Abstract
Objective
To characterize the relationship between neurophysiologic changes in the brain and behavioral response to constraint-induced language therapy (CILT) by using magnetoencephalography (MEG).
Design
Case series.
Setting
Medical school.
Participants
Patients (N = 23) with chronic aphasia after first-time unilateral stroke in the left hemisphere.
Interventions
Constraint-induced language therapy administered for 3 hours 4 times per week for 3 weeks. Language testing and functional imaging during a language comprehension task using MEG before, immediately after, and 3 months after CILT with a subgroup of patients undergoing additional MEG scanning and language testing 3 weeks before CILT.
Main Outcome Measures
The percent of correct information units and the number of late dipoles normalized to total activation.
Results
Three patterns of behavioral and neurophysiologic response to CILT were identified. Patients with significant improvement in language immediately after CILT who lost these gains at follow-up had greater right hemisphere activation than other patients at all MEG scanning sessions. Patients with significant improvement in language immediately after CILT who maintained these gains at follow-up exhibited an increase in left temporal activation after CILT, whereas patients who did not exhibit significant improvement in language after CILT exhibited comparably greater activation in left parietal areas.
Conclusions
Results suggest that although the right hemisphere may support recovery of language function in response to therapy, this recovery may not be stable, and some participation of perilesional areas of the left hemisphere may be necessary for a stable behavioral response.
Keywords: Aphasia, Cerebrovascular disorder, Language therapy, Magnetoencephalography, Rehabilitation, Stroke
Knowledge regarding the neural basis for response to therapy for aphasia secondary to stroke is of potential importance for prognosis and providing a rationale for the basis of treatment. In the current study, we examined the relationship between behavioral and neurophysiologic response to therapy for chronic aphasia by using MEG.
Previous functional imaging studies of the relationship between behavioral and neurophysiologic response to therapy for chronic aphasia provide a varied picture regarding the role of each hemisphere in supporting the recovery of function. Although some studies1,2 found a relationship between improvement in language function and increased activation in the right hemisphere after therapy, others found increased activity in the dominant hemisphere3–5 or bilateral increases in activation6–8 to be related to behavioral improvement. Breier et al,9 using MEG, and Richter et al,10 using fMRI, both found that improvement in language function after therapy was correlated with greater relative activation in the right hemisphere before therapy. There are several factors that potentially contribute to variance among findings, including aphasia type, lesion location and size, imaging modality, activation tasks used in the scanner, and therapy type and dosage.
In the current study, we used MEG to characterize the changes in neurophysiologic status in specific areas of the brain immediately before, immediately after, and 3 months after CILT in patients with chronic aphasia secondary to left hemisphere stroke. Unlike functional imaging modalities that index neuronal function indirectly through hemodynamic changes such as positron emission tomography or fMRI, MEG directly indexes neuronal discharge. Therefore, MEG provides complimentary data to other imaging modalities and may have some advantage in clinical populations in which complex structural and functional changes in vasculature may have occurred in response to injury. In addition, the temporal sensitivity of MEG presents the opportunity to directly remove early activity associated with primary sensory response to stimuli in any modality. We included a 3-month post-therapy follow-up language and imaging session in the current study to test the stability of both behavioral and neurophysiologic response to therapy, an approach that has not been commonly used in many previous studies but that may be crucial to explaining some of the variance in previous findings. In addition, a subgroup of patients underwent MEG scanning and language testing 3 weeks before therapy to test the specificity of behavioral changes to the therapy and the sensitivity of the MEG scanning task to the effects of therapy.
CILT is an approach to therapy for language dysfunction based on the principles of use-dependent learning. The approach was first described by Pulvermuller et al11 and is a modification of use-dependent learning applications in motor rehabilitation referred to as constraint-induced movement therapy. 12,13 The principles of constraint-induced approaches to therapy include (1) constraint to the impaired modality, (2) restraint of the unimpaired modality (in this case alternative modes of communication), and (3) massed practice occurring in an enriched environment using behavioral shaping. In the case of CILT, patients’ responses are limited to the speech modality only, and the patients are restrained from using any other means of communication (such as pointing, gesture, writing, and so on). Based on a previous study in a smaller group,9 we hypothesized that patients who exhibited an improvement in language function after CILT would exhibit more relative activation in right hemisphere areas homotopic to putative premorbid language areas within the left hemisphere before CILT. Although Breier et al9 did not find any relationship between changes in MEG activation after therapy and changes in language function, we hypothesized, in this larger group, that improvement in language function would be correlated with an increase in the relative degree of activation of the right hemisphere after CILT.
METHODS
Participants
Twenty-three patients ranging in age from 33 to 77 years of age (mean ± SD, 54±11) participated in the study (16 men, 7 women). Two patients dropped out of the study before undergoing the follow-up evaluation, but their data were retained for analyses comparing the pre- and immediately post-CILT testing sessions. Inclusion criteria included the following: (1) no preexisting condition that could affect language function (eg, dementia, developmental dysphasia by history), (2) no implanted metallic device or object (eg, cardiac pacemaker, gold teeth) that could interfere with MEG or MRI signals, and (3) no significant sensory impairments such as in significant unaided hearing loss or poor visual acuity that might preclude participation either in MEG imaging and/or the CILT intervention. Patients also had to be able to follow 1-step verbal commands and score at least 60% on the WAB yes/no section, and patients were excluded if they were unable to imitate any speech sounds or make any simple words with visual cues or if their speech was entirely or mostly jargon with no recognizable words and they could not be prompted to a correct response.
All patients had a history of first-time unilateral stroke in the left hemisphere, were at least 1 year post-stroke, and were right-handed premorbidly as assessed with the Edinburgh handedness Inventory.14 Stroke etiology was ischemic in most patients, but 2 had a history of hemorrhagic stroke. Strokes were generally in the distribution of the left middle cerebral artery and affected primarily posterior and/or anterior cortical areas, although all patients had evidence for some subcortical involvement. Two patients had only subcortical findings on MRI.
All patients were given standard audiometric screening at 500, 1000, and 2000Hz. As expected, hearing loss ranged from mild to moderate. Correlations between pure-tone averages and patterns of MEG activation were not significant. All patients provided informed consent before participation, and the protocols and procedures used were approved by the University of Texas Medical School/Houston Institutional Review Board.
