Abstract
The function of CUB domain-containing protein 1 (CDCP1), a recently described transmembrane protein expressed on the surface of hematopoietic stem cells and normal and malignant cells of different tissue origin, is not well defined. The contribution of CDCP1 to tumor metastasis was analyzed by employing HeLa carcinoma cells overexpressing CDCP1 (HeLa-CDCP1) and a high-disseminating variant of prostate carcinoma PC-3 naturally expressing high levels of CDCP1 (PC3-hi/diss). CDCP1 expression rendered HeLa cells more aggressive in experimental metastasis in immunodeficient mice. Metastatic colonization by HeLa-CDCP1 was effectively inhibited with subtractive immunization-generated, CDCP1-specific mAb 41-2, suggesting that CDCP1 facilitates relatively late stages of the metastatic cascade. In the chick embryo model, time- and dose-dependent inhibition of HeLa-CDCP1 colonization by mAb 41-2 was analyzed quantitatively in order to determine when and where CDCP1 functions during metastasis. Quantitative PCR and immunohistochemical analyses indicated that CDCP1 facilitated tumor cell survival soon after vascular arrest. Live cell imaging demonstrated that mAb 41-2’s function-blocking mechanism involved enhancement of tumor cell apoptosis, confirmed by attenuation of mAb 41-2-mediated effects with the caspase inhibitor, z-VAD-fmk. Under pro-apoptotic conditions in vitro, CDCP1 expression conferred HeLa-CDCP1 cells with resistance to doxorubicin-induced apoptosis, while ligation of CDCP1 with mAb 41-2 caused additional enhancement of the apoptotic response. The functional role of naturally-expressed CDCP1 was demonstrated by mAb 41-2-mediated inhibition of both experimental and spontaneous metastasis of PC3-hi/diss. These findings confirm that CDCP1 functions as an anti-apoptotic molecule and indicate that during metastasis CDCP1 facilitates tumor cell survival likely during or soon after extravasation.
Keywords: CDCP1, metastasis, extravasation, apoptosis, chick embryo model
Introduction
Recent years brought about a significant degree of interest in CUB domain-containing protein 1 (CDCP1), identified as a novel human tumor associated gene by differential cDNA analysis (1). The gene structure of CDCP1 suggested that the putative corresponding protein likely would be involved in cell interactions with the extracellular matrix (ECM). Functional importance of CDCP1 protein in vivo was initially indicated by the demonstration of differentially enhanced levels of CDCP1 in highly metastatic human tumor cells by the monoclonal antibody (mAb) 41-2, generated via subtractive immunization (2). The novel 135 kDa protein precipitated by mAb 41-2 was characterized as a transmembrane CUB domain-containing molecule and confirmed by amino acid sequencing to be CDCP1. The intracellular C-terminus of CDCP1 harbors several tyrosine residues and has been shown to be phosphorylated by Src family kinases (2). Phosphorylation of the C-terminus of CDCP1 by Src kinases along with evidence of CDCP1-mediated activation of several other kinases, suggested functional involvement of CDCP1 in outside-in signal transduction as a kinase docking molecule (3, 4). This conception was further affirmed by co-precipitation of PKCδ, a member of the PKC family, with CDCP1 (5). It has been also proposed that CDCP1 is involved in homotypic complex formation via its extracellular CUB domains (4); however, no such molecular interactions have been demonstrated directly. Recent findings also indicate that over-expression of CDCP1 leads to cell rounding and a loss of adhesion phenotype (3). In addition, CDCP1 expression renders anchorage-independent growth and resistance to anoikis of lung and gastric carcinoma cells (6, 7).
CDCP1 is expressed in many normal human tissues and cells, including hematopoietic stem and progenitor cells (2, 8, 9). Increased levels of CDCP1 were demonstrated in some aggressive epithelial cancers, correlating with poor prognosis, higher relapse rate and occurrence of metastases, and unfavorable overall survival of patients (10, 11). Therefore, CDCP1 emerges as a potential prognostic marker in several types of carcinomas as well as a possible target in cancer therapy. Thus, downregulation of CDCP1 by RNA interference in lung and gastric carcinoma cells resulted in suppressed invasion and experimental metastasis (6, 7). Treatment with anti-CDCP1 mAb 25A11 coupled with the cytotoxin saporin resulted in an inhibition of prostate cancer cell metastasis in a mouse xenograft model (12). However, the latter approach based on targeting of CDCP1-positive cancer cells is limited at least by two major considerations. First, the use of a toxin-conjugated antibody recognizing the cell surface molecule that is lacking in a xenogeneic host would kill CDCP1-expressing human cells by a general, likely toxin-antibody-internalization mechanism, not-related to the natural functions of CDCP1. Second, in cancer patients, the toxin-conjugated anti-CDCP1 antibodies may harm or kill normal cells due to almost ubiquitous expression of CDCP1 among human tissues. Thus, it appears that delivering of CDCP1-aimed therapeutics would require more focused, time-restricted or tissue-dependent approaches. In this regard, it becomes essential to mechanistically address specific aspects of CDCP1 functionality such as when and where in the metastatic cascade CDCP1 might function as a pro-metastatic molecule.
To characterize a pro-metastatic role of CDCP1, we generated carcinoma cells expressing high levels of CDCP1 by transfecting the CDCP1 cDNA into HeLa cells intrinsically lacking CDCP1 expression. In parallel, we have selected highly disseminating variants of prostate carcinoma PC-3 cells naturally expressing high levels of CDCP1. By employing these CDCP1-expressing cells and the CDCP1 function-blocking mAb 41-2 in quantitative experimental metastasis models, we have demonstrated that CDCP1 functions following cell arrest in the vasculature. Our findings also indicate that mAb 41-2-mediated inhibition of metastatic colonization is initiated during or soon after extravasation and is associated with a pronounced enhancement of tumor cell apoptosis involving caspase activation.
Results
Generation of HeLa-CDCP1 Cells
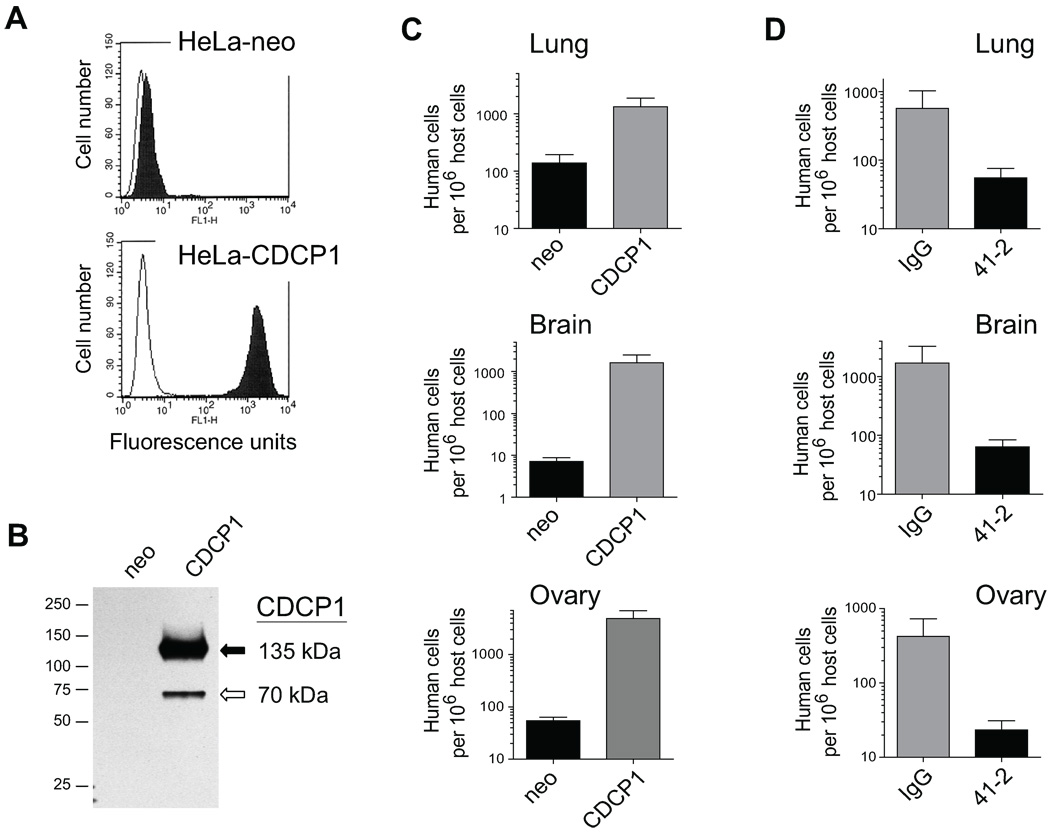
HeLa cells overexpressing CDCP1 protein were generated from the parental carcinoma cells, previously demonstrated to express no detectable CDCP1 (2). Flow cytometry confirmed high levels of cell surface CDCP1 in HeLa-CDCP1 cells and the corresponding lack of CDCP1 in control, HeLa-neo, cells (Fig. 1A). Analysis by western blotting indicated that HeLa-CDCP1 cells manifested the 135 kDa full length and a truncated 70 kDa species of CDCP1 (Fig. 1B).
FIGURE 1. Expression of CDCP1 in HeLa cells and experimental metastasis of HeLa-CDCP1 cells in immunodeficient mice.
A. Flow cytometry analysis of CDCP1 expression in HeLa cells. HeLa-neo and HeLa-CDCP1 cells were incubated with mAb 41-2 (closed histograms) or mouse IgG (open histograms), then with goat anti-mouse IgG conjugated with FITC and analyzed in FACScan. B. Western blot analysis of CDCP1 expressed in HeLa cells. CDCP1 is represented as two proteins bands of 135 kDa (solid arrow) and 70 kDa (open arrow) in HeLa-CDCP1 cells, but is undetectable in HeLa-neo cells. The position of mol wt standards (in kDa) is indicated on the left. C. Experimental metastasis of HeLa cells in SCID mice. 1×106 HeLa-neo and HeLa-CDCP1 cells were injected i.v. into SCID mice. Levels of experimental metastasis were analyzed 4 weeks following cell injections. Presented are data combined from two independent experiments employing 7 mice per cell variant. D. Inhibition of experimental metastasis of HeLa-CDCP1 cells by mAb 41-2. 1×106 HeLa-CDCP1 cells were injected i.v. into NOD-SCID mice with control IgG or mAb 41-2. Additional injections of corresponding antibodies were performed i.p. on days 1 and 2 after cell injection. Levels of experimental metastasis were determined 3 weeks after cell inoculation. Presented is one of two independent experiments each performed with 4 mice per variant. Data are means ± SEM of numbers of human cells per 106 host cells determined by qPCR in lungs, brain, and ovaries of sacrificed animals.
CDCP1-Mediated Colonization in the Mouse Experimental Metastasis Model
To determine how expression of CDCP1 affected the colonization potential of HeLa cells in an experimental metastasis model, immunodeficient SCID mice were injected i.v. with either control HeLa-neo or CDCP1-transfected HeLa cells (Fig. 1C). High levels of metastatic colonization were demonstrated four weeks later by human-specific qPCR in lungs, brain and ovaries for HeLa-CDCP1 cells (Fig. 1C, grey bars). Control HeLa-neo cells displayed low levels of lung colonization and essentially lacked the ability to efficiently metastasize/colonize the brain or ovaries (Fig. 1C, black bars). To confirm that the high metastatic potential of HeLa-CDCP1 cells was attributed to CDCP1-mediated functions, the cells were inoculated i.v. into mice along with function-blocking anti-CDCP1 mAb 41-2 or control mouse IgG. As determined by qPCR three weeks later, treatment with mAb 41-2 substantially reduced colonization of host organs (Fig. 1D), indicating that ligation of CDCP1 by mAb 41-2 negated the enhanced metastatic potential of HeLa-CDCP1 cells.
