Abstract
Acute lung injury (ALI) is a frequent pulmonary complication in critically ill patients. We characterized a murine model of LPS-induced ALI, focusing on T helper cells. Following LPS administration, BAL lymphocytes were increased as well as neutrophils, IL-6, TNF-α, and albumin. Analysis of LPS-induced T cells revealed increased T helper cell associated cytokines (IL-17A, IL-17F, and IL-22), expression of CD69, a cell activation marker, forkhead box P3 (Foxp3), and cytotoxic T lymphocyte antigen 4 (CTLA4) in CD4+ T cells. Administration of anti-CTLA4 antibody decreased LPS-induced BAL albumin and IL-17A, while increasing CD4+Foxp3+ cell number and Foxp3 expression in CD4+Foxp3+ cells. These data suggest that pulmonary LPS administration promotes CD4+ T cells and that T cell pathways involving CTLA4 contribute to ALI.
Keywords: T cells, CTLA4, LPS, acute lung injury
Introduction
Acute lung injury (ALI) is associated with acute respiratory distress syndrome (ARDS), a major cause of severe respiratory failure with high morbidity and mortality in critically ill patients (1, 2). The pathogenesis of ARDS remains ill-defined, and treatment of ARDS remains largely supportive. ALI models include characterization of lipopolysaccharide (LPS)-induced pulmonary immune responses (3). LPS, a component of gram-negative bacteria, binds to a signal-transducing integral membrane protein, Toll-like receptor 4 (TLR4), which is expressed on antigen-presenting cells (e.g., alveolar macrophages and lung dendritic cells). TLRs allow vertebrates to recognize a vast range of microbial products in a rapid innate immune response (4, 5). TLRs have recently been found to also be expressed on T cells, suggesting a potential pathway by which LPS may directly affect T cell activity (6-8). Innate and adaptive immune responses to invading microbes (e.g., gram-negative bacteria) are tightly interwoven and engage the total immunological capability of the host. Innate immunity is the first line of lung defense, includes structural barriers, alveolar macrophages, neutrophils, natural killer cells, and dendritic cells. Adaptive immunity, which is promoted by innate immunity, is composed of antigen specific lymphocytes that eliminate or prevent pathogenic challenges. Lymphocytes, including T and B cells, are major cells of the adaptive immune system. T lymphocytes (CD4+ T helper cells) have no cytotoxic or phagocytic activity and cannot kill infected cells or clear pathogens, but manage the immune response by directing other cells to perform these tasks.
Experimental depletion of CD4+ T lymphocytes in mice with Staphylococcus aureus pleural empyema is associated with decreased bacterial clearance (9). Sepsis induces a striking depletion of lymphocytes, leading to an inability of the host to combat the ongoing source of infection and predisposing to secondary opportunistic infections (10). Also, sepsis activates the remaining lymphocytes (11), and inhibition of lymphocyte apoptosis may improve sepsis outcomes (12). Infection is a precipitating factor for ARDS, and T cells are considered to play an important role in host defense for infection. Prior studies have shown that lymphocytes, in addition to neutrophils, infiltrate the lung in ALI models (13-15). Recently, D’Alessio et al. demonstrated that T regulatory (Treg) T cell subsets contribute to the resolution of ALI (16). Analysis of additional T cell subsets and T cell pathways in ALI remain ill-defined. In the present study, we characterize a model of LPS-induced ALI. To elucidate which T lymphocytes contribute to ALI, we examined parameters related to T cells, including T cell number, activity, and expression of cytotoxic T lymphocyte antigen 4 (CTLA4) and forkhead box P3 (Foxp3), which are T cell dependent suppressive molecules. Also, we tested whether administration of anti-CTLA4 antibody would impact ALI.
Materials and Methods
Mice
Female BALB/c mice (8 - 10 week-old) were purchased from Harlan Laboratories (Indianapolis, IN). T and B cell deficient recombination activating genes (RAG) knockout (RAG KO) mice (BALB/c background) were purchased from The Jackson Laboratory (Bar Harbor, ME). The mice were maintained according to the guidelines of the University of California, San Diego (UCSD) Animal Care Program.
Materials
Lipopolysaccharide (LPS) from Escherichia coli 055:B5 purified by gel-filtration chromatography was purchased from Sigma-Aldrich (St. Louis, MO). Mouse anti-CTLA4 antibody (αCTLA4, UC10-4F10-11) was purchased from Bio X Cell (West Lebanon, NH). Hamster IgG1, κ Isotype control (IgG, A19-3) was purchased from BD Biosciences (San Jose, CA).
Lipopolysaccharide-induced acute lung injury
Mice were anesthetized with isoflurane (Minrad Inc., Bethlehem, PA). The tongue was gently extended and the tip of an otoscope was introduced to reach the trachea. Once in the trachea, LPS (100 μg) in 50 μl of phosphate buffered saline (PBS) was administered through the cone of the otoscope. In the RAG KO mice experiments, LPS was administered intratracheally (i.t.) to wild type (WT) or RAG KO mice and the mice were harvested at day 2 after LPS administration. In the time-course experiments, LPS was administered i.t. to WT mice. PBS was administered to controls. Mice were harvested at indicated time points (day 1 – day 6) after LPS or PBS administration.
Treatment with anti-CTLA4 antibody
Mice were injected intraperitoneally with anti-CTLA4 antibody (αCTLA4, 100 μg) in 200 μl of PBS one day prior to intratracheal administration of LPS. Hamster IgG1 (IgG, 100 μg) was administered to controls. αCTLA4-treated mice were harvested at indicated time points (day 1 – day 6) after LPS administration. IgG-treated mice were harvested at days 2 and 4.
