammatory foci [119]. The infiltration of inflammatory cells correlates with the severity of liver disease, as these cells produce proinflammatory and cytotoxic mediators that exacerbate the liver damage initiated by virus-specific CTL [99]. Indeed, increased levels of CXCL9 and CXCL10 in patients with chronic HCV infection are associated with increased numbers of inflammatory cells in the liver and enhanced liver damage [120, 121]. Likewise, blocking IFN-γ-inducible chemokine expression during HBV infection was shown to suppress mononuclear cell recruitment and reduce liver damage without affecting the IFN-γ-dependent CTL response [119]. Hence, selectively targeting IFN-γ-inducible protein expression may represent a therapeutic strategy to minimize liver damage incurred during viral hepatitis without affecting the antiviral action of IFN-γ that is essential for viral clearance.
6.5. IFN-γ and liver fibrosis
Liver fibrosis refers to the accumulation of scar tissue that occurs as a wound healing response during most chronic liver diseases. Generally speaking, fibrosis results from excessive deposition of extracellular matrix proteins, such as collagen, by activated hepatic stellate cells (HSCs) in the liver, although other types of cells may also exhibit fibrogenic properties [122]. Widespread fibrosis and the emergence of regenerative nodules are characteristics of cirrhosis, which can lead to hepatic insufficiency and portal hypertension. In developed countries, liver fibrosis and cirrhosis are most commonly caused by alcohol abuse, nonalcoholic steatohepatitis, and chronic HCV infection [122].
While most HCV patients are asymptomatic, spontaneous progression to fibrosis occurs in about 20% of cases [123]. Interestingly, the rate of progression to fibrosis does not correlate with viral load [124] or HCV genotype [125], but instead depends on the generation of a Th1 response [126]. In fact, patients with intrahepatic, HCV-specific CD8+ T cells that produce IFN-γ were reported to have less fibrosis and lower fibrosis progression rates than patients without an intrahepatic IFN-γ response [127]. A similar correlation was found between fibrosis and the number of IFN-γ-secreting CD8+ T cells in the blood [120]. However, whereas IFN-γ was associated with decreased rates of fibrosis, the cytolytic activity of intrahepatic CD8+ T cells correlated with increased rates of fibrosis as well as decreased viral load [128]. These findings raise the intriguing possibility that CD8+ T cell effector functions may be dissociated during chronic HCV infection, resulting in either beneficial or detrimental effects to the liver [127].
The antifibrogenic effects of IFN-γ are well established. Exogenous IFN-γ treatment has been shown to suppress fibrosis in rodent models of liver fibrosis induced by carbon tetrachloride (CCl4) or dimethylnitrosamine [129, 130], and it may also improve fibrosis in patients with chronic HCV infection [131]. It is likely that IFN-γ protects the liver against fibrosis through several mechanisms, including direct inhibition of HSC activation. For example, treatment of cultured rat HSCs with exogenous IFN-γ was shown to inhibit proliferation and decrease extracellular matrix gene expression [132], and similar results were obtained in vivo [130]. These antifibrogenic effects of IFN-γ are mediated by Stat1, as IFN-γ did not suppress HSC activation in Stat1-deficient cells and failed to inhibit liver fibrosis in CCl4-treated Stat1−/− mice [133]. Inhibition of HSC activation by IFN-γ/Stat1 may result from suppression of transforming growth factor (TGF)-β signaling, which is a potent activator of these cells [134]. For instance, in CCl4-treated mice, serum levels of IFN-γ correlated with expression of Smad7 protein, a negative regulator of TGF-β signaling [135], whereas Stat1−/− mice displayed increased expression of activated Smad3 protein, a positive regulator of TGF-β signaling that is inhibited by Smad7 [133, 135].
Another possible mechanism by which IFN-γ protects against fibrosis is through activation of the immune system. For example, it has been demonstrated that NK cells can kill activated HSCs but not quiescent HSCs [136–138]. Furthermore, activation of NK cells by poly I:C induced HSC death and ameliorated liver fibrosis in a mouse model of liver fibrosis [138]. This effect was attributed to an IFN-γ-mediated increase in expression of the NK cell stimulatory receptor, NKG2D, and the death receptor activator, TRAIL [138]. Interestingly, ethanol-induced acceleration of liver fibrosis has been linked to inhibition of the antifibrotic effects of NK cells and IFN-γ, as HSCs from ethanol-fed mice are resistant to killing by NK cells, presumably due to decreased IFN-γ production, as well as diminished expression of NKG2D and TRAIL on NK cells [139]. Taken together, these observations highlight the importance of understanding the direct and indirect effects of IFN-γ on HSC activation and demonstrate the complexity associated with the IFN-γ-mediated antifibrogenic response.
6.6. IFN-γ and hepatocellular carcinoma
One disease that shows promising response to IFN-γ augmentation is hepatocellular carcinoma (HCC), which is the most common and lethal form of liver cancer and the third-leading cause of cancer deaths worldwide [140, 141]. Despite concerted treatment efforts over the past several decades, clinical studies of chemotherapy or hormone therapy fail to demonstrate improved survival in patients with advanced HCC [142]. However, recent studies demonstrate that IFN-γ supplementation elicits tumor-suppressive effects in models of HCC [51, 143–145]. In a study examining the expression of IFN-γ receptor and IFN-γ-inducible genes in human HCC tissues, the majority of noncancerous liver tissues displayed elevated IFN-γ receptor expression on hepatocytes compared to HCC tissues [145]. In IFNGR− cases of HCC, tumor size was larger and metastasis was more common than in IFNGR+ cases [145]. Another study established that human HCC cell lines possess fewer proteasome subunits and diminished antigen presentation capabilities compared to noncancerous hepatocytes [144]. Treatment with IFN-γ restored proteasome function and antigen presentation. These studies underscore the potential therapeutic use of IFN-γ in enhancing immune surveillance during cancer and implicate IFN-γ as a general tumor suppressor during HCC.
