Abstract
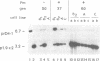
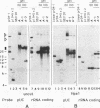
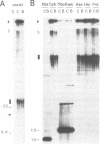
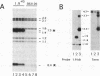
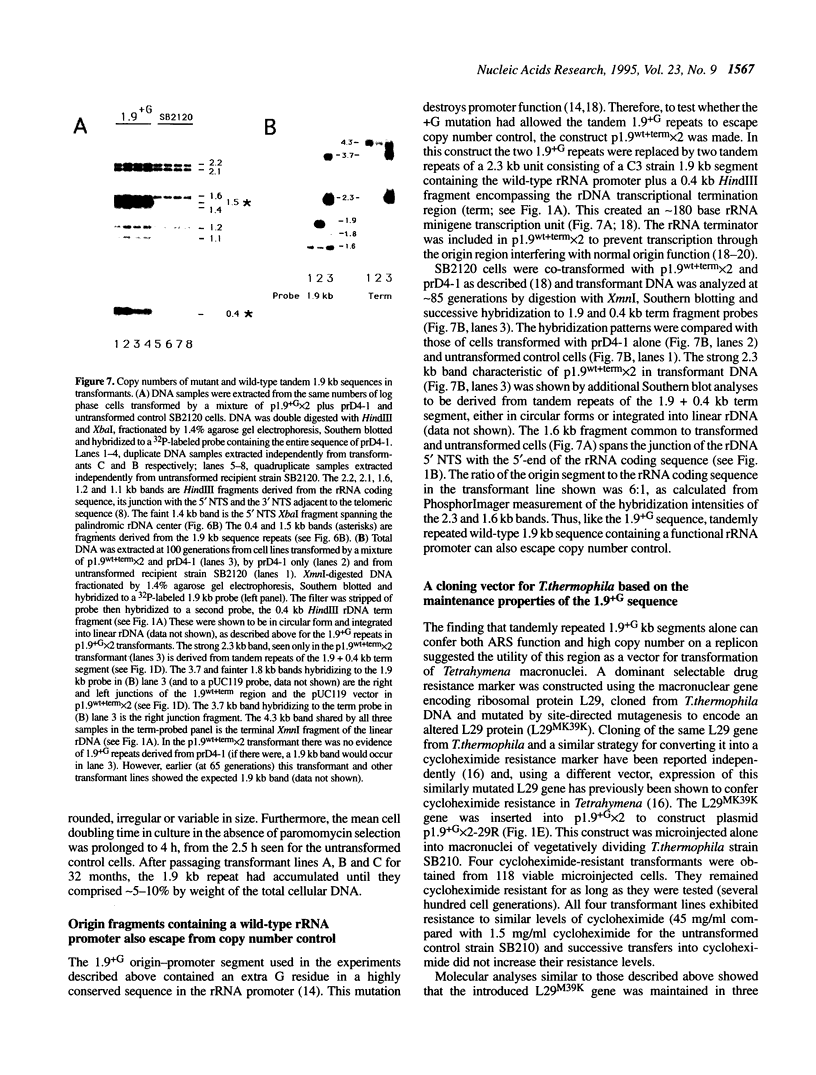
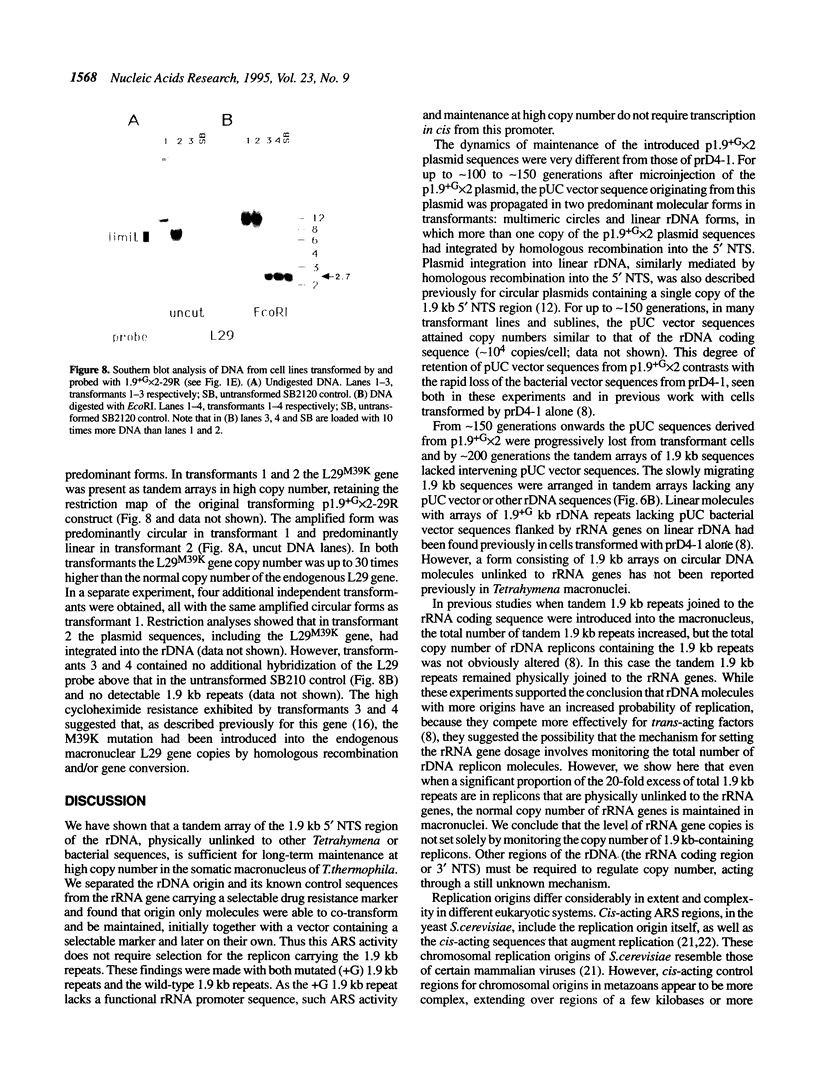
The rRNA genes in the somatic macronucleus of Tetrahymena thermophila are normally on 21 kb linear palindromic molecules (rDNA). We examined the effect on rRNA gene dosage of transforming T.thermophila macronuclei with plasmid constructs containing a pair of tandemly repeated rDNA replication origin regions unlinked to the rRNA gene. A significant proportion of the plasmid sequences were maintained as high copy circular molecules, eventually consisting solely of tandem arrays of origin regions. As reported previously for cells transformed by a construct in which the same tandem rDNA origins were linked to the rRNA gene [Yu, G.-L. and Blackburn, E. H. (1990) Mol. Cell. Biol., 10, 2070-2080], origin sequences recombined to form linear molecules bearing several tandem repeats of the origin region, as well as rRNA genes. The total number of rDNA origin sequences eventually exceeded rRNA gene copies by approximately 20- to 40-fold and the number of circular replicons carrying only rDNA origin sequences exceeded rRNA gene copies by 2- to 3-fold. However, the rRNA gene dosage was unchanged. Hence, simply monitoring the total number of rDNA origin regions is not sufficient to regulate rRNA gene copy number.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell S. P., Stillman B. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature. 1992 May 14;357(6374):128–134. doi: 10.1038/357128a0. [DOI] [PubMed] [Google Scholar]
- Bruns P. J., Katzen A. L., Martin L., Blackburn E. H. A drug-resistant mutation in the ribosomal DNA of Tetrahymena. Proc Natl Acad Sci U S A. 1985 May;82(9):2844–2846. doi: 10.1073/pnas.82.9.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Brehm S. L. Replication of the extrachromosomal ribosomal RNA genes of Tetrahymena thermophilia. Nucleic Acids Res. 1981 Jul 24;9(14):3531–3543. doi: 10.1093/nar/9.14.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B., Sniegowski P., Stephan W. The evolutionary dynamics of repetitive DNA in eukaryotes. Nature. 1994 Sep 15;371(6494):215–220. doi: 10.1038/371215a0. [DOI] [PubMed] [Google Scholar]
- Coverley D., Laskey R. A. Regulation of eukaryotic DNA replication. Annu Rev Biochem. 1994;63:745–776. doi: 10.1146/annurev.bi.63.070194.003525. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L. Eukaryotic DNA replication: anatomy of an origin. Annu Rev Biochem. 1993;62:29–63. doi: 10.1146/annurev.bi.62.070193.000333. [DOI] [PubMed] [Google Scholar]