Bronchoalveolar lavage harvest and cell count
Bronchoalveolar lavage (BAL) fluid was obtained by cannulating the trachea and lavaging the lungs three times with 1 ml of PBS containing 0.6 mM EDTA. BAL fluid cells were pelleted and the supernatant was stored at −80 °C until use.
BAL fluid cells were counted using a hemocytometer, and resuspended in RPMI-1640 (5 × 105 cells/ml). Slides for differential cell count were prepared with Cytospin (Thermo Scientific, Waltham, MA), and then fixed and stained with Diff-Quick (Imeb Inc., San Marcos, CA). For each sample, an investigator blinded to the treatment groups performed two counts of 100 cells.
Bronchoalveolar lavage albumin and cytokines
Albumin levels in BAL fluid were measured by ELISA (Alpco Diagnostics, Salem, NH). Samples of BAL fluid were aliquoted in duplicate into 96-well plates (100 μl/well) pre-coated with antibody to specific murine albumin and assayed according to the manufacturer’s instructions. Optical density was measured at 450 nm. Levels of interleukin (IL)-6, TNF-α, IL-2, IL-4, IL-13, and IL-17A in BAL fluid were measured by a multiplexed immunoassay (Millipore, Billerica, MA), and IL-17F, IL-22, IL-23, and IL-27 were measured by a multiplexed immunoassay (Biolegend Inc., San Diego, CA). Median fluorescent intensity was measured by Luminex 100 total system (Luminex, Austin, TX).
Preparation of lung homogenates
Following the BAL procedure, the lungs were perfused with 20 ml saline, and isolated. The lungs were cut into small pieces with scissors in a petri dish and finely chopped, then incubated at 37 °C for 60 minutes in 5 ml per lung of a digestion mixture consisting of collagenase from Clostridium histolyticum, type IV (1 mg/ml, Sigma-Aldrich, St. Louis, MO) and DNase I from bovine pancreas (0.5 mg/ml, Sigma-Aldrich, St. Louis, MO). Cell suspensions were obtained by passing the homogenate through a 70-μm nylon Falcon cell strainer (BD Biosciences, Bedford, MA) to remove pieces of tissue and debris. The pellets were resuspended in 5 ml of 10 mM EDTA in PBS. After lysis of erythrocytes with ammonium chloride potassium bicarbonate (ACK) lysing buffer, cell pellets were resuspended and washed in RPMI-1640 medium.
Flow Cytometric Analysis
Samples of lung homogenates were resuspended in staining buffer (PBS containing 2% fetal bovine serum and 0.05% sodium azide) to be 1 × 107 cells / ml, 100 μl of prepared cells (1 × 106 cells) were then added to each tube. After incubating for 30 min at 4 °C with the following anti-mouse antibodies: CD3 (PECy7), CD4 (FITC), CD8 (APC), CD25 (APC), CD19 (PE), CD69 (PE), or their respective immunoglobulin isotypes (eBioscience, San Diego, CA), samples were then washed twice with staining buffer, fixed by Fixation/Permeabilization solution (eBioscience, San Diego, CA), and incubated overnight. Intracellular staining with PE-conjugated anti-mouse CTLA4 and Foxp3 antibodies was performed using Mouse T regulatory cell staining kit (eBioscience, San Diego, CA). Samples were run on a BD FACSCalibur flow cytometry system (BD, Franklin Lakes, NJ). Data yielded were analyzed on FlowJo v8 (Tree Star, Ashland, Oregon).
Statistical analysis
Data are expressed as means ± SEM. All parameters were evaluated with the two tailed unpaired Student’s t-test or compared by one-way analysis of variance (ANOVA) followed by Bonferroni’s test. P value less than 0.05 was considered significant.