CDCP1-Mediated Colonization in the Chick Embryo Experimental Metastasis Model
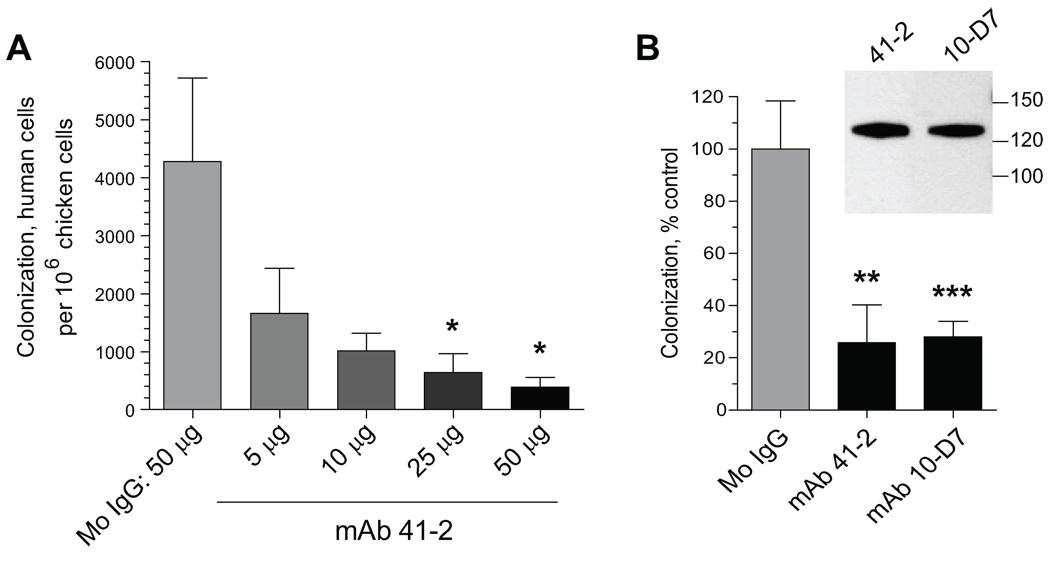
To examine mechanistically when and where during metastatic colonization CDCP1 exerts its pro-metastatic functions, we took advantage of our function-blocking anti-CDCP1 antibodies in combination with the chick embryo experimental model allowing for efficient morphological and quantitative analysis of tumor cell metastasis. HeLa-CDCP1 cells were injected i.v. into day 12 chick embryos with 50 µg control mouse IgG or increasing doses of mAb 41-2 (Fig. 2A). Five days later, portions of the CAM, a highly vascularized tissue serving as a preferential site for arrest, extravasation and expansion of tumor cells, were harvested and analyzed by qPCR to determine actual numbers of human cells in the host tissues. Metastatic colonization of HeLa-CDCP1 cells was diminished by mAb 41-2 in a dose-dependent manner and was sensitive to as low as 5 µg of antibody per embryo (Fig. 2A). Thus, the inhibitory effect of anti-CDCP1 mAb 41-2 on experimental metastasis observed in the mouse system was recapitulated in the quantitative chick embryo model. In addition to mAb 41-2, HeLa-CDCP1 colonization was efficiently inhibited by another CDCP1-specific mAb 10-D7, which similarly to mAb 41-2 was also generated by subtractive immunization. In HeLa-CDCP1 cells, mAb 10-D7 identified a protein band of the same 135 kDa mol. wt. as mAb 41-2 (Fig. 2B, inset) and exhibited identical cell surface binding (data not shown). Both anti-CDCP1 mAbs 41-2 and 10-D7 efficiently inhibited colonization of HeLa-CDCP1 cells, on average by 74% and 72% of control levels, respectively (Fig. 2B).
FIGURE 2. Experimental metastasis of HeLa-CDCP1 cells in the chick embryo is sensitive to anti-CDCP1 mAbs.
A. Dose-dependent inhibition of HeLa-CDCP1 experimental metastasis in the chick embryo. HeLa-CDCP1 cells (5×104) were injected i.v. into chick embryos with 50 µg of control IgG (8 embryos) or increasing amounts of mAb 41-2 ranging from 5 µg to 50 µg per embryo (7–8 embryos per dose). Five days later, the numbers of human cells in the CAM were determined by qPCR. *, P<0.05 compared to IgG control. B. Inhibition of experimental metastasis by CDCP1-specific mAbs 41-2 and 10-D7. HeLa-CDCP1 cells were injected i.v. with or without control IgG or anti-CDCP-1 mAbs 41-2 or 10-D7. The following day, each embryo received an additional i.v. inoculation of the corresponding antibody. Five days later, the levels of CAM colonization was determined by qPCR. The data are presented as percentage of number of human cells in control group (mean ± SEM) determined for mouse IgG (27 embryos), mAb 41-2 (23 embryos), and mAb 10-D7 (29 embryos) in 3 independent experiments. **, P<0.005 and ***, P< 0.001 compared to IgG control. Inset, Western blot analysis of CDCP1 in HeLa-CDCP1 cell lysates performed with mAb 41-2 or mAb 10-D7. Position of mol wt markers in kDa is indicated on the right.
Tyrosine Phosphorylation of CDCP1 and mAb 41-2-Mediated Inhibition of Experimental Metastasis
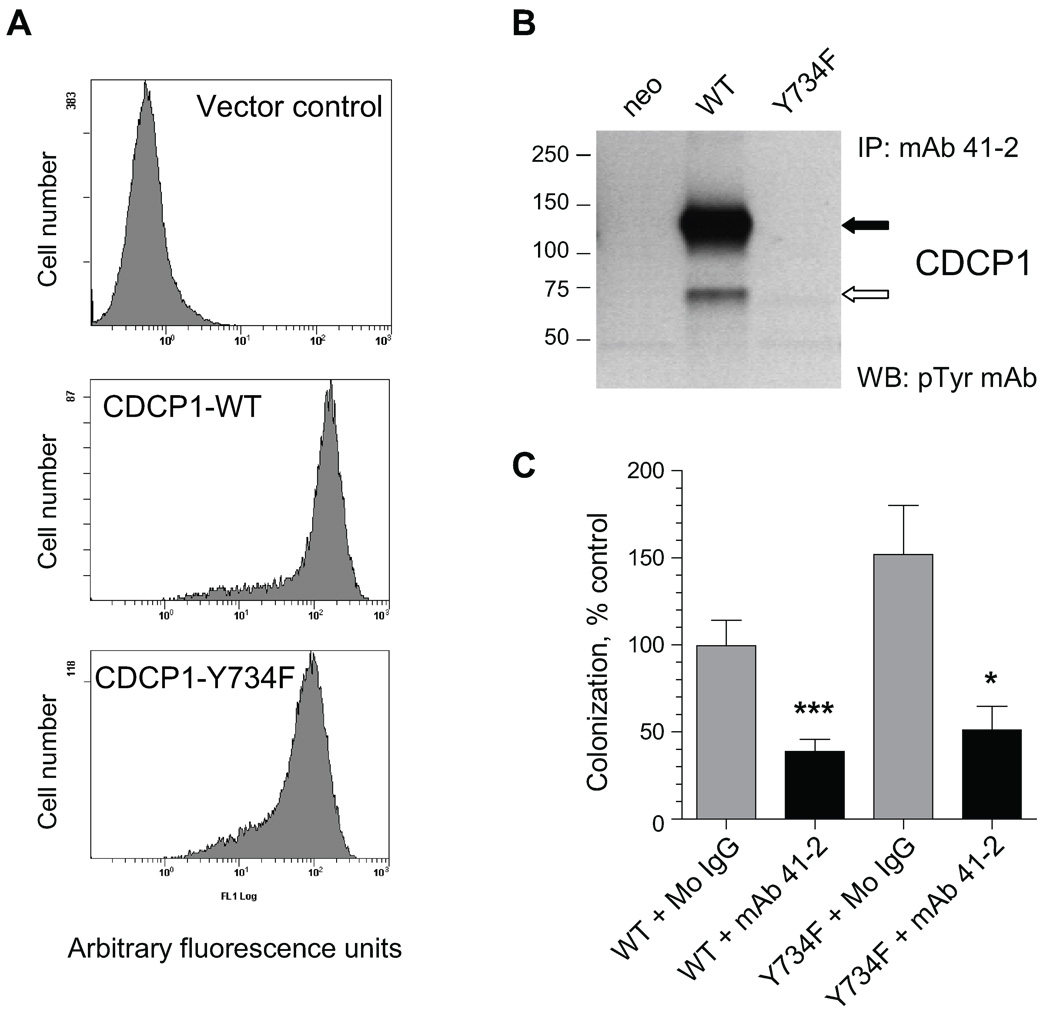
Functional activity of CDCP1 has been assumed to be closely associated with the C-terminus tyrosine phosphorylation. Therefore, we generated HeLa-CDCP1 cells where tyrosine residue Y734 was substituted for phenylalanine (Y734F), previously shown to completely abrogate tyrosine phosphorylation of the C-terminus (5). Similar levels of CDCP1 expression in HeLa cells expressing CDCP1-WT and CDCP1-Y734F constructs were demonstrated by flow cytometry (Fig. 3A), while western blotting indicated that the 135 kDa and 70 kDa species were both constitutively phosphorylated in HeLa-CDCP1-WT cells, but not in the HeLa-CDCP1-Y734F mutant (Fig. 3B).
FIGURE 3. Inhibition of experimental metastasis of HeLa-CDCP1 cells by mAb 41-2 does not depend on tyrosine phosphorylation of CDCP1 protein.
A. Flow cytometry analysis of HeLa cells expressing Y734F tyrosine mutant of CDCP1. HeLa cells transfected with an empty vector (control), wild type CDCP1 (CDCP1-WT) or CDCP1-Y734F mutant were analyzed in a FACScan for cell surface expression of CDCP1 after staining with mAb 41-2. B. Western blot analysis of tyrosine phosphorylation of CDCP1. CDCP1 was precipitated from HeLa-CDCP1-WT, HeLa-CDCP1-Y734F and HeLa-neo cells with mAb 41-2 and probed with anti-phosphotyrosine mAb 4G10. The position of mol wt standards (in kDa) is indicated on the left. Solid and open arrows indicate CDCP1 bands with approximate mol wt of 135 kDa and 70 kDa, respectively. C. Inhibition of experimental metastasis of HeLa-CDCP1-Y734F cells by mAb 41-2. HeLa-CDCP1-WT (WT) and HeLa-CDCP1-Y734F (Y734F) cells were injected i.v. into chick embryos along with control IgG or mAb 41-2. Levels of metastatic colonization in CAM were determined 5 days later. Data are presented as percentage (means ± SEM) of control colonization (HeLa-CDCP-WT cells, mouse IgG) determined from pooled data obtained in 4 independent experiments employing from 21 to 47 embryos per variable. *, P<0.05 and ***, P<0.0005 compared to IgG control.
HeLa-CDCP1-Y734F and HeLa-CDCP1-WT cells were injected i.v. into chick embryos along with mAb 41-2 or mouse IgG and analyzed 5 days later. As shown in Fig. 3C, HeLa-CDCP1-Y734F cells exhibited slightly enhanced levels of metastasis as compared with HeLa-CDCP1-WT cells. Importantly, HeLa-CDCP1-Y734F cell colonization was more than 50% inhibited by mAb 41-2, indicating that efficient inhibition of metastasis by mAb 41-2-mediated ligation of CDCP1 apparently does not require CDCP1 tyrosine phosphorylation.
