Summary
In vivo tumor cell migration through integrin-dependent pathways is key to the metastatic behavior of malignant cells. Using quantitative in vivo assays and intravital imaging we assessed the impact of cell migration, regulated by the integrin-associated tetraspanin CD151, on spontaneous human tumor cell metastasis. We demonstrate that promoting immobility through a CD151-specific metastasis blocking mAb prevents tumor cell dissemination by inhibiting intravasation without affecting primary tumor growth, tumor cell arrest, extravasation or growth at the secondary site. In vivo, this loss of migration is the result of enhanced tumor cell-matrix interactions, promoted by CD151, which prevent dissociation by individual cells and leads to a subsequent inhibition of invasion and intravasation at the site of the primary tumor.
Introduction
While tumor development at the primary site is cause for the onset and progression of neoplastic disease, the metastatic colonization of secondary tissues is clearly the most fatal aspect of clinical disease (Weiss, 1990, Hanahan and Weinberg, 2000). Tumor cell dissemination involves multiple sequential rate-limiting steps, including tumor cell mobilization, intravasation and extravasation, which ultimately result in the colonization of a secondary site (Bockhorn et al., 2007, Pantel and Brakenhoff, 2004, Bernards and Weinberg, 2002). Throughout the journey from its original location on route to a distant site, the metastasizing tumor cell interacts with its changing microenvironment. Together with transmembrane signaling receptors, adhesion complexes on the cell surface convey signals from the instructive microenvironment and regulate tumor cell behavior. The ability of the tumor cell to mobilize itself and leave the original tumor site is one of the prerequisite requirements of tumor cell dissemination (Stracke and Liotta, 1992, Fidler, 2003, Cameron et al., 2000, Hynes, 2003). Nevertheless, the role of active migration and invasion in metastasis remains undefined (Bockhorn et al., 2007).
While alternative, passive methods of dissemination have been proposed (Weidner, 2002) the identification of the pro-migratory factors Rho C and Twist as key contributors to the metastatic phenotype (Clark et al., 2000, Yang et al., 2004) implicates the need for a functional migration machinery. Cellular migration is mediated by the adhesion receptors (integrins) which regulate cellular behavior through cytoplasmic signaling pathways including Rho GTPases and kinases (Felding-Habermann et al., 2001, Guo and Giancotti, 2004). Adhesion receptor function is tightly controlled through regulatory pathways that include integrin-associated transmembrane proteins such as CD98 (Feral et al., 2005), CD47 (Brown and Frazier, 2001) and tetraspanins such as CD82 and CD151(Bienstock and Barrett, 2001, Hemler, 2005).
The integrin-associated protein CD151 is a member of the tetraspanin superfamily characterized by four transmembrane domains and well-conserved cysteine residues in a large extracellular loop (Hasegawa et al., 1998, Sincock et al., 1999, Hemler, 2005). CD151 has been shown to interact directly with the alpha subunit of several integrins through a small domain (QRD194–196) within the larger of its two extracellular loops (Kazarov et al., 2002). While associated with several integrins, the strongest CD151-integrin interactions are with the laminin-receptor integrins α3β1 and α6β1 (Serru et al., 1999, Lammerding et al., 2003). Like other tetraspanins, CD151 also interacts with itself thus forming small micro-domains on the cell surface that incorporate integrins together with other integrin-associated proteins (Claas et al., 2001, Charrin et al., 2003), Ig superfamily proteins (Le Naour et al., 2004), and cadherins (Chattopadhyay et al., 2003). CD151 has been shown to mediate integrin function by regulating cytoplasmic signaling. Specifically, CD151 modulates adhesion-dependent activation of RAS (Sawada et al., 2003), and integrin-associated CDC42 signaling (Shigeta et al., 2003). CD151 has been proposed as a cancer marker (Ang et al., 2004), however, while some components of the molecular mechanism of CD151 have been defined in vitro, very little is known about its in vivo functional role in tumor biology. We have previously demonstrated that an antibody directed to CD151 can inhibit spontaneous metastasis (Testa et al., 1999). However, thus far it has been unclear how, or at which step of the metastatic cascade CD151 contributes to tumor cell dissemination. To address these and more encompassing questions, we have developed quantitative methods of human tumor cell detection based on PCR amplification of alu sequences which allow for an extremely sensitive detection and quantification of each individual step in the metastatic process (Zijlstra et al., 2002). Furthermore, to assess motility of tumor cells in vivo, we have extended this model to now include a useful system for intra-vital imaging of individual tumor cell motility.
Using these methodologies, we have demonstrated that the immobilization of tumor cells with a unique function blocking anti-CD151 mAb prevents intravasation and subsequent metastasis. The real-time visualization of tumor cell migration in vivo demonstrates that motility at the secondary as well as the primary site is substantially inhibited by the anti-CD151 mAb. Real-time, intravital imaging indicated that the pronounced inhibition of migration was due to an inability of individual tumor cells to detach at the rear of the cell and depart from their original position within the tumor stroma. As a consequence of the inhibition of migration, a dramatic reduction of intravasation at the primary tumor site was observed which accounted completely for the diminished spontaneous metastasis of tumor cells.
Results
Anti-CD151 antibody (mAb 1A5) inhibits spontaneous metastasis of human tumor cells in vivo
We have demonstrated previously that human alu PCR in conjunction with the chick embryo spontaneous metastasis assay can be used to quantify metastatic behavior of human tumor cells in vivo (Zijlstra et al., 2002). By employing these methodologies in conjunction with a unique metastasis-blocking monoclonal antibody (mAb 1A5), the role of the tetraspanin CD151 in tumor cell dissemination was explored. Animals bearing HEp3 and HT1080 tumors were injected i.v. with control mAb 29-7 or with purified anti-CD151 monoclonal antibody, mAB 1A5. While both antibodies persist in the blood for the duration of the assay and localize to the tumor (Suppl. Fig. 1), only the anti-CD151 antibody inhibits spontaneous metastasis (Fig. 1A). This inhibition is target-specific because control antibodies, which also bind to the surface of HEp3 cells (29-7, Suppl. Fig. S1), do not interfere with metastasis (Fig 1A). Inhibition of metastasis is not cell-lineage specific since spontaneous dissemination of the epidermoid carcinoma HEp3 and the fibrosarcoma HT1080 are inhibited equally by mAb 1A5. In addition, large differences in CD151 expression between HEp3 and HT1080 (inset Fig. 1A) did not affect the ability of mAb 1A5 to inhibit metastasis, nor did the antibody recognize any antigen in normal chick tissue (Suppl. Fig. 1), further emphasizing the importance of tumor CD151 in metastasis. Importantly, the level of inhibition of HEp3 spontaneous metastasis in the SCID mouse by mAb 1A5 (>80%) is similar to that observed in the chick, affirming that the inhibition is not restricted to the chick model (Fig. 1B). In both models the tumor size is unaffected by antibody treatment (Chick: Control IgG = 345.7 mg ± 146.7, Anti-CD151 = ± 352.25 ± 145.6. Mouse: Control IgG = 2.87 g ± 1.29, Anti-CD151 = 2.86 ± 1.39) indicating that the inhibition of metastasis is independent of primary tumor expansion.
Figure 1. Treatment with anti-CD151 antibody (mAb 1A5) inhibits spontaneous metastasis of human tumor cells in vivo.
A) Control antibody (29-7) or anti-CD151 monoclonal antibody (1A5) was injected i.v. (100 ug) into HEp3 or HT1080 tumor-bearing chick embryos one day after applying the tumor cells. The level of metastasis to the chick lower CAM (LCAM) was determined by human alu PCR after 7 days as described in Materials and Methods. B) To confirm that CD151 also impacts metastasis in another in vivo model, human alu PCR was used to quantify the level of spontaneous metastasis in SCID mice bearing HEp3 tumors in response to systemic treatment (i.v. injection, 100 ug ×2) with mAb 1A5. Values are represented as mean ± SEM.
Matrix-mediated migration of tumor cells is inhibited by the mAb 1A5 independent of the type of matrix composition
Previous work has suggested that CD151 influences migration on laminin (Winterwood et al., 2006). To determine if the regulation of migration by CD151 is matrix component specific, the ability of mAb 1A5 to inhibit the matrix-mediated migration of HEp3 cells was assessed using a variety of matrixes. Considering the established association of CD151 with laminin-receptor integrins ((Serru et al., 1999) α3β1 and α6β1) it is not surprisingly that mAb 1A5 inhibited laminin-mediated migration (Fig 2A). However, mAb 1A5 was also capable of inhibiting migration on collagen type I (Fig 2B) which is mediated through the α1 and α2 integrin subunits not known to have strong interactions with CD151. In fact, for each of the tested matrix components (and thus their corresponding integrin adhesion receptors), antibody treatment reduced the level of migration to that on uncoated filters (Fig 2B). However, mAb 1A5 clearly does not inhibit matrix-independent migration on uncoated filters.
Figure 2. Anti-CD151 metastasis-blocking mAb 1A5 mediates inhibition of migration independent of the underlying matrix.
Transwell migration of HEp3 cells in response to an underlying matrix was assessed in the presence of mAb 1A5 or an unspecific control mAb. Haptotactic migration of HEp3 in response to laminin (LN, A) was assessed during a 16 hr incubation and compared to migration on control (uncoated) filters. B) Comparative summary of 1A5 inhibition of matrix mediated migration by HEp3 on LN, Fibronectin (FN), Vitronectin (VN), Col I, and Collagen type IV (Col IV). Values in A and B are represented as mean ± SEM C) Adhesion of HEp3 cells to collagen type I coated coverslips in the presence of control mAb or mAb 1A5 was visualized using phase contrast microscopy (upper panels). The formation of Paxillin containing focal adhesion complexes on a collagen type I substrata in the presence of a control mAb or mAb 1A5 (lower panels). A 2–3 fold enhancement of paxillin containing focal adhesion complexes was observed in the presence of mAb 1A5. Bars = 10μm.
The inhibition of matrix-mediated migration could be due to the disruption of adhesion to the matrix components. However, adhesion to, and spreading on collagen type I coated surfaces in vitro was not disrupted (Fig. 2C, upper panels). Furthermore, the formation of paxillin containing focal adhesions was enhanced (Fig. 2C, lower panels) suggesting that antibody treatment actually enhances matrix interactions rather than disrupting them.
In vivo migration controlled by CD151 is critical for tumor cell motility at the secondary site but not for the extravasation of arrested tumor cells
The observation that the function-blocking mAb 1A5 implements a broad inhibition of matrix-mediated migration suggests that it might also inhibit migration within a complex matrix substratum such as that found in vivo. To perform an analysis of tumor cell motility in vivo with respect to CD151, we developed the CAM as a biological platform for the visualization of tumor cell arrest, extravasation and migration. (Suppl. Fig. S2).
To assess if mAb 1A5 could interfere with extravasation and subsequent migration, GFP-expressing tumor cells were injected i.v. and their ability to arrest and disperse within the stroma was assessed by fluorescent microscopy. GFP expressing HEp3 and HT1080 cells readily arrested in the CAM vasculature in the presence and absence of anti-CD151 mAb (Fig. 3A, Day 1). In subsequent days the arrested tumor cell population in control animals expanded and was capable of disseminating widely throughout the CAM stroma. In contrast, the tumor cell population in mAb 1A5-treated animals proliferated but apparently failed to disseminate as they remained tightly clustered, suggesting these cells cannot migrate (Fig. 3A, Day 3 and 5). This inhibition is not Fc-dependent but requires the bivalent nature of the IgG (Suppl. Fig. S5). Both epidermoid carcinoma (HEp3) and fibrosarcoma (HT1080) demonstrate similar inhibition in motility.