Results
Lymphocytes are present and contribute to the severity of acute lung injury
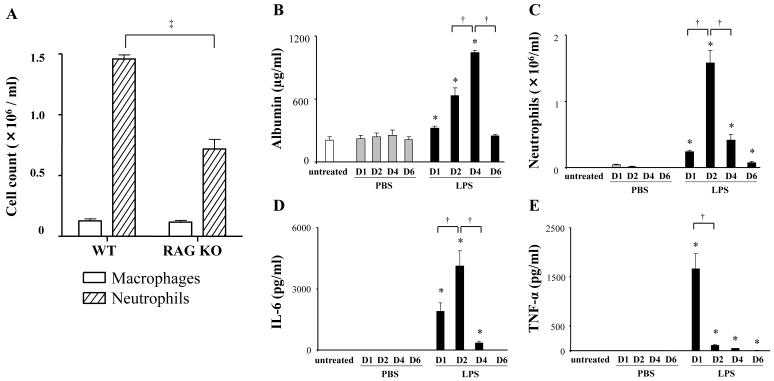
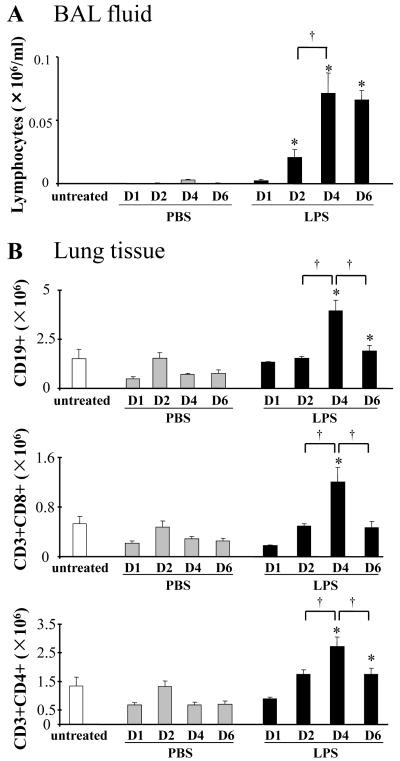
We analyzed a murine model of lipopolysaccharide (LPS)-induced acute lung injury (ALI) in wild type (WT) and lymphocyte-deficient (RAG KO, T and B cell deficient) mice. Compared to WT, RAG KO mice exhibited a significant decrease in bronchoalveolar lavage (BAL) neutrophils (Fig 1A). The decrease in LPS-induced neutrophils in RAG KO suggests that lymphocytes contribute to ALI. In further characterization of ALI in WT mice over 6 days, we analyzed increased pulmonary vascular permeability defined by increased BAL levels of albumin (Fig 1B). BAL albumin production increased at day 1, peaked at day 4, and returned to baseline by day 6. BAL neutrophils in LPS exposed mice also increased at day 1, but exhibited more rapid kinetics, peaking at day 2 and decreasing by day 4 (Fig 1C). LPS-induced ALI is associated with pro-inflammatory cytokines (e.g., IL-6 and TNF-α). LPS increased BAL IL-6, with similar kinetics to the neutrophil count (Fig 1D). LPS also increased TNF-α that decreased by day 2 (Fig 1E). We next examined LPS-induced BAL lymphocytes. In contrast to BAL neutrophils, lymphocytes peaked at day 4 and remained elevated at day 6 (Fig 2A). We also analyzed lymphocyte numbers in lung tissue from LPS exposed mice. The total number of CD19+ (B) cells, CD3+CD8+ (T cytotoxic) cells, and CD3+CD4+ (T helper) cells, respectively, was determined by flow cytometry (Fig 2B). Each subset increased at day 4 following LPS administration, which paralleled the increase in the number of BAL lymphocytes.
Figure 1. Analysis of pulmonary inflammatory responses in a model of LPS-induced ALI.
Lipopolysaccharide (LPS, 100 μg) was administered intratracheally to wild type (WT) Balb/c mice or RAG knockout (RAG KO) mice. Bronchoalveolar lavege (BAL) fluid was collected at day 2 after LPS administration and cell counts were determined by differential staining for neutrophils and macrophages (A). Data are shown as geometric mean ± SEM (n = 3 per group). ‡, RAG KO vs WT (p < 0.05). LPS (100 μg) or phosphate buffered saline (PBS) was administered to WT mice (Balb/c) intratracheally. BAL fluid was collected at indicated days (D1 - D6). Levels of albumin in the BAL fluid were determined by ELISA (B). Absolute neutrophil count in the BAL fluid was determined by differential staining of cells (C). Levels of IL-6, and TNF-α in BAL fluid were determined by a multiplexed immunoassay (D, E). Data are shown as geometric mean ± SEM (n = 3 - 6 per group). *, LPS group vs respective PBS group (p < 0.05); †, p < 0.05.
Figure 2. LPS increases the number of lymphocytes in the BAL and the lungs.
Lipopolysaccharide (LPS, 100 μg) or phosphate buffered saline (PBS) was administered to mice (Balb/c) intratracheally. BAL fluid and lungs were harvested at indicated days (D1 - D6). Lungs were digested as described in Methods. Absolute lymphocyte count in BAL fluid was determined by differential staining of cells (A). Absolute counts of CD19+ (B) cells, CD3+CD8+ (cytotoxic T) cells, and CD3+CD4+ (T helper) cells in lung tissue were determined by flow cytometry (B). Data are shown as geometrical mean ± SEM (n = 3 - 6 per group). *, LPS group vs respective PBS group (p < 0.05); †, p < 0.05.
LPS activates CD4+ T cells and induces Th17 cytokines
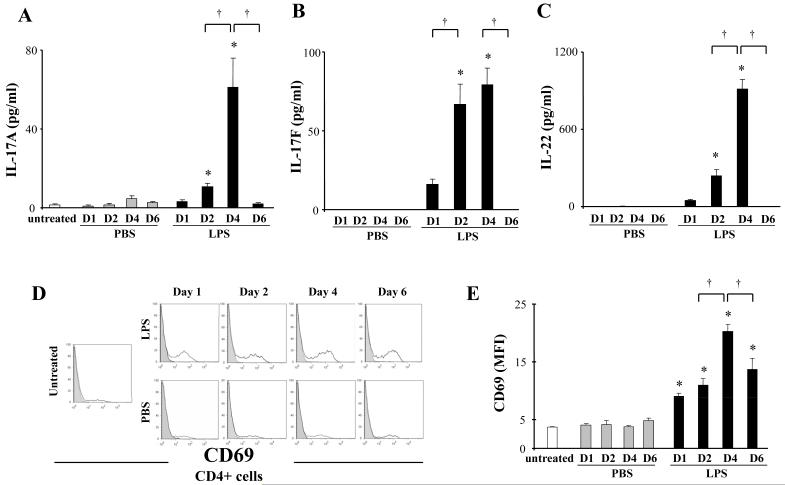
Based on the LPS-induced increase in T helper cells and their established role in inflammation, we focused on analysis of T helper cell associated cytokines (i.e., IL-2, IL-4, IL-13, and IL-17A). IL-17A increased in LPS exposed mice at day 4 (Fig 3A), whereas IL-2, IL-4 and IL-13 were not modified (not shown). We then analyzed other cytokines associated with Th17 cell response (i.e., IL-17F, IL-22, IL-23, and IL-27). IL-17F and IL-22 increased at day 4, similar to IL-17A (Fig 3B, 3C), whereas IL-23 and IL-27 were not detected throughout the time points analyzed (not shown). To evaluate the activities of CD4+ T cells, CD69, a marker of cell activation, was examined in the CD3+CD4+ cell population by flow cytometry. LPS administration increased CD69 expression on CD4+ cells that was highest at day 4, in parallel with increased IL-17A, IL-17F, and IL-22 (Fig 3D, 3E).
