Abstract
Purpose:
The aim of this study is to examine the epidemiologic and economic burden in surgically resected localized gastrointestinal stromal tumor (GIST) patients versus age- and gender-matched controls.
Method:
Two data sources were used to conduct a series of complementary analyses. First, the Surveillance, Epidemiology, and End Results (SEER) cancer registry was used to identify diagnosed GIST patients from 1993 to 2002 and determine incidence, prevalence, and 3-year survival. Second, using the SEER–Medicare linked database, a matched case-control analysis was conducted to determine resource utilization, GIST recurrence, and costs. Because GIST recurrence is not explicitly defined in the database, patterns in resource use were used to identify probable recurrence. Kaplan–Meier Sample Average (KMSA) Estimator technique was used to estimate costs of GIST and recurrence.
Results:
SEER registry results show over the 10-year time horizon average annual GIST incidence was 0.32 per 100,000 persons in the United States, 15-year limited-duration prevalence was 1.62 per 100,000 persons, and 3-year survival was 73%. A total of 292 GIST patients were included in the SEER–Medicare analyses; 35 were identified with probable recurrence. GIST patients had increased risk of mortality (hazard ratio: 1.23; 95% confidence intervals: 0.94–1.61) compared to controls. Median recurrence-free and postrecurrence survival was 45 and 46 months, respectively. GIST patients incurred significantly higher medical care costs in the first year after initial resection, with $23,221 attributable to GIST. GIST recurrence costs totaled $101,700 over 5 years after initial resection.
Conclusions:
GIST is associated with substantial medical care costs, estimated recurrence costs more than $100,000; treatments that delay or reduce recurrence could substantially reduce the burden of GIST.
Keywords: GIST, burden of illness, cancer
Introduction
Gastrointestinal stromal tumors (GIST) grow in the walls of the gastrointestinal tract, occurring most often in the stomach, although the small intestine and colon are also common sites.1 The incidence of GIST in the United States is estimated to be 3000–4000 cases per year,2 with the age of majority of the diagnosed patients being in the range of 40–80 and a median age of 60 years.3
Localized GIST has historically been treated with a combination of surgery and chemotherapy; however, because chemotherapy has since proven ineffective in the treatment of GIST, surgical resection is now the primary treatment for resectable tumors.4 Although surgical resection is currently the standard of care,5 complete gross resection of localized GIST is associated with a 5-year survival of 48%–65%.6–8 Poor survival is largely associated with high recurrence rates, which are >40% within the first 2 years following surgical resection.7 Several prognostic factors have been associated with risk of recurrence, including tumor size, location, and mitotic index.9–11 Few studies have specifically examined the burden of illness for surgically resected localized GIST. With the advent of new targeted therapy used in the prevention of GIST recurrence, it is important to understand the burden of this disease to quantify the potential benefits of these therapies. Therefore, we conducted a retrospective database analysis to examine resource utilization, recurrence, survival, and costs in surgically resected localized GIST patients.
Methods
A retrospective database analysis was conducted using data from the Surveillance, Epidemiology and End Results (SEER registry of the National Cancer Institute and the SEER–Medicare linked database to assess the epidemiologic and economic burden of surgically resected localized GIST. The SEER registry was used to estimate incidence, prevalence, and 3-year survival; the SEER–Medicare linked data were used to estimate survival, recurrence-free survival (RFS), resource utilization, and medical care costs.
SEER registry analyses
The SEER registry is a surveillance system that collects information on all newly diagnosed cancer cases in specified geographic areas. SEER Stat software (National Cancer Institute, Bethesda, MD) estimates incidence, prevalence, and survival directly by standardizing estimates to the US population and was used for analyses.
Sample selection
Patients included all those diagnosed with GIST between 1993 and 2002. Because the specific histology code identifying GIST (ICD-O-3 8936/3) was not introduced until 2001, an algorithm previously validated by Tran and colleagues12 along with the GIST histology code, where available, was used to identify GIST cases prior to 2001. Patients were categorized as having localized and resected GIST based on SEER Stat variables that identify cancer stage and surgery of the primary tumor site.