All patients presented with a persistent moderate to severe aphasia as indicated by the WAB15 (mean ± SD, 57±17), exhibiting deficits in both expressive and receptive language. In addition, all presented with significant word retrieval deficits as indicated by the Boston Naming Test16 (mean ± SD, 19±17). Detailed demographic data for each of the 3 groups described later are presented in table 1.
Table 1.
Group Means on Demographic Variables and Language Tests Immediately Pre-CILT
| Lost Response | Nonresponders | Responders | |
|---|---|---|---|
| Age (y) | 46.8±7.0 | 55.2±2.0 | 54.9±6.0 |
| Years of education (y) | 12±2.0 | 14±0.8 | 14±0.6 |
| Females (%) | 25 | 9 | 62 |
| Lesion volume (cubic cm) | 129.0±41.0 | 98.6±19.0 | 99.8±25.0 |
| WAB AQ (100*) | 55.5±17.0 | 56.5±19.0 | 61.9±14.0 |
| WAB Comprehension AQ (10*) | 7.2±1.0 | 7.6±2.0 | 8.0±1.0 |
| WAB Spontaneous Speech AQ (20*) | 11.3±2.0 | 10.2±4.0 | 11.0±4.0 |
| WAB Repetition AQ (10*) | 4.4±2.0 | 5.4±2.0 | 5.8±2.0 |
| Boston Naming Test (60*) | 17.8±19.0 | 17.4±19.0 | 23.0±17.0 |
NOTE. Values are mean ± SD unless otherwise noted.
Abbreviation: AQ, Aphasia Quotient.
Total possible.
Constraint-Induced Language Therapy
Therapy was administered to 2 patients in a dyad and consisted of 3-hour sessions 4 days a week for 3 weeks for a total of 36 hours of treatment. Constraint was operationally defined as limiting the response to spoken verbal production only. All other modes of communication were inhibited. The primary treatment task was a dual card task in which each patient took turns either requesting a matching card from a semantic category from the other patient or responding to that request. The forced use of spoken communication was accomplished by placing a visual barrier on the table between the patients so they could not see each other except for eye contact. Behavioral shaping, a reinforcement strategy consisting of successive approximation of goal behaviors in small steps, was used by increasing the communicative demands of the required request/response from single words to lengthy sentences. The therapist provided as much cuing as necessary for a successful response. In all cases, the patients ended each trial with a successful response using whatever means necessary (eg, phonemic or semantic cuing, repetition, and so on), with the amount of support reduced based on the patients’ needs.
Performance on the dual card task was used as the Tx response measure. The spoken request was scored for the number of CIUs. Each lexical item counted as 1 CIU except for the question phrase, for which all correct forms of the question were counted as 1 CIU, regardless of the number of words in the question phrase. For example: “Do you have a short pencil?” consists of a question phrase, “Do you have” equals 1 CIU, the adjective “short” equals 1 CIU, and the noun “pencil” equals 1 CIU for a total of 3 CIUs.
CIU data were collected immediately before, immediately after, and 3 months after therapy. At each of these time periods, there were 3 sessions; each were conducted on a different day. At each session, the patient was given a series of 20 cards chosen randomly from those used for CILT (10 cards containing pictures depicting high-frequency adjectives and nouns and 10 cards containing pictures depicting low-frequency adjectives and nouns) and asked to produce the 3 lexical items described earlier for each card.
Magnetoencephalography
The spatiotemporal patterns of brain activation specific to spoken word recognition17–22 and their stability or reproducibility over time23 have been previously established by using the same paradigm as used in the current study. The validity and topographic specificity of these maps have also been established by comparing them with the results of direct cortical stimulation mapping19,24 and with the results of the intracarotid amytal (Wada) procedure.25–27
At each MEG scanning session, the patient was given a recognition memory task for spoken words while data were collected in the scanner. The word list consisted of 165 English words with 5 words used as targets and the remaining 160 as distractors. Four blocks of 45 trials each were created for each scan, with the 5 targets presented in random order in each block among 40 new distractors for a total of 180 trials per scan. Two separate scans were obtained at each of the 3 scanning sessions. The target stimuli were presented consecutively for study immediately before the MEG scan as many times as was necessary for the patient to raise his/her left index finger to each of the 5 stimuli consecutively. The correct response rate during the scanning was low across all sessions (mean ± SD, 14.2%±10%). The number of button presses per session was about 40% of what was expected (mean ± SD, 8±7).
All participants were tested with a whole-head neuromagnetometera equipped with 248 gradiometer sensors and housed in a magnetically shielded room designed to reduce environmental magnetic noise. The recorded signals were filtered online with a bandpass between 0.1 and 20Hz, digitized at a rate of 254Hz, and adjusted relative to the mean amplitude in the 150-millisecond prestimulus period to remove direct current offset. The data were then submitted to an adaptive noise reduction procedure and, after artifact rejection, averaged across trials within each sensor. These averaged data were submitted to a set of fully automated procedures that scanned the averaged digitized evoked magnetic fields and identified at each point in time (every 4ms) the presence of single dipolar distributions, estimated the channel grouping that best covered each distribution, and used the portion of the flux distribution covered by such channel groupings to estimate the underlying dipolar source by using the standard model.28 Up to 4 sources could be estimated at each time point. Those dipolar sources that met the criteria of acceptability (correlation ≥0.9, confidence volume ≤30cm3) from each of the recording sessions were then compared according to their (1) degree of latency overlap and (2) spatial proximity to produce the final spatiotemporal maps corresponding to language function.