Kinetic Analysis of CDCP1-Mediated Tumor Cell Colonization
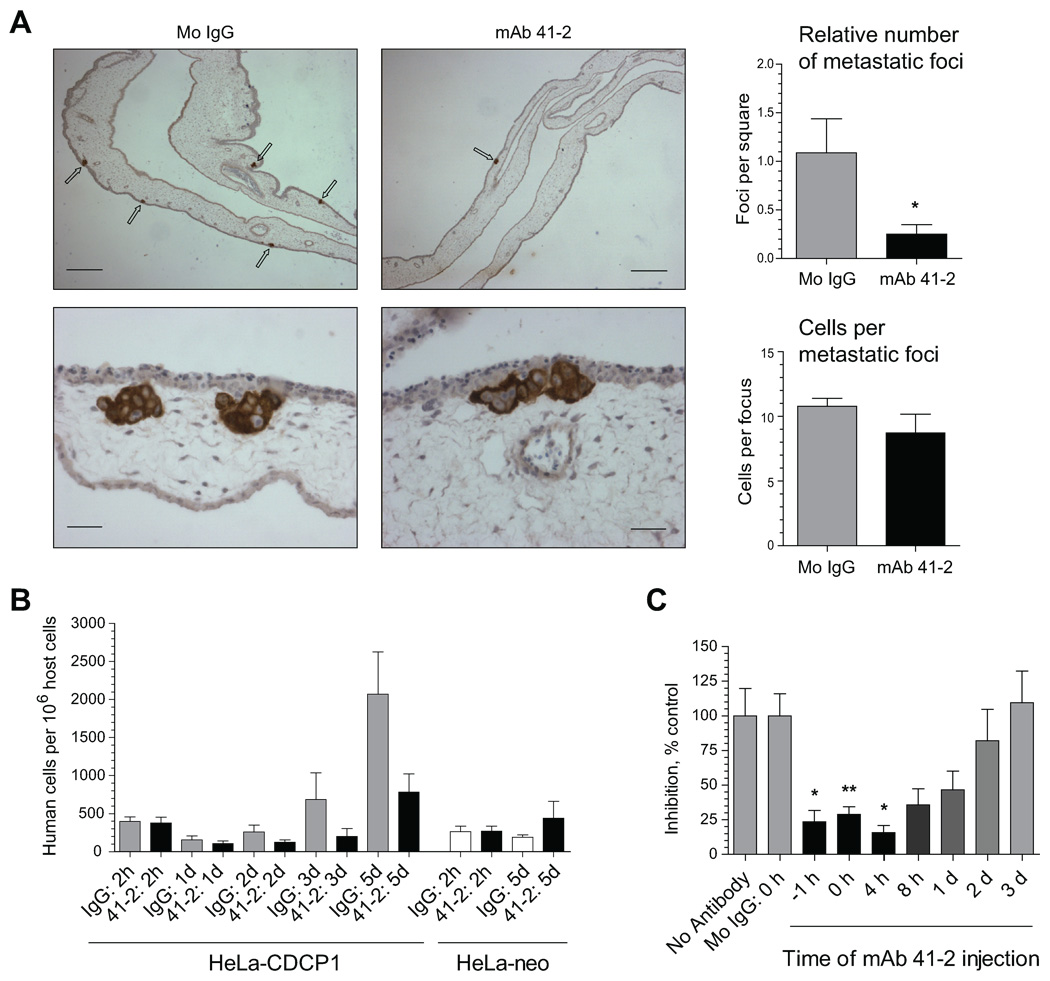
To elucidate the mechanisms underlying the inhibitory effects of CDCP1 ligation by mAb 41-2 on metastasis, we performed immunohistochemical analysis of tumor cells in the CAM tissue 5 days after i.v. inoculation of HeLa-CDCP1 cells along with mAb 41-2 or control IgG. The analysis indicated that treatment with mAb 41-2 resulted in reduction of metastatic microfoci formation (Fig. 4A, upper panels). Quantitative analysis confirmed a substantial decrease (>80%) in the foci density in the embryos treated with mAb 41-2 (Fig. 4A, upper bar graph). Quantitation of the relative number of tumor cells per individual metastatic focus indicated comparable cell numbers regardless of the antibody treatment (Fig. 4A, bottom panels and bar graph). These findings suggested that the inhibitory mechanisms of mAb 41-2 involve targeting single CDCP1-expressing tumor cells soon after their inoculation. The 10–20% of tumor cells that escaped initial 41-2 targeting generated near normal size metastatic foci.
FIGURE 4. Quantitative analysis of HeLa-CDCP1 experimental metastasis and its inhibition by mAb 41-2.
A. Immunohistochemical analysis of HeLa-CDCP1 colonization. HeLa-CDCP1 cells were injected i.v. into chick embryos along with control IgG or mAb 41-2. Five days later, distal portions of the CAM were harvested and processed for immunohistochemical analyses of human carcinoma cells stained (brown) with pan-cytokeratin-specific antibodies. Images were taken at original magnifications of 40X (upper panels; bar, 250 µm) and 400X (lower panels; bar, 25 µm). Arrows point to metastatic foci developed in the CAM mesoderm. The bar graphs on the right indicate the number of metastatic foci per arbitrary square filled with CAM tissue (upper graph) and the number of cells per foci within a given planar section (lower graph) were calculated from 6 to 9 specimens analyzed for each antibody treatment. *, P<0.0001. B. Kinetic analysis of HeLa-CDCP1 colonization. HeLa-CDCP1 and HeLa-neo cells were injected i.v. with control IgG or mAb 41-2. At indicated time points, CAM tissue was analyzed for numbers of human cells. Presented are means ± SEM determined for 5 embryos per time point. C. Time-course analysis of the sensitivity of HeLa-CDCP1 colonization to mAb 41-2. HeLa-CDCP1 cells were injected i.v. alone or with control mouse IgG (Mo IgG) or mAb 41-2 inoculated at indicated time points, i.e. 1 hr before cell injection (−1 h), simultaneously with the cells (0 h), or 4 hr, 8 hr, 1 day, 2 days and 3 days after cell injection. At five days following cell inoculation, all embryos were sacrificed and CAM analyzed by qPCR to determine numbers of human cells within chick embryo tissue. Data are presented as percentage (mean ± SEM) of experimental metastasis levels observed in antibody-treated embryos compared to no antibody control (100%). Presented are pooled data from 2 to 10 individual experiments employing a total of 8 to 74 embryos for each time point. *, P<0.05 and **, P<0.005 compared to no antibody control.
The contention that CDCP1 is functionally important at early stages of experimental metastasis was further verified in kinetic experiments where HeLa-CDCP1 cells were injected along with mAb 41-2 or control IgG and actual numbers of human cells within CAM tissue were determined at different time points following inoculations. As a negative control, HeLa-neo cells were also co-injected along with mAb 41-2 or control IgG and analyzed at the early (2 hr) and latest (5 days) time points. The qPCR analysis of CAM tissue (Fig. 4A) demonstrated similar numbers for both HeLa cell types at a 2 hr time point regardless of antibody treatment, indicating that CDCP1 ligation by the mAb 41-2 did not affect the initial arrest of HeLa-CDCP1 cells in the vasculature. However, the number of HeLa-CDCP1 cells was significantly diminished at 1 day, 2 days and 3 days in the embryos treated with mAb 41-2, leading to a significant delay in tumor cell colonization (black bars) as compared to IgG control (grey bars) (Fig. 4B). By day 5, this delay manifested itself as the 3–5-fold differential in colonization by HeLa-CDCP1 cells inoculated with mAb 41-2 versus control IgG. This analysis also indicated a lack of inhibitory effects of mAb 41-2 on HeLa-neo cells, further demonstrating the specificity of this function-blocking mAb. Additionally, the overall enhanced metastasis of HeLa-CDCP1 cells as compared with their CDCP1-negative counterparts was confirmed in these kinetic experiments by using an independent approach (Fig. 4B, grey bars versus white bars at 5 days).
To determine when injected HeLa-CDCP1 cells were most sensitive to ligation of CDCP1 by mAb 41-2, we varied the time of antibody injections (Fig. 4C). This kinetic analysis indicated that mAb 41-2 significantly blocked colonization of HeLa-CDCP1 cells within a narrow time frame during the first 24 hr following cell injection. More precisely, the most pronounced inhibition of metastasis occurred when mAb 41-2 was administered 1 hr before and within 4 hours after cell injections. The mAb 41-2-mediated inhibition of tumor cell colonization gradually diminished after 8 hr following cell inoculations until at 48–72 hr mAb 41-2 became completely ineffective (Fig. 4C), affirming that cell surface CDCP1 functions in the early stages of metastatic colonization following initial cell arrest in the vasculature.
Morphological Analysis of Inhibitory Effects of mAb 41-2 on HeLa-CDCP1 Experimental Metastasis
HeLa-CDCP1 cells were pre-labeled with green fluorescent CellTracker and injected into the chick embryo along with mAb 41-2 or control IgG. At different time points, non-fixed CAM was analyzed by fluorescence microscopy after highlighting the vasculature with rhodamine-conjugated LCA lectin (Fig. 5). As soon as 10 min after inoculation, HeLa-CDCP1 cells arrive in the terminal capillaries of the CAM (Fig. 5A, dotted ovals). The cells are clearly intravascular, approaching the tips of terminal capillaries (Fig. 5A, bottom). By 2 hr, most of the HeLa-CDCP1 cells have reached the ends of terminal capillaries, and appear arrested in narrow spaces of the capillary plexus (Fig. 5B). There were no apparent effects of mAb 41-2 on HeLa-CDCP1 cells at these early time points (left and right panels in Fig. 5B), affirming the nearly identical 2 hr quantitative cell arrest data presented in Fig. 4B.
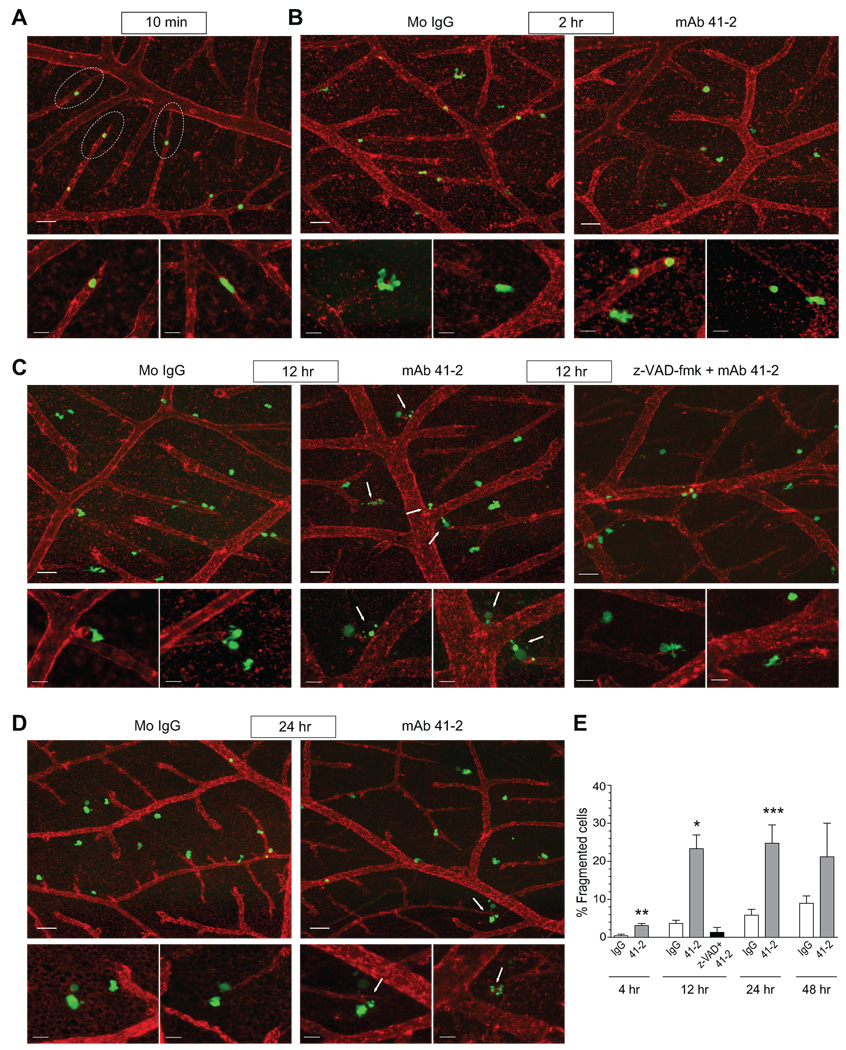
FIGURE 5. Kinetic analysis of live HeLa-CDCP1 cells during experimental metastasis.