Figure 3. LPS activates CD4+ T cells.
Lipopolysaccharide (LPS, 100 μg) or phosphate buffered saline (PBS) was administered to mice (Balb/c) intratracheally. BAL fluid and lungs were harvested at indicated days (D1 - D6). Lungs were digested as described in Methods. Levels of IL-17A, IL-17F, and IL-22 in BAL fluid were determined by a multiplexed immunoassay (A, B, C). Expression levels of CD69 in the CD3+CD4+ cell population in lung tissue were determined by flow cytometry; representative histograms of CD69 expression (shaded areas represent isotype control) (D), mean fluorescence intensity (MFI) for CD69 (E). Data are shown as geometrical mean ± SEM (n = 3 - 6 per group). *, LPS group vs respective PBS group (p < 0.05); †, p < 0.05.
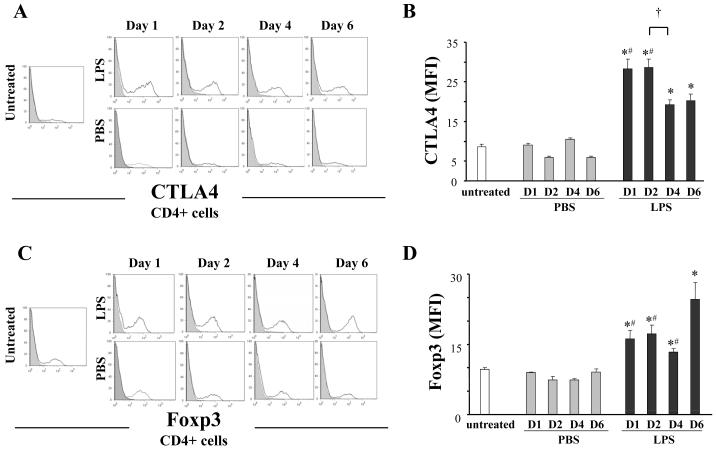
Thus, LPS exposure increased both CD4+ cell number and activity. To define the T cell population modified by LPS exposure, we analyzed lung tissue for 2 markers expressed by T cells, cytotoxic T lymphocyte antigen 4 (CTLA4) and forkhead box P3 (Foxp3). LPS exposure increased mean fluorescence intensity (MFI) of CTLA4 expression (Fig 4A, B). LPS exposure also increased Foxp3 expression which was highest at day 6 (Fig 4C, D).
Figure 4. LPS induces T helper cell-dependent suppressive pathways.
Lipopolysaccharide (LPS, 100 μg) or phosphate buffered saline (PBS) was administered to mice (Balb/c) intratracheally. Lungs were harvested at indicated days (D1 - D6). Lungs were digested as described in Methods. Expression levels of CTLA4 and Foxp3 in the CD3+CD4+ cell population in lung tissue were determined by flow cytometry: representative histograms of CTLA4 and Foxp3 expression (shaded areas represent isotype control) (A, C); mean fluorescence intensity (MFI) for CTLA4 and Foxp3 (B, D). Data are shown as geometrical mean ± SEM (n = 3 - 6 per group). *, LPS group vs respective PBS group (p < 0.05); #, vs LPS D6 (p < 0.05); †, p < 0.05.
