Abstract
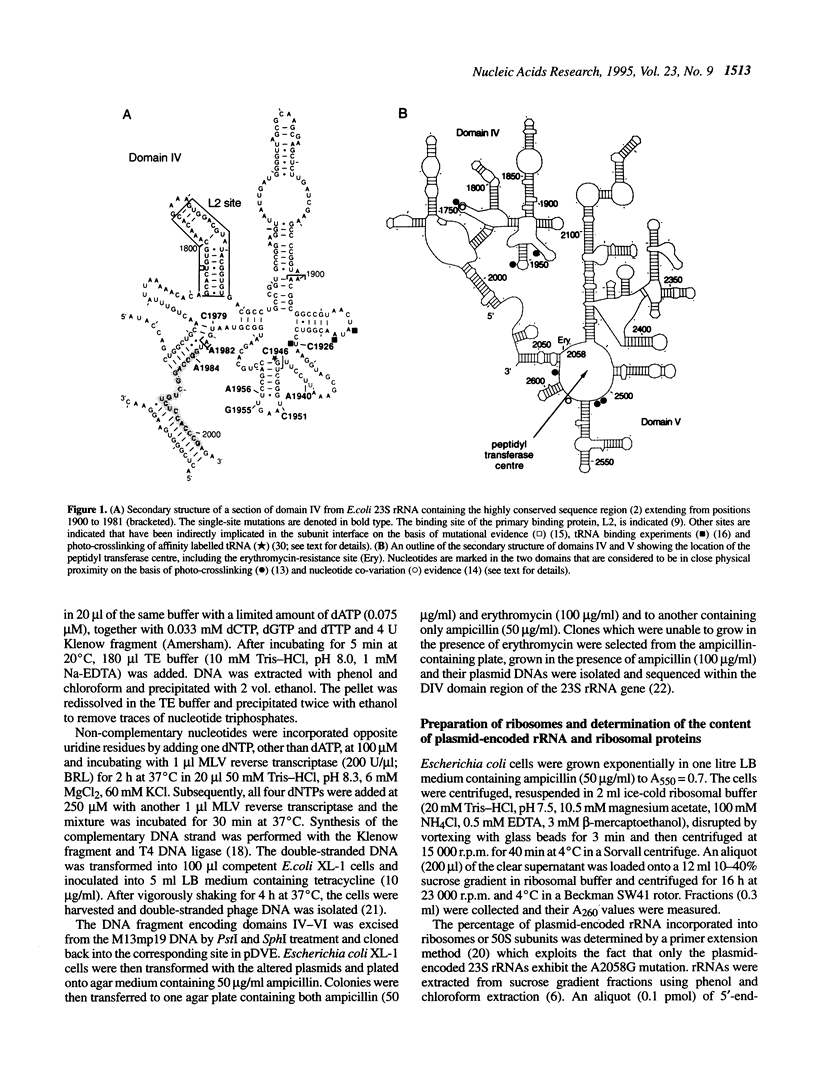
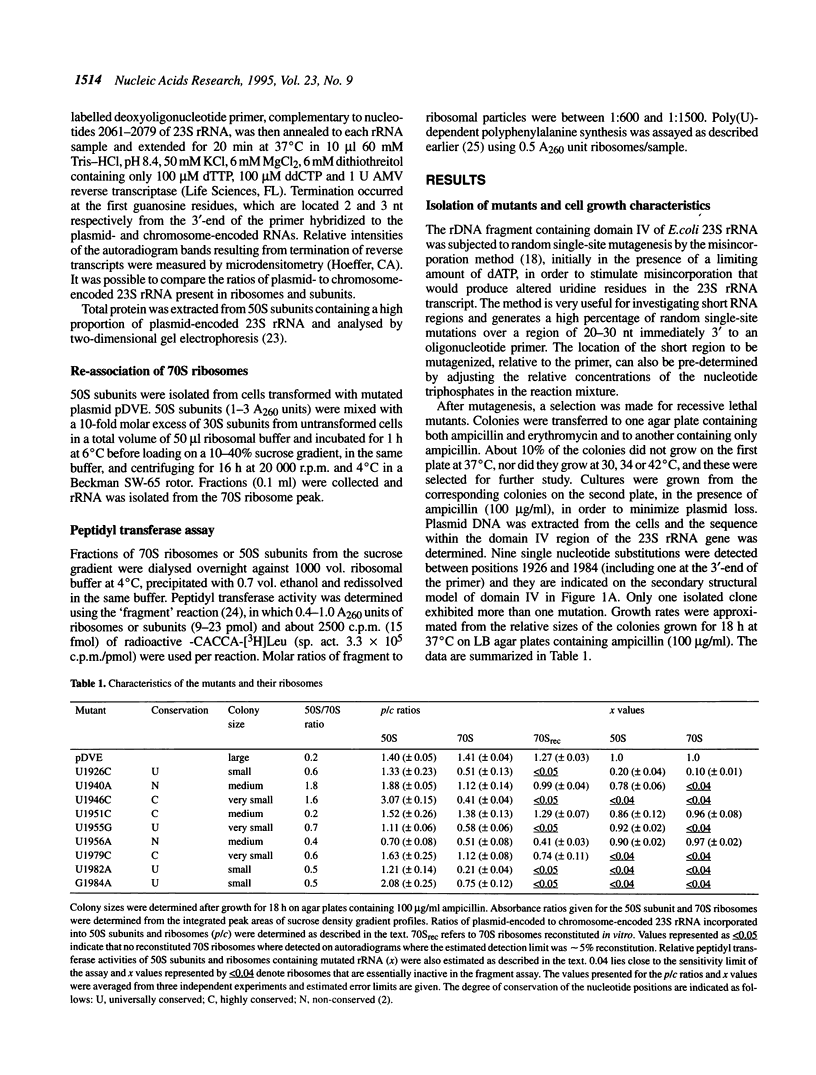
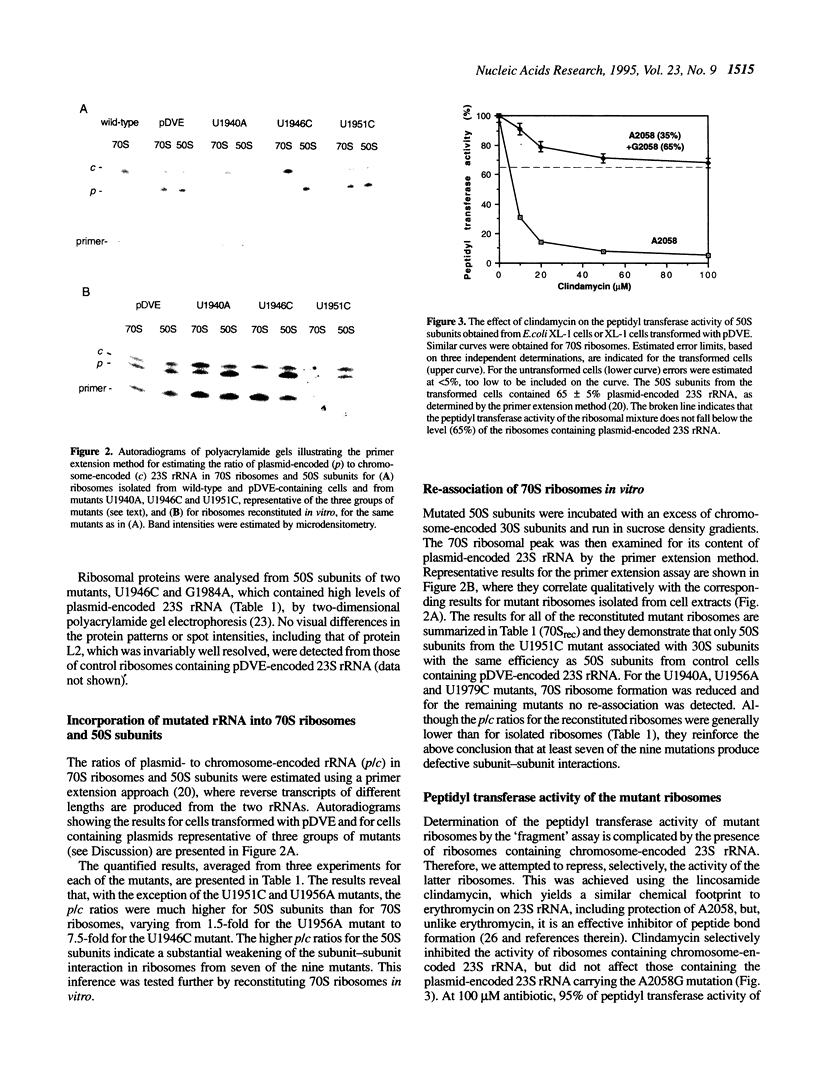
The function of the highly conserved and accessible region of domain IV of 23S rRNA (positions 1900-1981 in Escherichia coli 23S rRNA) was investigated by subjecting it to a random mutagenesis procedure that produced single-site mutations efficiently. Nine single-site mutants were selected that were recessive lethal. High levels of mutated 23S rRNA were expressed in E. coli and extracted ribosomes were investigated for their content of mutated rRNA. The peptidyl transferase activity of the ribosomes was also estimated using a newly developed method involving selective inhibition of chromosome-encoded ribosomes by clindamycin. Two of the mutants, U1940A and U1955G, yielded 50S subunits that were defective in subunit-subunit association but active in peptidyl transferase activity and five, U1926C, U1946C, U1979C, U1982A and G1984A, produced 50S subunits that were defective in both subunit-subunit interactions and peptidyl transferase activity. We infer that the large conserved rRNA region generates a complex structure that plays an essential role in maintaining and modulating subunit-subunit interactions and argue that its involvement in the peptidyl transferase centre is secondary, possibly involving the correct alignment of protein L2.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauclerk A. A., Cundliffe E. The binding site for ribosomal protein L2 within 23S ribosomal RNA of Escherichia coli. EMBO J. 1988 Nov;7(11):3589–3594. doi: 10.1002/j.1460-2075.1988.tb03236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. Translocation in protein synthesis: a hybrid structure model. Nature. 1968 May 18;218(5142):675–677. doi: 10.1038/218675a0. [DOI] [PubMed] [Google Scholar]