Study measures
Incidence was calculated as the number of new GIST cases per 100,000 population stratified by age group, gender, race, and tumor size. Cancer prevalence was defined as the number of individuals currently alive who have been diagnosed with cancer at any point in time, irrespective of time since diagnosis, current treatment, or cure. Limited-duration prevalence represents the proportion of people alive on a certain day who had a diagnosis of the disease within a specified time frame. According to SEER, registries of shorter duration, <40 or 50 years of data collection, can only estimate limited-duration prevalence. This analysis estimates 15-year limited-duration prevalence, which was calculated based on the number of patients diagnosed with GIST and alive on the first day of the year during the 15 years prior to any given year in the present study (ie, 1993–2002). Three-year survival was defined as the proportion of patients alive 3 years after a GIST diagnosis.
SEER–Medicare analyses
The SEER–Medicare database
The SEER–Medicare database is a collaborative effort of the National Cancer Institute, the SEER registries, and the Centers for Medicare and Medicaid Services. SEER–Medicare data are considered to be a limited-identifiable data set by the Health Insurance Portability and Accountability Act guidelines. Consequently, institutional review board approval was not required to obtain access to the data.
SEER–Medicare claims were used to identify resource utilization, survival, and differences in survival by tumor size and recurrence among GIST patients. In addition, medical care costs in the first year after surgical resection of the primary GIST diagnosis (initial resection) as well as the 5-year cumulative costs postinitial resection were estimated using the SEER–Medicare data. In addition, algorithms were used to identify an increased level of health care activity that could signify recurrence, allowing us to infer recurrence-free and postrecurrence survival (PRS), and recurrence costs.
Patient selection
Localized GIST cases were selected using the same diagnostic algorithm described in the SEER registry section. Patients were further limited to those aged above 65 years with continuous Medicare enrollment from 1 year prior to diagnosis through the end of the study period or death, whichever is earlier.
The SEER data used to identify surgical resection do have a corresponding date of surgery included in the database. Therefore, to obtain the date of surgical resection, we used the SEER–Medicare database to identify the surgical procedure and assign a date of service. Surgical resection was identified as an ICD-9 or CPT surgery procedure code (Table 1) appearing in the inpatient or outpatient records or a HCPCS surgery procedure code appearing in the inpatient records, from 30 days before to 90 days after the GIST diagnosis date. The surgical resection date was set equal to the procedure date where available. However, if the SEER variable indicated surgical resection and there was no corresponding procedure code present, then the date of resection was set equal to the GIST diagnosis date. The date of surgical resection was defined as the ‘index date’.
Table 1.
Administrative codes used to identify surgical resection of GIST
| Surgery site | Administrative codes (CPT, ICD-9, HCPCS) |
|---|---|
| Esophagus | CPT: 43100–43101, 43105, 43106, 43115, 43116, 43122, 43124, 34360, 43216–43217 |
| ICD-9: 42.3, 42.32, 42.33, 42.25, 42.41 | |
| Stomach | CPT: 43610–43611, 43250–43251, 43258 |
| ICD-9: 43.4, 43.41, 43.42, 44.15 | |
| Small intestine | CPT: 44110–44111, 44120–44121, 44125, 44130, 44202–44203, 44364–44365, 44369 |
| ICD-9: 45.3, 45.33, 45.6, 45.90–45.93, 45.95, 45.62, 45.6, 45.30, 45.31, 45.15 | |
| Colon, rectum, and anus | CPT: 44392–44394, 45308–45309, 45315, 45383–45385, 45160, 45170, 44140–44147, 44160, 44204–44208, 45113–45116, 45123, 45180, 45310, 45336, 45338, 45339, 46610, 46611, 46612 |
| ICD-9: 48.35, 48.3, 48.5, 45.41, 45.42, 45.94, 45.95, | |
| Peritoneum, omentum, and mesentery | CPT: 49200, 49201 |
| ICD-9: 54.4, 54.3, 54.22, 54.23 | |
| Any site | HCPCS: S2095, D7440–D7441, D7450–D7451, D7460–D7461, D7465, D7413–D7415 |
Abbreviations CPT, current procedure terminology; ICD, international classification of diseases; HCPCS, healthcare common procedure coding system.
Patients were excluded if they met any of the following criteria: any other cancer diagnosis prior to their index date, not eligible for Medicare Part A or B benefits starting 12 months before GIST diagnosis through follow-up, enrolled in health maintenance organization starting 12 months before GIST diagnosis through follow-up, Medicare eligibility based on end-stage renal disease or disability, date of death unknown or disparate by 3 months or more between SEER and Medicare, or first GIST diagnosis at time of death or autopsy.