Figure 3. In vivo, intra-stromal tumor cell migration but not the arrest, extravasation, or growth at the secondary site of metastasis is affected by anti-CD151 mAb 1A5.
A) The ability of anti-CD151 to impact in vivo mobility was determined by visualizing the intrastromal mobility of GFP-expressing HEp3 (upper panels) and HT1080 (lower panels) cells in the CAM when co-injected with 100μg anti-CD151 or a control IgG. Blood containing vessels are seen in black while GFP-expressing cells are bright green against the dull green glow of the eggshell. Each image is representative of twelve animals imaged in 4 separate experiments. B) Alterations in the ability of tumor cells to arrest, extravasate, and colonize were assessed by confocal imaging of GFP-expressing HEp3 (Green) cells in CAM with Lectin labeled vasculature (Red). Tumor cells coinjected with anti-CD151 or control IgG were imaged at 24hr and 5 days post injection. Merged topical view and vertical projection of a re-sliced Z-stack taken from a selected area (white box) illustrate the location of tumor cells relative to the vasculatrure. Tumor cells which remain inside the vasculature would be seen as yellow while extravasated cells are only seen in green (for further detail see Suppl. Fig. S2). Included images are representative of twelve animals from 4 separate experiments. C) Alu PCR analysis of experimental metastasis was used to quantify the affect of inhibited tumor cell migration on arrest (Day 1) and growth (Day 4) in the lung and the lower CAM (LCAM). HEp3 tumor cells (50,000) were injected into the allantoic vein of a 10 day old chick embryo. Tissue was harvested 3 hr after injection to quantify the number of arrested tumor cells or at 4 days after injection to assess the growth of the arrested cells. Similar results were obtained in 3 experiments. N=5. Values in C are represented as mean ± SEM. Bars in A = 200μm, in B = 20μm.
To determine if the failure of tumor cells to disseminate in mAb 1A5-treated animals was due to an inability to extravasate, we examined the vascular location of the GFP-expressing tumor cells in the presence and absence of mAb 1A5. At 24 hr postinjection all of the cells in animals treated either with control antibody or with mAb 1A5 had extravasated (N=300, Fig 3B, left panels, z-projection). At 5 days post injection, the extravasated cells had proliferated in both control and mAb 1A5 treated animals. However, in control animals these cells had migrated throughout the stroma while in mAb 1A5-treated animals the extravasated cells proliferated, but failed to disseminate and thus formed clusters or compact colonies immediately outside the vasculature (Fig 3B, right panels, z-projection). These observations indicate that the inhibition of migration by anti-CD151 mAb 1A5 appears not to be critical for vascular extravasation but prevents active migration of the tumor cells within the stroma after departure from the vasculature.
The tumor cell immobility mediated by CD151 upon antibody ligation does not reduce the arrest and growth of tumor cells in vivo
HEp3 cells were injected i.v. in the presence of control mAb or anti-CD151 mAb and their arrest in both the CAM and lung at 24hr and the subsequent proliferation of arrested cells four days post injection were determined quantitatively by Alu PCR. A comparable number of arrested cells (approx. 1000 cells in the LCAM and 200 cells in the lung) were detected in treated and control animals (Fig. 3C, Day 1). Furthermore a 5-fold increase in cell number occurs in the LCAM and a 2-fold increase in the lung for both mAb 1A5 and control mAb treated animals (Fig. 3C, Day 4). The tissue specific lag in tumor cell proliferation seen in the lung was observed previously (Zijlstra et al., 2002). These data confirm that the inhibition of motility by mAb 1A5 does not affect tumor cell arrest or growth in vivo.
Inhibition of motility by mAb 1A5 eliminates the invasive edge of the primary tumor
Although the loss of motility mediated by anti-CD151 prevents migration at the secondary site, it does not limit metastatic colonization by tumor cells injected intravenously (Fig. 3). This inability to influence post-intravasation metastatic events indicates that the inhibition of migration plays a critical role in the departure from the primary site. To monitor the migratory behavior of cells within the primary tumor we developed a distinct intravital imaging system (see Materials and Methods). GFP-expressing HEp3 cells (~5×104) were implanted into the CAM of ex ovo embryos and allowed to form a primary tumor. The animals were treated with a control mAb or mAb 1A5 at 24 hr post implantation and the tumors were imaged 7 days later. The hallmark of aggressive metastatic tumors is an irregular invasive front seen at the tumor-stromal interface (Friedl and Wolf, 2003, Hood and Cheresh, 2002, Geho et al., 2005, Liotta and Kohn, 2001). This invasive front is clearly visible in control HEp3 and HT1080 tumors (Fig. 4) where tumor cells have invaded the stroma surrounding the primary tumor, thereby generating an irregular border. High magnification (100×) imaging demonstrates that individual cells have migrated into the stromal tissue and appear particularly abundant along the stromal vasculature. It is not surprising that tumor cells indeed migrate rapidly along the vasculature (Suppl. Fig. S4 and Video V8), a phenomenon also reported in a mouse tumor model ((Wyckoff et al., 2000)). In contrast, mAb 1A5-treated tumors exhibit a very defined border with little or no invasion of the surrounding stroma nor migration along the vasculature for both HEp3 and HT1080 tumors (Fig. 4 lower panels).
Figure 4. The inhibition of migration prevents tumor cell invasion at the tumor-stroma interface.
GFP-expressing HEp3 and HT1080 CAM tumors were imaged intra-vitally using a newly developed intravital imaging system (see Materials and Methods) to visualize the invasive behavior of metastatic tumor cells. Chick embryos were injected i.v. with 100μg of mAb 1A5 or control mAb 24 hr after CAM implantation of GFP-expressing HEp3 cells. Images were taken 7 days after antibody treatment. The included images are representative of 10 individual tumors generated in 2 separate experiments with 5 animals each. Bars = 800μm (40×) and 100 μm (100×).
Inhibition of individual tumor cell migration by mAb 1A5 prevents invasion of the adjacent stroma
To determine if CD151-mediated control of migration was responsible for intra-tumoral cell motility in vivo, changes in velocity, distance and directionality of tumor cell motility in HEp3 tumors in response to mAb 1A5 were assessed using intravital microscopy. HEp3 tumor cells were implanted in the CAM and the motility of individual tumor cells was imaged over a 12.25 hr period in the presence and absence of mAb 1A5 (Fig. 5A and B). A representative population of motile cells, whose path could be traced during the entire time-period, was analyzed by tracking the centroid of individually selected cells. For each individual cell, the total distance migrated and the productive migration (distance from origin) was calculated (Fig 5C). In addition, the velocity, persistent forward motion (persistence) and changes in directionality (angle change) were determined for each cell.
Figure 5. Tumor cell motility is inhibited at the tumor-stroma interface in mAb 1A5 treated animals.
GFP-expressing HEp3 tumor cells were imaged intra-vitally for 12.25 hr in order to visualize, record and quantify the in vivo motility of individual tumor cell (see materials and methods). Antibody treatment was given i.v. 24 hrs after tumor cell implantation and imaging was initiated 48 hr later. Images were captured every 15 minutes. The motility of tumor cells in a tumor developing on a control animal (A) and a mAb 1A5-treated animal (B) is represented by comparing the first frame (0.00hr) and the last frame (12.25hr) of a 12.25 hr Video (see Suppl. Video V1 (control) and V2 (mAb 1A5)). The migration of individual cells is visualized as an overlay of dots and lines in each frame (left panels). The tracks of 10 representative cells are plotted in Wind-rose plots (right panels) with the initial position of each track superimposed at 0,0 to provide a relative overview of the migration tracks with a single point of origin. The temporal increase in the productive motility of 4 representative tracks from control and treated tumors are plotted in C). The most motile cell in the mAb 1A5 treated tumor (#5) is still far less motile than the average control cells. (D) The motility parameters were determined quantitatively for cells in a tumor from animals treated with either control mAb or mAb 1A5. Motility parameters were determined as an average of 30 cells/tumor with 48 measurements/cell. p values were calculated using Student’s t-test. A highly significant reduction in forward motility but no change in the ability to alter direction (turn angle) is apparent. Values are presented as mean ± SEM and representative of 3 distinct experiments. Scale bars in A and B = 100μm.
Individual HEp3 cells exhibit a high level of motility (22 μm/hr for a total distance of 288 μm, Suppl. Video V1, Fig. 5D). This average velocity compares favorably with the high speeds of 20–40 μm/hr recorded in 2 and 3 dimensional culture in vitro for cancer cells of epithelial origin (Soon et al., 2005, Harms et al., 2005). More important, HEp3 cells exhibit a persistence of 4.39 μm which facilitates a productive migration of 150 μm in untreated animals (Fig. 5C, D). Tracking of individual cells clearly demonstrates the ability of HEp3 cells to rapidly penetrate the stroma surrounding the primary tumor (Fig. 5A, control tracks #3 and 8). In contrast, the inhibition of motility is readily apparent in mAb 1A5-treated animals (Fig. 5B and Suppl. Video V2). Tumor cells in antibody-treated animals exhibit an approximate 4-fold inhibition of migration velocity (5.9 μm/hr), which, together with a 3.6 fold inhibition of persistence (1.21 μm) results in a 6.6 fold reduction in productive motility (23 μm, Fig. 5C, D). The substantial reduction in motility upon treatment with mAb 1A5 is apparent when the traces of individual tumor cells are plotted on a singular origin in a Wind-Rose plot (Fig. 5A and B, right panels).
In mAb 1A5-treated animals the tumor cells clearly exhibit some residual movement in the presence of mAb 1A5 and their ability to change direction is unaffected (Fig. 5D). Pseudopodial extensions and small forward and backward movements are readily observed within the tumor (Fig. 5B, tracks #1 and 2, Suppl. Video V2). However, considering an approximate body length of 20μm, on average, the mAb 1A5-treated tumor cells fail to travel more than one body length from their original position while in control animals, the average tumor cell travels 5–7 body lengths away from their original start position (Fig. 5A, B, D).
Inhibition of motility by anti-CD151 is mediated mechanistically by the inability of tumor cells to detach at the rear of the cell body
The migratory behavior of HEp3 cells in vivo was analyzed in greater detail by capturing enhanced images of individual migrating cells in control and mAb 1A5-treated animals (Fig. 6). In control tumors, migrating cells can be seen to invade the surrounding stroma as the leading edge of the cell penetrates the mesenchymal tissue (Fig. 6A, arrow). When the cell body moves forward in the direction of the invading pseudopodia, the trailing end of the cell (arrow-head) detaches and rapidly contracts towards the cell body (Fig. 6A, Cell #1 and #2, Suppl. Video V3 and V4). These motile control cells were compared to the most motile of the tumor cells in the mAb 1A5-treated animals (such as Fig. 5B track #5, represented as Cell #1 in Fig. 6B). The enlarged frames in Fig. 6B illustrate that the cells in the mAB 1A5-treated animals invade the stroma and appear to be capable of forward movement (see arrow, Fig. 6B, Cell #1 and #2). However, even though the centroid travels approximately 100μm from the original position, the rear of the cell fails to follow the movement of the cell body and remains attached close to the original location (see arrow-head, Fig. 6B, cell #1 and #2, Suppl. Video V5 and V6). A quantification of the migratory behavior of individual cells (n=250) at the tumor-stroma interface demonstrated that 83.3% of these boundary cells in control tumors were motile (move more than one body-length, >20μm) and only 1.7% of these motile cells failed to detach at the rear of the cell during the time of observation (12.5h). In contrast, in mAb 1A5-treated tumors, 24% of these boundary cells exhibited such motility but 88% of these “motile” cells failed to detach at the rear of the cell. As a consequence, 81.4% of control cells exhibited productive motility compared to less than 3% of the cells in mAb 1A5-treated tumor.