Administration of anti-CTLA4 antibody and analysis of CD4+Foxp3+ cell activation
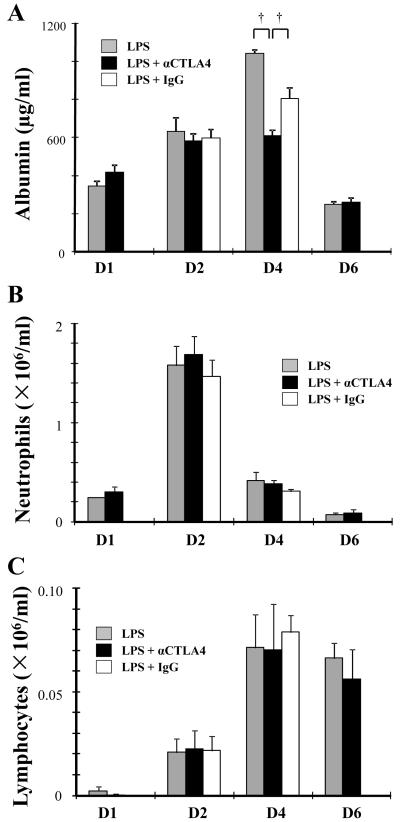
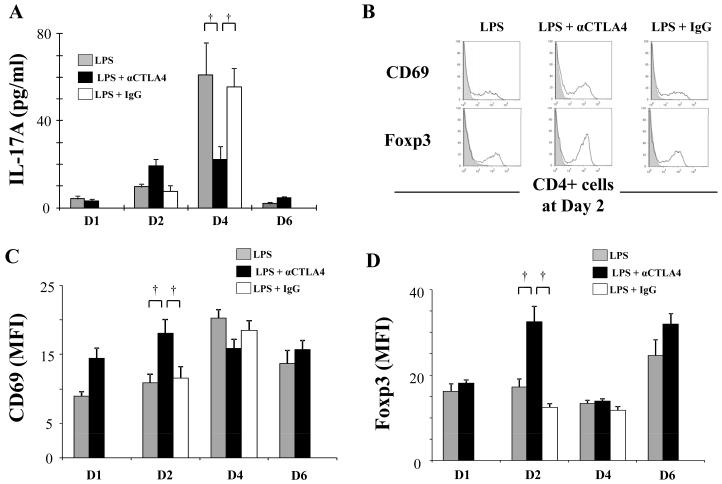
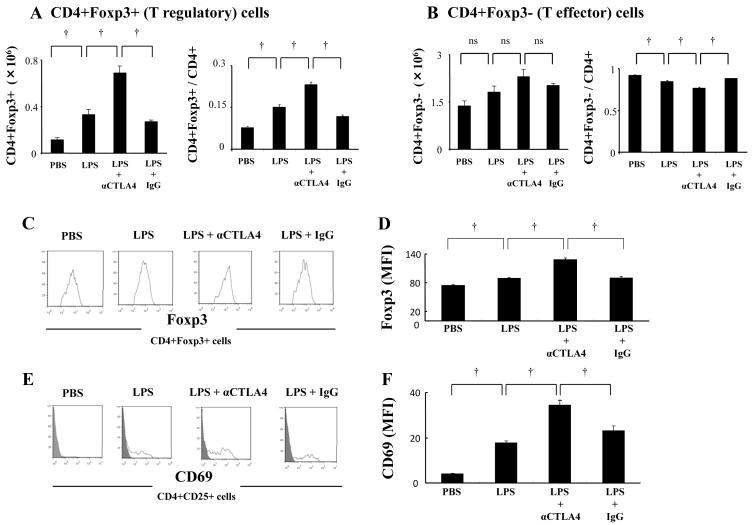
LPS exposure increased CTLA4 expression in CD4+ T cells. To examine the potential role of CTLA4 in ALI, we analyzed the impact of administration of anti-CTLA4 antibody (αCTLA4). BAL albumin, increased at day 4 in LPS exposed mice, was significantly decreased by αCTLA4 administration, suggesting that vascular permeability is reduced (Fig 5A). αCTLA4 significantly reduced LPS-induced BAL IL-17A (Fig 6A) without impacting neutrophil count, lymphocyte number (Fig 5B, C), IL-6, or TNF-α (not shown). Interestingly, the reduction in Th17 cells at day 4 by αCTLA4 was counterbalanced by a significant increase in both CD69 and Foxp3 expression in CD4+ T cells at day 2 (Fig 6B, C, D). We then focused on CD4+Foxp3+ cells at day 2 following LPS administration. Both absolute cell count and ratio of CD4+Foxp3+ cells in the CD4+ cell population were increased by αCTLA4 (Fig 7A). In contrast, the number of CD4+Foxp3− cells was not significantly increased. Also, the ratio of CD4+Foxp3− cells in the CD4+ cell population was decreased by αCTLA4 (Fig 7B). αCTLA4 also increased LPS-induced Foxp3 in CD4+Foxp3+ cells (Fig 7C, D) and LPS-induced CD69 in CD4+CD25+ cells (Fig 7E, F).
Figure 5. αCTLA4 decreases LPS-induced increase in albumin.
Mice were injected intraperitoneally with anti-CTLA4 antibody (αCTLA4, 100 μg) or hamster IgG (IgG, 100 μg) one day prior to intratracheal administration of LPS (100 μg). D6 group was reinjected intraperitoneally with αCTLA4 (100 μg) on day 3. BAL fluid was collected at indicated days (D1 - D6) after the LPS exposure. Levels of albumin in BAL were determined by ELISA (A). Neutrophil and lymphocyte counts in BAL fluid were determined by differential staining of cells (B, C). Data are shown as geometric mean ± SEM (n = 3 - 6 per group). †, p < 0.05.
Figure 6. αCTLA4 modifies LPS-induced cytokine production, CD69 and Foxp3 expression in CD4+ T cells.
Mice were injected intraperitoneally with anti-CTLA4 antibody (αCTLA4, 100 μg) or hamster IgG (IgG, 100 μg) one day prior to intratracheal administration of LPS (100 μg). D6 group was reinjected intraperitoneally with αCTLA4 (100 μg) on day 3. BAL fluid and lungs were collected at indicated days (D1 - D6) after the LPS exposure. Lungs were digested as described in Methods. Levels of IL-17A in BAL fluid were determined by a multiplexed immunoassay (A). Expression of CD69 and Foxp3 in the CD3+CD4+ cell population in lung tissue was determined by flow cytometry: representative histograms of CD69 and Foxp3 expression at day 2 (shaded areas represent isotype control) (B); mean fluorescence intensity (MFI) for CD69 and Foxp3 (C, D). Data are shown as geometric mean ± SEM (n = 3 - 6 per group). †, p < 0.05.