An age- and gender-matched control cohort of cancer-free patients was selected from Medicare enrollment files using the 5% sample of Medicare beneficiaries residing in SEER geographic areas. Control patients were not required to have used services and were not excluded if they developed non-GIST cancers after their index date. Control patients were excluded if they met any of the applicable exclusions for cases. Control patients were matched 1:1 with GIST patients on age and gender and assigned the same index date. After applying the study inclusion/exclusion criteria, the final GIST cohort was limited to those with localized disease.
Study measures
Gender and race were derived directly from SEER–Medicare, and race was categorized as white, black, other, and unknown (if values are missing). An adapted version of the Charlson comorbidity index13,14 was calculated using preindex date patient records, and chronic malignancy was excluded as a comorbidity. Length of Medicare follow-up (in months) was calculated by dividing the number of days between index and end dates by 30.25. The end date was the date of death or the end of Medicare claims (ie, December 31, 2005). Resource utilization per person-year was calculated by dividing the total resource use between index and end dates by total follow-up time in years. Number of hospital days reflected the number of days between admission and discharge, with both the admission and discharge dates inclusive and excluded hospice institutions and home health agencies.
For significance testing, the Student’s t-test was used for continuous variables and the χ2 test with categorical variables. An alpha level of 0.05 was used for all tests. All analyses were conducted using SAS version 9.1 (SAS Institute Inc, Cary, NC).
Cancer recurrence is not explicitly identified in the SEER–Medicare database; therefore, we constructed an algorithm which looks for additional surgical resections or other surgical procedures or gaps in chemotherapy treatment as a proxy for recurrence. Patients that satisfied at least one of the following criteria were considered to have a recurrence: surgical resection at least 90 days after the index date, radiofrequency ablation (CPT 43258, ICD-9 44.44) or embolization (CPT 61624, ICD-9 45.43, 42.33) at least 90 days after the index date, initial indication of chemotherapy treatment at least 90 days after the index date, or a gap in chemotherapy treatment of at least 90 days.
Median survival, RFS, and PRS were assessed using Kaplan–Meier analyses; differences between cases and controls were assessed using Cox proportional hazards models controlling for age, gender, income, and urban area. Survival was estimated from the index date until death. RFS was estimated from the index date until recurrence or death, and PRS was estimated from the date of recurrence until death. Patients with follow-up data ending before recurrence or death were censored, and the number of months spent alive was calculated as the difference between their last date of follow-up and the index date divided by 30.25. Differences in survival and RFS stratified by tumor size were also assessed using Cox proportional hazards models controlling for the same demographic characteristics as in the case-control analysis.
Health care costs in the first year after initial resection and 5-year cumulative costs were calculated using the KMSA estimator,15 a technique that accounts for variable follow-up due to death or censoring, and thereby provides an unbiased estimate of the average costs. Data were partitioned into 30-day intervals, where each month’s average cost was multiplied by the probability of surviving to the start of the month; the survival-adjusted costs were then summed to produce the total expected costs for the first year and over the 5-year period following initial resection. The total GIST-attributable costs were estimated as the difference in total costs between cases and controls. Costs attributable to GIST recurrence were estimated as the difference between costs among GIST patients with a recurrence (cases) and those GIST patients without a recurrence (controls).
Costs were estimated for each resource utilization category and reported overall and by tumor size. Bootstrapping methods (sampling with replacement) were used to obtain 95% credible intervals (95% confidence intervals [CI]) for the KMSA analyses.
Fewer recurrences were identified in our analyses (12% over the entire study period) compared with published recurrence rates (32%–40%).7,16 Therefore, we performed one-way sensitivity analyses using different algorithms to identify recurrence. First, we shortened the time interval between initial surgical resection and detection of a recurrence from 90 to 60 days using the original surgical procedures to identify recurrence. Second, keeping the days between the index date and identification of a recurrence fixed at 90 days, we added the following: 1) additional procedure/HCPCS codes for biopsy (32405), excision (11642, 22900, 49201), radiation (77413, 77300), chemotherapy (96408, 96410–96412, 96549, Q0085), and other surgical procedures (88172, 88305, 43235, 43239); 2) a surgical procedure coupled with an ICD-9 cancer diagnosis (140–239 excluding benign tumors) on the same date; and 3) identification of inpatient hospitalization with an ICD-9 cancer diagnosis (140–239 excluding benign tumors).