Figure 6. Anti-CD151 mediated inhibition of migration coincides with incomplete detachment at the rear of the cells.
Selected fields from real-time movies were captured, enhanced and processed to reveal the details of migration in the three-dimensional stroma of tumor-bearing animals treated with control mAb (A) or anti-CD151 mAb 1A5 (B). Also see Suppl. Video V3, V4, V5, and V6. A white arrow indicates the leading edge of the migrating cell and a white arrow-head indicates the trailing end of the same cell. The scale bare is 20μm and the time is marked on each frame. Note that, due to the differential in migration rates, the time line for the control cells is 0.5 hr/frame while the 1A5-treated cells are shown at 1.25hr/frame. Anti-CD151 induced loss of detachment was confirmed in vitro by visualization (C) and quantitation (D) of HEp3 cell migration on a collagen monolayer (200×, large arrow indicates start position of the rear and small arrow indicates the protrusive edge. Also see Suppl. Video V9). The impact on invasion was assessed in vitro using HEp3 cultivated in 3-dimensional collagen (E) where the loss of invasion (white arrow, left panel) and the formation of compact circular colonies was evident (right panel). Scale bars = 20μm (A, B and C) and 10μm (E).
Within a growing tumor, the inability of a proliferating cell to productively depart from its original position should result in a small compact colony of daughter cells. This was indeed true in primary tumors harvested from chick embryos treated with control mAb or mAb 1A5 (Suppl. Fig. S3). In addition, it was shown that in mAb 1A5-treated embryos, daughter cells fail to depart the location of the original parental cell during secondary site colonization (Fig. 3B, 3C).
The failure to detach induced by the anti-CD151 mAb is also observed in vitro (Fig. 6C and D). HEp3 cells treated with a control mAb clearly migrate and exhibit distinct detachment at the rear of the cell. In contrast, treatment with mAb 1A5 prevents detachment at the rear of the cells (white arrows) causing the cell to move back and forth in the same location without achieving productive movement. By quantifying detachment at the rear for individual tumor cells (Fig. 6D, N=30), the failure to detach and migrate productively is apparent (Fig. 6D). In a 3-dimensional collagen matrix, HEp3 cells which can invade the collagen matrix in the presence of a control mAb (Fig. 6E, arrow) are completely immobilized in the presence of mAb 1A5 and form compact colonies (Fig 6E right panel) as was also seen in the CAM in vivo (Fig. 3B).
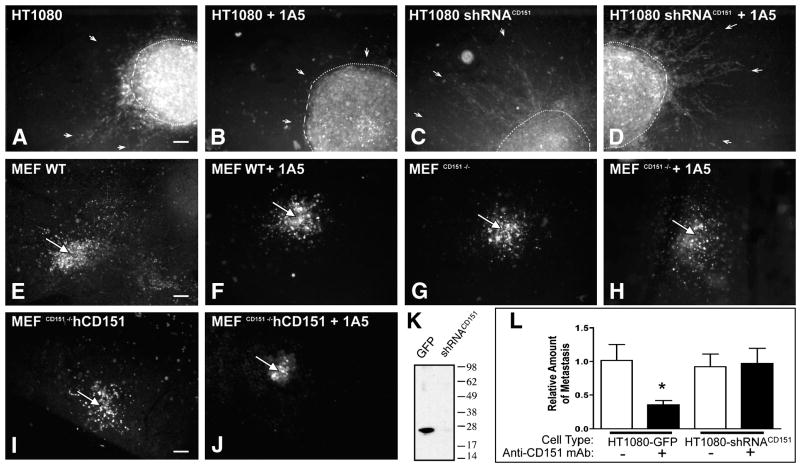
The loss of CD151 does not significantly impact in vivo motility while anti-CD151 mAb treatment mediates immobility only in the presence of human CD151
Recent reports of CD151 knockout mice have indicated that the genetic loss of CD151 does not result in dramatic developmental deficiencies (Wright et al., 2004, Sachs et al., 2006, Takeda et al., 2007), suggesting that migration is not critically impaired in these mice. To further explore the immobility mediated by CD151 we compared the in vivo migration of mouse embryonic fibroblasts (MEF) which lacked CD151 expression (MEFCD151−/−) with wild type MEF (MEFwt) and with MEFCD151−/− which were reconstituted with human CD151 (MEFCD151−/− hCD151). As seen in Figure 7E, G, and I, MEFwt, MEFCD151−/−, and MEFCD151−/− hCD151 all migrate into the stroma as individual cells, away from the point of CAM implantation (white arrow, E–J). These observations indicate that the mere absence of CD151 does not significantly impair the in vivo mobility of a migrating cell. Similarly, the down regulation of CD151 expression in HT1080 cells by shRNA (Fig. 7K) did not limit tumor migration in vivo (compare Fig. 7A and C) nor did it inhibit metastasis significantly (Fig. 7L). In contrast to the loss of CD151, treatment of HT1080 cells and MEFCD151−/− hCD151 with anti-CD151 mAb 1A5 blocks invasion of the stroma (Fig. 7B and J) and the cells remain tightly clustered at the CAM implantation site. However cells that lack human CD151 (MEF wt) or are substantially reduced in CD151 expression (HT1080 shRNACD151, MEFCD151−/−) are unaffected by the antibody (Fig. 7D, F and H) indicating further that the anti CD151 mAB is specific and is not targeting other molecules which might result in off-target inhibition of motility. In addition to these illustrative in vivo results, HT1080 cell migration assays carried out in vitro on collagen coated surfaces, also demonstrated that mAb 1A5 inhibits motility only when the CD151 antigen is present at normal levels on the cell surface (Supplemental Figure S6). These observations demonstrate that cell surface CD151, upon antibody binding, induces pronounced immobility of individual cells.
Figure 7. Anti-CD151 mAb ligation promotes immobility while the loss of CD151 fails to impact in vivo motility.
The necessity of CD151 expression for in vivo motility of tumor cells and MEF was assessed by implanting fluorescently labeled cells with reduced or ablated CD151 expression into the CAM and determining their ability to depart the implantation site. The ability of HT1080 cells stably transduced with an expression vector containing GFP (HT1080) or CD151 specific shRNA (HT1080 shRNACD151) to migrate out as individual cells was assessed in the presence of control antibody (A and C) or anti-CD151 mAb 1A5 (B and D) at 4 days after tumor cell implantation. The white dotted line demarks the border of the primary tumor while arrows indicate the stromal invasion of tumor cells. Likewise, the inability of anti-CD151 mAb 1A5 to prevent individual wild type MEF (E and F, MEF wt) and CD151 knockout MEF (G and H, MEFCD151−/−) from mobilizing, departing the implantation site and entering the stroma was illustrated by fluorescent microscopy 4 days after implantation. Similarly, the mobility of MEFCD151−/− reconstituted with hCD151 (I, MEFCD151−/− hCD151) was assessed in the CAM. To illustrate the ability of anti-CD151 to actively promote immobility, the ability of MEFCD151−/− hCD151 to disseminate from the implantation site was determined in the presence of mAb 1A5 (J). Each image is representative of 3 individual experiments with 3 animals each. A white arrow (E–J) indicates the injection site. Down-regulation of endogenous CD151 in HT1080 shRNACD151 is confirmed by Western blotting of whole cell lysates with anti-CD151 antibody (K). The impact of ablated CD151 expression on metastasis and the specificity of anti-CD151 treatment was further investigated by quantifying metastasis of HT1080 GFP or shRNACD151 in the presence or absence of anti-CD151 mAb 1A5 (L, Values represent the mean ± SEM where N=8). Scale bars =200μm.
Anti-CD151-mediated inhibition of in vivo motility results in complete inhibition of tumor cell intravasation
Since the anti-CD151-mediated inhibition of migration is a consequence of the inability of mAb 1A5-treated cells to detach at the rear of the cell, these tumor cells should not be able to escape from the primary tumor and enter the vasculature (intravasate). The arrival of intravasated human tumor cells in the lower CAM of tumor-bearing chick embryos is readily quantified (Fig. 8) using the human alu PCR-based assay (Zijlstra et al., 2002). Intravasating tumor cells are first observed in the lower CAM on day 3 and continue to arrive every day thereafter. The intravasated tumor cells which arrive in the CAM proliferate in the ensuing days with an approximate doubling time of 21–24 hr. In order to ascertain the number of intravasating cells arriving in the lower CAM within a single day, we determine the increase in the tumor cell population between day 5 and 6, and compare that with the number predicted solely on the basis of a doubling of those cells detected on day 5. The number of intravasated cells is the difference between the actual number cells present in the tissue and the number of cells expected due to cell doubling. In controls animals 6050 cells are present in the CAM on day 5 which should grow to ~12,000 cells by day 6 on the basis of their doubling time (dashed line in Fig. 8A). However, ~29,000 cells are actually measured on day 6, yielding a calculated value of 17,000 newly arriving cells between day 5 and 6 (area above dashed line, Fig. 8A). In contrast, there was no detectable intravasation over the same time period in mAb 1A5-treated animals as the approximately 1000 cells present in the lower CAM on day 5 failed to increase in numbers beyond a doubling (Fig. 8A). Tumor growth in all of these experiments (Fig. 8A and B) was not significantly different between control IgG and anti-CD151 treated animals and increased approximately 50mg from D5 to D6.
Figure 8. Function blocking anti-CD151 antibody (mAb 1A5) inhibits tumor cell intravasation.
A) Intravasation in animals treated i.v. on day 1 with mAb 1A5 or control mAb (mAb 29-7) as determined by alu PCR. The dashed line indicates the number of cells expected on day 6 based upon the growth (24 hr doubling time) of cells detected on day 5. The bar volume above the dashed line represent the number of intravasated cells. Similar results were obtained in 3 experiments. N=6. B) To assess the immediate impact of mAb 1A5-arrested intravasation on metastasis, HEp3 tumor bearing animals were injected 24 hr prior to harvesting the LCAM. The number of intravastated tumor cells in CAM tissue harvested on day 6 after a mAb treatment on day 5 was compared with the number of cells found in day 6 CAM from untreated animals. Values are represented as mean ± SEM. * P ≤ 0.01, ** P ≤ 0.001 Tumor sizes: A) Control: D5 143.5 ± 61, D6 216 ± 100, mAb 1A5: D5 167 ± 72, D6 200 ± 85. B) D5 115 ± 40, Control D6 200 ± 66, mAb 1A5: D6 191 ± 71
We proposed that the inhibition of motility on any given day would bring about an abrupt inhibition of intravasation. Indeed, when chick embryos bearing HEp3 tumors were injected with mAb 1A5 on day 5, the tumor cell population detected in the CAM on day 5 (2,150) only expands to 4,470 cells by day 6 in close accordance to the cell doubling rate, and no newly intravasating cells are detected (area above the dotted line, Fig. 8B). In contrast, control antibody-treated animals yield 11,500 cells on day 6, of which only 4,300 could be predicted from proliferation, thereby indicating a rate of intravasation of ~7,200/24hr. These quantitative tumor cell measurements are consistent with the in vivo motility and morphological observations and demonstrate that CD151-mediated inhibition of motility subsequently prevents intravasation and thereby ultimately inhibits tumor cell dissemination.