Investigations into the effects of IFN-γ on HCC cell survival and proliferation revealed that cells treated with IFN-γ undergo a G1 cell cycle arrest that coincides with diminished cyclin D activity and hypophosphorylation of the retinoblastoma tumor suppressor protein [51]. Furthermore, this phenomenon appears to be p21-dependent, since suppression of p21 abolished the anti-proliferative effects of IFN-γ on HCC cells. Concurrently, suppression of p21 resulted in HCC cell apoptosis upon IFN-γ treatment [51]. These pro-apoptotic effects of IFN-γ on HCC cells were corroborated in a different study that examined the tumor-suppressive effects of IL-12, a monocyte-derived cytokine known for stimulating lymphocytes to produce IFN-γ [143]. This study also demonstrated that IFN-γinduced apoptosis involved the activation of caspase-9, enhanced expression of Bax, and release of cytochrome c from the mitochondria, all of which are involved in the mitochondria-dependent apoptosis signaling pathway.
Interestingly, IFN-γ appears to be capable of functioning as a tumor suppressor in HCC by both inhibiting cell cycle progression and by initiating apoptosis, depending on the status of the cell cycle regulator p21. If p21 is functionally expressed, then IFN-γ elicits a cell cycle arrest. However, if p21 expression is somehow compromised, namely as a result of p53 disruption, then IFN-γ potentially activates pathways leading to mitochondria-dependent apoptosis [75]. Therefore, activation of apoptosis could be thought of as a “fail safe” tumor suppressive function of IFN-γ in HCC, activated only when p21 expression is insufficient. Collectively, these findings support further investigation into the use of IFN-γ as a chemotherapeutic agent for treating HCC.
7. Conclusion
In the liver, hepatocyte proliferation is essential for recovery after surgical resection or transplantation, whereas hepatocyte apoptosis is crucial for maintaining organ size and preventing uncontrolled proliferation, such as occurs during hepatocellular carcinoma. A growing body of evidence demonstrates that IFN-γ has anti-proliferative consequences on hepatocytes by stimulating apoptosis or eliciting a cell cycle arrest. The mechanisms by which IFN-γ orchestrates hepatocyte fate appear to be multi-faceted, yet they generally require the activation of both Stat1 and IRF-1 transcription factors. In the whole liver, identifying mechanisms of IFN-γ action is complicated by the presence of other cells that may also be targets for IFN-γ, such as Kupffer cells and hepatic stellate cells, the activation of which may further modify hepatocyte fate and function. Furthermore, under conditions of injury and inflammation, other soluble mediators, such as growth factors and proinflammatory cytokines, may synergize or antagonize IFN-γ signaling pathways, which further confounds our understanding of how IFN-γ affects hepatocyte fate and liver disease in general. Nevertheless, recent evidence demonstrates that the use of IFN-γ therapy may be a promising strategy to modulate in vivo hepatocyte activity during liver regeneration, liver transplantation and hepatocellular carcinoma and to inhibit the development of fibrosis. These findings provide a rationale for further investigating mechanisms by which IFN-γ regulates hepatocyte fate in the diseased liver and for the continued development of IFN-γ as a therapeutic approach to enhance recovery from liver disease.
Acknowledgments
This work was supported by National Institutes of Health grants P20RR016454 from the INBRE Program of the National Center for Research Resources and R15DK088749 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Biographies
 Christopher J. Horras is a graduate student in the Department of Biological Sciences at Boise State University. He holds a B.S. in Biology from the College of Idaho and a M.A. in Teaching from The University of Portland. His research interests lie in understanding how the innate immune system regulates liver regeneration.
Christopher J. Horras is a graduate student in the Department of Biological Sciences at Boise State University. He holds a B.S. in Biology from the College of Idaho and a M.A. in Teaching from The University of Portland. His research interests lie in understanding how the innate immune system regulates liver regeneration.
 Cheri L. Lamb is a graduate student in the Department of Biological Sciences at Boise State University, where she also earned a B.S. in Health Sciences. Her research interests include understanding how exposure to aryl hydrocarbon receptor ligands modulates Stat1 activation in response to IFN-γ-treatment.
Cheri L. Lamb is a graduate student in the Department of Biological Sciences at Boise State University, where she also earned a B.S. in Health Sciences. Her research interests include understanding how exposure to aryl hydrocarbon receptor ligands modulates Stat1 activation in response to IFN-γ-treatment.
 Kristen A. Mitchell, PhD, is an Assistant Professor in the Department of Biological Sciences at Boise State University. She received her PhD in Pharmacology and Toxicology from Washington State University (laboratory of Dr. B. Paige Lawrence) and completed postdoctoral training at the University of Texas Medical Branch in Galveston (laboratory of Dr. Cornelis Elferink). Her research interests include investigating how the aryl hydrocarbon receptor regulates cell cycle progression and how exogenous ligands for this receptor cause immunotoxicity and elicit a cell cycle arrest. She holds memberships in the American Physiological Society and the Society of Toxicology.
Kristen A. Mitchell, PhD, is an Assistant Professor in the Department of Biological Sciences at Boise State University. She received her PhD in Pharmacology and Toxicology from Washington State University (laboratory of Dr. B. Paige Lawrence) and completed postdoctoral training at the University of Texas Medical Branch in Galveston (laboratory of Dr. Cornelis Elferink). Her research interests include investigating how the aryl hydrocarbon receptor regulates cell cycle progression and how exogenous ligands for this receptor cause immunotoxicity and elicit a cell cycle arrest. She holds memberships in the American Physiological Society and the Society of Toxicology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christopher J. Horras, Email: chorras@yahoo.com.
Cheri L. Lamb, Email: cherilamb@u.boisestate.edu.