- Daignan-Fornier B., Bolotin-Fukuhara M. Mutational study of the rRNA in yeast mitochondria: functional importance of T1696 in the large rRNA gene. Nucleic Acids Res. 1988 Oct 11;16(19):9299–9306. doi: 10.1093/nar/16.19.9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohme F., Fahnestock S. R. Identification of proteins involved in the peptidyl transferase activity of ribosomes by chemical modification. J Mol Biol. 1979 Mar 25;129(1):63–81. doi: 10.1016/0022-2836(79)90060-3. [DOI] [PubMed] [Google Scholar]
- Douthwaite S. Interaction of the antibiotics clindamycin and lincomycin with Escherichia coli 23S ribosomal RNA. Nucleic Acids Res. 1992 Sep 25;20(18):4717–4720. doi: 10.1093/nar/20.18.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egebjerg J., Christiansen J., Garrett R. A. Attachment sites of primary binding proteins L1, L2 and L23 on 23 S ribosomal RNA of Escherichia coli. J Mol Biol. 1991 Nov 20;222(2):251–264. doi: 10.1016/0022-2836(91)90210-w. [DOI] [PubMed] [Google Scholar]
- Geyl D., Böck A., Isono K. An improved method for two-dimensional gel-electrophoresis: analysis of mutationally altered ribosomal proteins of Escherichia coli. Mol Gen Genet. 1981;181(3):309–312. doi: 10.1007/BF00425603. [DOI] [PubMed] [Google Scholar]
- Hampl H., Schulze H., Nierhaus K. H. Ribosomal components from Escherichia coli 50 S subunits involved in the reconstitution of peptidyltransferase activity. J Biol Chem. 1981 Mar 10;256(5):2284–2288. [PubMed] [Google Scholar]
- Herr W., Noller H. F. Protection of specific sites in 23 S and 5 S RNA from chemical modification by association of 30 S and 50 S ribosomes. J Mol Biol. 1979 Jun 5;130(4):421–432. doi: 10.1016/0022-2836(79)90432-7. [DOI] [PubMed] [Google Scholar]