Source locations, which were initially computed in reference to the MEG Cartesian coordinate system mentioned previously, were coregistered on the T1-weighted MRI (TR 13.6ms; TE 4.8ms; recording matrix 256 × 256 pixels, 1 excitation, 240-mm field of view, and 1.4-mm slice thickness) obtained from the patient. Transformation of the MEG coordinate system into MRI-defined space was achieved with the aid of 3 lipid capsules inserted into the ear canals and attached to the nasion, which were easily visualized on the MRIs when using the MRI overlay tool, which is part of the 4-D Neuroimaging software.a The location of individual dipoles was determined with the use of a standard MRI atlas of the human brain.29
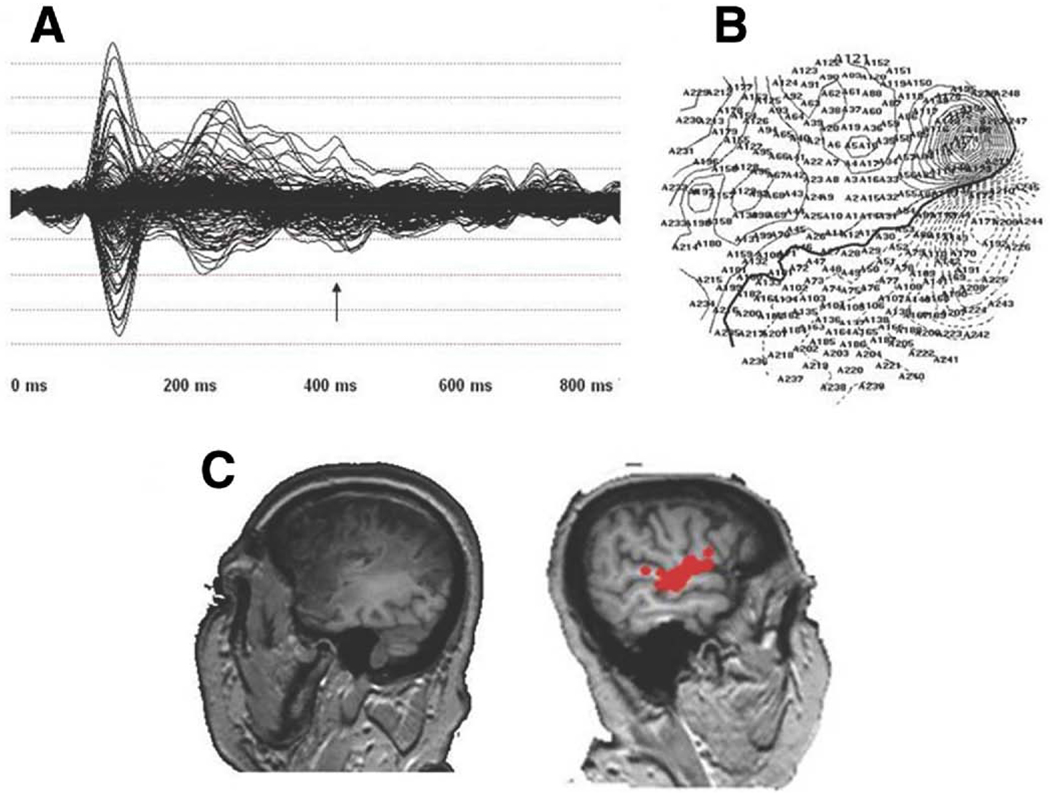
The averaged waveforms for one of the patients for 1 run is presented in figure 1A along with the isofield contour map as recorded at the head surface at 420 milliseconds (arrow) (fig 1B) and the MEG MRI coregistered scan for dipoles thresholded between 150 milliseconds after stimulus onset (offset of the N1m) and approximately 800 milliseconds after stimulus onset within the left and right hemispheres (fig 1C), by which time the late activation has ended.
Fig 1.
(A) The averaged waveform for a single run for a single patient for all sensors, (B) isofield contour map at 420 milliseconds for the patient, and (C) thresholded dipoles between 150 milliseconds and 800 milliseconds in the left (left side of image) and right (right side of image, red circles) hemispheres.
Lesion Volume Estimation
Using Brain Extraction Tool v2.1 within FSLv4.1.2 software, b brain and nonbrain areas were automatically identified; a brain mask was generated; and brain components including gray matter, white matter, and CSF were extracted from the T1-weighted image for each subject. Subsequently, the output of Brain Extraction Tool (eg, T1_brain) was provided to FASTb (FMRIB’s Automated Segmentation Tool v3.53) for generating an intensity-based, 4-class binary segmentation of the brain into gray matter, white matter, CSF, and lesion for each subject. Each tissue class was loaded separately for reviewing the segmentation results from FAST. Necessary edits were made to the CSF and lesion masks before moving on to quantification of volumes for each tissue class. Command line utilities included with FSL were used for generating volumetric data for each tissue class in each subject.
RESULTS
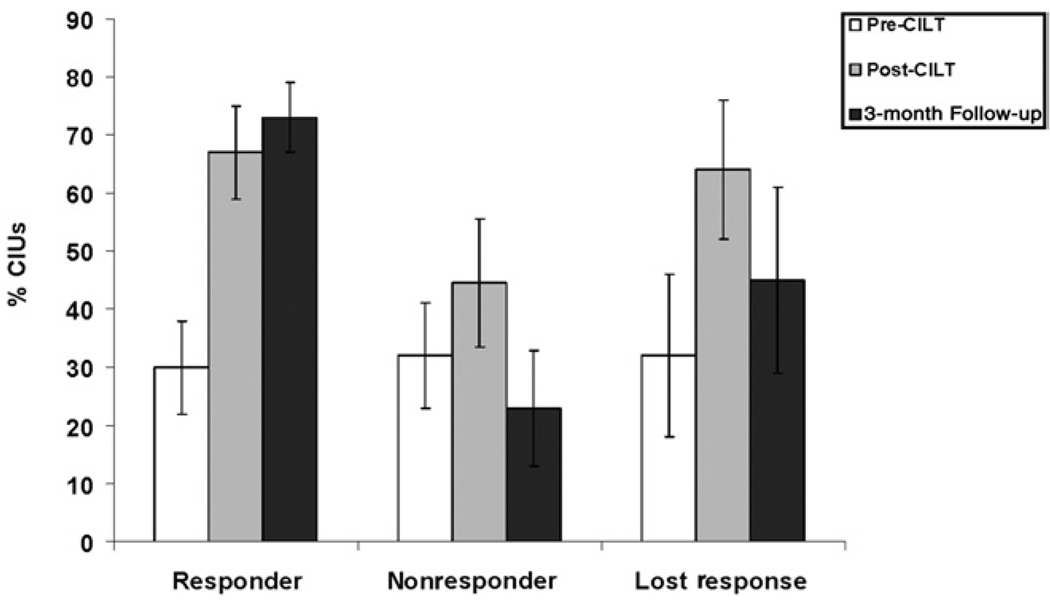
Patients were divided into 3 groups based on the pattern of change in percent CIUs: (1) those that exhibited an improvement of >24% CIUs (1 SD in pre-CILT data across all patients) or more over pre-CILT testing and did not lose this status at the 3-month follow-up testing session (responder; n = 8), (2) those that fell into the responder group immediately after therapy but lost this status at the 3-month follow-up (lost-response, n = 4), and (3) those that did not exhibit this degree of improvement over pre-CILT performance at either post-therapy assessment (nonresponder, n = 11). The mean percent CIUs for the pre-CILT, post-CILT, and follow-up time periods are presented for each group in figure 2. There were no significant group differences on pre-CILT percent CIUs (F2,20=.05, P>.95).