Figure 7. αCTLA4 increases LPS-induced T regulatory cell number and activation.
Mice were injected intraperitoneally with anti-CTLA4 antibody (αCTLA4, 100 μg) or hamster IgG (IgG, 100 μg) one day prior to intratracheal administration of LPS (100 μg). Lungs were collected at day 2 after the LPS exposure and digested as described in Methods. Absolute cell counts and ratio of CD4+Foxp3+ (T regulatory) and CD4+Foxp3− (T effector) cells in the CD4+ cell population were determined by flow cytometry (A, B). Expression of Foxp3 in the CD4+Foxp3 cell population and CD69 in the CD4+CD25+ cell population in lung tissue was determined by flow cytometry: representative histograms of CD69 and Foxp3 expression (shaded areas represent isotype control) (C, E); mean fluorescence intensity (MFI) for Foxp3 and CD69 (D, F). Data are shown as geometric mean ± SEM (n = 3 - 6 per group). †, p < 0.05; ns, not significant.
Discussion
Acute lung injury (ALI) is classically characterized as an innate response, predominantly involving neutrophils. In a murine model of ALI, we focused on the analysis of T cell dependent pathways. Consistent with lung injury models, LPS administration induces BAL neutrophils in wild type mice. Lymphocyte deficient mice were unable to increase neutrophils in response to LPS, suggesting the concept that T cells may contribute to ALI. LPS also increased a number of T cell associated parameters, including lymphocyte number, activity, and IL-17A production. Notably, LPS exposure, an antigen-independent pathway, also increased two molecules found primarily on T cells, cytotoxic T lymphocyte antigen 4 (CTLA4) and forkhead box P3 (Foxp3). Testing the functional role of CTLA4, anti-CTLA4 antibody (αCTLA4) administration to LPS exposed mice resulted in decreased IL-17A and albumin production. These findings suggest that CTLA4 and T cells may contribute to pulmonary inflammatory pathways in ALI.
We analyzed LPS-induced T cells over a 6-day time course, providing information on the early and late influx of cells and secretion of cytokines. As expected, LPS administration increased BAL neutrophils and inflammatory cytokines (i.e., IL-6, TNF-α) within 2 days (early phase). LPS administration increased lymphocytes within 4 - 6 days (late phase). Other groups have shown that LPS induced TNF-α secretion in macrophages is decreased when CD4+ T cells are depleted (17), and that γδ T cells increase BAL neutrophils by producing IL-17A (18), suggesting that lymphocytes may facilitate neutrophil migration to the lungs. In our study, the kinetic analysis of neutrophil and lymphocytes support the concept that lymphocytes may play other roles in addition to facilitation of neutrophil migration, perhaps in dampening inflammation in ALI. In a prior study, adrenaline attenuated ALI by diminishing the recruitment of CD4+ cells to lung (19). The acute phase of ALI is characterized mainly by the influx of protein-rich edema fluid into the air space as a consequence of increased permeability of the alveolar-capillary barrier (20). Following LPS exposure, albumin levels peaked later than neutrophils, indicating that neutrophil infiltration may not directly correspond with lung vascular permeability. The time difference between the LPS-induced neutrophil influx and albumin increase may be due to several possibilities. First, proteolytic enzymes released from neutrophils, such as elastase and free oxygen radicals require time to exert their effects. Second, the peak of tissue damage may be earlier than the peak of albumin levels. The kinetics of ALI indicate that lung inflammation peaks at day 2 (acute phase) with the resolution process starting at day 4. Third, severity of ALI may not be determined solely by neutrophils. Different cell types may contribute to the lung damage in ALI. Expression of CD69, a cell activation marker, on T cells was increased by LPS throughout the time points analyzed. Together, these data suggest that T cells may impact both acute and late phases of inflammation in ALI.
Th17 cell related cytokines (i.e., IL-17A, IL-17F, and IL-22) were increased following LPS exposure. Interestingly, LPS induced Th17 cell related cytokines increased in a pattern similar to increased lymphocyte number and CD69 expression. Other T helper cell related cytokines (i.e., IL-2, IL-4, and IL-13) were not increased (not shown), which implies that, among the subsets of T helper cells, Th17 cells may play a role in ALI. IL-17 is important for host defense against infection as well as neutrophil recruitment (21). Indeed, IL-17 receptor-deficient mice succumb to infection (22). In contrast, elevated and prolonged expression of IL-17 is found in autoimmune disease (23). In murine models of LPS-induced airway inflammation, previous reports showed that neutralization of IL-17 significantly reduces neutrophil infiltration (24-26). And recently, others have demonstrated that IL-17 receptor antagonist KO mice show reduced neutrophilic inflammation of ALI in the setting of influenza infection induced ALI (18). Together, these studies in ALI suggest that IL-17 is increased at an early phase, induces inflammatory cytokines, and affects neutrophil kinetics. In our model, the time course for LPS-induced IL-17 is delayed compared to the increase of neutrophil infiltration. IL-17 is primarily considered a pro-inflammatory cytokine, but, in an allergic model, IL-17 suppresses eosinophil migration (27). In a bleomycin-induced lung injury model, IL-17 produced by γδ T cells controls resolution of inflammation (28). In a Helicobacter pylori-induced gastritis model, IL-17 exerts anti-inflammatory effects (29). Thus, IL-17 may play both pathogenic and protective roles in immune responses. Of note, the kinetics of LPS-induced IL-17 family members and Foxp3 differ, with IL-17 secreted earlier than Foxp3, which may impact their biological activities.