- Larsen N. Higher order interactions in 23s rRNA. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5044–5048. doi: 10.1073/pnas.89.11.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffers H., Egebjerg J., Andersen A., Christensen T., Garrett R. A. Domain VI of Escherichia coli 23 S ribosomal RNA. Structure, assembly and function. J Mol Biol. 1988 Dec 5;204(3):507–522. doi: 10.1016/0022-2836(88)90351-8. [DOI] [PubMed] [Google Scholar]
- Lehtovaara P. M., Koivula A. K., Bamford J., Knowles J. K. A new method for random mutagenesis of complete genes: enzymatic generation of mutant libraries in vitro. Protein Eng. 1988 Apr;2(1):63–68. doi: 10.1093/protein/2.1.63. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Osswald M., Schueler D., Brimacombe R. Selective isolation and detailed analysis of intra-RNA cross-links induced in the large ribosomal subunit of E. coli: a model for the tertiary structure of the tRNA binding domain in 23S RNA. Nucleic Acids Res. 1990 Aug 11;18(15):4325–4333. doi: 10.1093/nar/18.15.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Chloramphenicol, erythromycin, carbomycin and vernamycin B protect overlapping sites in the peptidyl transferase region of 23S ribosomal RNA. Biochimie. 1987 Aug;69(8):879–884. doi: 10.1016/0300-9084(87)90215-x. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Intermediate states in the movement of transfer RNA in the ribosome. Nature. 1989 Nov 9;342(6246):142–148. doi: 10.1038/342142a0. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Sites of interaction of the CCA end of peptidyl-tRNA with 23S rRNA. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3725–3728. doi: 10.1073/pnas.88.9.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Robertson J. M., Noller H. F. Interaction of elongation factors EF-G and EF-Tu with a conserved loop in 23S RNA. Nature. 1988 Jul 28;334(6180):362–364. doi: 10.1038/334362a0. [DOI] [PubMed] [Google Scholar]