Fig 2.
The mean percent CIUs at the pre-CILT (white bars), post-CILT (gray bars), and 3-month follow-up (black bars) testing sessions for the responder, nonresponder, and lost-response groups. Error bars represent the standard error of the mean.
Group means on demographic variables as well as pre-CILT means for each of the groups on the WAB; Aphasia Quotient; WAB Spontaneous Speech, Repetition, and Comprehension subtests; and the Boston Naming Test are presented in table 1. Differences among groups on these measures were assessed by using analysis of variance with the group (lost-response, responder, nonresponder) as the between-subjects variable for continuous variables and the Fisher exact test for discrete variables. There were no group differences in age (F2,20=.76, P<.48), years of education (F2,20=.95, P<.40), or lesion volume (F2,20=.31, P<.74). The percentage of women was significantly higher in the responder group (Fisher exact test, P<.01). There were no significant group differences on the WAB Aphasia Quotient (F2,20=.29, P>.75), WAB Spontaneous Speech subtest (F2,20=.16, P>.85), the WAB Comprehension subtest (F2,20=.20, P>.80), the WAB Repetition subtest (F2,20=.53, P>.55), and the Boston Naming Test (F2,20=.24, P>.75), indicating groups were relatively well matched in terms of aphasia severity and type before CILT. The WAB was administered at the same time points as the imaging. There was a small (<3 points) increase in the WAB after CILT across all 3 groups that was statistically significant (F2,17=7.85, P<.004). There were no group differences for the change in the WAB score after CILT.
The Relationship Between Behavioral and Neurophysiologic Response to CILT
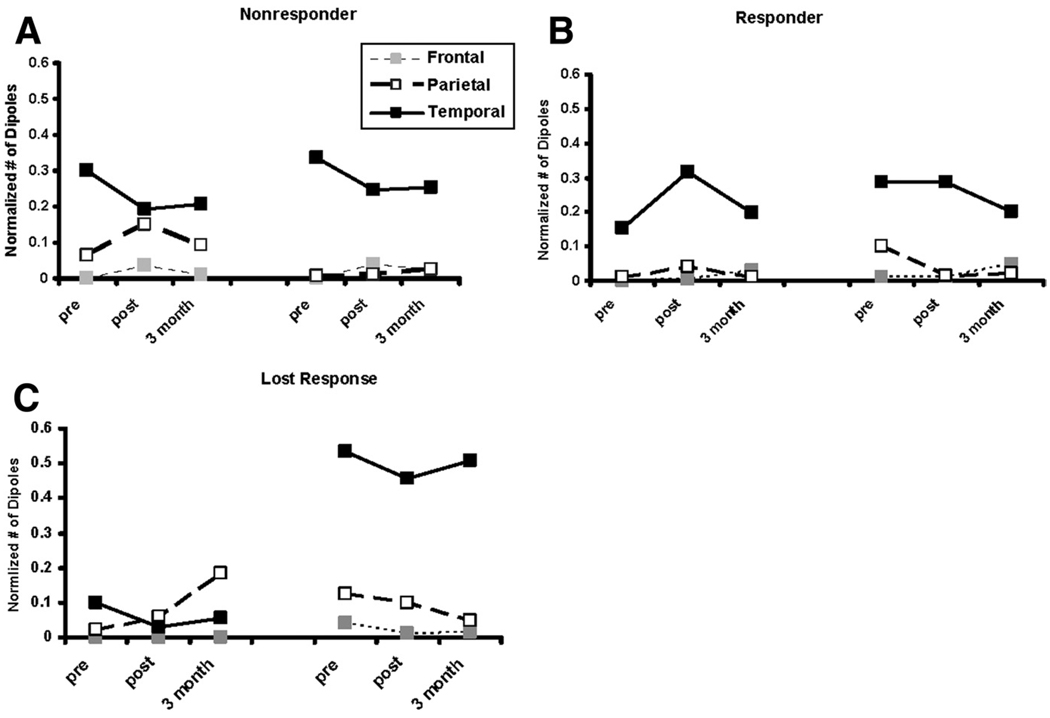
Speech processing occurs in a distributed network of areas in the brain. Analyses of recent imaging and lesion data have led to the suggestion that one of these networks is a ventral stream devoted to speech analysis and comprehension and includes the superior temporal gyrus, superior temporal sulcus, middle temporal gyrus, and a posterior-inferior area of the inferior temporal gyrus. A second network of areas devoted to speech-motor function runs from the temporoparietal junction to the frontal speech motor areas, including Broca’s area.30 Although the relative degree of activation observed in the areas comprising these networks during functional imaging is dependent on task and imaging modality, areas within both of these networks are active during speech perception.31–40 Therefore, the total number of thresholded late (after the resolution of the N1m) dipoles (between 150ms and 800ms after stimulus onset) in each of the 3 regions of interest, including the inferior frontal gyrus, the lateral temporal cortex, and the inferior parietal (including supramarginal and angular gyri) cortex in each hemisphere, was determined for each subject and normalized to the total number of thresholded dipoles observed during this time period. These regions of interest contained approximately 75% of the late activation across both hemispheres. The only remaining areas with significant (>1% of total) activation were in primary motor and primary somatosensory and visual areas. Group means for normalized activity in the chosen regions of interest in each hemisphere at each time point are presented in figure 3A through C.
Fig 3.
Mean normalized late MEG activation in the frontal (dotted lines, gray boxes), parietal (dashed lines, white boxes), and temporal (solid lines, black boxes) lobes in the left (left side of each graph) and right (right side of each graph) hemispheres at the pre- and post- CILT and 3-month follow-up scanning sessions for the (A) non-responder, (B) responder, and (C) lost-response groups.