A–D. HeLa-CDCP1 cells were pre-labeled with green fluorescent CellTracker and injected i.v. into chick embryos with control IgG or mAb 41-2. The vasculature was highlighted with rhodamine-conjugated LCA. At indicated time points, the non-fixed CAM tissue was visualized in a fluorescent AxioVision microscope. Images were taken at original magnifications of 100X (upper panels; bar, 50 µm) and 400X (smaller lower panels, bar, 20 µm) at 10 min (A), 2 hr (B), 12 hr (C), and 24 hr (D) after cell injection. Dotted ovals in (A) delineate the tips of terminal capillaries with arrested HeLa cells. In (C), a group of embryos was inoculated with the cells pre-treated with z-VAD-fmk. Arrows in (C) and (D) point to fragmented HeLa cells. E. Fragmented cells were counted among all green fluorescent cells in the images taken with 40x objective. Control IgG; open bars, mAb 41-2, grey bars; pre-treatment with z-VAD-fmk, followed by injection with mAb 41-2, black bar. Data are presented as the percentage of fragmented cells from total number of analyzed cells and means ± SEM calculated from up to 30 images pooled from 3 to 5 independent experiments per time point each employing from 3 to 5 embryos per condition. *, P<0.05 and **, P<0.01, and ***, P<0.005 compared to IgG control for corresponding time point.
By 12 hours after injection with control IgG, the HeLa-CDCP1 cells appear to scatter from the terminal capillaries, presenting cell protrusions characteristic of motile behavior (Fig. 5C, left panels). However in the embryos injected with mAb 41-2, HeLa-CDCP1 cells are still positioned close to the ends of terminal capillaries (Fig. 5C, middle panels), indicative of their inefficient outward motility. More importantly, many of mAb 41-2-treated HeLa-CDCP1 cells displayed morphology typical of HeLa cells that underwent apoptosis (13), with cell retraction, plasma membrane blebbing, and condensed and fragmented nuclei, i.e. resembling apoptotic bodies (Fig. 5C, middle panels, arrows). At the 24 hr time point, HeLa-CDCP1 cells in control embryos continue their motile scattering into the areas between arteriolar and venous capillaries (Fig. 5D, left panels), while in the embryos treated with mAb 41-2, the majority of tumor cells seem to be halted at the tips of the terminal capillaries with many of them having undergone blebbing and/or fragmentation (Fig. 5D, right panels, arrows).
Quantitative analysis of the percentage of fragmented cells (apoptotic bodies) was performed on multiple digital images of the CAM at different time points (Fig. 5E). This kinetic analysis demonstrated a slow, time-dependent increase of cell fragmentation in embryos injected with control IgG, reaching approximately 8% by 48 hr after cell inoculations. However, treatment with mAb 41-2 caused a steep, 20–25% increase in cell fragmentation at 12 hr and 24 hr, thereby constituting a substantial, 3–5-fold differential over IgG control.
To confirm that fragmented cells actually represented apoptotic bodies and that the increase in their numbers mediated by mAb 41-2 was indeed attributed to actual apoptosis, HeLa-CDCP1 cells were first pre-treated with the caspase inhibitor z-VAD-fmk and then injected into embryos along with mAb 41-2 and the inhibitor. Treatment with z-VAD-fmk resulted in a dramatic decrease in the percentage of fragmented cells in the CAM tissue below control levels (Fig. 5E, black bar), consistent with inhibition of apoptosis and release of apoptotic bodies by blocking caspase activation (14). Morphologically, z-VAD-fmk treatment reverted appearance of HeLa-CDCP1 cells injected with mAb 41-2 to that observed in the presence of control antibody manifesting very few fragmented cells (Fig. 5C, right panels vs. left panels). Sensitivity of HeLa-CDCP1 cell fragmentation to z-VAD-fmk indicates a general involvement of caspase activation and suggests that at the early stages of experimental metastasis, inoculated cells undergo naturally occurring apoptosis, the levels of which are substantially enhanced by ligation with anti-CDCP1 mAb 41-2.
Effects of mAb 41-2 on Functions of HeLa-CDCP1 Cells in vitro
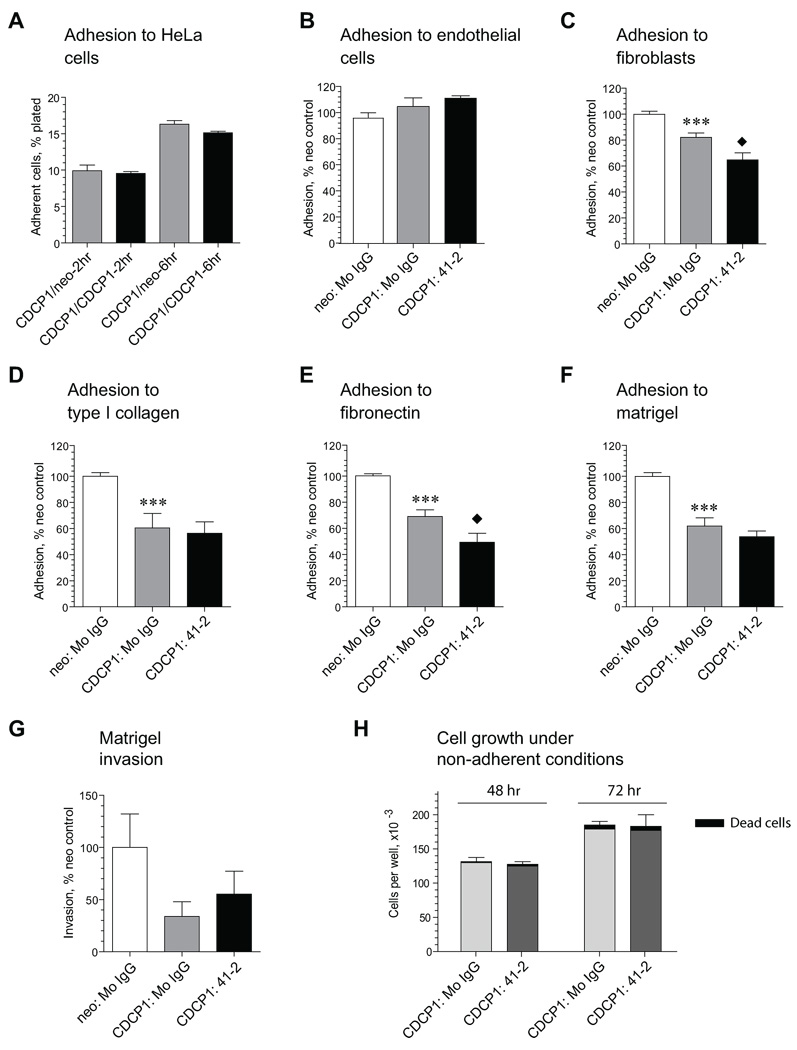
Z-VAD-fmk-sensitive fragmentation of HeLa-CDCP1 cells, enhanced by mAb 41-2 as the cells exit the vasculature, suggested that this function-blocking antibody might induce anoikis events due to inhibition of CDCP1-mediated cell-cell adhesion or cell adhesion to ECM proteins. This suggestion was analyzed in a series of in vitro assays addressing different aspects of cell functionality. First, we analyzed whether CDCP1 expression rendered HeLa cells with enhanced levels of homotypic interactions. As shown in Fig. 6A, the expression of CDCP1 did not enhance homotypic adherence of HeLa-CDCP1 cells. Adherence of HeLa-CDCP1 cells was equal to HeLa-neo and HeLa-CDCP1 cells both 2 hr following cell plating when cells had just attached, or later, after 6 hr, when plated cells had firmly adhered and spread on the underlying cellular layers. We next investigated the possibility that CDCP1 enhanced heterotypic adherence to vascular endothelial cells and mesenchymal fibroblasts and whether these heterotypic interactions were sensitive to mAb 41-2. The expression of CDCP1 did not confer HeLa-CDCP1 cells with enhanced capability of heterotypic adhesion to endothelial cells (Fig. 6B) or fibroblasts (Fig. 6C) as compared to HeLa-neo control. In addition, mAb 41-2 did not affect HeLa-CDCP1 cell adhesion to endothelial cells, but slightly inhibited adhesion to fibroblasts (Fig. 6, B and C, black bars). In ECM-mediated adhesion, HeLa-CDCP1 cells demonstrated significantly less adhesion to type I collagen, fibronectin and Matrigel as compared to HeLa-neo cells (Fig. 6, D–F), but the levels of cell adhesion were not significantly inhibited by mAb 41-2 with the exception of a slight inhibition of attachment to fibronectin (Fig. 6, D–F, black bars). Similarly, HeLa-CDCP1 cells did not demonstrate significant levels of chemotactic invasion in Matrigel assays compared to HeLa-neo control, and HeLa-CDCP1 invasion levels were not inhibited by mAb 41-2 (Fig. 6G). Finally, ligation of CDCP1 by mAb 41-2 did not affect significantly the motility of HeLa-CDCP1 cells in Transwell assays where haptotactic migration was mediated by type I collagen, fibronectin or Matrigel, and chemotactic migration was induced by serum (data not shown).
FIGURE 6. Effects of CDCP1 expression and mAb 41-2 treatment on functional characteristics of HeLa cells in vitro.
A. Homotypic adhesion of HeLa-CDCP1 cells to HeLa-neo or HeLa-CDCP1 cells. Data are means ± SEM of percentage of cells adhered after 2 hr and 6 hr incubation periods. Presented is one out of 2 independent experiments performed in triplicate. B and C. Heterotypic adhesion of HeLa-neo and HeLa-CDCP1 cells to human endothelial (B) or chicken fibroblasts (C) in the presence of control IgG or mAb 41-2. Presented is one out of 5 (B) and 3 (C) independent experiments performed in triplicate. Data are percent (means ± SEM) of HeLa-neo cell adhesion in the presence of control IgG. ***, P<0.005 compared to neo control; black diamond, P<0.05 compared to adhesion of HeLa-CDCP1 cells in the presence of control IgG. D-F. Haptotactic adhesion of HeLa-neo and HeLa-CDCP1 cells. Cells were allowed to adhere for 45 min to type I collagen (D), fibronectin (E) or Matrigel (F) in the presence of control IgG or mAb 41-2. Presented is one from 4 independent experiments performed in quadruplicate. Data are percentage (mean ± SEM) of control HeLa-neo cell adhesion in the presence of mouse IgG. ***, P<0.0001 compared to HeLa-neo control; black diamond, P<0.05 compared to adhesion of HeLa-CDCP1 cells in the presence of mouse IgG. G. Chemotactic migration of HeLa-neo and HeLa-CDCP1 cells. Cells were plated in SF-DMEM into Transwell inserts pre-coated with Matrigel and stimulated to migrate for 48 hr towards 5% FCS placed into the outer chamber with control mouse IgG or mAb 41-2. Data are presented as percentage (mean ± SEM) of migration observed with HeLa-neo cells. Presented is one from 3 independent experiments performed in triplicate. H. HeLa-CDCP1 growth under adhesion-free conditions. Cells were plated into ultra-low adhesion plates in D-10 supplemented with mouse IgG or mAb 41-2 and cells were counted at 48 and 72 hr. Presented is one from 4 independent experiments performed in triplicate. Data are means ± SEM of total cell numbers per well.