References
- 1.Billiau A, Matthys P. Interferon-gamma: a historical perspective. Cytokine & Growth Factor Reviews. 2009;20:97–113. doi: 10.1016/j.cytogfr.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Kano A, Watanabe Y, Takeda N, Aizawa S, Akaike T. Analysis of IFN-gamma-induced cell cycle arrest and cell death in hepatocytes. Journal of Biochemistry. 1997;121:677–683. doi: 10.1093/oxfordjournals.jbchem.a021639. [DOI] [PubMed] [Google Scholar]
- 3.Shinagawa T, Yoshioka K, Kakumu S, et al. Apoptosis in cultured rat hepatocytes: the effects of tumor-necrosis-factor-alpha and interferon-gamma. Journal of Pathology. 1991;165:247–253. doi: 10.1002/path.1711650309. [DOI] [PubMed] [Google Scholar]
- 4.Michalopoulos GK. Liver regeneration after partial hepatectomy critical analysis of mechanistic dilemmas. American Journal of Pathology. 2010;176:2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lalor PF, Shields P, Grant AJ, Adams DH. Recruitment of lymphocytes to the human liver. Immunology and Cell Biology. 2002;80:52–64. doi: 10.1046/j.1440-1711.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 6.Valente G, Ozmen L, Novelli F, et al. Distribution of interferon-gamma receptor in human tissues. Eur J Immunol. 1992;22:2403–2412. doi: 10.1002/eji.1830220933. [DOI] [PubMed] [Google Scholar]
- 7.Lai HS, Lin WH, Hsu WM, Chen CN, Chang KJ, Lee PH. Variations in interferon gamma receptor gene expression during liver regeneration after partial hepatectomy in rats. American Surgeon. 2009;75:49–54. [PubMed] [Google Scholar]
- 8.Crispe IN. The liver as a lymphoid organ. Annual Review of Immunology. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 9.Wullaert A, van Loo G, Heyninck K, Beyaert R. Hepatic tumor necrosis factor signaling and nuclear factor-kappa B: Effects on liver homeostasis and beyond. Endocrine Reviews. 2007;28:365–386. doi: 10.1210/er.2006-0031. [DOI] [PubMed] [Google Scholar]
- 10.Farrar MA, Schreiber RD. The molecular cell biology of interferon-gamma and its receptor. Annual Review of Immunology. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 11.Shuai K, Stark GR, Kerr IM, Darnell JE. A single phosphotyrosine residue of stat91 required for gene activation by interferon-gamma. Science. 1993;261:1744–1746. doi: 10.1126/science.7690989. [DOI] [PubMed] [Google Scholar]
- 12.Wen ZL, Zhong Z, Darnell JE. Maximal activation of transcription by stat1 and stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 13.Ihle JN, Witthuhn BA, Quelle FW, et al. Signaling by the cytokine receptor superfamily - jaks and stats. Trends in Biochemical Sciences. 1994;19:222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 14.Sato T, Selleri C, Young NS, Maciejewski JP. Inhibition of interferon regulatory factor-1 expression results in predominance of cell growth stimulatory effects of interferon-gamma due to phosphorylation of Stat1 and Stat3. Blood. 1997;90:4749–4758. [PubMed] [Google Scholar]
- 15.Saha B, Prasanna SJ, Chandrasekar B, Nandi D. Gene modulation and immunoregulatory roles of interferon gamma. Cytokine. 2010;50:1–14. doi: 10.1016/j.cyto.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annual Review of Immunology. 2008;26:535–584. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- 17.Gough DJ, Levy DE, Johnstone RW, Clarke CJ. IFN-gamma signaling: does it mean JAK-STAT? Cytokine & Growth Factor Reviews. 2008;19:383–394. doi: 10.1016/j.cytogfr.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Ramana CV, Grammatikakis N, Chernov M, et al. Regulation of c-myc expression by IFN-gamma through Stat1-dependent and -independent pathways. Embo Journal. 2000;19:263–272. doi: 10.1093/emboj/19.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gil MP, Bohn E, O’Guin AK, et al. Biologic consequences of Stat1-independent IFN signaling. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6680–6685. doi: 10.1073/pnas.111163898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deb A, Haque SJ, Mogensen T, Silverman RH, Williams BRG. RNA-dependent protein kinase PKR is required for activation of NF-kappa B by IFN-gamma in a STAT1-independent pathway. Journal of Immunology. 2001;166:6170–6180. doi: 10.4049/jimmunol.166.10.6170. [DOI] [PubMed] [Google Scholar]
- 21.Morita M, Watanabe Y, Akaike T. Protective effect of hepatocyte growth-factor on interferon-gamma-induced cytotoxicity in mouse hepatocytes. Hepatology. 1995;21:1585–1593. [PubMed] [Google Scholar]
- 22.Kano A, Haruyama T, Akaike T, Watanabe Y. IRF-1 is an essential mediator in IFN-gamma-induced cell cycle arrest and apoptosis of primary cultured hepatocytes. Biochemical and Biophysical Research Communications. 1999;257:672–677. doi: 10.1006/bbrc.1999.0276. [DOI] [PubMed] [Google Scholar]
- 23.Romeo G, Fiorucci G, Chiantore MV, Percario ZA, Vannucchi S, Affabris E. IRF-1 as a negative regulator of cell proliferation. Journal of Interferon and Cytokine Research. 2002;22:39–47. doi: 10.1089/107999002753452647. [DOI] [PubMed] [Google Scholar]
- 24.Kroger A, Koster M, Schroeder K, Hauser H, Mueller PP. Activities of IRF-1. Journal of Interferon and Cytokine Research. 2002;22:5–14. doi: 10.1089/107999002753452610. [DOI] [PubMed] [Google Scholar]
- 25.Sanceau J, Hiscott J, Delattre O, Wietzerbin J. IFN-beta induces serine phosphorylation of Stat-1 in Ewing’s sarcoma cells and mediates apoptosis via induction of IRF-1 and activation of caspase-7. Oncogene. 2000;19:3372–3383. doi: 10.1038/sj.onc.1203670. [DOI] [PubMed] [Google Scholar]