These trends were analyzed by using a multivariate approach to a within-subjects design with time point (pre-CILT, post-CILT, follow-up), region of interest (temporal, inferior parietal, frontal), and hemisphere (left, right) as the within-subjects factors and group (responder, nonresponder, lostresponse) as the between-subjects variable. There was a significant group × hemisphere interaction (F2,18=3.65, P<.05). Looking within hemispheres, there were no significant effects for the left hemisphere. For the right hemisphere, there was a significant effect of group across all time points (F2,18=5.27, P<.02). Multiple comparisons using the Tukey test indicated a significant difference between the lost-response groups and both the responder and nonresponder groups but no difference between the latter 2 groups of patients. As can be seen in figure 3, the lost-response group has greater right hemisphere activation than the other 2 groups across all scanning sessions.
To determine if there were differences in the neurophysiologic response to therapy between the responder and nonresponder groups, we focused on the changes that occurred between the pre- and immediately post-CILT scanning sessions, removing the lost-response group and the 3-month follow- up scanning session from the analyses. A multivariate approach to a within-subjects design similar to the one described earlier, with time point (pre-CILT, post-CILT), region of interest (temporal, inferior parietal, frontal), and hemisphere (left, right) as the within-subjects factors and group (responder, nonresponder) as the between-subjects variable, was used. There was a significant group × time point by region of interest interaction (F2,16=3.87, P<.04). Looking within the region of interest, for parietal areas, there was a significant group × hemisphere interaction (F1,17=4.72, P<.04), whereas there were significant time point × group interactions for temporal (F1,17=4.68, P<.05) and frontal (F1,17=5.92, P<.025) regions of interest. As can be seen in figure 3A through C, the nonresponder group exhibited greater left as compared with right parietal activation, whereas the responder group showed the opposite asymmetry pre-CILT and a bilateral profile after therapy. In addition, the nonresponder group exhibited a decrease in left temporal activation after therapy, whereas the responder group exhibited an increase in left temporal activation as well as a decrease in right frontal activation not apparent in the nonresponder group.
Sensitivity of MEG Activation to Therapy
Nine subjects underwent additional MEG imaging and language testing 3 weeks before CILT with no therapy within the time period between this additional session and the pre-CILT session. Percent CIUs and MEG activation data at the 3-weeks before, immediately pre-, and post-CILT sessions are presented in table 2. The trends for percent CIUs were analyzed by using a multivariate approach to a within-subjects design with time point (3 weeks before CILT, immediately pre-CILT, post-CILT) as the within-subjects factor. A contrast transformation was used to compare the immediately pre-CILT time point with the 3-weeks before and post-CILT sessions. There was a significant effect of time point (F2,7=11.14, P<.007), with a significant difference between the immediately pre- and post-CILT sessions (F1,8=24.3, P<.001) but not the 3-weeks before and immediately pre-CILT sessions (P>.05). For the MEG activation data, trends were analyzed in a similar manner with time point (3 weeks before CILT, immediately pre-CILT, post-CILT), region of interest (temporal, parietal, frontal), and hemisphere (left, right) as within-subjects factors. There was a significant time point by hemisphere interaction (F2,7=5.25, P<.04). Looking within the hemisphere, the only significant effects were in the left hemisphere, with a significant difference between the immediately pre-CILT and post-CILT scans (F1,8=8.26, P<.02) but not the 3-weeks before CILT and immediately pre-CILT sessions (P>.05). As can be seen in table 2, both performance on the Tx task and the degree of activation in the left hemisphere increased between the immediately pre-CILT and post-CILT sessions but not the 3-weeks before CILT and immediately pre-CILT sessions.
Table 2.
Percent Correct CIUs and Left Hemisphere MEG Activation for the Deferred Therapy Group
| 3 Weeks Before CILT |
Immediately Pre-CILT |
Immediately Post-CILT |
|
|---|---|---|---|
| Correct CIUs | 30.8±24.0 | 33.4±4.0 | 57.3±30.0* |
| Dipoles | .13±.22 | .14±.20.0 | .36±.30* |
NOTE. Values are mean percent ± SD.
Significant (P<.05) difference from pre-CILT levels.
The Relationship Between Scanner Performance and Comprehension and Group Membership
The relationship between performance on the scanning task and group membership was examined by using a repeated-measures multivariate analysis of variance as described earlier, with time point (3 weeks before CILT, immediately pre-CILT, post-CILT) as the within-subjects variable and group (responder, nonresponder, lost-response) as the between-subjects variable. There were no significant main or interaction effects of group (P>.12). Similarly, there were no group effects either within or across testing sessions for the WAB Comprehension Index (P>.70).
DISCUSSION
By including a 3-month follow-up assessment in the current study, we were able to identify 3 distinct behavioral responses to CILT. Immediately after therapy, approximately 50% of the patients exhibited a significant response to CILT; however, approximately one third fell out of the responder group at the 3-month follow-up assessment. Although this lost-response group was small and their findings require further confirmation in larger study samples, the members exhibited a distinct and consistent profile of activation that was relatively stable across all 3 MEG scanning sessions and was characterized by significantly greater activation in areas within the right hemisphere homotopic to putative premorbid language areas than the responder and nonresponder groups. Therefore, the hypothesis that improvement in language function after CILT would be associated with greater premorbid activation in the right hemisphere was only partially supported by the results of this study. The hypothesis that improvement in language function after CILT would be associated with an increase in right hemisphere activation was not supported. To the contrary, a stable positive response to therapy was associated with an increase in activation in left temporal areas after CILT.
Breier et al,9 using MEG, and Richter et al,10 using fMRI, both reported that positive response to aphasia therapy was associated with greater right hemisphere activation before therapy during language activation tasks. Both studies also reported no association between change in language function across therapy and change in activation in either hemisphere. Rather, it was the degree of pretherapy activity elicited in the scanner during a verbal task that correlated with change in language performance in response to therapy. The Breier et al41 study was relatively small (n = 5) and did not include a follow- up period. Although the Richter et al10 study, which reported essentially the same results as that of Breier et al,41 was larger (n = 16), a follow-up period to assess maintenance was not included either. With the inclusion of a follow-up assessment, we were able to show that pre-CILT activation in the right hemisphere predicted behavioral improvement immediately after therapy but not at follow-up. Rather, the patients with the greatest degree of pre-CILT activation in the right hemisphere lost their initial responder status at follow-up.