Results
SEER registry results
Incidence, prevalence, and 3-year overall survival are displayed in Table 2. Between 1993 and 2002, 1466 new cases of surgically resected localized GIST were identified in the SEER database. The average annual incidence was 0.32 per 100,000, and the average annual 15-year prevalence was 1.62 per 100,000. The average 3-year survival for GIST cases that were diagnosed between 1993 and 2002 was 73%.
Table 2.
Incidence, prevalence and 3-year survival for GIST patients from the SEER registry
| Incidence/100,000 | Prevalence/100,000 | 3-year survival | |
|---|---|---|---|
| Overall | 0.32 | 1.62 | 73% |
| Age (years): | |||
| <5 | 0.06 | 0.24 | 86% |
| 05–14 | 0.01 | 0.28 | 100% |
| 15–24 | 0.03 | 0.17 | 81% |
| 25–34 | 0.06 | 0.33 | 83% |
| 35–44 | 0.18 | 0.94 | 86% |
| 45–54 | 0.40 | 2.05 | 84% |
| 55–64 | 0.80 | 3.87 | 70% |
| 65–74 | 1.10 | 5.38 | 72% |
| 75–84 | 1.47 | 7.81 | 61% |
| 85+ | 0.94 | 7.50 | 49% |
| Sex: | |||
| Male | 0.34 | 1.44 | 70% |
| Female | 0.31 | 1.80 | 75% |
| Race: | |||
| White | 0.29 | 1.59 | 73% |
| Black | 0.50 | 1.45 | 65% |
| Other | 0.43 | 2.01 | 76% |
| Hispanic ethnicity: | |||
| Non-Spanish-Hispanic-Latino | 0.33 | N/A | 72% |
| Spanish-Hispanic-Latino | 0.29 | N/A | 75% |
| Tumor size (cm): | |||
| 0–<3 | 0.02 | 0.10 | 76% |
| 3–<6 | 0.07 | 0.32 | 77% |
| 6–10 | 0.10 | 0.41 | 77% |
| >10 | 0.10 | 0.38 | 66% |
| Unknown | 0.02 | 0.11 | 67% |
Note: GIST identification based on Tran algorithm or the ICD-O-3 GIST code (8936). Prevalence was not available for those of Hispanic origin.
SEER–Medicare results
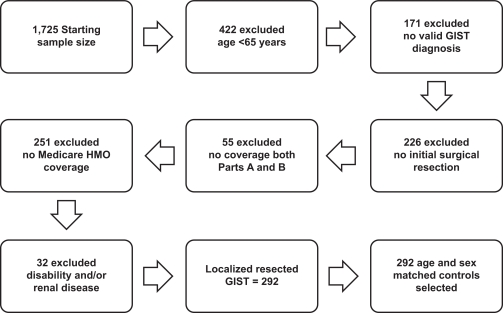
From an initial sample of 1725 GIST patients, we identified 292 patients newly diagnosed with GIST between 1993 and 2002 who met all of the inclusion criteria (Figure 1). The final sample consisted of these 292 GIST patients with an equal number of age- and gender-matched controls; 35 GIST cases experienced a GIST recurrence during the 10-year follow-up. Out of the 292 GIST patients, 15 presented with tumors <3 cm, 73 with tumors 3 to <6 cm, 86 with tumors 6 to <10 cm, 99 with tumors ≥10 cm, and the remaining 19 patients had unknown tumor size.
Figure 1.
SEER–Medicare analysis sample selection.
Table 3 presents the patient characteristics for the GIST cohort and their matched controls. There were no statistically significant differences found for age, gender, geographic region, location of residence, or median income, but there were significantly more white controls than cases. The Charlson comorbidity index score was significantly higher for cases than controls, and significantly longer Medicare follow-up was seen for controls than cases.
Table 3.