- 26.Tamura T, Ishihara M, Lamphier MS, et al. An IRF-1-dependent pathway of DNA damage-induced apoptosis in mitogen-activated T lymphocytes. Nature. 1995;376:596–599. doi: 10.1038/376596a0. [DOI] [PubMed] [Google Scholar]
- 27.Chow WA, Fang JJ, Yee JK. The IFN regulatory factor family participates in regulation of Fas ligand gene expression in T cells. Journal of Immunology. 2000;164:3512–3518. doi: 10.4049/jimmunol.164.7.3512. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe Y, Suzuki O, Haruyama T, Akaike T. Interferon-gamma induces reactive oxygen species and endoplasmic reticulum stress at the hepatic apoptosis. Journal of Cellular Biochemistry. 2003;89:244–253. doi: 10.1002/jcb.10501. [DOI] [PubMed] [Google Scholar]
- 29.Kanki K, Kawamura T, Watanabe Y. Control of ER stress by a chemical chaperone counteracts apoptotic signals in IFN-gamma-treated murine hepatocytes. Apoptosis. 2009;14:309–319. doi: 10.1007/s10495-009-0318-x. [DOI] [PubMed] [Google Scholar]
- 30.Melen K, Keskinen P, Lehtonen A, Julkunen I. Interferon-induced gene expression and signaling in human hepatoma cell lines. Journal of Hepatology. 2000;33:764–772. doi: 10.1016/s0168-8278(00)80308-6. [DOI] [PubMed] [Google Scholar]
- 31.Vadrot N, Legrand A, Nello E, Bringuier AF, Guillot R, Feldmann G. Inducible nitric oxide synthase (iNOS) activity could be responsible for resistance or sensitivity to IFN gamma-induced apoptosis in several human hepatoma cell lines. Journal of Interferon and Cytokine Research. 2006;26:901–913. doi: 10.1089/jir.2006.26.901. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Saavedra JE, Lu T, et al. O-2-Vinyl 1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate protection against D-galactosamine/endotoxin-induced hepatotoxicity in mice: Genomic analysis using microarrays. Journal of Pharmacology and Experimental Therapeutics. 2002;300:18–25. doi: 10.1124/jpet.300.1.18. [DOI] [PubMed] [Google Scholar]
- 33.Ou JH, Carlos TM, Watkins SC, et al. Differential effects of nonselective nitric oxide synthase (NOS) and selective inducible NOS inhibition on hepatic necrosis, apoptosis, ICAM-1 expression, and neutrophil accumulation during endotoxemia. Nitric Oxide-Biology and Chemistry. 1997;1:404–416. doi: 10.1006/niox.1997.0136. [DOI] [PubMed] [Google Scholar]
- 34.Rai RM, Lee FYJ, Rosen A, et al. Impaired liver regeneration in inducible nitric oxide synthase-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13829–13834. doi: 10.1073/pnas.95.23.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yagnik GP, Takahashi Y, Tsoulfas G, Reid K, Murase N, Geller DA. Blockade of the L-arginine/NO synthase pathway worsens hepatic apoptosis and liver transplant preservation injury. Hepatology. 2002;36:573–581. doi: 10.1053/jhep.2002.35058. [DOI] [PubMed] [Google Scholar]
- 36.Gao JJ, Morrison DC, Parmely TJ, Russell SW, Murphy WJ. An interferon-gamma-activated site (GAS) is necessary for full expression of the mouse iNOS gene in response to interferon-gamma and lipopolysaccharide. Journal of Biological Chemistry. 1997;272:1226–1230. doi: 10.1074/jbc.272.2.1226. [DOI] [PubMed] [Google Scholar]
- 37.Kamijo R, Harada H, Matsuyama T, et al. Requirement for transcription factor irf-1 in no synthase induction in macrophages. Science. 1994;263:1612–1615. doi: 10.1126/science.7510419. [DOI] [PubMed] [Google Scholar]
- 38.Martin E, Nathan C, Xie QW. Role of interferon regulatory factor-1 in induction of nitric-oxide synthase. Journal of Experimental Medicine. 1994;180:977–984. doi: 10.1084/jem.180.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vodovotz Y, Kirn PKM, Bagci EZ, et al. Inflammatory modulation of hepatocyte apoptosis by nitric oxide: In vivo, in vitro, and in silico studies. Current Molecular Medicine. 2004;4:753–762. doi: 10.2174/1566524043359944. [DOI] [PubMed] [Google Scholar]
- 40.Dimmeler S, Haendeler J, Nehls M, Zeiher AM. Suppression of apoptosis by nitric oxide via inhibition of interleukin-1 beta-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. Journal of Experimental Medicine. 1997;185:601–607. doi: 10.1084/jem.185.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim YM, Talanian RV, Billiar TR. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. Journal of Biological Chemistry. 1997;272:31138–31148. doi: 10.1074/jbc.272.49.31138. [DOI] [PubMed] [Google Scholar]
- 42.Li JR, Billiar TR, Talanian RV, Kim YM. Nitric oxide reversibly inhibits seven members of the caspase family via S-nitrosylation. Biochemical and Biophysical Research Communications. 1997;240:419–424. doi: 10.1006/bbrc.1997.7672. [DOI] [PubMed] [Google Scholar]
- 43.Li JR, Yang SF, Billiar TR. Cyclic nucleotides suppress tumor necrosis factor alpha-mediated apoptosis by inhibiting caspase activation and cytochrome c release in primary hepatocytes via a mechanism independent of Akt activation. Journal of Biological Chemistry. 2000;275:13026–13034. doi: 10.1074/jbc.275.17.13026. [DOI] [PubMed] [Google Scholar]
- 44.Mannick JB, Miao XQ, Stamler JS. Nitric oxide inhibits Fas-induced apoptosis. Journal of Biological Chemistry. 1997;272:24125–24128. doi: 10.1074/jbc.272.39.24125. [DOI] [PubMed] [Google Scholar]
- 45.Ogasawara J, Watanabefukunaga R, Adachi M, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 46.Leist M, Gantner F, Bohlinger I, Germann PG, Tiegs C, Wendel A. Murine hepatocyte apoptosis induced in-vitro and in-vivo by TNF-alpha requires transcriptional arrest. Journal of Immunology. 1994;153:1778–1788. [PubMed] [Google Scholar]