Discussion
The regulation of metastasis through individual tumor cell migration
This work identifies the role of tumor cell migration in metastasis as regulated through the tetraspanin CD151. Using highly sensitive detection of human tumor cells by PCR, along with intravital visualization of tumor cell migration, we determined that CD151 can control de-adhesion at the rear of the cell which thereby promotes immobility of tumor cells and, as a consequence, the successful dissemination of tumor cells from the primary tumor. Contrary to the ablation of CD151 expression, the ligation of CD151 with its cognate mAb 1A5 results in immobilization of CD151 expressing tumor cells. These observations demonstrate that metastasis requires migration as an active component of dissemination and intravasation.
The immobilization mediated by CD151 upon antibody ligation prevents invasion and dissemination at a number of points in the metastatic cascade, including migration within the primary tumor and at the secondary location. However, with regards to the full metastatic ability of human tumor cells, individual cell migration was critical only in the departure from the primary tumor and entry into the vasculature (intravasation). In contrast, arrest, extravasation and proliferation at the secondary location appeared unaffected by the lack of productive motility. Tumor cells introduced directly into the circulation in the presence of anti-CD151 during experimental metastasis were able to arrest, extravasate, and proliferate within the surrounding stroma.
The impact of tumor cell immobilization by anti-CD151 on the progression through individual stages of metastasis
It is of interest that extravasation, a process that involves active motility by the tumor cells (Luzzi et al., 1998, Chambers et al., 1992), Suppl. Video V7) is not prevented by the inhibition of CD151-mediated migration. However, since extravasation requires arrested tumor cells to pass through a thin layer of endothelial cells and basement membrane without dissociating from any previously formed matrix contacts, this process can be achieved in a single body length. Individual cells in mAb 1A5-treated animals exhibit the ability to extend one body length (~15–20μm) even though they fail to dissociate from their original location (Fig 6). This distance would be sufficient to pass through the vascular wall and enter the surrounding stroma. Since in vivo growth is not affected by mAb treatment, no diminishment of secondary site colonization occurs during experimental metastasis.
In contrast to experimental metastasis, spontaneous metastasis is inhibited >80%, thereby emphasizing the importance of CD151 in the departure of tumor cells from the primary tumor. Cells within the primary tumor exhibit a continuous mobility, illustrating that no tumor cell is “static” or entirely immotile. While much of the movement appears random, in fact a portion of the cells exhibit a persistent forward movement that facilitates invasion of the stroma (Fig 5A). This forward mobility is not always outwards but is also directed into the tumor mass (track 7 in Fig 5A) indicating that the migratory clues are not a simple, unidirectional chemotactic stimulus from the stroma. When plotting the productive migration of tumor cells we observe that many migratory cells move forward in bursts of activity preceded or followed by a period of non-productive movement. This migration appears to be guided at least in part by the vasculature (Suppl. Fig. S4 and Video V8).
The 3–4 fold reduction in overall (total) tumor cell motility would not account for the near absence of invasion and the >80% reduction in spontaneous metastasis. However, a quantitative analysis of the motion parameters demonstrates that both the velocity and the persistence of movement are reduced 3–4 fold. Together they are responsible for a 6–7 fold reduction in productive movement away from the point of origin (Fig. 5D). Furthermore, because the mAb 1A5-treated tumor cell fails to depart its original position, even as it possesses some minimal movement, no significant productive departure from the primary tumor can be achieved, and thus little or no metastasis can occur.
The inhibition of migration, it should be emphasized, does not result in the disruption of adhesive interactions but rather in the prevention of de-adhesion. Our direct in vivo and in vitro observations of this behavior are corroborated by the quantitative measurements of migration. A disruption of adhesive capacity would have resulted in a complete loss of any movement, thus yielding a “0” value for all migration parameters. Conversely, loss of chemotactic or directional movement would lead to a reduction in persistence and a subsequent reduction in productive motility without changes in velocity or even total migration. In contrast, the joint decrease in velocity and persistence observed in mAb 1A5-treated animals corresponds with the reduced ability to de-adhere. Rear detachment of migrating cells has previously been suggested to be the rate-limiting step in 2D migration in vitro (Gallant et al., 2005, Palecek et al., 1998). We present an observation that specifically demonstrates that an integrin-associated tetraspanin can control the cell’s adhesive machinery such that it fails to disengage and thus prevents productive motility of a tumor cell in vivo. That this can occur within a complex ECM such as the tumor stroma with multiple adhesion ligands, indicates the importance of de-adhesion in tumor cell motility.
While the exact mechanism by which CD151 controls migration is currently unknown, the direct interaction of CD151 with the laminin-receptor integrins (α3β1 and α6β1) suggests that the tetraspanin might regulate migration through direct control of integrin function. Indeed the expression of CD151 correlates positively with migration on laminin (Winterwood et al., 2006, Sterk et al., 2002) and CD151 has been shown to potentiate ligand binding activity of α3β1 and α6β1 (Nishiuchi et al., 2005, Lammerding et al., 2003). Most importantly, CD151 can facilitate adhesion strengthening (Nishiuchi et al., 2005, Lammerding et al., 2003), a process that can reduce migration by inhibiting detachment of the cell (DiMilla et al., 1993, Palecek et al., 1997). Our in vitro and in vivo observations suggest that CD151 can promote immobility on a variety of matrixes in addition to laminin. Furthermore, the mere expression of CD151 does not appear to be sufficient for regulating migration in vivo (Fig. 7), an observation which is supported by the normal embryonic development of three independently generated CD151 knockout mice (Wright et al., 2004, Sachs et al., 2006, Takeda et al., 2007). Human patients with CD151 deficiencies suffer principally of regional skin blistering, sensorineural deafness and kidney failure. The latter is also found in knockout mice. However, neither human nor mouse fails to form organs or heal skin wounds in the absence of CD151 suggesting that, at a cellular level, cells which lack CD151 retain a functional migration machinery. The migration of MEFCD151−/− in CAM stroma (Fig. 7) confirms this hypothesis. Conversely, the near complete failure of CD151 positive human tumor cells and MEFCD151−/− expressing human CD151 to detach in response to anti-CD151 mAb demonstrates that antibody-bound cell surface CD151 can promote tumor cell immobility through a dominant negative effect on migration. This action is likely to be mediated through an associated effector molecule because tetraspanins act primarily as scaffolding proteins in the membrane.
While direct interaction between integrins and CD151 is evident, several non-integrin membrane proteins which can regulate motility are also associated with tetraspanin enriched microdomains (Claas et al., 2001, Hemler, 2005). These membrane proteins include growth factor receptors (Klosek et al., 2005), Ig superfamily proteins and cell adhesion molecules (Le Naour et al., 2006), and E-Cadherin (Chattopadhyay et al., 2003) each of which is capable of impacting migration indirectly. The continued ability of CD151 negative cells to migrate in vitro and in vivo indicates that CD151 is neither sufficient nor necessary for migration. In contrast, the inhibition of migration in CD151 expressing tumor cells with the anti-CD151 mAb 1A5 indicates that the tetraspanin can promote immobility. This immobilization does not appear to depend on steric hindrance because monomeric Fab fragment of 1A5 binds to CD151 on tumor cells but does not promote immobility (Suppl. Fig. 5). In the absence of catalytic domains in CD151 it is likely that CD151 recruits elements that promote immobility or sequesters elements needed for deadhesion upon ligation with the cognate antibody. The identification of the partner(s) that associate with CD151 and mediate control over cellular (de)adhesion should reveal the molecular mechanism responsible for controlling de-adhesion. Such identification studies are now ongoing.
Preventing metastatic dissemination through the inhibition of migration
The microenvironment to which a metastasizing tumor cell is exposed en route to a secondary site is both an architectural as well as a signaling framework that is continually instructive with regards to cellular behavior (Bissell and Labarge, 2005). The primary approach to intervening with the invasive nature of cancer has been focused on the identification and disruption of extracellular signals that promote migration (Wyckoff et al., 2004, Clark et al., 2000), the disruption of adhesive properties (Murphy-Ullrich, 2001, Czekay et al., 2003), or the disruption of migration mediated signaling downstream of the cell surface sensors (Guo and Giancotti, 2004). Since adhesive interactions with these highly instructive surroundings are in large part responsible for migration, it may seem intuitive that disruption of such adhesions would prevent tumor cell dissemination. However, our data present a case study in which the contrary, the inhibition of de-adhesion, can be an equal, or even more powerful approach to preventing metastasis.
The disruption of a single adhesive function through blocking antibodies or peptides directed towards the ligand or its receptor (integrin) can readily disrupt in vitro adhesion and migration (Ruoslahti, 1996) or alter the survival (Stupack et al., 2006) and morphology (Kenny and Bissell, 2003) of tumor cells within the matrix. However, within the complexity of the in vivo stroma it is difficult to disrupt the multitude of adhesive interactions effectively. In fact, disabling or blocking a single proteolytic or adhesive event often causes a tumor cell to alter their mechanism of migration without the loss of productive motility (Friedl and Wolf, 2003). In contrast, a single interfering step that prevents dissociation can immobilize the target cell without having to out-compete newly available adhesion sites, continuously renewing chemotactic stimuli or matrix modifying enzymatic activities. This is apparent in our ability to promote immobility on multiple matrixes to which the tumor cells adhere (Fig 2). The immobilization that occurs does not prevent localized protrusion and forward movement of the cells. In contrast, immobilized cells can be seen protruding into the immediate surroundings and adhering to the newly invaded space. However, as the rear of the cell fails to dissociate, the cell halts upon extending one body length and remains immotile or returns to its original location (Fig. 6). Ultimately, the consequence of this inability to dissociate blocks the invasive behavior of tumor cells and prevents intravasation and thereby the metastasis of primary tumor cells.
The role of migration as an active contributor to metastasis is a central question at the forefront of metastasis research (Bockhorn et al., 2007). The immobility mediated by CD151 upon antibody ligation is in vivo proof of principle that migration is an active mechanism that contributes to metastasis. Furthermore, it is to our knowledge the first direct evidence demonstrating that regulation of de-adhesion can function as a powerful anti-metastasis target. Improving the stabilizing interactions of tumor cells with the microenvironment has already proven successful in reverting the malignant phenotype independent of its genetic instructions (Weaver et al., 1997). Negative regulators of migration, which enhance adhesion of tumor cells to their surroundings, could be highly effective in preventing the invasive behavior of tumors as well as inhibiting metastasis. The CD151-mediated inhibition of migration demonstrates clearly the role of migration in tumor cell departure from the primary tumor and the role of CD151 and cell de-adhesion in tumor cell motility, intravasation and subsequent metastasis.