The early stages of T cell activation are regulated by interaction of costimulatory molecules CD80 and CD86 with their counter-receptors CD28 on the T cell surface (30). Once activated, these T cells transiently increase CTLA4 on their cell surface, interacting with the same CD80 and CD86 costimulatory molecules, but now inhibiting cell cycle progression and IL-2 production (31). Thus, CTLA4 signaling provides negative feedback to activated T cells, thereby dampening an immune response. In our model, LPS increased CTLA4 expression from day 1 though day 6, indicating that T helper cells were activated and susceptible to downregulation from the early to the late phase, concomitant with increased CD69 expression. Mattern et al. demonstrated that monocyte-dependent activation of human T cells by LPS requires costimulatory signals via CD28 and/or CTLA4 but is not major histocompatibility complex restricted (32). TLRs are expressed on T cells and TLR ligands act directly upon T cells (6-8). Together, our findings in an ALI model suggest that T cells may be regulated, in part, by antigen-independent pathways.
In vivo analysis of anti-CTLA4 antibody (αCTLA4) administration resulted in decreased albumin and IL-17A levels, suggesting a beneficial effect in ALI of manipulating a T cell pathway. While BAL cell counts were not altered by aCTLA4 administration, the severity of ALI may not always be determined solely by neutrophils. Multiple cells may contribute to the lung damage, and CD4+ T cells may impact other cells, e.g., other lymphocyte subsets, macrophages, or epithelial cells. Specific subset of lymphocytes may act as anti-inflammatory cells, not pro-inflammatory cells, i.e., Tregs. D’Alessio et al. recently demonstrated that Tregs play critical roles during the resolution phase in an ALI model (16). They showed that Tregs resolve lung inflammation by inducing TGF-b and neutrophil apoptosis. The contribution of other T cell subsets, the impact of T cells on the early phase in ALI, and the potential role of CTLA4 were not examined. In our study, we focused on additional T cell subsets and the early phase of ALI. We also analyzed αCTLA4 which may activate not only T effector cells but also Tregs. Since Tregs constitutively express CTLA4 on their cell surface (33), αCTLA4 may impair the suppressive function of Tregs. Prior studies in cancer patients show that αCTLA4 increases both activated effector CD4 T cells as well as Tregs (34). Inhibiting CTLA4 signaling with αCTLA4 enhances Treg proliferation and overall Treg frequency (35). Notably, we demonstrated that αCTLA4 increased LPS-induced Foxp3 in CD4+ cells, suggesting that the decrease of albumin levels by αCTLA4 may relate to Treg activation. This concept is supported by an increased absolute CD4+Foxp3 cell number, but not CD4+Foxp3− (effector) cells in the αCTLA4 treated group. Further, LPS-induced Foxp3 expression in CD4+Foxp3+ cells and LPS-induced CD69 expression on CD4+CD25+ cells were increased by αCTLA4. Our study suggests that αCTLA4 may activate Tregs more than T effector cells, perhaps suppressing inflammation or accelerating resolution. If so, the severity of ALI may depend on the T effector cell/Treg balance. Also, αCTLA4 suppression of IL-17A synthesis is associated with decreased albumin. Tregs, which are activated by αCTLA4, may suppress Th17 cell activation, Thus, the Th17/ Treg balance may be important in ALI pathogenesis and warrants further analysis.
In summary, we focus on analysis of T cells in a model of ALI. Notably, LPS induces increased number and activation of T cells, as well as other T cell associated markers, i.e., IL-17A, Foxp3, and CTLA4. Testing the functional role of CTLA4, anti-CTLA4 antibody decreased both LPS-induced albumin and IL-17A, while increasing number of CD4+Foxp3+ cells and Foxp3 expression in CD4+Foxp3+ cells. These data are consistent with a role for CTLA4+ T cells in impacting LPS-induced inflammation. The specific pathways by which T cells are activated in ALI remain to be determined. That potential therapeutic impact of manipulating T cell pathways involving CTLA4 in ALI merits further investigation.
Acknowledgments
We thank Koichiro Asano, Mark Fuster, David Rothstein, and Timothy Bigby for critical review of the manuscript.
Footnotes
This work was supported by National Institutes of Health Grant 5R01HL081663-05.