- 47.Rolfe M, James NH, Roberts RA. Tumour necrosis factor alpha (TNF alpha) suppresses apoptosis and induces DNA synthesis in rodent hepatocytes: a mediator of the hepatocarcinogenicity of peroxisome proliferators? Carcinogenesis. 1997;18:2277–2280. doi: 10.1093/carcin/18.11.2277. [DOI] [PubMed] [Google Scholar]
- 48.Huh C, Factor V, Sánchez A, Uchida K, Conner E, Thorgeirsson S. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci U S A. 2004;101:4477–4482. doi: 10.1073/pnas.0306068101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishiki Y, Ohnishi H, Muto Y, Matsumoto K, Nakamura T. Direct evidence that hepatocyte growth-factor is a hepatotrophic factor for liver-regeneration and has a potent antihepatitis effect invivo. Hepatology. 1992;16:1227–1235. [PubMed] [Google Scholar]
- 50.McCullough CT, Tura BJ, Harrison DJ. Growth factor attenuation of IFN gamma-mediated hepatocyte apoptosis requires p21(waf-1) International Journal of Experimental Pathology. 2006;87:275–281. doi: 10.1111/j.1365-2613.2006.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Detjen KM, Murphy D, Welzel M, Farwig K, Wiedenmann B, Rosewicz S. Downregulation of p21(waf/cip-1) mediates apoptosis of human hepatocellular carcinoma cells in response to interferon-gamma. Experimental Cell Research. 2003;282:78–89. doi: 10.1016/s0014-4827(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 52.Yang G, Xu Y, Chen X, Hu G. IFITM1 plays an essential role in the antiproliferative action of interferon-gamma. Oncogene. 2007;26:594–603. doi: 10.1038/sj.onc.1209807. [DOI] [PubMed] [Google Scholar]
- 53.Sun R, Park O, Horiguchi N, et al. STAT1 contributes to dsRNA inhibition of liver regeneration after partial hepatectomy in mice. Hepatology. 2006;44:955–966. doi: 10.1002/hep.21344. [DOI] [PubMed] [Google Scholar]
- 54.Chin YE, Kitagawa M, Su WCS, You ZH, Iwamoto Y, Fu XY. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21(WAF1/CIP1) mediated by STAT1. Science. 1996;272:719–722. doi: 10.1126/science.272.5262.719. [DOI] [PubMed] [Google Scholar]
- 55.Macleod KF, Sherry N, Hannon G, et al. P53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes & Development. 1995;9:935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- 56.Townsend PA, Scarabelli TM, Davidson SM, Knight RA, Latchman DS, Stephanou A. STAT-1 interacts with p53 to enhance DNA damage-induced apoptosis. Journal of Biological Chemistry. 2004;279:5811–5820. doi: 10.1074/jbc.M302637200. [DOI] [PubMed] [Google Scholar]
- 57.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 58.Takami K, Takuwa N, Okazaki H, et al. Interferon-gamma inhibits hepatocyte growth factor-stimulated cell proliferation of human bronchial epithelial cells upregulation of p27(kip1) cyclin-dependent kinase inhibitor. American Journal of Respiratory Cell and Molecular Biology. 2002;26:231–238. doi: 10.1165/ajrcmb.26.2.4643. [DOI] [PubMed] [Google Scholar]
- 59.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 60.Michalopoulos GK. Liver regeneration. Journal of Cellular Physiology. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun R, Gao B. Negative regulation of liver regeneration by innate immunity (natural killer cells/interferon-gamma) Gastroenterology. 2004;127:1525–1539. doi: 10.1053/j.gastro.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 62.Habu S, Fukui H, Shimamura K, et al. In vivo effects of anti-asialo gm1 1. reduction of NK activity and enhancement of transplanted tumor-growth in nude-mice. Journal of Immunology. 1981;127:34–38. [PubMed] [Google Scholar]
- 63.Tamura F, Masuhara A, Sakaida I, Fukumoto E, Nakamura T, Okita K. FK506 promotes liver regeneration by suppressing natural killer cell activity. Journal of Gastroenterology and Hepatology. 1998;13:703–708. doi: 10.1111/j.1440-1746.1998.tb00717.x. [DOI] [PubMed] [Google Scholar]
- 64.Tanigawa K, Sakaida I, Masuhara M, Hagiya M, Okita K. Augmenter of liver regeneration (ALR) may promote liver regeneration by reducing natural killer (NK) cell activity in human liver diseases. Journal of Gastroenterology. 2000;35:112–119. doi: 10.1007/s005350050023. [DOI] [PubMed] [Google Scholar]
- 65.Vujanovic NL, Polimeno L, Azzarone A, et al. Changes of liver-resident nk cells during liver-regeneration in rats. Journal of Immunology. 1995;154:6324–6338. [PubMed] [Google Scholar]
- 66.Sun R, Park O, Horiguchi N, et al. STAT1 contributes to dsRNA inhibition of liver regeneration after partial hepatectomy in mice. Hepatology. 2006;44:955–966. doi: 10.1002/hep.21344. [DOI] [PubMed] [Google Scholar]
- 67.Sun R, Gao B. Negative regulation of liver regeneration by innate immunity (natural killer cells/interferon-gamma) Gastroenterology. 2004;127:1525–1539. doi: 10.1053/j.gastro.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 68.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappa B by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 69.Dong Z, Zhang J, Sun R, Wei H, Tian Z. Impairment of liver regeneration correlates with activated hepatic NKT cells in HBV transgenic mice. Hepatology. 2007;45:1400–1412. doi: 10.1002/hep.21597. [DOI] [PubMed] [Google Scholar]
- 70.Moon DB, Lee SG. Liver Transplantation. Gut and Liver. 2009;3:145–165. doi: 10.5009/gnl.2009.3.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Issa F, Schiopu A, Wood KJ. Role of T cells in graft rejection and transplantation tolerance. Expert Review of Clinical Immunology. 2010;6:155–169. doi: 10.1586/eci.09.64. [DOI] [PubMed] [Google Scholar]