Materials and Methods
Cells, antibodies and reagents
The epidermoid carcinoma cells (HEp3) and fibrosarcoma cells (HT1080) were maintained as described previously (Zijlstra et al., 2002). CD151−/− mouse embryonic fibroblasts (MEFCD151−/− were kindly provided by the laboratory of Dr. Sonnenberg (Netherlands Cancer Institute). Wild type MEF and MEFCD151−/− were immortalized using pBRSV (ATCC) encoding SV40 Large T antige, and human CD151-GFP expression was reconstituted in MEFCD151−/− (MEFCD151−/− hCD151) by transduction with PLXN containing CD151-GFP. Transduction with shRNA containing the target sequence GCCTCAAGTACCTGCTGTTTAC was used to down regulated CD151 expression in HT1080 cells (HT1080shRNACD151). Positive and negative cell sorting was used to collect MEFCD151−/− hCD151 and HT1080shRNACD151 respectively. The anti-CD151 monoclonal antibody 1A5 (mAb 1A5) and control antibodies directed to the surface of HEp3 cells were obtained by subtractive immunization (Zijlstra et al., 2003, Testa et al., 1999) and purified endotoxin free by protein-G sepharose affinity purification. To generate cells lines expressing green fluorescent protein (GFP), cultured cells were infected with PLXN-GFP and selected by neomycin selection without clonal propagation. Selected cells were passaged in vivo to maintain the metastatic phenotype.
The chick embryo metastasis model
The quantitative spontaneous and experimental metastasis assays were performed as described previously (Zijlstra et al., 2002). In spontaneous metastasis assays the tumor cells are placed directly on the CAM where a primary tumor forms over a period of 7 days while in experimental metastasis the tumor cells are injected i.v. and allowed to colonize organs for 7 days. At the end of indicated time periods, tumors are harvested, weighed and processed for histology. Metastasis is determined by harvesting lower CAM (LCAM), lung and liver and processing the tissue for the detection of human DNA by alu PCR. For i.v. delivery of antibodies, the injections were performed the day after tumor cell application unless indicated otherwise. To analyze arrest, extravasation, growth and colonization, 5×104 cells were injected i.v. into each egg at developmental day 12. Human tumor cell metastasis in the ex ovo model was developed on the basis of our previously established CAM angiogenesis model (Seandel et al., 2001). Human tumor cell behavior was analyzed in shell-less embryos as described below after i.v. injection using a glass capillary needle or during primary tumor formation after topical application of tumor cells directly onto the CAM.
Mouse SCID spontaneous metastasis
To assess the metastasis of HEp3 cells in mice, 3×106 cells were injected subcutaneously and tumors were allowed to form for 17 days. Control mAb or mAb 1A5 was administered i.v. at 5 days and 13 days after tumor cell injection. The lungs of tumor bearing animals were harvested at the end of the experiment and 50mg of lung tissue was processed as described for chick lungs (Zijlstra et al., 2002). All animals (mice and chick embryos) were housed, maintained and treated by procedures approved by our Institutional Animal Core and Use Committee (IACUC) and state and federal guidelines for the humane treatment of laboratory animals.
Detection of human tumor cell arrest, growth, intravasation and metastasis
The detection of human tumor cell metastasis in chick tissue was based upon the quantitative detection of human alu sequences present in chick tissue DNA extracts (Zijlstra et al., 2002). To approximate the actual number of tumor cells present in each tissue sample the alu signal from experimental samples was interpolated with a standard curve. A separate standard curve was generated for mice and chicken. The actual number of tumor cells/50mg lung tissue could be determined over a range of 50 to 100,000 cells/50mg tissue. Tumor cell arrest was determined by quantifying the number of cells present in a tissue within 24hr after i.v. injection. In vivo tumor cell growth was quantified by determining the expansion of arrested cells from day 1 to day 4. Intravasation was determined by comparing the number of cells that appeared in lung, liver or lower CAM of tumor bearing animals on day 5 vs. day 6. The increase in cell number that could not be accounted for by growth of the cells present on day 5 is considered the number of intravasated cells.
Human Tumor Cell Motility
In vitro Migration assays
Cellular migration in vitro was analyzed using a Boyden chamber system as previously described (Degryse et al., 1999). 8μm pore filters were coated with indicated matrix components and matrix mediated migration was analyzed 16 hr after 50,000 cells were applied to the top chamber. Antibodies were applied at 10μg/ml unless stated otherwise. Migrating cells were quantified in 10 random fields of three replicate wells.
End point in vivo extravasation and motility assays
The ability of tumor cells to extravasate was analyzed during experimental metastasis by imaging the location of GFP expressing tumor cells with respect to the vasculature by which they arrived. To achieve this, 1×105 GFP-expressing HEp3 and HT1080 cells were i.v. injected and allowed to arrest and colonize the CAM for 24 hr to 5 days at which time the CAM was harvested and mounted under a glass coverslip in standard IF mounting medium. Immediately prior to harvesting, 50μg of Rhodamine conjugated Lens Culinaris Agglutinin (LCA, Vector Laboratories Inc., Burlingame CA)) was injected i.v. to label the chick vasculature (Jilani et al., 2003). Tumor cells were considered to have extravasated when the GFP signal was no longer inside the Rhodamine labeled vasculature, as determined by confocal imaging (MRC1024 laser scanning confocal microscope (Biorad, Hercules CA)).
The motility of human tumor cells during experimental metastasis in vivo was assessed by imaging tumor cells in the CAM after i.v. injection. 1×105 GFP-expressing tumor cells were injected i.v. on embryonic day 12 and colonization of the CAM as well as tumor cell dissemination within the CAM stroma was assessed by harvesting CAM from 3 animals at indicated time points and imaging the tumor cell population by epifluorescent microscopy (Olympus BX60, Melville NY). Digital images were acquired using the C-view imaging software (DVC, Austin, TX) and further processed using Photoshop (Adobe, San Jose CA) As the arrested tumor cell population grows, the expanding tumor cell population disseminates throughout the CAM stroma. Failure of the proliferating tumor cells to migrate results in compact colony formation.
Intra-vital real-time imaging of tumor cell motility
To achieve real-time imaging of tumor cells migrating within the tissue stroma and the primary tumor itself a chick-embryo imaging unit was constructed. This unit was designed for the purpose of maintaining the chick embryo under proper temperature and humidity as well as immobilizing the tissue to be imaged, while providing a self-contained unit that prevents exposure of the microscope electronics and objectives to humidity and heat. This chick embryo-imaging unit was built around the shell-less (ex ovo) system developed previously (Seandel et al., 2001) in which chick embryos are maintained without the shell in a plastic dish. The CAM, which is normally located immediately underneath the eggshell in ovo, is now exposed as a free-floating organ atop the developing embryo. Migration of cells within the primary tumor was monitored by imaging a tumor generated within the CAM underneath a coverslip. This was achieved by implanting a mixture of GFP-expressing and non-GFP tumor cells directly into the CAM mesenchyme using a capillary needle at developmental day 10 and covering the area with a sterile coverslip. The animal was subsequently returned to the incubator and imaged at predetermined time-points by immobilizing the coverslip in the chick embryo-imaging unit.
Image capture and processing
Real-time imaging of tumor cell migration was achieved by capturing a four-dimensional image series of the entire tumor within the CAM. A 150μm image stack was captured with a 15μm step size every 15 minutes for 12.5 hr (50 frames) using an upright epifluorescent microscope with a motorized z-stage (Carl Zeiss, Thornwood NY) controlled by Openlab™(Improvision, Lexington MA). For each time-point, a 60μm stack containing the in-focus images of the tumor, was selected and projected as an “extended focus” image using Volocity™(Improvision, Lexington MA). Image drift and rotation was correct by using the Stack_Reg plugin (Biomedical Imaging Group http://bigwww.epfl.ch/) running in the open-source software ImageJ (NIH). Cell tracking and motility quantitation was completed by visually determining the centroid (center of the cell body) at every 15 minute interval and tracing it from one frame to the next using the Manual_Tracking plugin running in ImageJ. These traces were used for migration analysis of the motion parameters velocity, turn angle, and persistence as described previously (Mandeville et al., 1995). Total migration was defined as the total distance migrated while productive migration was defined as the absolute distance from the point of origin. The ability of a cell to mobilize itself (motility) is defined as centroid movement in excess of one body length (>20μm).
Histological analysis of chick tissues
For fluorescent staining each tissue was frozen in Tissue-Tec mounting media and 6μm sections were fixed in Zinc-Formalin and processed for immunological detection of tumor antigens. To detect antigen expression, tumor tissue was incubated with tumor specific mouse monoclonal antibodies and primary antibody binding was visualized with fluorescently labeled secondary (anti-mouse) antibody. To visualize in vivo tumor localization of tumor specific antibodies, tumor tissue and control tissues from antibody-injected animals were incubated with fluorescently labeled secondary anti-mouse antibodies only.
Supplementary Material
Supplemental Figure S1. Tumor specific antibodies injected i.v. persist in the blood and localize to the tumor. The ability of human tumor specific antibodies mAb 1A5 and mAb 29-7 to localize to the tumor in vivo was assessed using IF microscopy. Tumor tissue and whole blood was collected for analysis 24 hr after tumor bearing animals were injected i.v. with 100μg of antibody. The localization of tumor specific i.v. injected antibodies is evident in the tumor tissue (defined by dashed lines, inset contains higher magnification) of mAb 29-7(A) and 1A5 (C) injected animals but not normal tissue (indicated by white arrows in the insets) nor uninjected animals (E) when staining with fluorescently labeled anti-mouse antibodies (Red = antibody stain, Blue = nuclear stain). Standard immunofluorescent staining with 29-7 (B) or 1A5 (D) illustrates specificity of these antibodies for tumor tissue and the total antigen distribution in tumor tissue. Western blotting of normal CAM tissue and tumor tissue with the mAB 1A5 was used to confirm the absence of reactive antigen in normal chick tissue (F). The persistence of these antibodies in the blood of the chick embryo was assessed by quantitative mouse IgG ELISA of plasma at 1 and 4 days after i.v. injection (G). Values are represented as mean ± SEM. Bars = 100μm.
Supplemental Figure S2. Intravital visualization of tumor cell arrest, extravasation, and dissemination in the chick CAM. The CAM is ideal for imaging arrested and extravasating tumor cells because it contains an expansive vascular bed, which can be labeled with a fluorophore-conjugated lectin (Lewis et al., 2006), and the entire depth of the CAM (60–80μm) can be imaged in a single confocal Z-stack. Confocal imaging of arrested tumor cells allows one to determine the precise location of a tumor cell relative to the vasculature. A) A schematic representation of the extravasation and migration assessment in the chick embryo. GFP-expressing HEp3 cells injected i.v. into the Allantoic vein arrest in vasculature throughout the body of the chick embryo as well as the CAM itself. To assess the extravasation of arrested tumor cells, the vasculature of chick embryos can be labeled with Rhodamine linked lectin (LCA) and harvested at distinct time-points after injection of the tumor cells. Confocal imaging and Z-projection of a re-sliced topical view of Rhodamine-Lectin labeled CAM containing GFP-expressing tumor cells can be used to distinguish between intra-vascular tumor cells and extravasated tumor cells. No circulating tumor cells could be seen within minutes after i.v. injection. Furthermore, a detailed time-course analysis (0.5hr –6days) of arrested tumor cells demonstrated that no tumor cells could be found intravascular after 24 hr (data not shown). B) The topical view of a CAM section 6 hr after GFP-HEp3 cell injection. #1 and #2 are two fields selected for further image analysis. The white arrow heads indicate individual cells C) Z-projections of re-sliced View #1 and #2 seen topically in B. Note that the green GFP-cell in #1 is seen as a yellow object because it is within the plane of the capillary bed thus indicating that it is still intravascular. In contrast the green GFP-cells in #2 are above the plane of the red vasculature indicating that they have extravasated. Bars = 20μm.