These in vitro results indicate that mAb 41-2-mediated inhibition of HeLa-CDCP1 colonization apparently does not involve significant abrogation of cell-cell or cell-ECM interactions, which in turn could elicit anoikis. Whether ligation of CDCP1 would increase cell death under anoikis-inducing conditions was verified in a series of in vitro experiments where HeLa-CDCP1 cells were incubated with or without mAb 41-2: Neither cell growth nor percentage of dead cells (varying from 1.5 to 6% for mouse IgG control and from 3.4 to 4.5% for mAb 41-2-treated cells) was significantly affected under adhesion-free conditions in ultra-low adhesion plates (Fig. 6H). Similarly, mAb 41-2 did not significantly affect HeLa-CDCP1 colony formation in soft agar cultures (data not shown).
Opposing Effects of CDCP1 Expression and mAb 41-2 Treatment on Drug-Induced Apoptosis
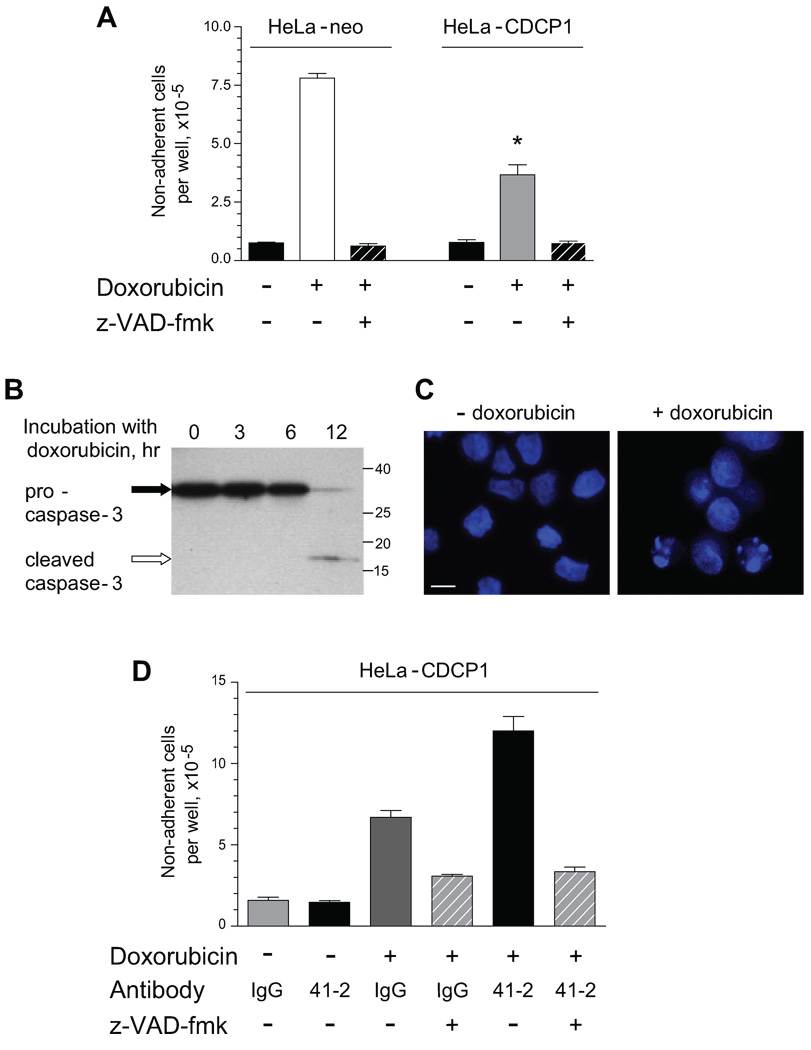
As indicated in the in vivo live cell imaging experiments (Fig. 5), anoikis or apoptosis might occur under conditions where mAb 41-2-treated CDCP1-positive cells encounter stress or a pro-apoptotic environment. Therefore, we further analyzed the in vitro effects of mAb 41-2 on HeLa-CDCP1 cells under pro-apoptotic conditions, which were induced by incubation with doxorubicin, an inducer of caspase activation-mediated cell death (15–18). Drug-induced apoptosis causes cell retraction, rounding and cell detachment (13), and therefore the numbers of detached cells served as a measurement of apoptotic response. Treatment of either HeLa-CDCP1 cells or their control HeLa-neo counterparts with doxorubicin caused apoptosis accompanied by detachment of dead cells (Fig. 7A). As expected, the doxorubicin-treated cells exhibited caspase activation confirmed by western blotting (Fig. 7B) and nuclei fragmentation visualized by DAPI staining (Fig. 7C). Importantly, the levels of doxorubicin-induced apoptosis were approximately 50% lower in HeLa-CDCP1 cells compared to identically-treated HeLa-neo control, thus demonstrating that the expression of CDCP1 provided significant resistance to apoptosis (Fig. 7A). Doxorubicin-induced apoptosis was abrogated by pre-treatment of the cells with z-VAD-fmk, confirming the involvement of caspase activation (Fig. 7A, hatched bars).
FIGURE 7. mAb 41-2 enhances drug-induced apoptosis of HeLa-CDCP1 cells.
A. CDCP1 expression protects HeLa cells from doxorubicin-induced apoptosis. HeLa-neo cells and HeLa-CDCP1 cells were treated with doxorubicin (+) or corresponding volume of diluent (−). Where indicated, the cells were pre-treated for 2 hr with z-VAD-fmk (+) before addition of doxorubicin. Data are presented as the number of non-adherent cells representing detached apoptotic cells counted 48 hr later. B. Doxorubicin-induced activation of caspase 3. HeLa-CDCP1 cells were treated with doxorubicin for indicated time (hr). Both adherent and non-adherent cells were harvested and caspase activation status was analyzed by western blotting. Positions of pro-caspase 3 and activated cleaved caspase 3 are indicated on the left. Position of mol. wt. markers is indicated on the right (kDa). C. Nuclei fragmentation caused by doxorubicin treatment. HeLa-CDCP1 cells were treated with vehicle or doxorubicin for 24 hr, fixed and stained with DAPI. D. mAb 41-2 enhanced doxorubicin-induced apoptosis. HeLa-CDCP1 cells were treated with vehicle (−) or doxorubicin (+) in the presence of control IgG or mAb 41-2. Where indicated, HeLa-CDCP1 cells were pretreated with z-VAD-fmk (+) before adding doxorubicin (hatched bars). Following 48 hr incubation, non-adherent apoptotic cells from individual wells were counted. Presented are means ± SEM from one out of 4 independent experiments performed in triplicate. *, P<0.05 compared to HeLa-neo cells, **, P<0.05 compared to HeLa-CDCP1 cells incubated in the doxorubicin and IgG.
We next verified whether addition of mAb 41-2 to HeLa-CDCP1 cells cultured in pro-apoptotic conditions enhanced drug-induced cell death and detachment (Fig. 7D). While mAb 41-2 did not induce any negative effects in the absence of doxorubicin, treatment with mAb 41-2 in the presence of the drug resulted in an additional 2-fold increase in the number of detached cells compared to IgG control, and this differential was essentially abrogated by z-VAD-fmk (Fig. 7D, hatched bars). The specificity of the mAb 41-2-induced differential was demonstrated by the complete lack of any increase in doxorubicin-induced cell detachment in the presence of another HeLa-CDCP1 cell surface-reacting mAb 29-7 recognizing CD44 (data not shown).
CDCP1 Naturally Expressed in Tumor Cells Facilitates Experimental and Spontaneous Metastasis
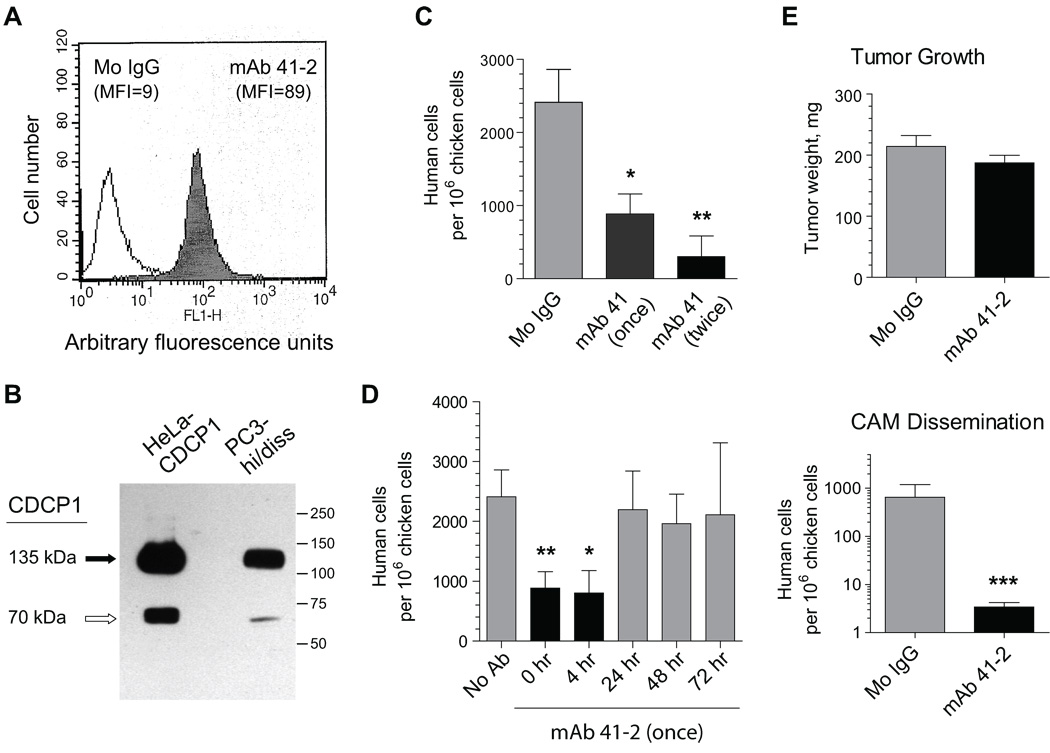
To expand our findings to tumor cells endogenously expressing CDCP1, we employed a recently-selected variant of the CDCP1-positive human prostate PC-3 carcinoma cell line, PC3-hi/diss, which is capable of high levels of dissemination in both spontaneous and experimental metastasis models (Conn et al., unpublished results). Cell surface staining demonstrated relatively high levels of CDCP1 expression in PC3-hi/diss cells (Fig. 8A), while western blotting confirmed that similar to CDCP1 overexpressed in HeLa cells, the naturally expressed CDCP1 was also represented mainly by the 135 kDa species and a 65–70 kDa fragment (Fig. 8B).
FIGURE 8. Functional analysis of CDCP1 naturally expressed in prostate PC3 carcinoma variant.
