Abstract
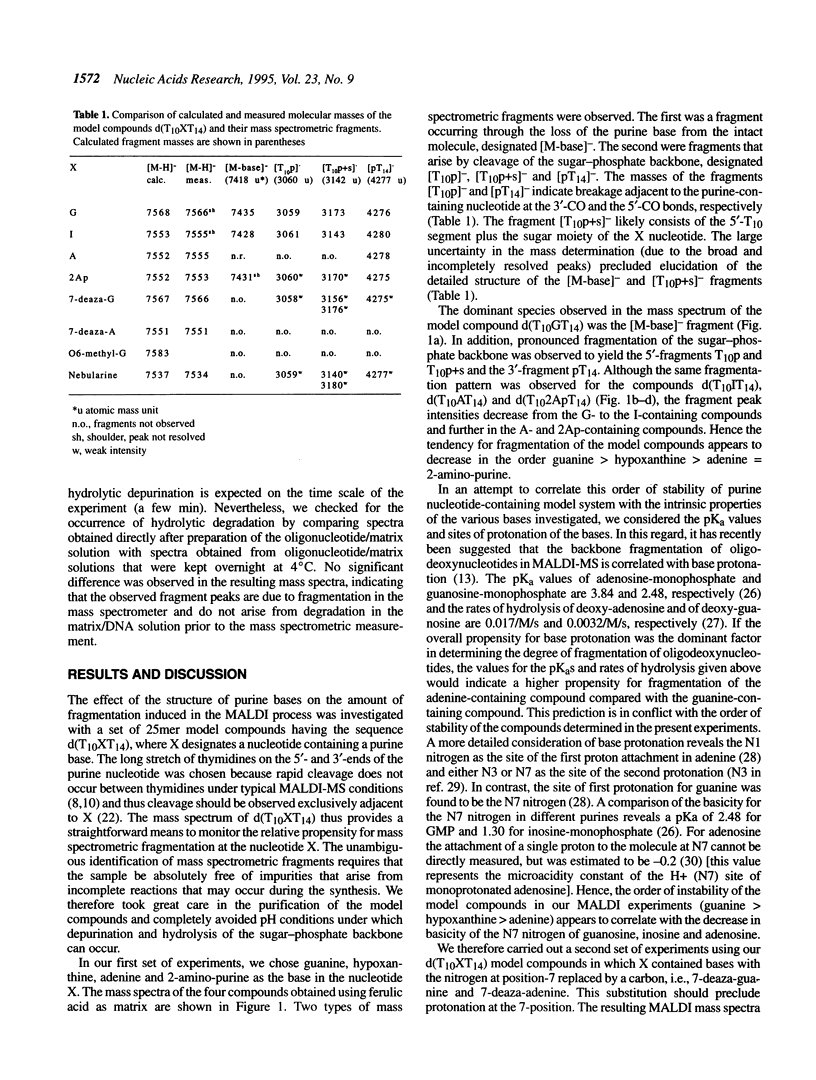
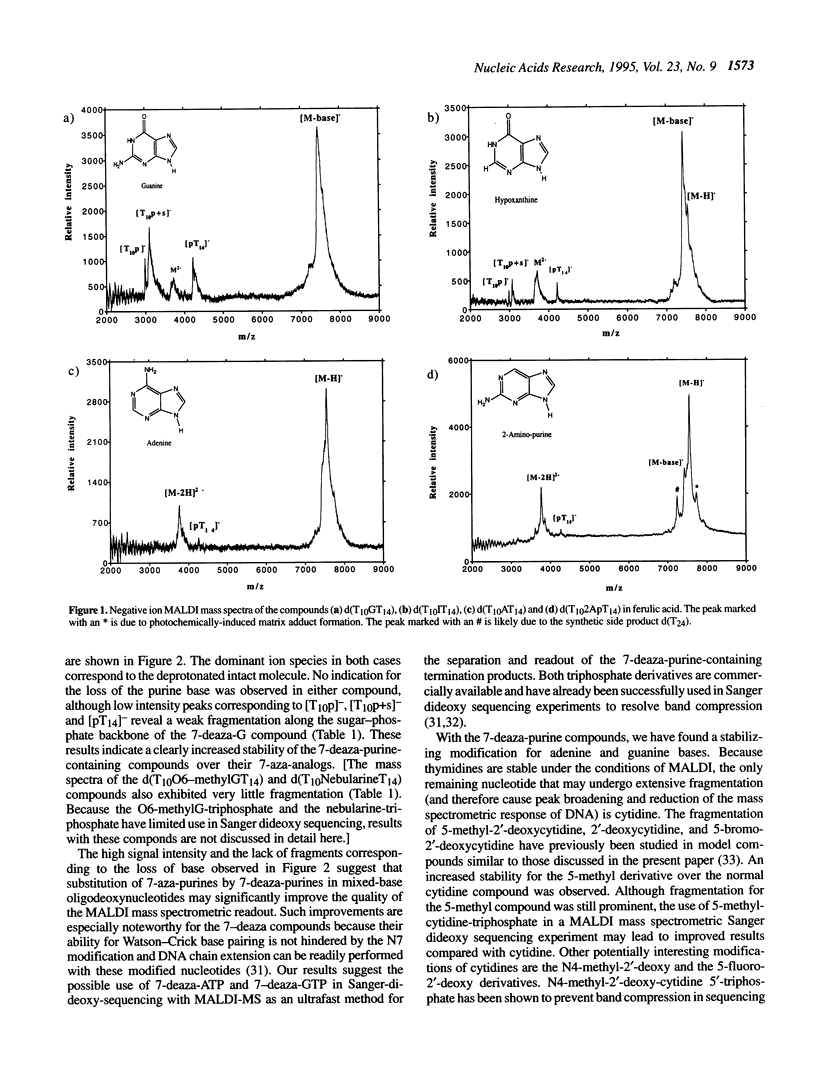
The use of matrix-assisted laser desorption mass spectrometry (MALDI-MS) has been suggested as an ultrafast readout of Sanger DNA sequencing ladders in a manner analogous to that used with sequencing gels. Currently, a serious limitation of MALDI-MS for the analysis of DNA results from the tendency for oligonucleotides to undergo facile fragmentation in the gas phase. The present study was undertaken to gain an understanding of the influence of various chemical structural features of purine bases on the stability of oligodeoxynucleotide ions produced by MALDI. The study focused on the stability of model compounds of the type d(TTTTTTTTTTXTTTTTTTTT TTTT), where T designates deoxythymidine and X a purine-containing 2'-deoxynucleotide. A variety of different purine derivatives were chosen as the base in the nucleotide X. The mass spectra of the model compounds containing 7-deaza analogues of guanine and adenine reveal a significantly increased stability compared to the 7-aza analogues under the conditions of MALDI-MS. The previously reported incorporation of the 7-deaza-2'-deoxy-adenosine triphosphate and the 7-deaza-2'-deoxy-guanosine triphosphate into DNA by polymerases suggests their use in a Sanger dideoxy sequencing experiment. The dideoxy termination products with the 7-deaza-purines instead of the 7-aza-purines might be sufficiently stable to allow separation and detection of the sequencing ladder by MALDI-MS. Thus, an ultrafast (seconds) read-out of DNA sequence may become feasible.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. Mass spectrometry of proteins and peptides in biotechnology. Curr Opin Biotechnol. 1993 Aug;4(4):412–419. doi: 10.1016/0958-1669(93)90006-i. [DOI] [PubMed] [Google Scholar]