- 72.Roayaie S, Sheiner PA, Emre S, et al. Cytokine profiles in early rejection following OKT3 treatment in liver transplant patients. Mediators of Inflammation. 2000;9:141–146. doi: 10.1080/09629350020002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shen K, Zheng SS, Park O, Wang H, Sun Z, Gao B. Activation of innate immunity (NK/IFN-gamma) in rat allogeneic liver transplantation: contribution to liver injury and suppression of hepatocyte proliferation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1070–1077. doi: 10.1152/ajpgi.00554.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tiegs G. Cellular and cytokine-mediated mechanisms of inflammation and its modulation in immune-mediated liver injury. Zeitschrift Fur Gastroenterologie. 2007;45:63–70. doi: 10.1055/s-2006-927397. [DOI] [PubMed] [Google Scholar]
- 75.Hardtke-Wolenski M, Jaeckel E. Mouse Models for Experimental Autoimmune Hepatitis: Limits and Chances. Digestive Diseases. 2010;28:70–79. doi: 10.1159/000282067. [DOI] [PubMed] [Google Scholar]
- 76.Kim KM, Kim YM, Park M, et al. A broad-spectrum caspase inhibitor blocks concanavalin A-induced hepatitis in mice. Clinical Immunology. 2000;97:221–233. doi: 10.1006/clim.2000.4939. [DOI] [PubMed] [Google Scholar]
- 77.Kunstle G, Hentze H, Germann PG, Tiegs G, Meergans T, Wendel A. Concanavalin A hepatotoxicity in mice: Tumor necrosis factor-mediated organ failure independent of caspase-3-like protease activation. Hepatology. 1999;30:1241–1251. doi: 10.1002/hep.510300517. [DOI] [PubMed] [Google Scholar]
- 78.Tagawa Y, Kakuta S, Iwakura Y. Involvement of Fas/Fas ligand system-mediated apoptosis in the development of concanavalin A-induced hepatitis. European Journal of Immunology. 1998;28:4105–4113. doi: 10.1002/(SICI)1521-4141(199812)28:12<4105::AID-IMMU4105>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 79.Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. Journal of Clinical Investigation. 1992;90:196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tiegs G. Experimental hepatitis and role of cytokines. Acta Gastro-Enterologica Belgica. 1997;60:176–179. [PubMed] [Google Scholar]
- 81.Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hong F, Jaruga B, Kim WH, et al. Opposing roles of STAT1 and STAT3 in T cell-mediated hepatitis: regulation by SOCS. Journal of Clinical Investigation. 2002;110:1503–1513. doi: 10.1172/JCI15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ksontini R, Colagiovanni DB, Josephs MD, et al. Disparate roles for TNF-alpha and Fas ligand in concanavalin A-induced hepatitis. Journal of Immunology. 1998;160:4082–4089. [PubMed] [Google Scholar]
- 84.Kusters S, Gantner F, Kunstle G, Tiegs G. Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology. 1996;111:462–471. doi: 10.1053/gast.1996.v111.pm8690213. [DOI] [PubMed] [Google Scholar]
- 85.Siebler J, Wirtz S, Klein S, et al. A key pathogenic role for the STAT1/T-bet signaling pathway in T-cell-mediated liver inflammation. Hepatology. 2003;38:1573–1580. doi: 10.1016/j.hep.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 86.Mizuhara H, Uno M, Seki N, et al. Critical involvement of interferon gamma in the pathogenesis of T-cell activation-associated hepatitis and regulatory mechanisms of interleukin-6 for the manifestations of hepatitis. Hepatology. 1996;23:1608–1615. doi: 10.1053/jhep.1996.v23.pm0008675184. [DOI] [PubMed] [Google Scholar]
- 87.Nicoletti F, Zaccone P, Xiang M, et al. Essential pathogenetic role for interferon (IFN-)gamma in concanavalin A-induced T cell-dependent hepatitis: Exacerbation by exogenous IFN-gamma and prevention by IFN-gamma receptor-immunoglobulin fusion protein. Cytokine. 2000;12:315–323. doi: 10.1006/cyto.1999.0561. [DOI] [PubMed] [Google Scholar]
- 88.Jaruga B, Hong F, Kim WH, Gao B. IFN-gamma/STAT1 acts as a proinflammatory signal in T cell-mediated hepatitis via induction of multiple chemokines and adhesion molecules: a critical role of IRF-1. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2004;287:G1044–G1052. doi: 10.1152/ajpgi.00184.2004. [DOI] [PubMed] [Google Scholar]
- 89.Senaldi G, Shaklee CL, Guo J, et al. Protection against the mortality associated with disease models mediated by TNF and IFN-gamma in mice lacking IFN regulatory factor-1. Journal of Immunology. 1999;163:6820–6826. [PubMed] [Google Scholar]
- 90.Streetz K, Fregien B, Plumpe J, et al. Dissection of the intracellular pathways in hepatocytes suggests a role for Jun kinase and IFN regulatory factor-1 in Con A-induced liver failure. Journal of Immunology. 2001;167:514–523. doi: 10.4049/jimmunol.167.1.514. [DOI] [PubMed] [Google Scholar]
- 91.Grad JM, Zeng XR, Boise LH. Regulation of Bcl-x(L): a little bit of this and a little bit of STAT. Current Opinion in Oncology. 2000;12:543–549. doi: 10.1097/00001622-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 92.Naka T, Fujimoto M, Kishimoto T. Negative regulation of cytokine signaling: STAT-induced STAT inhibitor. Trends in Biochemical Sciences. 1999;24:394–398. doi: 10.1016/s0968-0004(99)01454-1. [DOI] [PubMed] [Google Scholar]
- 93.Nicola NA, Nicholson SE, Metcalf D, et al. Negative regulation of cytokine signaling by the SOCS proteins. Cold Spring Harbor Symposia on Quantitative Biology. 1999;64:397–404. doi: 10.1101/sqb.1999.64.397. [DOI] [PubMed] [Google Scholar]
- 94.Colletti LM, Kunkel SL, Green M, Burdick M, Strieter RM. Hepatic inflammation following 70% hepatectomy may be related to up-regulation of epithelial neutrophil activating protein-78. Shock. 1996;6:397–402. doi: 10.1097/00024382-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 95.Colletti LM, Green M, Burdick MD, Kunkel SL, Strieter RM. Proliferative effects of CXC chemokines in rat hepatocytes in vitro and in vivo. Shock. 1998;10:248–257. doi: 10.1097/00024382-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 96.Gujral JS, Liu J, Farhood A, Hinson JA, Jaeschke H. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2004;286:G499–G507. doi: 10.1152/ajpgi.00318.2003. [DOI] [PubMed] [Google Scholar]