The role of the right hemisphere in recovery from aphasia secondary to stroke is still being debated. Some researchers42,43 suggest that the increase in right hemisphere activation observed after stroke may be related to transcallosal disinhibition and is not functional in nature because it does not always correlate with the level of recovery. Interference with language function, however, using transcranial magnetic stimulation applied to the right hemisphere44 or after right hemisphere anesthesia during the Wada procedure45 after stroke does suggest that right hemisphere activation may be functional in some patients. In addition, at least some prior studies1,8,46,47 report a correlation between an increase in right hemisphere activation after therapy for aphasia and a positive response to therapy. Very few imaging studies of patients who have undergone aphasia therapy have assessed maintenance over a longer period of time. By assessing maintenance of behavioral gains across a 3-month period in the current study, we were able to identify a group of patients who exhibited a significant loss of post-CILT gains and found that these patients were the most likely to have strong right hemisphere lateralization of neurophysiologic response during the performance of a linguistic task. These data suggest that although the right hemisphere may be able to support language gains during the chronic period after stroke, these gains may be short-lived and not necessarily stable.
Contrary to our hypothesis that improvement in language function after therapy would be associated with an increase in right hemisphere activation, the responder and nonresponder groups exhibited differing changes in neurophysiologic responses in both hemispheres after CILT. The responder group exhibited an increase in left temporal activation after therapy, whereas the nonresponder group exhibited an increase in left parietal activation and a decrease in left temporal activation. Left parietal areas may be activated in the nonresponder group to assume the function of damaged temporal areas, albeit less efficiently. In contrast, the responder group appears to be able to respond to therapy with an increase in left temporal activity, and this increase was associated with stable improvement in language function in response to CILT.
The groups formed on the basis of the Tx response measure were relatively well matched in terms of aphasia severity and type as well as age and years of education before CILT. In addition, there were no significant group differences either within or across language testing and scanning sessions in comprehension as measured by the WAB or scanner performance. There was a significantly higher percentage of women in the responder group. This study was not designed to assess sex differences in response to CILT; however, a greater degree of bilateral language representation in women, as has been suggested,48,49 would be consistent with these results. Although group differences in lesion volume did not reach significance, the lost-response group had larger lesions, a finding that is consistent with greater activation of the right hemisphere during the scanner task compared with the other groups.
The number of correct responses in the MEG recognition memory task was low in this study sample, although this was not unexpected because the patients were aphasic; however, even passive listening to spoken words has been shown to reliably activate language processing networks,50–53 and the great preponderance of activation was detected in temporoparietal and frontal areas of the left hemisphere known to be involved in language function and/or their homotopic counterparts in the right hemisphere. In addition, a subgroup of 9 patients underwent scanning and language testing 3 weeks before CILT in addition to the other time points, with no intervention in between the 3 weeks before and immediately before CILT sessions. Changes in behavioral and MEG activation were apparent only across the therapy session and not before therapy, suggesting that the MEG activation task was sensitive to the effects of therapy.
Study Limitations
Although CILT training has both receptive and expressive components, the training and the Tx outcome measure are focused on speech production. Speech production, however, cannot be directly imaged in the MEG scanner because the artifact produced by speech muscle movement renders the signal uninterpretable. Therefore, a covert task was chosen by necessity. Because the hypotheses tested in this study involved characterizing changes in the relative degree of engagement of the hemispheres during language function in response to therapy, we chose an MEG task that had been well validated for this specific purpose. Speech production as trained in the CILT involves lexical access, both on a phonologic and semantic basis. Both of these processes have been shown to involve posterior language areas also involved in speech comprehension, 54,55 and both the CILT and the scanner task involved processing of single words. Our findings are, of course, specific to the methodology used, and it would be important to repeat the design used in this study using a task that more directly imaged the neural representation of speech production. For example, the use of a receptive language task in the scanner likely accounts for the relatively reduced degree of activity in inferior frontal areas and may have had an effect on the degree of activity observed in inferior parietal areas as well. A task that produced a greater degree of activation in motor speech areas in parietal and frontal areas might produce different, possibly complimentary, results. This would be a matter for further research.
CONCLUSIONS
Although the current study sample was relatively large for studies of this type, the individual subgroups were relatively small. This is particularly true of the lost-response group. Although the findings for this group were quite consistent, corroboration of the findings in larger groups of patients is necessary. In addition, because of imaging limitations, the scanner and treatment tasks were not matched, and different tasks and task demands, in addition to different imaging modalities, might well produce different and potentially complimentary results. The current findings, however, do support suggestions that the efficacy of at least some interventions for chronic aphasia is related to the ability of perilesional areas to reorganize in support of language function,3,4,5,8 and that, although other areas in the dominant hemisphere may show increased activity after stroke, this activity may be associated with less efficient processing. Furthermore, current findings indicate that interventions similar to that used in the current study may produce less stable results in which language function appears to have reorganized predominantly to the right hemisphere after stroke. These findings also suggest a potential role for noninvasive functional imaging modalities such as fMRI and MEG in prescribing rehabilitative strategies for chronic aphasia. Further research using experimental designs similar to that described previously may eventually enable practitioners to identify patients that might benefit the most from a specific rehabilitative approach on the basis of patterns of brain activity obtained during functional imaging.
Acknowledgments
Supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke (grant no. P51-NS046588; Papanicolaou).
List of Abbreviations
- CILT
constraint-induced language therapy
- CIU
correct information unit
- CSF
cerebral spinal fluid
- fMRI
functional magnetic resonance imaging
- MEG
magnetoencephalography
- MRI
magnetic resonance imaging
- Tx
treatment
- WAB
Western Aphasia Battery
Footnotes
Presented in part at the Annual Meeting of the International Neuropsychological Society, February 11, 2009, Atlanta, GA.
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
Suppliers
4-D Neuroimaging, San Diego, CA 92121. (No longer available for commercial use.)
Software Library, University of Oxford, FMRIB Centre, University of Oxford, Dept of Clinical Neurology, John Radcliffe Hospital, Headington, Oxford OX3 9DU, UK.