- Delidakis C., Kafatos F. C. Amplification enhancers and replication origins in the autosomal chorion gene cluster of Drosophila. EMBO J. 1989 Mar;8(3):891–901. doi: 10.1002/j.1460-2075.1989.tb03450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs D. L., Shaiu W. L., Benbow R. M. Modular sequence elements associated with origin regions in eukaryotic chromosomal DNA. Nucleic Acids Res. 1994 Jul 11;22(13):2479–2489. doi: 10.1093/nar/22.13.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg J., Nielsen H. Complete sequence of the extrachromosomal rDNA molecule from the ciliate Tetrahymena thermophila strain B1868VII. Nucleic Acids Res. 1990 Dec 11;18(23):6915–6919. doi: 10.1093/nar/18.23.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaertig J., Gorovsky M. A. Efficient mass transformation of Tetrahymena thermophila by electroporation of conjugants. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9196–9200. doi: 10.1073/pnas.89.19.9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase S. B., Heinzel S. S., Calos M. P. Transcription inhibits the replication of autonomously replicating plasmids in human cells. Mol Cell Biol. 1994 Apr;14(4):2516–2524. doi: 10.1128/mcb.14.4.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapler G. M. Developmentally regulated processing and replication of the Tetrahymena rDNA minichromosome. Curr Opin Genet Dev. 1993 Oct;3(5):730–735. doi: 10.1016/s0959-437x(05)80091-7. [DOI] [PubMed] [Google Scholar]
- Kapler G. M., Orias E., Blackburn E. H. Tetrahymena thermophila mutants defective in the developmentally programmed maturation and maintenance of the rDNA minichromosome. Genetics. 1994 Jun;137(2):455–466. doi: 10.1093/genetics/137.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysan P. J., Smith J. G., Calos M. P. Autonomous replication in human cells of multimers of specific human and bacterial DNA sequences. Mol Cell Biol. 1993 May;13(5):2688–2696. doi: 10.1128/mcb.13.5.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson D. D., Blackburn E. H., Yaeger P. C., Orias E. Control of rDNA replication in Tetrahymena involves a cis-acting upstream repeat of a promoter element. Cell. 1986 Oct 24;47(2):229–240. doi: 10.1016/0092-8674(86)90445-9. [DOI] [PubMed] [Google Scholar]
- Larson D. D., Umthun A. R., Shaiu W. L. Copy number control in the Tetrahymena macronuclear genome. J Protozool. 1991 May-Jun;38(3):258–263. doi: 10.1111/j.1550-7408.1991.tb04439.x. [DOI] [PubMed] [Google Scholar]
- Marahrens Y., Stillman B. A yeast chromosomal origin of DNA replication defined by multiple functional elements. Science. 1992 Feb 14;255(5046):817–823. doi: 10.1126/science.1536007. [DOI] [PubMed] [Google Scholar]