A. Flow cytometry analysis of CDCP1 expression in PC3-hi/diss variant. PC3-hi/diss cells were incubated with control IgG (open histogram) or mAb 41-2 (closed histogram). MFI, mean fluorescence intensity. B. Western blot analysis of CDCP1 expression in PC3-hi/diss and HeLa-CDCP1 cells was performed by probing SDS-PAGE-separated lysates with goat anti-CDCP1 antibody. Solid and open arrows indicate 135 kDa and 70 kDa CDCP1 protein bands, respectively. Position of mol wt markers in kDa is indicated on the right. C. Inhibition of PC3-hi/diss experimental metastasis with mAb 41-2. PC3-hi/diss cells were injected i.v. into day 12 chick embryos along with mouse IgG (Mo IgG) or mAb 41-2. The following day, a group of embryos received an additional injection of mAb 41-2. Colonization by PC3-hi/diss cells is presented as means ± SEM calculated from pooled data obtained in 2 independent experiments involving from 6 to 12 embryos per antibody treatment. *, P<0.05 and **, P<0.005 compared to IgG control. D. Kinetic analysis of the sensitivity of PC3-hi/diss experimental metastasis to mAb 41-2. PC3-hi/diss cells were injected i.v. into chick embryos with no antibody (No Ab) or with mAb 41-2 injected at the indicated time points. Colonization by PC3-hi/diss cells, determined 5 days after cell injection, is presented as numbers of human cells (means ± SEM) in one out of 2 independent experiments employing from 4 to 12 embryos per time point. P<0.05 in two-tailed (*) and one-tailed (**) t-tests compared to the no antibody control. E. Inhibition of spontaneous metastasis of PC3-hi/diss cells with mAb 41-2. PC3-hi/diss cells were grafted on the CAM of day 10 chick embryos. The following day, the embryos were injected i.v. with mouse IgG (Mo IgG) or mAb 41-2. Presented are means ± SEM of tumor weights (top) and numbers of disseminated human cells (bottom) determined 7 days after cell inoculations by qPCR. ***, P< 0.005 compared to control IgG in Mann-Whitney test.
In the experimental metastasis model, PC3-hi/diss cells were injected into chick embryos along with one or two injections of mAb 41-2 or control IgG. Single and double treatments with mAb 41-2 resulted in a more than 50% and 80% inhibition of experimental metastasis, respectively (Fig. 8C). Thus, similar to the CDCP1 molecule over-expressed in transfected cells, naturally-expressed CDCP1 was also sensitive in vivo to the function-blocking mAb 41-2. We also carried out a kinetic analysis of inhibition of PC3-hi/diss colonization by timed additions of mAb 41-2 and demonstrated that inoculated PC3-hi/diss cells were sensitive to mAb 41-2 during the first 4 hours following injection (Fig. 8D). Importantly, this kinetics of inhibition by mAb 41-2 confirmed our findings observed in the HeLa-CDCP1 model system (Fig. 4C) and clearly pointed to a specific time-frame available for targeting CDCP1 soon after vascular arrest and initial extravasation of tumor cells.
We also determined whether CDCP1-mediated functions can be blocked by mAb 41-2 in a spontaneous metastasis model. When grafted on the CAM of the chick embryos, PC3-hi/diss cells form primary tumors and spontaneously disseminate to the distal CAM and internal organs. Administration o f function-blocking mAb 41-2 did not significantly affect primary tumor formation by PC3-hi/diss cells (Fig. 8E, top), but almost completely abrogated their spontaneous dissemination (Fig. 8E, bottom). Therefore, yet another functional feature of endogenously-expressed CDCP1 was verified in the PC3-hi/diss model, confirming that CDCP1 plays an important mechanistic role in tumor cell dissemination.
Discussion
High expression levels of CUB domain-containing protein 1 (CDCP1) on the cell surface of hematopoietic stem cells/cell progenitors (8, 9) and malignant cells of different tissue origin suggested a functional importance of the antigen in cell differentiation, homing and dissemination. However the precise role of CDCP1 in these processes is still not well defined. The involvement of CDCP1 in cancer has been investigated in several studies indicating that CDCP1 has a functional role in tumor cell metastasis. Different aspects of CDCP1 functionality have been highlighted including elevated expression in several metastatic cancers (1, 2, 8–11, 19), CDCP1-mediated signaling (3–7, 20), and inhibition of CDCP1-mediated metastasis by toxin-conjugated anti-CDCP1 mAb (12). Nevertheless, the question whether CDCP1 expression facilitates early stages of tumor cell dissemination such as primary tumor formation, tumor cell escape, and intravasation or later stages such as vascular survival, arrest, extravasation, and metastatic foci formation, remained largely unexplored. Taking advantage of our unique function-blocking mAbs, 41-2 and 10-D7, specifically targeting cell surface CDCP1, herein we sought to identify specific steps of the metastatic cascade in which CDCP1 functions as a pro-metastatic molecule as well as to define the mechanisms by which ligation of cell surface CDCP1 with a specific anti-CDCP1 mAb inhibits metastasis. To facilitate these studies, tumor cells overexpressing CDCP1 were generated from the parental CDCP1-negative HeLa carcinoma.
CDCP1 expression in HeLa cells appeared to confer these relatively non-aggressive cells with higher efficiency in experimental metastasis in mice, where substantial colonization by HeLa-CDCP1 cells was demonstrated in the lungs, brain and ovaries. Importantly, HeLa-CDCP1 metastatic colonization in mice was extremely sensitive to early treatment with function-blocking mAb 41-2, suggesting that CDCP1 functions soon after tumor cell inoculation into the vasculature. To further investigate which cellular steps of experimental metastasis are promoted by CDCP1, we took advantage of the chick embryo metastasis model, which facilitates detailed kinetic studies and microscopic analyses of live tumor cells disseminating in vivo. We confirmed that HeLa cells overexpressing CDCP1 were more efficient in metastatic colonization in chick embryos and that similar to the mouse model, this colonization was sensitive to anti-CDCP1 mAb 41-2. In addition to mAb 41-2, another anti-CDCP1 mAb, 10-D7, also blocked colonization of HeLa-CDCP1 cells. Interestingly, both 41-2 and 10-D7 mAbs were generated independently via subtractive immunization, an approach that seems to result in high-frequency production of function-blocking antibodies exhibiting exceptional affinity towards their respective antigens (2, 21–24).
Binding of mAb 41-2 to HeLa-CDCP1 cells resulting in ligation of cell surface CDCP1, could trigger antigen clustering and signal transduction. The existence of several tyrosine phosphorylation sites in the C-terminus of CDCP1 originally suggested outside-in signaling through phosphorylation by tyrosine kinases (2), which was then ascribed later to members of the src kinase family (4, 5) and then to tumor cell functions such as anoikis resistance and metastatic potential of lung and gastric carcinoma cells (6, 7). To address these relevant issues, we generated HeLa cells expressing a critical CDCP1 mutant, HeLa-CDCP1-Y734F. In contrast to HeLa cells expressing wild type of CDCP1, their Y734F counterpart completely lacks tyrosine phosphorylation, confirming the singular importance of Y734 for phosphorylation of the whole native molecule (5, 6). However, despite complete lack of tyrosine phosphorylation, HeLa-CDCP1-Y734F mutant exhibited high levels of metastatic colonization as well as sensitivity to mAb 41-2. Therefore, with regard to the inhibitory mechanisms of the anti-CDCP1 mAb 41-2, blocking of CDCP1 functions in our in vivo models apparently does not involve outside-in signaling mediated via tyrosine phosphorylation of CDCP1.
To delineate the in vivo mechanisms by which mAb 41-2 inhibits experimental metastasis of tumor cells expressing CDCP1, we performed quantitative analyses of metastatic colonization by HeLa-CDCP1 cells in the absence or presence of mAb 41-2 over a detailed time course. First, immunohistological studies indicated that mAb 41-2 targeted individual Hela-CDCP1 cells following inoculation rather than inhibited outgrowth of established metastatic foci. This conclusion was corroborated by qPCR analysis demonstrating a clear delay in colonization kinetics produced by mAb 41-2 versus control mouse IgG. Furthermore, mAb 41-2 injections performed at different time intervals after HeLa-CDCP1 cell inoculations clearly pointed to the narrow, approximately 8-hour window open for the inhibition of CDCP1-dependent experimental metastasis. Importantly, this finding was confirmed by kinetic studies performed with the prostate carcinoma PC3-hi/diss cells naturally expressing CDCP1. Altogether, these findings for the first time indicated that CDCP1 is likely involved in early stages of metastatic colonization, namely after initial cell arrest in the vasculature and before the beginning of multicellular foci formation.
Microscopy studies of live HeLa-CDCP1 cells were performed to address the question of which precise steps in the early stages of experimental metastasis were affected by mAb 41-2. The findings demonstrated that mAb 41-2 induced enhanced fragmentation of HeLa-CDCP1 cells soon after their extravasation from terminal capillaries, where the cells initially arrest after inoculation. These fragmented HeLa-CDCP1 cells, the percentage of which increased substantially upon in vivo mAb 41-2 treatment, closely resembled apoptotic bodies characteristic of cells that underwent apoptosis (14, 25) and appeared as cells with blebbed cytoplasm and condensed, disintegrated nuclei. These morphological characteristics are the same features observed in HeLa cells that were induced by drugs to undergo apoptosis in vitro (13). Early stages of experimental metastasis in mice are associated with a substantial clearing of inoculated tumor cells, partially due to apoptotic events involving caspase activation in approximately 10% of cells that reached the lung vasculature (26). Similarly, natural apoptotic fragmentation of HeLa-CDCP1 cells was observed in vivo in our chick embryo experimental metastasis model where quantification of apoptotic bodies demonstrated their gradual increase to 8–9% within 48 hr after cell inoculation with control IgG. However, if HeLa-CDCP1 cells were co-injected with mAb 41-2, the percentage of in vivo fragmented cells increased more than 6-fold by 12 hr. Importantly, this increase was completely abrogated with z-VAD-fmk, which brought the percentage of apoptotic bodies to levels even lower than those observed in control. Our results are consistent with findings demonstrating that prevention of caspase activation during chemotherapeutic agent-induced apoptosis in vitro leads to the inhibition of apoptotic body formation (14). Furthermore, metastatic colonization in mice, which is normally accompanied by 5–10% levels of apoptosis of i.v. inoculated cells, could be substantially inhibited upon an additional 2–2.5 fold increase of tumor cell apoptosis (26), providing a clear precedent for a direct link between enhanced apoptosis and diminished metastasis.
Inhibition of experimental metastasis by mAb 41-2 is fundamentally different from the recently-described, not unexpected inhibition of prostate carcinoma metastasis by the highly cytotoxic saporin conjugated to human CDCP1-specific mAb 25A11 (12). In the case of function-blocking mAb 41-2, inhibition of tumor cell metastasis is CDCP1-specific and likely enhances the apoptotic rate of tumor cells during or soon after extravasation. Enhancing the apoptotic rate of tumor cells that reached the blood stream and arrested in the vasculature, appears to constitute an important approach to regulate the outcome of metastatic events since extravasation was recently demonstrated to be rate-limiting in the metastatic cascade (27). The contention that mAb 41-2 can enhance apoptosis in CDCP1-positive cells experiencing stress was further validated in vitro, under pro-apoptotic conditions induced by doxorubicin where mAb 41-2 specifically increased 2–2.5 times the percentage of detached apoptotic cells while another mAb, specifically binding to cell surface CD44 did not produce such effect. Conversely, CDCP1 expression was demonstrated to confer HeLa cells with the ability to better withstand apoptosis-inducing conditions. Although pro-apoptotic conditions were drug-induced in this in vitro system, it allowed us to highlight anti-apoptotic properties of CDCP1. Furthermore, these results supported our suggestion that ligation of CDCP1 with function-blocking mAb 41-2, resulting in the inhibition of experimental metastasis, is linked to events involved in natural apoptosis triggered in vivo by stress during the traumatic extravasation step, as was indicated by our live cell imaging kinetic studies.