Supplemental Figure S3. Promoting immobility with anti-CD151 prevents dispersion of tumor cells within the primary tumor. Cryo-sections of 7-day HEp3 tumors from in ovo embryos were imaged by fluorescent microscopy to assess the impact of inhibited tumor cell motility on the dispersion of tumor cells within the primary tumor. HEp3 tumors were generated on the CAM in ovo using a mixture of GFP-expressing and non-expressing cells at a ratio of 1:100. Tumor-bearing animals were treated with 1A5 or a control IgG 24 hr after tumor cell application. The primary tumor was resected at day 7, stained with DAPI (blue) and imaged using standard epifluorescent microscopy. The dotted line demarks the tumor. Bars = 200μm.
Supplemental Figure S4. Tumor cell migration along established blood vessels indicates a vascular guidance of tumor cell migration. In the invasive front of HEp3 tumors the invading tumor cells are most abundant in areas adjacent to the stromal vasculature. To determine if HEp3 tumor cells utilize the stromal vasculature for guidance, the intra-stromal tumor cell migration was imaged intra-vitally. GFP-expressing HEp3 tumors were generated underneath a coverslip in the CAM of ex ovo chick embryos as described in materials and methods (GFP vs. non-GFP expressing cells at 1:10 ratio). Three days after tumor cell implantation, the migration of tumor cells was recorded for 4.5 hr. A fluorescent image (488 nm) and a bright field image was captured every 15 minutes (see Suppl. Video V8). The vasculature was visualized by inverting the bright field image and merging it with the fluorescent image in the red channel. Still images were captured with the 4 and 10× objective. A selected field of cells migrating along the stromal vasculature (outlined in 10× image) is enlarged and shown both in the black and white GFP-channel as well as the merged GFP (green cells) and bright field (red vessels) images. Time is indicated in hours and minutes. Bars = 800μm (4×) or 100μm (10×).
Supplemental Figure S5. The inhibition of mobility by anti-CD151 (mAb 1A5) requires bivalency of antigen binding. Control IgG (A), intact mAb 1A5 (B), bivalent 1A5 Fab’2 (C), or monovalent 1A5 Fab fragments (D) were injected i.v. together with GFP-expressing HEp3 cells to address whether antigen binding by the antibody simply physically disrupts CD151 function or may involve a more functionally related signaling through CD151. Even though each of the 1A5 components bind strongly to the cell surface when compared by flow cytometry (E), only bivalent IgG or Fab’2 of mAb 1A5 can inhibit motility of the tumor cells. This observation indicates that binding of the antibody to CD151 is not sufficient to disrupt function. Bars = 200μm.
Supplemental Figure S6. HT1080 cells that lack CD151 expression can no longer be immobilized by anti-CD151 mAb 1A5. The inability of anti-CD151 mAb 1A5 to promote immobility in the absence of CD151 expression was confirmed in vitro by visualizing the migration of HT1080 and HT1080 shRNACD151on a collagen monolayer in the presence of control IgG (mAb 29-7) or anti-CD151 (mAb 1A5). The initial starting point is indicated by the large white arrow while the protruding edge is indicated by the small white arrow. The addition of mAb 1A5 to wt HT1080 cells inhibits their detachment and they remain at their starting point (3rd panel down), while the addition of mAb 1A5 to HT1080 cells downregulated in their expression of CD151 has no effect on the migration of the cells as they actively detach and migrate (4th panel down) similar to HT1080 cells treated with control antibody (1st and 2nd panels). Bars = 10μm.
Supplemental Video V1. Intravital imaging of in vivo tumor cell motility in control HEp3 tumors. GFP-expressing HEp3 tumor cells in control primary tumors were imaged intra-vitally for 12.25 hr in order to visualize, record and quantify individual tumor cell motility.
Supplemental Video V2. Intravital imaging of tumor cell motility in mAb 1A5-treated HEp3 tumors demonstrates a loss of in vivo motility. GFP-expressing HEp3 tumor cells in primary tumors from mAb 1A5-treated animals were imaged intra-vitally for 12.25 hr in order to visualize, record and quantify individual tumor cell motility.
Supplemental Video V3. Individual tumor cell motility of a cell in a tumor from a control animal (#1). An individual HEp3 cell can be seen migrating in this cropped video selection of a tumor from a control animal.
Supplemental Video V4. Individual tumor cell motility of a cell in a tumor from a control animal (#2). An individual HEp3 cell can be seen migrating in this cropped video selection of a tumor from a control animal.
Supplemental Video V5. Individual tumor cell motility of a cell in a tumor from a mAb 1A5-treated animal (#1). The inability of a HEp3 cell to detach at the rear of the cell as it enters the stroma can be seen migrating in this cropped video selection of a tumor from a mAb 1A5-treated animal.
Supplemental Video V6. Individual tumor cell motility of a cell in a tumor from a mAb 1A5-treated animal (#2). The inability of a HEp3 cell to detach at the rear of the cell as it enters the stroma can be seen migrating in this cropped video selection of a tumor from a mAb 1A5-treated animal.
Supplemental Video V7. The extravasation of HEp3 cells from the chick embryo vasculature. HEp3 cells arrested in the CAM vasculature after i.v. injection are seen migration in the vasculature and extravasate from the vasculature into the stroma below. Seven cell (1–7) are in the field of view. Cell#1 extravasates while cell# 4–7 have already extravasated. Cell# 2 and 3 remain in the vasculature. “*” indicates the location where the protruding/leading edge of the cell migrates out of the vasculature to a lower focal plane. “^” indicates the trailing end of the cell as it departs the vasculature.
Supplemental Video V8. The vascular guidance of HEp3 cell migration in vivo. GFP-expressing tumor cells in a small primary tumor can be seen migrating rapidly along the vasculature illustrating the possibility of vascular guidance of tumor cell migration.
Supplemental Video V9. Individual tumor cell motility of HEp3 cells treated with control mAb or mAb 1A5 while migrating on collagen type I coated coverslips. In vitro, individual HEp3 cells migrating on collagen type I detach readily at the rear in the presence of a control mAb while mAb 1A5 treatment prevents detachment at the rear. As a result, the cell to moves back and forth without achieving productive forward movement. The white asterisk indicates the initial attachment at the rear of the cell.
Acknowledgments
This work was supported by National Institutes of Health grants R01 HL068648 (to H.S.), R01 CA105412 (to J.P.Q.), NSERC PDF-313420 (to J.L) and American Heart Fellowship 0225103y (to A.Z.) We thank Dr. Neil Dilley for assessing motility parameters, Chenxing Li for tissue processing, Rebecca Mellor for technical assistance and Alicia Palestini for administrative assistance. TSRI manuscript no.: 17769-CB.
Footnotes
Significance
The most lethal aspect of a malignancy, metastatic dissemination, is thought to involve migration machinery that mobilizes the tumor cell. The aspect of the metastatic cascade that is strictly dependent upon cell motility has thus far not been defined clearly. Here we demonstrate that specific mAb-mediated inhibition of migration, through the integrin-associated tetraspanin, CD151, prevents tumor cells from entering the vasculature and subsequent colonization of secondary organs. The inhibition of migration is due to a dramatic increase in adhesion that prevents cell dissociation and promotes pronounced immobilization of the tumor cell. The described work illustrates the contribution of tumor cell motility to intravasation and defines induced enhancement of malignant cell adhesion within the primary tumor as a potential therapeutic approach.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ANG J, LIJOVIC M, ASHMAN LK, KAN K, FRAUMAN AG. CD151 protein expression predicts the clinical outcome of low-grade primary prostate cancer better than histologic grading: a new prognostic indicator? Cancer Epidemiol Biomarkers Prev. 2004;13:1717–21. [PubMed] [Google Scholar]
- BERNARDS R, WEINBERG RA. A progression puzzle. Nature. 2002;418:823. doi: 10.1038/418823a. [DOI] [PubMed] [Google Scholar]
- BIENSTOCK RJ, BARRETT JC. KAI1, a prostate metastasis suppressor: prediction of solvated structure and interactions with binding partners; integrins, cadherins, and cell-surface receptor proteins. Mol Carcinog. 2001;32:139–53. doi: 10.1002/mc.1073. [DOI] [PubMed] [Google Scholar]
- BISSELL MJ, LABARGE MA. Context, tissue plasticity, and cancer: are tumor stem cells also regulated by the microenvironment? Cancer Cell. 2005;7:17–23. doi: 10.1016/j.ccr.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOCKHORN M, JAIN R, MUNN L. Active versus passive mechanisms in metastasis: do cancer cells crawl into vessels, or are they pushed? Lancet Oncol. 2007;8:444–8. doi: 10.1016/S1470-2045(07)70140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN EJ, FRAZIER WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–5. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- CAMERON MD, SCHMIDT EE, KERKVLIET N, NADKARNI KV, MORRIS VL, GROOM AC, CHAMBERS AF, MACDONALD IC. Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res. 2000;60:2541–6. [PubMed] [Google Scholar]
- CHAMBERS AF, SCHMIDT EE, MACDONALD IC, MORRIS VL, GROOM AC. Early steps in hematogenous metastasis of B16F1 melanoma cells in chick embryos studied by high-resolution intravital videomicroscopy. J Natl Cancer Inst. 1992;84:797–803. doi: 10.1093/jnci/84.10.797. [DOI] [PubMed] [Google Scholar]
- CHARRIN S, MANIE S, BILLARD M, ASHMAN L, GERLIER D, BOUCHEIX C, RUBINSTEIN E. Multiple levels of interactions within the tetraspanin web. Biochemical & Biophysical Research Communications. 2003;304:107–12. doi: 10.1016/s0006-291x(03)00545-x. [DOI] [PubMed] [Google Scholar]
- CHATTOPADHYAY N, WANG Z, ASHMAN LK, BRADY-KALNAY SM, KREIDBERG JA. alpha3beta1 integrin-CD151, a component of the cadherin-catenin complex, regulates PTPmu expression and cell-cell adhesion. Journal of Cell Biology. 2003;163:1351–62. doi: 10.1083/jcb.200306067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAAS C, STIPP CS, HEMLER ME. Evaluation of prototype transmembrane 4 superfamily protein complexes and their relation to lipid rafts. Journal of Biological Chemistry. 2001;276:7974–84. doi: 10.1074/jbc.M008650200. [DOI] [PubMed] [Google Scholar]
- CLARK EA, GOLUB TR, LANDER ES, HYNES RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–5. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- CZEKAY RP, AERTGEERTS K, CURRIDEN SA, LOSKUTOFF DJ. Plasminogen activator inhibitor-1 detaches cells from extracellular matrices by inactivating integrins. J Cell Biol. 2003;160:781–91. [Google Scholar]
- DEGRYSE B, RESNATI M, RABBANI SA, VILLA A, FAZIOLI F, BLASI F. Src-dependence and pertussis-toxin sensitivity of urokinase receptor-dependent chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. Blood. 1999;94:649–62. [PubMed] [Google Scholar]
- DIMILLA P, STONE J, QUINN J, ALBELDA S, LAUFFENBURGER D. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J Cell Biol. 1993;122:729–37. doi: 10.1083/jcb.122.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDING-HABERMANN B, O’TOOLE TE, SMITH JW, FRANSVEA E, RUGGERI ZM, GINSBERG MH, HUGHES PE, PAMPORI N, SHATTIL SJ, SAVEN A, MUELLER BM. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci U S A. 2001;98:1853–8. doi: 10.1073/pnas.98.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERAL CC, NISHIYA N, FENCZIK CA, STUHLMANN H, SLEPAK M, GINSBERG MH. CD98hc (SLC3A2) mediates integrin signaling. Proc Natl Acad Sci U S A. 2005;102:355–60. doi: 10.1073/pnas.0404852102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIDLER IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- FRIEDL P, WOLF K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- GALLANT ND, MICHAEL KE, GARCIA AJ. Cell adhesion strengthening: contributions of adhesive area, integrin binding, and focal adhesion assembly. Mol Biol Cell. 2005;16:4329–40. doi: 10.1091/mbc.E05-02-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEHO DH, BANDLE RW, CLAIR T, LIOTTA LA. Physiological mechanisms of tumor-cell invasion and migration. Physiology (Bethesda) 2005;20:194–200. doi: 10.1152/physiol.00009.2005. [DOI] [PubMed] [Google Scholar]