- ALI
- acute lung injury
- αCTLA4
- anti-CTLA4 antibody
- Tregs
- T regulatory cells
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Hirano S. Quantitative time-course profiles of bronchoalveolar lavage cells following intratracheal instillation of lipopolysaccharide in mice. Ind Health. 1997;35:353–358. doi: 10.2486/indhealth.35.353. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 5.Barton GM, Medzhitov R. Control of adaptive immune responses by Toll-like receptors. Curr Opin Immunol. 2002;14:380–383. doi: 10.1016/s0952-7915(02)00343-6. [DOI] [PubMed] [Google Scholar]
- 6.Matsuguchi T, Takagi K, Musikacharoen T, Yoshikai Y. Gene expressions of lipopolysaccharide receptors, toll-like receptors 2 and 4, are differently regulated in mouse T lymphocytes. Blood. 2000;95:1378–1385. [PubMed] [Google Scholar]
- 7.Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsland BJ, Nembrini C, Grun K, Reissmann R, Kurrer M, Leipner C, Kopf M. TLR ligands act directly upon T cells to restore proliferation in the absence of protein kinase C-theta signaling and promote autoimmune myocarditis. J Immunol. 2007;178:3466–3473. doi: 10.4049/jimmunol.178.6.3466. [DOI] [PubMed] [Google Scholar]
- 9.Mohammed KA, Nasreen N, Ward MJ, Antony VB. Induction of acute pleural inflammation by Staphylococcus aureus. I. CD4+ T cells play a critical role in experimental empyema. J Infect Dis. 2000;181:1693–1699. doi: 10.1086/315422. [DOI] [PubMed] [Google Scholar]
- 10.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr., Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 11.Schwulst SJ, Muenzer JT, Chang KC, Brahmbhatt TS, Coopersmith CM, Hotchkiss RS. Lymphocyte phenotyping to distinguish septic from nonseptic critical illness. J Am Coll Surg. 2008;206:335–342. doi: 10.1016/j.jamcollsurg.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 12.Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, Korsmeyer SJ, Karl IE. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci U S A. 1999;96:14541–14546. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie YC, Dong XW, Wu XM, Yan XF, Xie QM. Inhibitory effects of flavonoids extracted from licorice on lipopolysaccharide-induced acute pulmonary inflammation in mice. Int Immunopharmacol. 2009;9:194–200. doi: 10.1016/j.intimp.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Harris JF, Aden J, Lyons CR, Tesfaigzi Y. Resolution of LPS-induced airway inflammation and goblet cell hyperplasia is independent of IL-18. Respir Res. 2007;8:24. doi: 10.1186/1465-9921-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris PE, Glass J, Cross R, Cohen DA. Role of T-lymphocytes in the resolution of endotoxin-induced lung injury. Inflammation. 1997;21:269–278. doi: 10.1023/a:1027393715300. [DOI] [PubMed] [Google Scholar]
- 16.D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, King LS. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009 doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Souza NB, Mandujano FJ, Nelson S, Summer WR, Shellito JE. CD4+ T lymphocyte depletion attenuates lipopolysaccharide-induced tumor necrosis factor secretion by alveolar macrophages in the mouse. Lymphokine Cytokine Res. 1994;13:359–366. [PubMed] [Google Scholar]
- 18.Crowe CR, Chen K, Pociask DA, Alcorn JF, Krivich C, Enelow RI, Ross TM, Witztum JL, Kolls JK. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol. 2009;183:5301–5310. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Philippakis GE, Lazaris AC, Papathomas TG, Zissis C, Agrogiannis G, Thomopoulou G, Nonni A, Xiromeritis K, Nikolopoulou-Stamati P, Bramis J, Patsouris E, Perrea D, Bellenis I. Adrenaline attenuates the acute lung injury after intratracheal lipopolysaccharide instillation: an experimental study. Inhal Toxicol. 2008;20:445–453. doi: 10.1080/08958370801903891. [DOI] [PubMed] [Google Scholar]
- 20.Suratt BT, Parsons PE. Mechanisms of acute lung injury/acute respiratory distress syndrome. Clin Chest Med. 2006;27:579–589. doi: 10.1016/j.ccm.2006.06.005. abstract viii. [DOI] [PubMed] [Google Scholar]
- 21.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 22.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prause O, Bossios A, Silverpil E, Ivanov S, Bozinovski S, Vlahos R, Sjostrand M, Anderson GP, Linden A. IL-17-producing T lymphocytes in lung tissue and in the bronchoalveolar space after exposure to endotoxin from Escherichia coli in vivo--effects of anti-inflammatory pharmacotherapy. Pulm Pharmacol Ther. 2009;22:199–207. doi: 10.1016/j.pupt.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Miyamoto M, Prause O, Sjostrand M, Laan M, Lotvall J, Linden A. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J Immunol. 2003;170:4665–4672. doi: 10.4049/jimmunol.170.9.4665. [DOI] [PubMed] [Google Scholar]
- 26.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170:2106–2112. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- 27.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braun RK, Ferrick C, Neubauer P, Sjoding M, Sterner-Kock A, Kock M, Putney L, Ferrick DA, Hyde DM, Love RB. IL-17 producing gammadelta T cells are required for a controlled inflammatory response after bleomycin-induced lung injury. Inflammation. 2008;31:167–179. doi: 10.1007/s10753-008-9062-6. [DOI] [PubMed] [Google Scholar]
- 29.Otani K, Watanabe T, Tanigawa T, Okazaki H, Yamagami H, Watanabe K, Tominaga K, Fujiwara Y, Oshitani N, Arakawa T. Anti-inflammatory effects of IL-17A on Helicobacter pylori-induced gastritis. Biochem Biophys Res Commun. 2009;382:252–258. doi: 10.1016/j.bbrc.2009.02.107. [DOI] [PubMed] [Google Scholar]
- 30.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 31.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattern T, Flad HD, Brade L, Rietschel ET, Ulmer AJ. Stimulation of human T lymphocytes by LPS is MHC unrestricted, but strongly dependent on B7 interactions. J Immunol. 1998;160:3412–3418. [PubMed] [Google Scholar]
- 33.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 34.Kavanagh B, O’Brien S, Lee D, Hou Y, Weinberg V, Rini B, Allison JP, Small EJ, Fong L. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112:1175–1183. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang AL, Teijaro JR, Njau MN, Chandran SS, Azimzadeh A, Nadler SG, Rothstein DM, Farber DL. CTLA4 expression is an indicator and regulator of steady-state CD4+ FoxP3+ T cell homeostasis. J Immunol. 2008;181:1806–1813. doi: 10.4049/jimmunol.181.3.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]