- 97.Park JW, Gruys ME, McCormick K, et al. Primary hepatocytes from mice treated with IL-2/IL-12 produce T cell chemoattractant activity that is dependent on monokine induced by IFN-gamma (Mig) and chemokine responsive to gamma-2 (Crg-2) Journal of Immunology. 2001;166:3763–3770. doi: 10.4049/jimmunol.166.6.3763. [DOI] [PubMed] [Google Scholar]
- 98.Selzner N, Selzner M, Odermatt B, Tian YH, Van Rooijen N, Clavien PA. ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell-dependent release of TNF-alpha/IL-6 in mice. Gastroenterology. 2003;124:692–700. doi: 10.1053/gast.2003.50098. [DOI] [PubMed] [Google Scholar]
- 99.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annual Review of Pathology-Mechanisms of Disease. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 100.Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annual Review of Immunology. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 101.Shoukry NH, Cawthon AG, Walker CM. Cell-mediated immunity and the outcome of hepatitis C virus infection. Annual Review of Microbiology. 2004;58:391–424. doi: 10.1146/annurev.micro.58.030603.123836. [DOI] [PubMed] [Google Scholar]
- 102.Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:S21–S29. doi: 10.1053/jhep.2002.36227. [DOI] [PubMed] [Google Scholar]
- 103.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: Function and regulation by innate cytokines. Annual Review of Immunology. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 104.Bigger CB, Brasky KM, Lanford RE. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. Journal of Virology. 2001;75:7059–7066. doi: 10.1128/JVI.75.15.7059-7066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6669–6674. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. Journal of Experimental Medicine. 2001;194:1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thimme R, Wieland S, Steiger C, et al. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. Journal of Virology. 2003;77:68–76. doi: 10.1128/JVI.77.1.68-76.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakamoto Y, Guidotti LG, Pasquetto V, Schreiber RD, Chisari FV. Differential target cell sensitivity to CTL-activated death pathways in hepatitis B virus transgenic mice. Journal of Immunology. 1997;158:5692–5697. [PubMed] [Google Scholar]
- 109.Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annual Review of Immunology. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- 110.Frese M, Schwarzle V, Barth K, et al. Interferon-gamma inhibits replication of subgenomic and genomic hepatitis C virus RNAs. Hepatology. 2002;35:694–703. doi: 10.1053/jhep.2002.31770. [DOI] [PubMed] [Google Scholar]
- 111.Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 112.McClary H, Koch R, Chisari FV, Guidotti LG. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. Journal of Virology. 2000;74:2255–2264. doi: 10.1128/jvi.74.5.2255-2264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Guidotti LG, McClary H, Loudis JM, Chisari FV. Nitric oxide inhibits hepatitis B virus replication in the livers of transgenic mice. Journal of Experimental Medicine. 2000;191:1247–1252. doi: 10.1084/jem.191.7.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guidotti LG, Morris A, Mendez H, et al. Intertlueron-regulated pathways that control hepatitis B virus replication in transgenic mice. Journal of Virology. 2002;76:2617–2621. doi: 10.1128/JVI.76.6.2617-2621.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Steimle V, Siegrist CA, Mottet A, Lisowskagrospierre B, Mach B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science. 1994;265:106–109. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 116.Zhou F. Molecular mechanisms of IFN-gamma to upregulate MHC class I antigen processing and presentation. International Reviews of Immunology. 2009;28:239–260. doi: 10.1080/08830180902978120. [DOI] [PubMed] [Google Scholar]
- 117.Van den Eynde BJ, Morel S. Differential processing of class-I-restricted epitopes by the standard proteasome and the immunoproteasome. Current Opinion in Immunology. 2001;13:147–153. doi: 10.1016/s0952-7915(00)00197-7. [DOI] [PubMed] [Google Scholar]
- 118.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. Journal of Experimental Medicine. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kakimi K, Lane TE, Wieland S, et al. Blocking chemokine responsive to gamma-2/interferon (IFN)-gamma inducible protein and monokine induced by IFN-gamma activity in vivo reduces the pathogenetic but not the antiviral potential of hepatitis B virus-specific cytotoxic T lymphocytes. Journal of Experimental Medicine. 2001;194:1755–1766. doi: 10.1084/jem.194.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Prezzi C, Casciaro MA, Francavilla V, et al. Virus-specific CD8+ T cells with type 1 or type 2 cytokine profile are related to different disease activity in chronic hepatitis C virus infection. European Journal of Immunology. 2001;31:894–906. doi: 10.1002/1521-4141(200103)31:3<894::aid-immu894>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 121.Shields PL, Morland CM, Salmon M, Qin SX, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. Journal of Immunology. 1999;163:6236–6243. [PubMed] [Google Scholar]