- GUO W, GIANCOTTI FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–26. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- HANAHAN D, WEINBERG RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- HARMS BD, BASSI GM, HORWITZ AR, LAUFFENBURGER DA. Directional persistence of EGF-induced cell migration is associated with stabilization of lamellipodial protrusions. Biophys J. 2005;88:1479–88. doi: 10.1529/biophysj.104.047365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASEGAWA H, NOMURA T, KISHIMOTO K, YANAGISAWA K, FUJITA S. SFA-1/PETA-3 (CD151), a member of the transmembrane 4 superfamily, associates preferentially with alpha 5 beta 1 integrin and regulates adhesion of human T cell leukemia virus type 1-infected T cells to fibronectin. Journal of Immunology. 1998;161:3087–95. [PubMed] [Google Scholar]
- HEMLER ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–11. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- HOOD JD, CHERESH DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- HYNES RO. Metastatic potential: generic predisposition of the primary tumor or rare, metastatic variants-or both? Cell. 2003;113:821–3. doi: 10.1016/s0092-8674(03)00468-9. [DOI] [PubMed] [Google Scholar]
- JILANI SM, MURPHY TJ, THAI SN, EICHMANN A, ALVA JA, IRUELA-ARISPE ML. Selective binding of lectins to embryonic chicken vasculature. J Histochem Cytochem. 2003;51:597–604. doi: 10.1177/002215540305100505. [DOI] [PubMed] [Google Scholar]
- KAZAROV AR, YANG X, STIPP CS, SEHGAL B, HEMLER ME. An extracellular site on tetraspanin CD151 determines alpha 3 and alpha 6 integrin-dependent cellular morphology. Journal of Cell Biology. 2002;158:1299–309. doi: 10.1083/jcb.200204056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNY PA, BISSELL MJ. Tumor reversion: correction of malignant behavior by microenvironmental cues. Int J Cancer. 2003;107:688–95. doi: 10.1002/ijc.11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLOSEK SK, NAKASHIRO K, HARA S, SHINTANI S, HASEGAWA H, HAMAKAWA H. CD151 forms a functional complex with c-Met in human salivary gland cancer cells. Biochem Biophys Res Commun. 2005;336:408–16. doi: 10.1016/j.bbrc.2005.08.106. [DOI] [PubMed] [Google Scholar]
- LAMMERDING J, KAZAROV AR, HUANG H, LEE RT, HEMLER ME. Tetraspanin CD151 regulates alpha6beta1 integrin adhesion strengthening. Proc Natl Acad Sci U S A. 2003;100:7616–21. doi: 10.1073/pnas.1337546100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LE NAOUR F, ANDRE M, GRECO C, BILLARD M, SORDAT B, EMILE JF, LANZA F, BOUCHEIX C, RUBINSTEIN E. Profiling of the tetraspanin web of human colon cancer cells. Mol Cell Proteomics. 2006;5:845–57. doi: 10.1074/mcp.M500330-MCP200. [DOI] [PubMed] [Google Scholar]
- LE NAOUR F, CHARRIN S, LABAS V, LE CAER JP, BOUCHEIX C, RUBINSTEIN E. Tetraspanins connect several types of Ig proteins: IgM is a novel component of the tetraspanin web on B-lymphoid cells. Cancer Immunology, Immunotherapy. 2004;53:148–52. doi: 10.1007/s00262-003-0477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS JD, DESTITO G, ZIJLSTRA A, GONZALEZ MJ, QUIGLEY JP, MANCHESTER M, STUHLMANN H. Viral nanoparticles as tools for intravital vascular imaging. Nat Med. 2006;12:354–60. doi: 10.1038/nm1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIOTTA LA, KOHN EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–9. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- LUZZI KJ, MACDONALD IC, SCHMIDT EE, KERKVLIET N, MORRIS VL, CHAMBERS AF, GROOM AC. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol. 1998;153:865–73. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDEVILLE JT, GHOSH RN, MAXFIELD FR. Intracellular calcium levels correlate with speed and persistent forward motion in migrating neutrophils. Biophys J. 1995;68:1207–17. doi: 10.1016/S0006-3495(95)80336-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURPHY-ULLRICH JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest. 2001;107:785–90. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIUCHI R, SANZEN N, NADA S, SUMIDA Y, WADA Y, OKADA M, TAKAGI J, HASEGAWA H, SEKIGUCHI K. Potentiation of the ligand-binding activity of integrin alpha3beta1 via association with tetraspanin CD151. Proc Natl Acad Sci U S A. 2005;102:1939–44. doi: 10.1073/pnas.0409493102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALECEK S, LOFTUS J, GINSBERG M, LAUFFENBURGER D, HORWITZ A. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–40. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- PALECEK SP, HUTTENLOCHER A, HORWITZ AF, LAUFFENBURGER DA. Physical and biochemical regulation of integrin release during rear detachment of migrating cells. J Cell Sci. 1998;111 (Pt 7):929–40. doi: 10.1242/jcs.111.7.929. [DOI] [PubMed] [Google Scholar]
- PANTEL K, BRAKENHOFF RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–56. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- RUOSLAHTI E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- SACHS N, KREFT M, VAN DEN BERGH WEERMAN M, BEYNON A, PETERS T, WEENING J, SONNENBERG A. Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol. 2006;175:33–9. doi: 10.1083/jcb.200603073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAWADA S, YOSHIMOTO M, ODINTSOVA E, HOTCHIN NA, BERDITCHEVSKI F. The tetraspanin CD151 functions as a negative regulator in the adhesion-dependent activation of Ras. J Biol Chem. 2003;278:26323–6. doi: 10.1074/jbc.C300210200. [DOI] [PubMed] [Google Scholar]
- SEANDEL M, NOACK-KUNNMANN K, ZHU D, AIMES RT, QUIGLEY JP. Growth factor-induced angiogenesis in vivo requires specific cleavage of fibrillar type I collagen. Blood. 2001;97:2323–32. doi: 10.1182/blood.v97.8.2323. [DOI] [PubMed] [Google Scholar]
- SERRU V, LE NAOUR F, BILLARD M, AZORSA DO, LANZA F, BOUCHEIX C, RUBINSTEIN E. Selective tetraspan-integrin complexes (CD81/alpha4beta1, CD151/alpha3beta1, CD151/alpha6beta1) under conditions disrupting tetraspan interactions. Biochemical Journal. 1999;340:103–11. [PMC free article] [PubMed] [Google Scholar]
- SHIGETA M, SANZEN N, OZAWA M, GU J, HASEGAWA H, SEKIGUCHI K. CD151 regulates epithelial cell-cell adhesion through PKC- and Cdc42-dependent actin cytoskeletal reorganization. Journal of Cell Biology. 2003;163:165–76. doi: 10.1083/jcb.200301075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINCOCK PM, FITTER S, PARTON RG, BERNDT MC, GAMBLE JR, ASHMAN LK. PETA-3/CD151, a member of the transmembrane 4 superfamily, is localised to the plasma membrane and endocytic system of endothelial cells, associates with multiple integrins and modulates cell function. Journal of Cell Science. 1999;112:833–44. doi: 10.1242/jcs.112.6.833. [DOI] [PubMed] [Google Scholar]
- SOON L, MOUNEIMNE G, SEGALL J, WYCKOFF J, CONDEELIS J. Description and characterization of a chamber for viewing and quantifying cancer cell chemotaxis. Cell Motil Cytoskeleton. 2005;62:27–34. doi: 10.1002/cm.20082. [DOI] [PubMed] [Google Scholar]
- STERK LM, GEUIJEN CA, VAN DEN BERG JG, CLAESSEN N, WEENING JJ, SONNENBERG A. Association of the tetraspanin CD151 with the laminin-binding integrins alpha3beta1, alpha6beta1, alpha6beta4 and alpha7beta1 in cells in culture and in vivo. J Cell Sci. 2002;115:1161–73. doi: 10.1242/jcs.115.6.1161. [DOI] [PubMed] [Google Scholar]
- STRACKE ML, LIOTTA LA. Multi-step cascade of tumor cell metastasis. In Vivo. 1992;6:309–16. [PubMed] [Google Scholar]
- STUPACK DG, TEITZ T, POTTER MD, MIKOLON D, HOUGHTON PJ, KIDD VJ, LAHTI JM, CHERESH DA. Potentiation of neuroblastoma metastasis by loss of caspase-8. Nature. 2006;439:95–9. doi: 10.1038/nature04323. [DOI] [PubMed] [Google Scholar]
- TAKEDA Y, KAZAROV AR, BUTTERFIELD CE, HOPKINS BD, BENJAMIN LE, KAIPAINEN A, HEMLER ME. Deletion of tetraspanin Cd151 results in decreased pathologic angiogenesis in vivo and in vitro. Blood. 2007;109:1524–32. doi: 10.1182/blood-2006-08-041970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TESTA JE, BROOKS PC, LIN JM, QUIGLEY JP. Eukaryotic expression cloning with an antimetastatic monoclonal antibody identifies a tetraspanin (PETA-3/CD151) as an effector of human tumor cell migration and metastasis. Cancer Res. 1999;59:3812–20. [PubMed] [Google Scholar]
- WEAVER VM, PETERSEN OW, WANG F, LARABELL CA, BRIAND P, DAMSKY C, BISSELL MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–45. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDNER N. New paradigm for vessel intravasation by tumor cells. Am J Pathol. 2002;160:1937–9. doi: 10.1016/S0002-9440(10)61141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS L. Metastatic inefficiency. Adv Cancer Res. 1990;54:159–211. doi: 10.1016/s0065-230x(08)60811-8. [DOI] [PubMed] [Google Scholar]
- WINTERWOOD NE, VARZAVAND A, MELAND MN, ASHMAN LK, STIPP CS. A Critical Role for Tetraspanin CD151 in {alpha}3{beta}1 and {alpha}6{beta}4 Integrin-dependent Tumor Cell Functions on Laminin-5. Mol Biol Cell. 2006 doi: 10.1091/mbc.E05-11-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRIGHT MD, GEARY SM, FITTER S, MOSELEY GW, LAU LM, SHENG KC, APOSTOLOPOULOS V, STANLEY EG, JACKSON DE, ASHMAN LK. Characterization of mice lacking the tetraspanin superfamily member CD151. Molecular & Cellular Biology. 2004;24:5978–88. doi: 10.1128/MCB.24.13.5978-5988.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYCKOFF J, WANG W, LIN EY, WANG Y, PIXLEY F, STANLEY ER, GRAF T, POLLARD JW, SEGALL J, CONDEELIS J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–9. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- WYCKOFF JB, JONES JG, CONDEELIS JS, SEGALL JE. A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res. 2000;60:2504–11. [PubMed] [Google Scholar]
- YANG J, MANI SA, DONAHER JL, RAMASWAMY S, ITZYKSON RA, COME C, SAVAGNER P, GITELMAN I, RICHARDSON A, WEINBERG RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- ZIJLSTRA A, MELLOR R, PANZARELLA G, AIMES RT, HOOPER JD, MARCHENKO ND, QUIGLEY JP. A quantitative analysis of rate-limiting steps in the metastatic cascade using human-specific real-time polymerase chain reaction. Cancer Research. 2002;62:7083–92. [PubMed] [Google Scholar]
- ZIJLSTRA A, TESTA JE, QUIGLEY JP. Targeting the proteome/epitome, implementation of subtractive immunization. Biochemical & Biophysical Research Communications. 2003;303:733–44. doi: 10.1016/s0006-291x(03)00357-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Tumor specific antibodies injected i.v. persist in the blood and localize to the tumor. The ability of human tumor specific antibodies mAb 1A5 and mAb 29-7 to localize to the tumor in vivo was assessed using IF microscopy. Tumor tissue and whole blood was collected for analysis 24 hr after tumor bearing animals were injected i.v. with 100μg of antibody. The localization of tumor specific i.v. injected antibodies is evident in the tumor tissue (defined by dashed lines, inset contains higher magnification) of mAb 29-7(A) and 1A5 (C) injected animals but not normal tissue (indicated by white arrows in the insets) nor uninjected animals (E) when staining with fluorescently labeled anti-mouse antibodies (Red = antibody stain, Blue = nuclear stain). Standard immunofluorescent staining with 29-7 (B) or 1A5 (D) illustrates specificity of these antibodies for tumor tissue and the total antigen distribution in tumor tissue. Western blotting of normal CAM tissue and tumor tissue with the mAB 1A5 was used to confirm the absence of reactive antigen in normal chick tissue (F). The persistence of these antibodies in the blood of the chick embryo was assessed by quantitative mouse IgG ELISA of plasma at 1 and 4 days after i.v. injection (G). Values are represented as mean ± SEM. Bars = 100μm.