Overall patient characteristics and demographics for GIST patients and their matched controls in SEER-Medicine
| Characteristic | All GIST patients 1993–2002 | Matched controls |
Difference (cases-controls) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P-value* | ||||||||||
| Number of patients | 292 | 292 | 0 | N/A | ||||||
| Age as of initial GIST diagnosis: | ||||||||||
| Mean (SD) | 76 | (6.4) | N/A | N/A | N/A | N/A | ||||
| Median | 75 | N/A | N/A | N/A | ||||||
| Interquartile range | 65 | 91 | N/A | N/A | N/A | N/A | ||||
| Age as of initial GIST diagnosis (n[%]): | ||||||||||
| 65–74 years | 130 | (44.5) | N/A | N/A | N/A | N/A | ||||
| 75–84 years | 133 | (45.6) | N/A | N/A | N/A | N/A | N/A | |||
| 85+ years | 29 | (9.9) | N/A | N/A | N/A | N/A | ||||
| Male (n, %) | 125 | (42.8) | N/A | N/A | N/A | N/A | N/A | |||
| Race (n, %): | ||||||||||
| Caucasian | 215 | (73.6) | 258 | (88.4) | −43 | −(14.7) | ||||
| Black | 33 | (11.3) | 19 | (6.5) | 14 | (4.8) | <0001 | |||
| Other | 44 | (15.1) | 15 | (5.1) | 29 | (9.9) | ||||
| Charlson comorbidity index: | ||||||||||
| Mean (SD) | 1.6 | (2.0) | 1.2 | (2.0) | 0.4 | (0.0) | 0.037 | |||
| Median | 1.0 | 1.0 | 0.0 | |||||||
| Interquartile range | 0.0 | 11.0 | 0.0 | 10.0 | 0.0 | 1.0 | ||||
| Geographic region (n, %): | ||||||||||
| Northeast | 42 | (14.4) | 44 | (15.1) | −2 | −(0.7) | ||||
| Midwest | 74 | (25.3) | 82 | (28.1) | −8 | −(2.7) | 0.8115 | |||
| South | 23 | (7.9) | 19 | (6.5) | 4 | (1.4) | ||||
| West | 153 | (52.4) | 147 | (50.3) | 6 | (2.1) | ||||
| Median household income by zip code (n, %): | ||||||||||
| Less than $39,999 | 122 | (42.0) | 119 | (41.0) | 3 | (1.0) | ||||
| $40,000–$79,999 | 153 | (52.0) | 148 | (51.0) | 5 | (1.0) | 0.439 | |||
| Greater or equal to $80,000 | 17 | (6.0) | 25 | (8.0) | −8 | (−2) | ||||
| Length of Medicare follow-up (months): | ||||||||||
| Mean (SD) | 33.3 | (29.8) | 39.2 | (32.0) | −5.9 | −(2.3) | 0.022 | |||
| Median | 25.2 | 30.6 | −5.4 | |||||||
| Interquartile range | 0.1 | 119.7 | 0.3 | 119.7 | −0.2 | 0.0 | ||||
GIST cases experienced significantly more hospital stays and hospital days than their matched controls. Mean annual hospitalization for cases was 1.6 and 0.7 for controls (P < 0.001); mean number of days were 13.8 for cases and 6.0 for controls (P < 0.001).
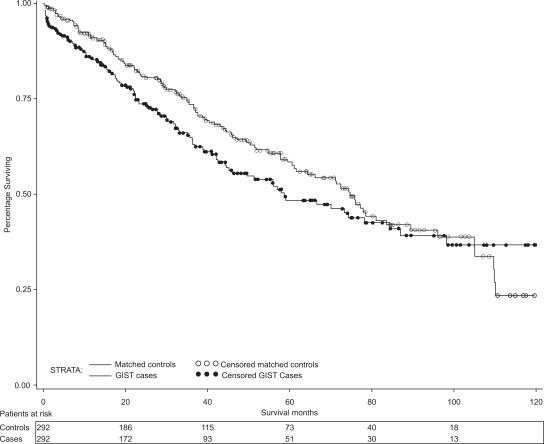
Figure 2 displays the Kaplan–Meier survival curves for GIST cases and their matched controls. Median overall survival for cases was 58.7 months and 74.7 months for controls. When controlling for age, gender, income, and urban area, the mortality hazard ratio for cases relative to controls is 1.23 (95% CI: 0.94–1.61). Median survival for patients with tumors 3 to <6 cm was 87.6 months, 6 to <10 cm was 74 months, ≥10 cm was 50 months, and unknown tumor size was 42.8 months. The median survival could not be estimated for tumor sizes <3 cm due to small numbers of events. When controlling for age, gender, income, and urban area, survival did not significantly differ by tumor size (P = 0.117).
Figure 2.
Overall survival for GIST patients and their matched controls.
The median RFS for GIST cases was 44.9 months. When stratifying by tumor size, RFS was lowest among patients with tumors <3 cm (32.3 months) and tumors ≥10 cm (35.7 months) and higher for patients with tumors 3 to <6 cm (87 months) and tumors 6 and <10 cm (46.5 months) (Table 4). Controlling for age, gender, income, and urban area, RFS did not significantly differ by tumor size for tumors >3 cm (P = 0.08). Median PRS was estimated at 46.3 months for all tumor size; for those with tumors 3 to <6 cm, it was 11.8 months, and for those with tumors ≥10 cm, it was 35.4 months.