- Noller H. F. Ribosomal RNA and translation. Annu Rev Biochem. 1991;60:191–227. doi: 10.1146/annurev.bi.60.070191.001203. [DOI] [PubMed] [Google Scholar]
- Powers T., Noller H. F. Evidence for functional interaction between elongation factor Tu and 16S ribosomal RNA. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1364–1368. doi: 10.1073/pnas.90.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Fonseca C., Amils R., Garrett R. A. Fine structure of the peptidyl transferase centre on 23 S-like rRNAs deduced from chemical probing of antibiotic-ribosome complexes. J Mol Biol. 1995 Mar 24;247(2):224–235. doi: 10.1006/jmbi.1994.0135. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmund C. D., Ettayebi M., Borden A., Morgan E. A. Antibiotic resistance mutations in ribosomal RNA genes of Escherichia coli. Methods Enzymol. 1988;164:673–690. doi: 10.1016/s0076-6879(88)64077-8. [DOI] [PubMed] [Google Scholar]
- Vester B., Garrett R. A. A plasmid-coded and site-directed mutation in Escherichia coli 23S RNA that confers resistance to erythromycin: implications for the mechanism of action of erythromycin. Biochimie. 1987 Aug;69(8):891–900. doi: 10.1016/0300-9084(87)90217-3. [DOI] [PubMed] [Google Scholar]
- Wower J., Hixson S. S., Zimmermann R. A. Labeling the peptidyltransferase center of the Escherichia coli ribosome with photoreactive tRNA(Phe) derivatives containing azidoadenosine at the 3' end of the acceptor arm: a model of the tRNA-ribosome complex. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5232–5236. doi: 10.1073/pnas.86.14.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]