Supplemental Figure S2. Intravital visualization of tumor cell arrest, extravasation, and dissemination in the chick CAM. The CAM is ideal for imaging arrested and extravasating tumor cells because it contains an expansive vascular bed, which can be labeled with a fluorophore-conjugated lectin (Lewis et al., 2006), and the entire depth of the CAM (60–80μm) can be imaged in a single confocal Z-stack. Confocal imaging of arrested tumor cells allows one to determine the precise location of a tumor cell relative to the vasculature. A) A schematic representation of the extravasation and migration assessment in the chick embryo. GFP-expressing HEp3 cells injected i.v. into the Allantoic vein arrest in vasculature throughout the body of the chick embryo as well as the CAM itself. To assess the extravasation of arrested tumor cells, the vasculature of chick embryos can be labeled with Rhodamine linked lectin (LCA) and harvested at distinct time-points after injection of the tumor cells. Confocal imaging and Z-projection of a re-sliced topical view of Rhodamine-Lectin labeled CAM containing GFP-expressing tumor cells can be used to distinguish between intra-vascular tumor cells and extravasated tumor cells. No circulating tumor cells could be seen within minutes after i.v. injection. Furthermore, a detailed time-course analysis (0.5hr –6days) of arrested tumor cells demonstrated that no tumor cells could be found intravascular after 24 hr (data not shown). B) The topical view of a CAM section 6 hr after GFP-HEp3 cell injection. #1 and #2 are two fields selected for further image analysis. The white arrow heads indicate individual cells C) Z-projections of re-sliced View #1 and #2 seen topically in B. Note that the green GFP-cell in #1 is seen as a yellow object because it is within the plane of the capillary bed thus indicating that it is still intravascular. In contrast the green GFP-cells in #2 are above the plane of the red vasculature indicating that they have extravasated. Bars = 20μm.
Supplemental Figure S3. Promoting immobility with anti-CD151 prevents dispersion of tumor cells within the primary tumor. Cryo-sections of 7-day HEp3 tumors from in ovo embryos were imaged by fluorescent microscopy to assess the impact of inhibited tumor cell motility on the dispersion of tumor cells within the primary tumor. HEp3 tumors were generated on the CAM in ovo using a mixture of GFP-expressing and non-expressing cells at a ratio of 1:100. Tumor-bearing animals were treated with 1A5 or a control IgG 24 hr after tumor cell application. The primary tumor was resected at day 7, stained with DAPI (blue) and imaged using standard epifluorescent microscopy. The dotted line demarks the tumor. Bars = 200μm.
Supplemental Figure S4. Tumor cell migration along established blood vessels indicates a vascular guidance of tumor cell migration. In the invasive front of HEp3 tumors the invading tumor cells are most abundant in areas adjacent to the stromal vasculature. To determine if HEp3 tumor cells utilize the stromal vasculature for guidance, the intra-stromal tumor cell migration was imaged intra-vitally. GFP-expressing HEp3 tumors were generated underneath a coverslip in the CAM of ex ovo chick embryos as described in materials and methods (GFP vs. non-GFP expressing cells at 1:10 ratio). Three days after tumor cell implantation, the migration of tumor cells was recorded for 4.5 hr. A fluorescent image (488 nm) and a bright field image was captured every 15 minutes (see Suppl. Video V8). The vasculature was visualized by inverting the bright field image and merging it with the fluorescent image in the red channel. Still images were captured with the 4 and 10× objective. A selected field of cells migrating along the stromal vasculature (outlined in 10× image) is enlarged and shown both in the black and white GFP-channel as well as the merged GFP (green cells) and bright field (red vessels) images. Time is indicated in hours and minutes. Bars = 800μm (4×) or 100μm (10×).
Supplemental Figure S5. The inhibition of mobility by anti-CD151 (mAb 1A5) requires bivalency of antigen binding. Control IgG (A), intact mAb 1A5 (B), bivalent 1A5 Fab’2 (C), or monovalent 1A5 Fab fragments (D) were injected i.v. together with GFP-expressing HEp3 cells to address whether antigen binding by the antibody simply physically disrupts CD151 function or may involve a more functionally related signaling through CD151. Even though each of the 1A5 components bind strongly to the cell surface when compared by flow cytometry (E), only bivalent IgG or Fab’2 of mAb 1A5 can inhibit motility of the tumor cells. This observation indicates that binding of the antibody to CD151 is not sufficient to disrupt function. Bars = 200μm.
Supplemental Figure S6. HT1080 cells that lack CD151 expression can no longer be immobilized by anti-CD151 mAb 1A5. The inability of anti-CD151 mAb 1A5 to promote immobility in the absence of CD151 expression was confirmed in vitro by visualizing the migration of HT1080 and HT1080 shRNACD151on a collagen monolayer in the presence of control IgG (mAb 29-7) or anti-CD151 (mAb 1A5). The initial starting point is indicated by the large white arrow while the protruding edge is indicated by the small white arrow. The addition of mAb 1A5 to wt HT1080 cells inhibits their detachment and they remain at their starting point (3rd panel down), while the addition of mAb 1A5 to HT1080 cells downregulated in their expression of CD151 has no effect on the migration of the cells as they actively detach and migrate (4th panel down) similar to HT1080 cells treated with control antibody (1st and 2nd panels). Bars = 10μm.
Supplemental Video V1. Intravital imaging of in vivo tumor cell motility in control HEp3 tumors. GFP-expressing HEp3 tumor cells in control primary tumors were imaged intra-vitally for 12.25 hr in order to visualize, record and quantify individual tumor cell motility.
Supplemental Video V2. Intravital imaging of tumor cell motility in mAb 1A5-treated HEp3 tumors demonstrates a loss of in vivo motility. GFP-expressing HEp3 tumor cells in primary tumors from mAb 1A5-treated animals were imaged intra-vitally for 12.25 hr in order to visualize, record and quantify individual tumor cell motility.
Supplemental Video V3. Individual tumor cell motility of a cell in a tumor from a control animal (#1). An individual HEp3 cell can be seen migrating in this cropped video selection of a tumor from a control animal.
Supplemental Video V4. Individual tumor cell motility of a cell in a tumor from a control animal (#2). An individual HEp3 cell can be seen migrating in this cropped video selection of a tumor from a control animal.
Supplemental Video V5. Individual tumor cell motility of a cell in a tumor from a mAb 1A5-treated animal (#1). The inability of a HEp3 cell to detach at the rear of the cell as it enters the stroma can be seen migrating in this cropped video selection of a tumor from a mAb 1A5-treated animal.
Supplemental Video V6. Individual tumor cell motility of a cell in a tumor from a mAb 1A5-treated animal (#2). The inability of a HEp3 cell to detach at the rear of the cell as it enters the stroma can be seen migrating in this cropped video selection of a tumor from a mAb 1A5-treated animal.
Supplemental Video V7. The extravasation of HEp3 cells from the chick embryo vasculature. HEp3 cells arrested in the CAM vasculature after i.v. injection are seen migration in the vasculature and extravasate from the vasculature into the stroma below. Seven cell (1–7) are in the field of view. Cell#1 extravasates while cell# 4–7 have already extravasated. Cell# 2 and 3 remain in the vasculature. “*” indicates the location where the protruding/leading edge of the cell migrates out of the vasculature to a lower focal plane. “^” indicates the trailing end of the cell as it departs the vasculature.
Supplemental Video V8. The vascular guidance of HEp3 cell migration in vivo. GFP-expressing tumor cells in a small primary tumor can be seen migrating rapidly along the vasculature illustrating the possibility of vascular guidance of tumor cell migration.
Supplemental Video V9. Individual tumor cell motility of HEp3 cells treated with control mAb or mAb 1A5 while migrating on collagen type I coated coverslips. In vitro, individual HEp3 cells migrating on collagen type I detach readily at the rear in the presence of a control mAb while mAb 1A5 treatment prevents detachment at the rear. As a result, the cell to moves back and forth without achieving productive forward movement. The white asterisk indicates the initial attachment at the rear of the cell.