Table 4.
Recurrence-free survival estimates
| Characteristic |
Tumor size |
||||
|---|---|---|---|---|---|
| 0−<3 cm | 3−<6 cm | 6−<10 cm | ≥10 cm | unknown | |
| Number of patients | 15 | 73 | 86 | 99 | 19 |
| Number of patients with recurrence | 1 | 6 | 11 | 14 | 3 |
| Recurrence-free survival (months) | |||||
| Median | 32.3 | 87.0 | 46.5 | 35.7 | 17.6 |
| Interquartile range (25–75) | (18.6–N/A) | (25.6–N/A) | (27.4–N/A) | (13.8–72.2) | (3.9–62.7) |
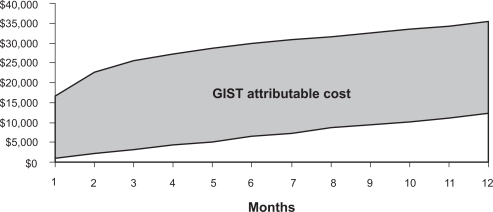
Total costs in the first year after initial resection were ∼$23,200 (95% CI: $16,600–$30,600) higher for cases than controls ($35,500 vs $12,300) (Figure 3). Costs by resource utilization category are presented in Table 5. GIST-related hospital costs accounted for the majority of the total costs and were estimated to be $16,900 (95% CI: $13,500–$20,500); outpatient costs were $4250 (95% CI: $55.9–$9580). When stratifying by tumor size, patients with tumors 3 to <6 cm had the largest GIST-attributable costs ($28,200) compared to those with tumors <3 cm ($2650) or 6 to <10 cm ($21,800) or ≥10 cm ($23,900). Cost differences between cases and controls were significant for all tumor groups, except those with tumors <3 cm ($2650, 95% CI: $19,800–$28,300). Finally, GIST-related hospital costs ranged from $6630 (95% CI: $6590–$24,100) for patients with tumors <3 cm to $18,100 (95% CI: $9890–$25,500) for patients with tumors 3 to <6 cm.
Figure 3.
GIST attributable cost first-year after initial resection.
Table 5.
First-year medical costs by resource utilization category for GIST patients and their matched controls in SEER-Medicare
| GIST cases | Matched controls | Difference (cases-controls) | |
|---|---|---|---|
| Hospital | $22,042 | $5,173 | $16,900 |
| Outpatient | $8,369 | $4,121 | $4,200 |
| Hospice | $66 | $367 | −$300 |
| Home health | $1,265 | $611 | $700 |
| Skilled nursing facility | $1,426 | $246 | $1,200 |
| Physician | $1,804 | $1,488 | $300 |
| Durable medical equipment | $506 | $251 | $300 |
The 5-year cumulative costs were ∼$24,800 (95% CI: $3140–$55,200) higher for cases than controls ($98,900 vs $74,100). GIST-related hospital costs were estimated to be $14,100 (95% CI: $4930–$23,200), and outpatient costs were $12,700 (95% CI: $8390–$37,400). When stratifying by tumor size, the 5-year cumulative costs are significantly higher for individuals with tumors between 3 and 6 cm compared to their controls ($107,500, 95% CI: $4790–$251,500). GIST-related hospital costs ranged from $10,200 (95% CI: $30,500–$12,700) for patients with tumors <3 cm to $27,400 (95% CI: $11,200–$48,600) for patients with tumors 3 to <6 cm.
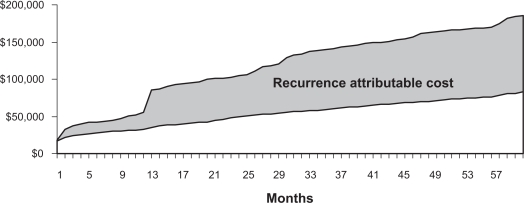
Five-year cumulative costs for patients without a recurrence were estimated to be $83,400; those with a recurrence incurred costs of $185,100. These results indicate that GIST recurrence adds an additional $101,700 (95% CI: $8840–$219,200) to the cost of care over the 5-year period after initial surgical resection (Figure 4).