- Metzenberg S., Agabian N. Mitochondrial minicircle DNA supports plasmid replication and maintenance in nuclei of Trypanosoma brucei. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5962–5966. doi: 10.1073/pnas.91.13.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Johnston C. G., Spradling A. C. The role of ACE3 in Drosophila chorion gene amplification. EMBO J. 1989 Dec 20;8(13):4153–4162. doi: 10.1002/j.1460-2075.1989.tb08600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M., Sapolsky R. J., Davis R. W. Transcription interferes with elements important for chromosome maintenance in Saccharomyces cerevisiae. Mol Cell Biol. 1988 May;8(5):2184–2194. doi: 10.1128/mcb.8.5.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virta-Pearlman V. J., Gunaratne P. H., Chinault A. C. Analysis of a replication initiation sequence from the adenosine deaminase region of the mouse genome. Mol Cell Biol. 1993 Oct;13(10):5931–5942. doi: 10.1128/mcb.13.10.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaeger P. C., Orias E., Shaiu W. L., Larson D. D., Blackburn E. H. The replication advantage of a free linear rRNA gene is restored by somatic recombination in Tetrahymena thermophila. Mol Cell Biol. 1989 Feb;9(2):452–460. doi: 10.1128/mcb.9.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C., Yao C. H. Accurate processing and amplification of cloned germ line copies of ribosomal DNA injected into developing nuclei of Tetrahymena thermophila. Mol Cell Biol. 1989 Mar;9(3):1092–1099. doi: 10.1128/mcb.9.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C., Yao C. H. Transformation of Tetrahymena to cycloheximide resistance with a ribosomal protein gene through sequence replacement. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9493–9497. doi: 10.1073/pnas.88.21.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G. L., Blackburn E. H. Amplification of tandemly repeated origin control sequences confers a replication advantage on rDNA replicons in Tetrahymena thermophila. Mol Cell Biol. 1990 May;10(5):2070–2080. doi: 10.1128/mcb.10.5.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G. L., Blackburn E. H. Transformation of Tetrahymena thermophila with a mutated circular ribosomal DNA plasmid vector. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8487–8491. doi: 10.1073/pnas.86.21.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G. L., Hasson M., Blackburn E. H. Circular ribosomal DNA plasmids transform Tetrahymena thermophila by homologous recombination with endogenous macronuclear ribosomal DNA. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5151–5155. doi: 10.1073/pnas.85.14.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel S. G., Fangman W. L. Creation of ARS activity in yeast through iteration of non-functional sequences. Yeast. 1990 May-Jun;6(3):179–186. doi: 10.1002/yea.320060302. [DOI] [PubMed] [Google Scholar]