The mechanisms of antibody-mediated inhibition of metastasis can involve blocking of functionally active adhesion sites in cell-associated matrices. Thus anti-laminin single chain antibody was demonstrated to effectively inhibit establishment and growth subcutaneous tumors in mice (28). Therefore, in a search of alternative mechanisms involved in the inhibition of tumor cell metastasis by CDCP1 antibody ligation, we investigated whether mAb 41-2 is capable of blocking tumor cell adhesion to cells and ECM proteins. However, our in vitro findings indicated that mAb 41-2-mediated inhibition of tumor cell metastasis apparently did not involve blocking interactions between the cell surface expressed CDCP1 and putative ligands on endothelial cells and mesenchymal fibroblasts, i.e. the two major cell types that would be encountered by tumor cells during vascular arrest and extravasation. Our data indicate that strong CDCP1-specific receptors are apparently not present on the surface of HeLa-CDCP1 cells, endothelial cells, or fibroblasts. The data also indicate that type I collagen and the components of Matrigel do not present strong ligand-like motifs to cell surface-expressed CDCP1. However, slight but statistically significant inhibition of HeLa-CDCP1 cell adhesion to fibronectin in a short in vitro assay may indicate that ligation of CDCP1 in vivo during much longer time frame of experimental metastasis could be associated with abrogation of ligand binding. This abrogation could be due to diminishment of cell surface CDCP1 due to antibody-induced internalization, which in turn, could elicit anoikis-like mechanisms ultimately leading to cell death at early stages of experimental metastasis. In addition to the established mechanism of antibody-induced apoptosis via binding to death receptors (25), destabilization of actin cytoskeleton also has been implemented as one of the possible mechanisms of antibody-mediated apoptosis (29). Induction of death via a classic apoptotic pathway has been recently shown for mAb SC104 raised against a cell surface antigen expressed in colorectal tumors (30). Interestingly, mAb SC104 demonstrated additive tumor cell killing when used in combination with cytotoxic drugs. Direct apoptosis-inducing abilities have also been ascribed for mAb Pro 1.5 generated by subtractive immunization against prostate cancer cells (24). The precise intracellular mechanisms triggered by ligation of cell surface CDCP1 with mAb 41-2 and ultimately leading to inhibition of experimental metastasis, are not yet established, but are under active investigation.
Our novel findings implicating CDCP1 in the extravasation step of tumor metastasis were further extended to tumor cells naturally expressing CDCP1, i.e. PC3-hi/diss cells. Similarly to HeLa-CDCP1 cells, experimental metastasis of PC3-hi/diss cells was efficiently inhibited by mAb 41-2 during a narrow time frame, again indicating the likely functional importance of CDCP1 during tumor cell extravasation. In addition to experimental metastasis in the chick embryo model system, PC3-hi/diss cells form sizable tumors and spontaneously intravasate and disseminate into secondary organs. Therefore, this model system allowed us to address whether ligation of CDCP1 with mAb 41-2 also inhibited natural spontaneous metastasis of tumor cells. Remarkably, one i.v. inoculation of mAb 41-2 dramatically reduced dissemination of PC3-hi/diss cells from primary tumors. In conjunction with the likely role of CDCP1 in extravasation, indicated by our experimental metastasis data, kinetic analysis of spontaneous dissemination by live cell imaging microscopy would allow us in the future to determine whether ligation of CDCP1 by mAb 41-2 also prevents intravasation of CDCP1-expressing tumor cells, one of the earliest and possibly rate-limiting steps in the metastatic cascade. By employing quantitative and kinetic in vivo analyses, our analytical probing for the function of CDCP1 in cancer progression now provides new insights as to where and when the cell surface CDCP1 functions as a pro-survival molecule in the metastatic cascade.
Materials and Methods
Cell Lines and Culture Conditions
The HeLa cells and PC-3 cells were purchased from ATCC and maintained in DMEM-10% FCS (HyClone) (D10). HeLa cells were transfected using Lipofectamine 2000 with CDCP1 cDNA (2) in the pcDNA3.1-neo vector (Invitrogen). Several CDCP1-positive clones were combined to generate a HeLa-CDCP1 cell line. Control HeLa-neo cells were generated by transfection of parental cells with the empty vector. HeLa cells stably expressing tyrosine-to-phenylalanine mutant of CDCP1 (CDCP1-Y734F) were generated by transfection with Flag-tagged construct created by site-directed mutagenesis of full length wild type CDCP1 (CDCP1-WT) using PfuUltra (Stratagen). A high disseminating variant of the prostate carcinoma PC-3 cell line (PC3-hi/diss) was generated by serial passaging of primary tumors developed on the CAM of chick embryos. Subconfluent cell cultures were briefly treated with trypsin-EDTA, washed with D10 and serum-free DME (SF-DMEM), and resuspended in SF-DMEM.
Antibodies
The following CDCP1-specific antibodies were used in the study: mAb 41-2, generated by subtractive immunization with a non-metastatic population versus a metastatic population of epidermoid HEp-3 carcinoma (2); mAb 10-D7, also generated by subtractive immunization, but using HeLa-neo and HeLa-CDCP1 cells as tolerogen and immunogen, respectively; and goat antibody ab1377, generated against the C-terminal peptide of CDCP1 (Abcam). Murine anti-phosphotyrosine mAb 4G10 and rabbit antibody #9662 recognizing pro- and activated species of caspase-3 were purchased from Cell Signaling.
Flow Cytometry
Cells were incubated first with 2 µg/mL of mAb 41-2 or 10-D7, then with FITC-conjugated goat anti-mouse antibody (Sigma), and analyzed in a flow cytometer (Becton Dickinson).
Experimental Metastasis in Mice
Female 6–8 week-old immunodeficient SCID or NOD-SCID mice (TSRI breeding facility) were injected i.v. with 1×106 HeLa-CDCP1 or HeLa-neo cells. Where indicated, 100 µg of mAb 41-2 or mouse IgG (Jackson ImmunoResearch) were inoculated with cell suspensions. Additional antibody injections were performed i.p. on days 2 and 3 following cell inoculations. Three (NOD-SCID) or four weeks (SCID) later, the mice were sacrificed and internal organs were harvested to determine numbers of human tumor cells.
Experimental and Spontaneous Metastasis in Chick Embryos
Experimental metastasis was performed in chick embryos developed from fertilized SPAFAS White Leghorn eggs (Charles River). On day 12 of incubation, the embryos were injected i.v. with 5×104 or 1×105 cells, as described (31). CDCP1-specific mAbs 41-2 or 10-D7 or control IgG were inoculated i.v. at 50 µg per embryo at indicated time points. On day 5, portions of the CAM were harvested to determine numbers of human tumor cells.
Spontaneous metastasis in chick embryos was performed as described (31). On day 10 of incubation, 2×106 PC3-hi/diss cells were grafted on the CAM. The following day, the embryos were injected i.v. with 50 µg mAb 41-2 or control IgG. On day 7 after cell grafting, the primary tumors were excised and weighed, and portions of the CAM were harvested and analyzed for numbers of human cells.
Quantitation of Human Tumor Cells by Real-Time Quantitative PCR (qPCR)
Numbers of human cells within murine or chick embryo tissues were determined by qPCR performed exactly as described (31) using a standard curve generated by serial dilutions of human tumor cells within a constant number (106) of chick embryo or mouse fibroblasts.
Immunohistochemistry
CAM samples were fixed in Zn-formalin and embedded in paraffin. HeLa-CDCP1 cells were visualized by immunostaining with anti-human pan-cytokeratin mAbs (C 2562, Sigma) essentially as described (31). Digital images were captured using Olympus BX60 microscope equipped with a digital DVC video camera and processed with SC3 Adobe Photoshop software (Adobe Systems, Inc.).
Live Cell Imaging
HeLa cells were labeled with 2 µM green CellTracker CMFDA and injected i.v. at 1×105 cells per embryo with 50 µg mAb 41-2 or control mouse IgG. Where indicated, HeLa-CDCP1 cells were pre-incubated for 2 hr in 50 µM z-VAD-fmk (R&D). To highlight vasculature, the embryos were injected with 50 µg of rhodamine-conjugated Lens culinaris agglutinin (LCA, Vector). At indicated time points, the embryos were sacrificed and the CAM was stretched on glass slides, and examined in a Carl Zeiss Axio Imager.M1m microscope. Digital images were taken with Axiovision Rel. 4.6 software (Carl Zeiss MicroImaging GmbH) and processed with CS3 Adobe Photoshop software.
Western Blotting and Immunoprecipitation
The cells were lysed in modified RIPA (mRIPA) buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA) with protease inhibitors (Sigma). Equivalent amounts of protein (20 µg) were separated on SDS-PAGE 4–20% pre-cast gels (Invitrogen) and transferred onto Immobilon-P membranes. The membranes were blocked with 5% non-fat dry milk in PBS-0.05% Tween-20 (PBS-Tw) and incubated with 1 µg/mL of primary antibodies. To analyze tyrosine phosphorylation, CDCP1 was immunoprecipitated from cell lysates (0.5–1 mg) with 2 µg mAb 41-2. Precipitated proteins were eluted with SDS buffer, separated by SDS-PAGE, and transferred to Immobilon-P membranes. The blots were blocked with 5% BSA/PBS-Tw, incubated with 1 µg/mL anti-phosphotyrosine mAb 4G10, followed by incubation with anti-mouse (Bio-Rad) or anti-goat (Pierce) HRP-conjugated antibodies. Bound antibodies were visualized using SuperSignal West Pico Chemiluminescent substrate (Pierce).
Cell-Cell Interactions and Cell Adhesion
To analyze homotypic adhesion of HeLa-CDCP1 cells, confluent layers of HeLa-neo or HeLa-CDCP1 cells grown in D10 in 24-well clusters, were washed in SF-DMEM and overlaid with 1×105 HeLa-CDCP1 cells pre-labeled with green fluorescent CellTracker CMFDA (Invitrogen Corp.). To analyze heterotypic cell adhesion, HeLa-neo and HeLa-CDCP1 cells were pre-labeled with green fluorescent CellTracker and plated in SF-DMEM at 5×105 cells per well onto confluent layers of human lung microvascular endothelial cells (Cambrex) or chicken CAM fibroblasts in the presence of 50 µg/mL of control mouse IgG (Jackson ImmunoResearch Laboratories) or mAb 41-2. Following 2 hr or 6 hr incubation, non-adherent cells were gently washed out and remaining cells were detached with trypsin/EDTA and analyzed in a flow cytometer to determine the percentage of green fluorescent cells among total detached cells.
To analyze haptotactic adhesion, HeLa-neo and HeLa-CDCP1 cells were resuspended in SF-DMEM supplemented with 50 µg/mL control mouse IgG or mAb 41-2 and plated onto layers of type I collagen, fibronectin, or growth factor reduced Matrigel, each pre-coated at 5, 10 and 10 µg/mL, respectively. Following incubation for 45 min, non-adherent cells were washed out and adherent cells fixed and stained with 0.2% crystal violet solution in 10% ethanol. After washing with PBS, incorporated dye was extracted from the cells with sodium phosphate (100 mM, pH 4.5) in 50% ethanol and optical density measured at 560 nm.