Figure 4.
Cumulative recurrence attributable cost over five years.
Sensitivity analyses
The first sensitivity analysis, shortening the gap days between services to 60 days, identified 10 additional GIST cases with recurrence (45); when these patients were included in the analysis, median PRS increased to 70.2 months and costs attributable to recurrence over the 5-year period after surgical resection also increased. The 5-year recurrence-attributable cumulative costs were $161,700 (95% CI: $66,100–$276,300). The second sensitivity analysis (with additional procedure codes) identified 104 additional GIST cases with recurrence (139). With the inclusion of these additional patients in the analyses, median PRS decreased to 28 months, and 5-year recurrence costs also decreased. Cumulative 5-year costs attributable to recurrence were $89,100 (95% CI: $51,100–$131,200) over the 5-year period after initial surgical resection in this analysis.
Discussion
A retrospective database analysis was conducted to examine the epidemiologic and economic burden of GIST. The results showed that localized resected GIST affects ∼1000 people each year, and the burden of GIST recurrence is a major driver of the economic impact of disease. GIST-attributable costs in the first year after surgical resection were estimated to be $23,300. The 5-year GIST-attributable cumulative costs were estimated to be $24,800. These results indicate that the majority of GIST-attributable costs are accrued in the first year of disease. In addition, $16,900 (73%) of the GIST-attributable costs in the first year were related to the hospital, indicating that the majority of costs in the first year are likely related to surgical resection as treatment for the disease. The 5-year cumulative cost for GIST patients with a recurrence was estimated to be almost twice as much as the cost for GIST patients without a recurrence ($185,100 vs $83,400), respectively.
We were not able to identify any other published studies that examine costs accrued by patients with GIST after initial surgical resection. However, similar methodology was used in another study assessing costs of recurrence in breast cancer patients. Stokes et al17 used SEER–Medicare data to estimate breast cancer recurrence costs and survival. Their study reported substantial increases in costs due to breast cancer recurrence. To our knowledge, our study is the first study to examine GIST-attributable costs and costs related to GIST recurrence. The results of this study may be useful to individuals conducting economic assessments of GIST treatments.
Any interpretations of the findings from the present study are subject to several limitations. The SEER registry data represent about 30% of the US population; therefore, results may not be representative of all GIST patients. Although a validated algorithm was used to identify GIST patients before 2001, grouping stromal tumors may inadvertently include diseases other than GIST.18 Also certain groups are under or overrepresented in SEER data (eg, black and ‘other’ races).19
The Medicare database does not directly identify a cancer recurrence. We used an algorithm that looks for additional surgical resections, procedures, or gaps in chemotherapy treatment as a proxy for recurrence. We performed sensitivity analyses around identification of recurrence; however, the sensitivity and specificity of the algorithm to appropriately identify recurrences is unknown. Published literature suggests that ∼40% of patients with localized disease experience a recurrence,7 whereas only 12% of GIST patients in our study were identified as having a recurrence, suggesting that our main algorithm may have underestimated GIST recurrence. In our two sensitivity analyses, the recurrence rates were 15% and 48%.
Further limitations result from using Medicare claims data to estimate utilization, costs, and recurrence because the population is restricted to patients aged 65 years and older. Consequently, our results may not be applicable to the entire localized GIST population whose mean age is 63 years.12 We acknowledge that because higher medical costs are accrued by older than younger patients (ie, ≥65 vs <65 years old), cost differences between cases and controls would be expected to be greater for patients <65 years old. Using data restricted to persons ≥65 years of age may also impact our estimates of survival. Because our population is elderly, a large portion of the sample is likely dying of causes other than GIST; this may complicate the interpretation of survival differences between cases and controls, especially when analyzing survival over a long period of time (5 years). In addition, SEER–Medicare data do not include some healthcare services, such as oral prescription medications, which could pose another limitation, except that oral medications were not used in the treatment of localized GIST during this time period and would likely not affect our findings. Finally, costs were evaluated from a limited perspective (ie, Medicare-covered patients only), although because Medicare is a primary payer for older patients, we expect that the bulk of costs were captured in our analysis.