- 122.Bataller R, Brenner DA. Liver fibrosis. Journal of Clinical Investigation. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dore GJ, Freeman AJ, Law M, Kaldor JM. Is severe liver disease a common outcome for people with chronic hepatitis C? Journal of Gastroenterology and Hepatology. 2002;17:423–430. doi: 10.1046/j.1440-1746.2002.02730.x. [DOI] [PubMed] [Google Scholar]
- 124.Serfaty L, Chazouilleres O, PoujolRobert A, et al. Risk factors for cirrhosis in patients with chronic hepatitis C virus infection: Results of a case-control study. Hepatology. 1997;26:776–779. doi: 10.1002/hep.510260334. [DOI] [PubMed] [Google Scholar]
- 125.Benvegnu LB, Pontisso P, Cavalletto D, Noventa F, Chemello L, Alberti A. Lack of correlation between hepatitis C virus genotypes and clinical course of hepatitis C virus-related cirrhosis. Hepatology. 1997;25:211–215. doi: 10.1053/jhep.1997.v25.pm0008985292. [DOI] [PubMed] [Google Scholar]
- 126.Sobue S, Nomura T, Ishikawa T, et al. Th1/Th2 cytokine profiles and their relationship to clinical features in patients with chronic hepatitis C virus infection. Journal of Gastroenterology. 2001;36:544–551. doi: 10.1007/s005350170057. [DOI] [PubMed] [Google Scholar]
- 127.Bonilla N, Barget N, Andrieu M, et al. Interferon gamma-secreting HCV-specific CD8+T cells in the liver of patients with chronic C hepatitis: relation to liver fibrosis - ANRS HC EP07 study. Journal of Viral Hepatitis. 2006;13:474–481. doi: 10.1111/j.1365-2893.2005.00711.x. [DOI] [PubMed] [Google Scholar]
- 128.Nelson DR, Marousis CG, Davis GL, et al. The role of hepatitis C virus-specific cytotoxic T lymphocytes in chronic hepatitis C. Journal of Immunology. 1997;158:1473–1481. [PubMed] [Google Scholar]
- 129.Baroni GS, Dambrosio L, Curto P, et al. Interferon gamma decreases hepatic stellate cell activation and extracellular matrix deposition in rat liver fibrosis. Hepatology. 1996;23:1189–1199. doi: 10.1002/hep.510230538. [DOI] [PubMed] [Google Scholar]
- 130.Rockey DC, Chung JJ. Interferon-gamma inhibits lipocyte activation and extracellular-matrix messenger RNA expression during experimental liver injury: implications for treatment of hepatic-fibrosis. Journal of Investigative Medicine. 1994;42:660–670. [PubMed] [Google Scholar]
- 131.Muir AJ, Sylvestre PB, Rockey DC. Interferon gamma-1b for the treatment of fibrosis in chronic hepatitis C infection. Journal of Viral Hepatitis. 2006;13:322–328. doi: 10.1111/j.1365-2893.2005.00689.x. [DOI] [PubMed] [Google Scholar]
- 132.Rockey DC, Maher JJ, Jarnagin WR, Gabbiani G, Friedman SL. Inhibition of rat hepatic lipocyte activation in culture by interferon-gamma. Hepatology. 1992;16:776–784. doi: 10.1002/hep.1840160325. [DOI] [PubMed] [Google Scholar]
- 133.Jeong WI, Park O, Radaeva S, Gao B. STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity. Hepatology. 2006;44:1441–1451. doi: 10.1002/hep.21419. [DOI] [PubMed] [Google Scholar]
- 134.Breitkopf K, Godoy P, Ciuclan L, Singer MV, Dooley S. TGF-beta/Smad signaling in the injured liver. Zeitschrift Fur Gastroenterologie. 2006;44:57–66. doi: 10.1055/s-2005-858989. [DOI] [PubMed] [Google Scholar]
- 135.Ulloa L, Doody J, Massague J. Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-gamma/STAT pathway. Nature. 1999;397:710–713. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- 136.Mehal WZ. Activation-induced cell death of hepatic stellate cells by the innate immune system. Gastroenterology. 2006;130:600–603. doi: 10.1053/j.gastro.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 137.Melhem A, Muhanna N, Bishara A, et al. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. Journal of Hepatology. 2006;45:60–71. doi: 10.1016/j.jhep.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 138.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian ZG, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 139.Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248–258. doi: 10.1053/j.gastro.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Worns MA, Weinmann A, Schuchmann M, Galle PR. Systemic therapies in hepatocellular carcinoma. Digestive Diseases. 2009;27:175–188. doi: 10.1159/000218351. [DOI] [PubMed] [Google Scholar]
- 141.Schutte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma: epidemiological trends and risk factors. Digestive Diseases. 2009;27:80–92. doi: 10.1159/000218339. [DOI] [PubMed] [Google Scholar]
- 142.Zhu AX. Systemic treatment of hepatocellular carcinoma: dawn of a new era? Annals of Surgical Oncology. 2010;17:1247–1256. doi: 10.1245/s10434-010-0975-6. [DOI] [PubMed] [Google Scholar]
- 143.Komita H, Homma S, Saotome H, Zeniya M, Ohno T, Toda G. Interferon-gamma produced by interleukin-12-activated tumor infiltrating CD8(+)T cells directly induces apoptosis of mouse hepatocellular carcinoma. Journal of Hepatology. 2006;45:662–672. doi: 10.1016/j.jhep.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 144.Matsui M, Machida S, Itani-Yohda T, Akatsuka T. Downregulation of the proteasome subunits, transporter, and antigen presentation in hepatocellular carcinoma, and their restoration by interferon-gamma. Journal of Gastroenterology and Hepatology. 2002;17:897–907. doi: 10.1046/j.1440-1746.2002.02837.x. [DOI] [PubMed] [Google Scholar]
- 145.Nagao M, Nakajima Y, Kanehiro H, et al. The impact of interferon gamma receptor expression on the mechanism of escape from host immune surveillance in hepatocellular carcinoma. Hepatology. 2000;32:491–500. doi: 10.1053/jhep.2000.16470. [DOI] [PubMed] [Google Scholar]