References
- 1.Musso M, Weiller C, Kiebel S, Müller SP, Bülau P, Rijntjes M. Training-induced brain plasticity in aphasia. Brain. 1999;122(Pt 9):1781–1790. doi: 10.1093/brain/122.9.1781. [DOI] [PubMed] [Google Scholar]
- 2.Raboyeau G, De Boissezon X, Marie N, et al. Right hemisphere activation in recovery from aphasia: lesion effect or function recruitment? Neurology. 2008;70:290–298. doi: 10.1212/01.wnl.0000287115.85956.87. [DOI] [PubMed] [Google Scholar]
- 3.Belin P, Van Eeckhout P, Zilbovicius M, et al. Recovery from nonfluent aphasia after melodic intonation therapy: a PET study. Neurology. 1996;47:1504–1511. doi: 10.1212/wnl.47.6.1504. [DOI] [PubMed] [Google Scholar]
- 4.Leger A, Démonet JF, Ruff S, et al. Neural substrates of spoken language rehabilitation in an aphasic patient: an fMRI study. Neuroimage. 2002;17:174–183. doi: 10.1006/nimg.2002.1238. [DOI] [PubMed] [Google Scholar]
- 5.Meinzer M, Flaisch T, Breitenstein C, et al. Functional re-recruitment of dysfunctional brain areas predicts language recovery in chronic aphasia. Neuroimage. 2008;39:2038–2046. doi: 10.1016/j.neuroimage.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Fridriksson J, Morrow L. Cortical activation and language task difficulty in aphasia. Aphasiology. 2005;19:239–250. doi: 10.1080/02687030444000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulvermuller F, Hauk O, Zohsel K, et al. Therapy-related reorganization of language in both hemispheres of patients with chronic aphasia. Neuroimage. 2005;28:481–489. doi: 10.1016/j.neuroimage.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 8.Vitali P, Abutalebi J, Tettamanti M, et al. Training-induced brain remapping in chronic aphasia: a pilot study. Neurorehabil Neural Repair. 2007;21:152–160. doi: 10.1177/1545968306294735. [DOI] [PubMed] [Google Scholar]
- 9.Breier JI, Maher LM, Novak B. Papanicolaou AC. Functional imaging before and after constraint-induced language therapy for aphasia using magnetoencephalography. Neurocase. 2006;12:322–331. doi: 10.1080/13554790601126054. [DOI] [PubMed] [Google Scholar]
- 10.Richter M, Miltner WH, Straube T. Association between therapy outcome and right-hemispheric activation in chronic aphasia. Brain. 2008;131:1391–1401. doi: 10.1093/brain/awn043. [DOI] [PubMed] [Google Scholar]
- 11.Pulvermuller F, Neininger B, Elbert T, et al. Constraint-induced therapy of chronic aphasia after stroke. Stroke. 2001;32:1621–1626. doi: 10.1161/01.str.32.7.1621. [DOI] [PubMed] [Google Scholar]
- 12.Taub E, Uswatte G, Pidikiti R. Constraint-Induced movement therapy: a new family of techniques with broad application to physical rehabilitation—a clinical review. J Rehabil Res Dev. 1999;36:237–251. [PubMed] [Google Scholar]
- 13.Taub E. Harnessing brain plasticity through behavioral techniques to produce new treatments in neurorehabilitation. Am Psychol. 2004;59:692–704. doi: 10.1037/0003-066X.59.8.692. [DOI] [PubMed] [Google Scholar]
- 14.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 15.Kertesz A. Western Aphasia Battery. San Antonio: The Psychological Corp; 1982. [Google Scholar]
- 16.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lee & Febiger; 2000. [Google Scholar]
- 17.Breier JI, Simos PG, Zouridakis G, Papanicolaou AC. Relative timing of neuronal activity in distinct temporal lobe areas during a recognition memory task for words. J Clin Exp Neuropsychol. 1998;20:782–790. doi: 10.1076/jcen.20.6.782.1116. [DOI] [PubMed] [Google Scholar]
- 18.Breier JI, Simos PG, Zouridakis G, Papanicolaou AC. Lateralization of cerebral activation in auditory verbal and non-verbal memory tasks using magnetoencephalography. Brain Topogr. 1999;12:89–97. doi: 10.1023/a:1023458110869. [DOI] [PubMed] [Google Scholar]
- 19.Papanicolaou AC, Simos PG, Breier JI, et al. Magnetoencephalographic mapping of the language-specific cortex. J Neurosurg. 1999;90:85–93. doi: 10.3171/jns.1999.90.1.0085. [DOI] [PubMed] [Google Scholar]
- 20.Papanicolaou AC, Pazo-Alvarez P, Castillo EM, et al. Functional neuroimaging with MEG: normative language profiles. Neuroimage. 2006;33:326–342. doi: 10.1016/j.neuroimage.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Simos PG, Breier JI, Zouridakis G, et al. Identification of language-specific brain activity using magnetoencephalography. J Clin Exp Neuropsychol. 1998;20:706–722. doi: 10.1076/jcen.20.5.706.1127. [DOI] [PubMed] [Google Scholar]
- 22.Simos PG, Breier JI, Zouridakis G, et al. Assessment of functional cerebral laterality for language using magnetoencephalography. J Clin Neurophysiol. 1998;15:364–372. doi: 10.1097/00004691-199807000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Breier JI, Simos PG, Zouridakis G, Papanicolaou AC. Lateralization of activity associated with language function using magnetoencephalography: a reliability study. J Clin Neurophysiol. 2000;17:503–510. doi: 10.1097/00004691-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Simos PG, Papanicolaou AC, Breier JI, et al. Localization of language-specific cortex by using magnetic source imaging and electrical stimulation mapping. J Neurosurg. 1999;91:787–796. doi: 10.3171/jns.1999.91.5.0787. [DOI] [PubMed] [Google Scholar]
- 25.Breier JI, Simos PG, Zouridakis G, et al. Language dominance determined by magnetic source imaging: a comparison with the Wada procedure. Neurology. 1999;53:938–945. doi: 10.1212/wnl.53.5.938. [DOI] [PubMed] [Google Scholar]
- 26.Breier JI, Simos PG, Wheless JW, et al. Language dominance in children as determined by magnetic source imaging and the intra-carotid amobarbital procedure: a comparison. J Child Neurol. 2001;16:124–130. doi: 10.1177/088307380101600211. [DOI] [PubMed] [Google Scholar]
- 27.Papanicolaou AC, Simos PG, Castillo EM, et al. Magnetocephalography: a noninvasive alternative to the Wada procedure. J Neurosurg. 2004;100:867–876. doi: 10.3171/jns.2004.100.5.0867. [DOI] [PubMed] [Google Scholar]