Our findings provide an increased understanding of the burden of localized GIST and suggest that recurrence contributes substantially to the economic impact of GIST. In light of these results, delaying or preventing recurrence should be a priority in treating GIST patients. Presently, the only known way to delay or prevent GIST recurrence after surgical resection is the use of a tyrosine kinase inhibitor. Imatinib (Gleevec/Glivec; Novartis, Basel, Switzerland), a tyrosine kinase inhibitor that targets the KIT protein, is currently approved by the US Food and Drug Administration as adjuvant therapy in surgically resected Kit (CD117)-positive GIST patients. Our analysis may be under or overestimating the burden of the disease given the current use of imatinib as adjuvant treatment. The economic impact of such treatment is yet to be determined.
Acknowledgments
This research was supported by Novartis Pharmaceuticals. We thank Virginia M Rosen, PhD, from the i3 Innovus Clinical Services Group for her assistance in the writing of this manuscript.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.D’Amato G, Steinert DM, McAuliffe JC, Trent JC. Update on the biology and therapy of gastrointestinal stromal tumors. Cancer Control. 2005;12(1):44–56. doi: 10.1177/107327480501200106. [DOI] [PubMed] [Google Scholar]
- 2.Gold JS, DeMatteo RP. Combined surgical and molecular therapy: the gastrointestinal stromal tumor model. Ann Surg. 2006;244(2):176–184. doi: 10.1097/01.sla.0000218080.94145.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van der Zwan SM, DeMatteo RP. Gastrointestinal stromal tumor: 5 years later. Cancer. 2005;104(9):1781–1788. doi: 10.1002/cncr.21419. [DOI] [PubMed] [Google Scholar]
- 4.Demetri GD. Identification and treatment of chemoresistant inoperable or metastatic GIST: experience with the selective tyrosine kinase inhibitor imatinib mesylate (STI571) Eur J Cancer. 2002;38(Suppl 5):S52–S59. doi: 10.1016/s0959-8049(02)80603-7. [DOI] [PubMed] [Google Scholar]
- 5.NCCN.org [homepage on the Internet] The NCCN Clinical Practice Guidelines in Oncology™ Soft Tissue Carcinoma (Version I.2009) ©2009 National Comprehensive Cancer Network, Inc. Available from: http://www.NCCN.org. Accessed May 5, 2009.
- 6.Ng EH, Pollock RE, Munsell MF, Atkinson EN, Romsdahl MM. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg. 1992;215(1):68–77. doi: 10.1097/00000658-199201000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231(1):51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiu MH, Farr GH, Papachristou DN, Hajdu SI. Myosarcomas of the stomach: natural history, prognostic factors and management. Cancer. 1982;49(1):177–187. doi: 10.1002/1097-0142(19820101)49:1<177::aid-cncr2820490136>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 9.DeMatteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST) Cancer. 2008;112(3):608–615. doi: 10.1002/cncr.23199. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson B, Bumming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era–a population-based study in western Sweden. Cancer. 2005;103(4):821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 11.Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130(10):1466–1478. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 12.Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol. 2005;100(1):162–168. doi: 10.1111/j.1572-0241.2005.40709.x. [DOI] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 15.Lin DY, Feuer EJ, Etzioni R, Wax Y. Estimating medical costs from incomplete follow-up data. Biometrics. 1997;53(2):419–434. [PubMed] [Google Scholar]
- 16.Martin J, Poveda A, Llombart-Bosch A, et al. Spanish Group for Sarcoma Research. Deletions affecting codons 557–558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: a study by the Spanish Group for Sarcoma Research (GEIS) J Clin Oncol. 2005;23(25):6190–6198. doi: 10.1200/JCO.2005.19.554. [DOI] [PubMed] [Google Scholar]
- 17.Stokes M, Thompson D, Montoya E, Weinstein MC, Winer EP, Earle CC. Ten-year survival and cost following breast cancer recurrence: estimates from SEER-medicare data. Value Health. 2008;11(2):213–220. doi: 10.1111/j.1524-4733.2007.00226.x. [DOI] [PubMed] [Google Scholar]
- 18.Howe JR, Karnell LH, Scott-Conner C. Small bowel sarcoma: analysis of survival from the National Cancer Data Base. Ann Surg Oncol. 2001;8(6):496–508. doi: 10.1007/s10434-001-0496-4. [DOI] [PubMed] [Google Scholar]
- 19.Frey CM, McMillen MM, Cowan CD, Horm JW, Kessler LG. Representativeness of the surveillance, epidemiology, and end results program data: recent trends in cancer mortality rates. J Natl Cancer Inst. 1992;84(11):872–877. doi: 10.1093/jnci/84.11.872. [DOI] [PubMed] [Google Scholar]