For haptotactic migration, Transwell filter inserts of 6.5 mm diameter and 8 µm pore size (Corning) were coated on the underside with type I collagen, fibronectin and growth factor reduced Matrigel (Becton Dickinson) in PBS at 5, 10 and 10 µg/mL, respectively, overnight at 4°C. HeLa-neo and HeLa-CDCP1 cells were plated at 1×105 cells per Transwell insert and allowed to migrate in SF-DMEM to the pre-coated surfaces for 24 hr. For chemotactic migration, HeLa cells were placed into uncoated Transwell inserts in SF-DMEM and stimulated to migrate for 48 hr towards 5% FCS placed into the outer Transwell chamber in the presence of 25 µg/mL of control mouse IgG or mAb 41-2. The transmigrated cells were detached with trypsin/EDTA and counted. Matrigel invasion was performed exactly as described for chemotactic migration, but Transwell inserts were pre-coated each with 2 µg of low growth factor Matrigel.
Growth of HeLa-CDCP1 cells under non-adherent conditions was evaluated 48 and 72 hours after plating 5×104 cells in 1 mL D-10 per well of 12-well ultra-low adhesion cluster (Nunc) in the presence of 25 µg/mL mouse IgG or mAb 41-2. Single cell suspensions were prepared by treatment with trypsin/EDTA and cell viability was determined by trypan blue exclusion.
Induction of Apoptosis in vitro
HeLa cells were seeded at 0.5×106 cells per well of a 12 well plate. After overnight incubation, the D10 was changed to SF-DMEM supplemented with indicated antibodies (50 µg/mL) in the presence or absence of 5 µM doxorubicin (Sigma). Where indicated, HeLa-CDCP1 cells were pre-incubated for 2 hr with 50 µM z-VAD-fmk before addition of mAb 41-2 and/or doxorubicin. The cells that had undergone apoptosis detach from the bottom of the plate (13) and therefore the percentage of non-adherent cells was used as a measure of apoptosis. The detached apoptotic cells were harvested and counted after 48-hr incubation. To confirm apoptotic status of doxorubicin-treated cells, caspase activation was verified by western blotting and nuclei fragmentation by fluorescent microscopy after staining of non-adherent methanol-fixed cells with 1 µg/mL DAPI (Invitrogen).
Data Analysis and Statistics
Data processing and statistical analysis were performed using GraphPad Prizm® (GraphPad Software). All experiments were performed at least twice and the numbers of experiments, animals and samples are indicated in the text or figure legends. Data are presented as means ± SEM calculated from numerical data from a representative or pooled experiments. Where indicated, fold differences were determined from pooled data as the ratios of numerical values for individual embryos over a mean of the control in the corresponding experiment. Student’s two-tailed and one-tailed t-tests and Mann-Whitney test were used to determine P value of differences between the means of experimental data sets; P<0.05 was considered as statistically significant.
Acknowledgements
This work was supported by the NIH and NCI grants CA129484 and CA105412 (JPQ), T32CA77109-08 (EMC) and T32HL07195 (VCA), and the National Health and Medical Research Council of Australia 339732 (JDH). We thank Chenxing Li for her excellent technical assistance.
References
- 1.Scherl-Mostageer M, Sommergruber W, Abseher R, Hauptmann R, Ambros P, Schweifer N. Identification of a novel gene, CDCP1, overexpressed in human colorectal cancer. Oncogene. 2001;20(32):4402–4408. doi: 10.1038/sj.onc.1204566. [DOI] [PubMed] [Google Scholar]
- 2.Hooper JD, Zijlstra A, Aimes RT, et al. Subtractive immunization using highly metastatic human tumor cells identifies SIMA135/CDCP1, a 135 kDa cell surface phosphorylated glycoprotein antigen. Oncogene. 2003;22(12):1783–1794. doi: 10.1038/sj.onc.1206220. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt AS, Erdjument-Bromage H, Tempst P, Craik CS, Moasser MM. Adhesion signaling by a novel mitotic substrate of src kinases. Oncogene. 2005;24(34):5333–5343. doi: 10.1038/sj.onc.1208582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvares SM, Dunn CA, Brown TA, Wayner EE, Carter WG. The role of membrane microdomains in transmembrane signaling through the epithelial glycoprotein Gp140/CDCP1. Biochim Biophys Acta. 2008;1780(3):486–496. doi: 10.1016/j.bbagen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benes CH, Wu N, Elia AE, Dharia T, Cantley LC, Soltoff SP. The C2 domain of PKCdelta is a phosphotyrosine binding domain. Cell. 2005;121(2):271–280. doi: 10.1016/j.cell.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Uekita T, Jia L, Narisawa-Saito M, Yokota J, Kiyono T, Sakai R. CUB domain-containing protein 1 is a novel regulator of anoikis resistance in lung adenocarcinoma. Mol Cell Biol. 2007;27(21):7649–7660. doi: 10.1128/MCB.01246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uekita T, Tanaka M, Takigahira M, et al. CUB-domain-containing protein 1 regulates peritoneal dissemination of gastric scirrhous carcinoma. Am J Pathol. 2008;172(6):1729–1739. doi: 10.2353/ajpath.2008.070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conze T, Lammers R, Kuci S, et al. CDCP1 is a novel marker for hematopoietic stem cells. Ann N Y Acad Sci. 2003;996:222–226. doi: 10.1111/j.1749-6632.2003.tb03249.x. [DOI] [PubMed] [Google Scholar]
- 9.Buhring HJ, Kuci S, Conze T, et al. CDCP1 identifies a broad spectrum of normal and malignant stem/progenitor cell subsets of hematopoietic and nonhematopoietic origin. Stem Cells. 2004;22(3):334–343. doi: 10.1634/stemcells.22-3-334. [DOI] [PubMed] [Google Scholar]
- 10.Awakura Y, Nakamura E, Takahashi T, et al. Microarray-based identification of CUB-domain containing protein 1 as a potential prognostic marker in conventional renal cell carcinoma. J Cancer Res Clin Oncol. 2008;134(12):1363–1369. doi: 10.1007/s00432-008-0412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda JI, Oda T, Inoue M, et al. Expression of CUB domain containing protein (CDCP1) is correlated with prognosis and survival of patients with adenocarcinoma of lung. Cancer Sci. 2008 doi: 10.1111/j.1349-7006.2008.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siva AC, Wild MA, Kirkland RE, et al. Targeting CUB domain-containing protein 1 with a monoclonal antibody inhibits metastasis in a prostate cancer model. Cancer Res. 2008;68(10):3759–3766. doi: 10.1158/0008-5472.CAN-07-1657. [DOI] [PubMed] [Google Scholar]
- 13.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9(3):231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Reedy MC, Hannun YA, Obeid LM. Inhibition of caspases inhibits the release of apoptotic bodies: Bcl-2 inhibits the initiation of formation of apoptotic bodies in chemotherapeutic agent-induced apoptosis. J Cell Biol. 1999;145(1):99–108. doi: 10.1083/jcb.145.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gamen S, Anel A, Perez-Galan P, et al. Doxorubicin treatment activates a Z-VAD-sensitive caspase, which causes deltapsim loss, caspase-9 activity, and apoptosis in Jurkat cells. Exp Cell Res. 2000;258(1):223–235. doi: 10.1006/excr.2000.4924. [DOI] [PubMed] [Google Scholar]
- 16.Panaretakis T, Laane E, Pokrovskaja K, et al. Doxorubicin requires the sequential activation of caspase-2, protein kinase Cdelta, and c-Jun NH2-terminal kinase to induce apoptosis. Mol Biol Cell. 2005;16(8):3821–3831. doi: 10.1091/mbc.E04-10-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202(12):1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huigsloot M, Tijdens IB, Mulder GJ, van de Water B. Differential regulation of doxorubicin-induced mitochondrial dysfunction and apoptosis by Bcl-2 in mammary adenocarcinoma (MTLn3) cells. J Biol Chem. 2002;277(39):35869–35879. doi: 10.1074/jbc.M200378200. [DOI] [PubMed] [Google Scholar]
- 19.Perry SE, Robinson P, Melcher A, et al. Expression of the CUB domain containing protein 1 (CDCP1) gene in colorectal tumour cells. FEBS Lett. 2007;581(6):1137–1142. doi: 10.1016/j.febslet.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Brown TA, Yang TM, Zaitsevskaia T, et al. Adhesion or plasmin regulates tyrosine phosphorylation of a novel membrane glycoprotein p80/gp140/CUB domain-containing protein 1 in epithelia. J Biol Chem. 2004;279(15):14772–14783. doi: 10.1074/jbc.M309678200. [DOI] [PubMed] [Google Scholar]
- 21.Brooks PC, Lin JM, French DL, Quigley JP. Subtractive immunization yields monoclonal antibodies that specifically inhibit metastasis. J Cell Biol. 1993;122(6):1351–1359. doi: 10.1083/jcb.122.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zijlstra A, Testa JE, Quigley JP. Targeting the proteome/epitome, implementation of subtractive immunization. Biochem Biophys Res Commun. 2003;303(3):733–744. doi: 10.1016/s0006-291x(03)00357-7. [DOI] [PubMed] [Google Scholar]
- 23.Zijlstra A, Lewis J, Degryse B, Stuhlmann H, Quigley JP. The inhibition of tumor cell intravasation and subsequent metastasis via regulation of in vivo tumor cell motility by the tetraspanin CD151. Cancer Cell. 2008;13(3):221–234. doi: 10.1016/j.ccr.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldstein NI, Fisher PB. Surface-epitope masking (SEM): an immunological subtraction approach for developing monoclonal antibodies targeting surface-expressed molecules. Methods Mol Biol. 2007;383:245–258. doi: 10.1007/978-1-59745-335-6_16. [DOI] [PubMed] [Google Scholar]
- 25.Huerta S, Goulet EJ, Huerta-Yepez S, Livingston EH. Screening and detection of apoptosis. J Surg Res. 2007;139(1):143–156. doi: 10.1016/j.jss.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 26.Tsai YC, Mendoza A, Mariano JM, et al. The ubiquitin ligase gp78 promotes sarcoma metastasis by targeting KAI1 for degradation. Nat Med. 2007;13(12):1504–1509. doi: 10.1038/nm1686. [DOI] [PubMed] [Google Scholar]
- 27.Kouros-Mehr H, Bechis SK, Slorach EM, et al. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell. 2008;13(2):141–152. doi: 10.1016/j.ccr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanz L, Kristensen P, Blanco B, et al. Single-chain antibody-based gene therapy: inhibition of tumor growth by in situ production of phage-derived human antibody fragments blocking functionally active sites of cell-associated matrices. Gene Ther. 2002;9(15):1049–1053. doi: 10.1038/sj.gt.3301725. [DOI] [PubMed] [Google Scholar]
- 29.Tezel G, Wax MB. The mechanisms of hsp27 antibody-mediated apoptosis in retinal neuronal cells. J Neurosci. 2000;20(10):3552–3562. doi: 10.1523/JNEUROSCI.20-10-03552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durrant LG, Harding SJ, Green NH, Buckberry LD, Parsons T. A new anticancer glycolipid monoclonal antibody, SC104, which directly induces tumor cell apoptosis. Cancer Res. 2006;66(11):5901–5909. doi: 10.1158/0008-5472.CAN-05-3812. [DOI] [PubMed] [Google Scholar]
- 31.Deryugina EI, Zijlstra A, Partridge JJ, et al. Unexpected effect of matrix metalloproteinase down-regulation on vascular intravasation and metastasis of human fibrosarcoma cells selected in vivo for high rates of dissemination. Cancer Res. 2005;65(23):10959–10969. doi: 10.1158/0008-5472.CAN-05-2228. [DOI] [PubMed] [Google Scholar]