- 28.Sarvas J. Basic mathematical and electromagnetic concepts of the biomagnetic inverse problem. Phys Med Biol. 1987;32:11–22. doi: 10.1088/0031-9155/32/1/004. [DOI] [PubMed] [Google Scholar]
- 29.Damasio H. Human brain anatomy in computerized images. New York, NY: Oxford University Press; 1995. [Google Scholar]
- 30.Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- 31.Benson RR, Whalen DH, Richardson M, et al. Parametrically dissociating speech and nonspeech perception in the brain using fMRI. Brain Lang. 2001;78:364–396. doi: 10.1006/brln.2001.2484. [DOI] [PubMed] [Google Scholar]
- 32.Binder JR. Neuroanatomy of language processing studied with functional MRI. Clin Neurosci. 1997;4:87–94. [PubMed] [Google Scholar]
- 33.Binder J. Functional magnetic resonance imaging. Language mapping. Neurosurg Clin N Am. 1997;8:383–392. [PubMed] [Google Scholar]
- 34.Binder JR, Frost JA, Hammeke TA, et al. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demonet JF, Chollet F, Ramsay S, et al. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992;115(Pt 6):1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- 36.Frith CD, Friston KJ, Liddle PF, Frackowiak RS. A PET study of word finding. Neuropsychologia. 1991;29:1137–1148. doi: 10.1016/0028-3932(91)90029-8. [DOI] [PubMed] [Google Scholar]
- 37.Poeppel D. A critical review of PET studies of phonological processing. Brain Lang. 1996;55:317–351. doi: 10.1006/brln.1996.0108. [DOI] [PubMed] [Google Scholar]
- 38.Price CJ, Wise RJ, Warburton EA, et al. Hearing and saying. The functional neuro-anatomy of auditory word processing. Brain. 1996;119(Pt 3):919–931. doi: 10.1093/brain/119.3.919. [DOI] [PubMed] [Google Scholar]
- 39.Sabri M, Binder JR, Desai R, et al. Attentional and linguistic interactions in speech perception. Neuroimage. 2008;39:1444–1456. doi: 10.1016/j.neuroimage.2007.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vannest JJ, Karunanayaka PR, Altaye M, et al. Comparison of fMRI data from passive listening and active-response story processing tasks in children. J Magn Reson Imaging. 2009;29:971–976. doi: 10.1002/jmri.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Breier JI, Maher LM, Schmadeke S, Hasan KM, Papanicolaou AC. Changes in language-specific brain activation after therapy for aphasia using magnetoencephalography: a case study. Neurocase. 2007;13:169–177. doi: 10.1080/13554790701448200. [DOI] [PubMed] [Google Scholar]
- 42.Blank SC, Bird H, Turkheimer F, et al. Speech production after stroke: the role of the right pars opercularis. Ann Neurol. 2003;54:310–320. doi: 10.1002/ana.10656. [DOI] [PubMed] [Google Scholar]
- 43.Rosen HJ, Petersen SE, Linenweber MR, et al. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55:1883–1894. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- 44.Winhuisen L, Thiel A, Schumacher B, et al. The right inferior frontal gyrus and poststroke aphasia: a follow-up investigation. Stroke. 2007;38:1286–1292. doi: 10.1161/01.STR.0000259632.04324.6c. [DOI] [PubMed] [Google Scholar]
- 45.Kinsbourne M. The minor cerebral hemisphere as a source of aphasic speech. Trans Am Neurol Assoc. 1971;96:141–145. [PubMed] [Google Scholar]
- 46.Crosson B, Moore AB, Gopinath K, et al. Role of the right and left hemispheres in recovery of function during treatment of intention in aphasia. J Cogn Neurosci. 2005;17:392–406. doi: 10.1162/0898929053279487. [DOI] [PubMed] [Google Scholar]
- 47.Peck KK, Moore AB, Crosson BA, et al. Functional magnetic resonance imaging before and after aphasia therapy: shifts in hemodynamic time to peak during an overt language task. Stroke. 2004;35:554–559. doi: 10.1161/01.STR.0000110983.50753.9D. [DOI] [PubMed] [Google Scholar]
- 48.Clements AM, Rimrodt SL, Abel JR, et al. Sex differences in cerebral laterality of language and visuospatial processing. Brain Lang. 2006;98:150–158. doi: 10.1016/j.bandl.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 49.Shaywitz BA, Shaywitz SE, Pugh KR, et al. Sex differences in the functional organization of the brain for language. Nature. 1995;373:607–609. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- 50.Fernandez-Espejo D, Junque C, Vendrell P, et al. Cerebral response to speech in vegetative and minimally conscious states after traumatic brain injury. Brain Inj. 2008;22:882–890. doi: 10.1080/02699050802403573. [DOI] [PubMed] [Google Scholar]
- 51.Londei A, D’Ausilio A, Basso D, et al. Brain network for passive word listening as evaluated with ICA and Granger causality. Brain Res Bull. 2007;72:284–292. doi: 10.1016/j.brainresbull.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 52.Smits M, Visch-Brink E, Schraa-Tam CK, et al. Functional MR imaging of language processing: an overview of easy-to-implement paradigms for patient care and clinical research. Radiographics. 2006;26 Suppl 1:S145–S158. doi: 10.1148/rg.26si065507. [DOI] [PubMed] [Google Scholar]
- 53.Wu J, Cai C, Kochiyama T, et al. Function segregation in the left inferior frontal gyrus: a listening functional magnetic resonance imaging study. Neuroreport. 2007;18:127–131. doi: 10.1097/WNR.0b013e328010a07e. [DOI] [PubMed] [Google Scholar]
- 54.Graves WW, Grabowski TJ, Mehta S, et al. The left posterior superior temporal gyrus participates specifically in accessing lexical phonology. J Cogn Neurosci. 2008;20:1698–1710. doi: 10.1162/jocn.2008.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Graves WW, Grabowski TJ, Mehta S, et al. A neural signature of phonological access: distinguishing the effects of word frequency from familiarity and length in overt picture naming. J Cogn Neurosci. 2007;19:617–631. doi: 10.1162/jocn.2007.19.4.617. [DOI] [PubMed] [Google Scholar]