- Beavis R. C., Chait B. T. Cinnamic acid derivatives as matrices for ultraviolet laser desorption mass spectrometry of proteins. Rapid Commun Mass Spectrom. 1989 Dec;3(12):432–435. doi: 10.1002/rcm.1290031207. [DOI] [PubMed] [Google Scholar]
- Beavis R. C., Chait B. T. Factors affecting the ultraviolet laser desorption of proteins. Rapid Commun Mass Spectrom. 1989 Jul;3(7):233–237. doi: 10.1002/rcm.1290030708. [DOI] [PubMed] [Google Scholar]
- Caruthers M. H. Gene synthesis machines: DNA chemistry and its uses. Science. 1985 Oct 18;230(4723):281–285. doi: 10.1126/science.3863253. [DOI] [PubMed] [Google Scholar]
- Chait B. T., Kent S. B. Weighing naked proteins: practical, high-accuracy mass measurement of peptides and proteins. Science. 1992 Sep 25;257(5078):1885–1894. doi: 10.1126/science.1411504. [DOI] [PubMed] [Google Scholar]
- Chait B. T., Wang R., Beavis R. C., Kent S. B. Protein ladder sequencing. Science. 1993 Oct 1;262(5130):89–92. doi: 10.1126/science.8211132. [DOI] [PubMed] [Google Scholar]
- Hillenkamp F., Karas M., Beavis R. C., Chait B. T. Matrix-assisted laser desorption/ionization mass spectrometry of biopolymers. Anal Chem. 1991 Dec 15;63(24):1193A–1203A. doi: 10.1021/ac00024a002. [DOI] [PubMed] [Google Scholar]
- Jensen M. A., Zagursky R. J., Trainor G. L., Cocuzza A. J., Lee A., Chen E. Y. Improvements in the chain-termination method of DNA sequencing through the use of 7-deaza-2'-deoxyadenosine. DNA Seq. 1991;1(4):233–239. doi: 10.3109/10425179109020778. [DOI] [PubMed] [Google Scholar]
- Keough T., Baker T. R., Dobson R. L., Lacey M. P., Riley T. A., Hasselfield J. A., Hesselberth P. E. Antisense DNA oligonucleotides. II: The use of matrix-assisted laser desorption/ionization mass spectrometry for the sequence verification of methylphosphonate oligodeoxyribonucleotides. Rapid Commun Mass Spectrom. 1993 Mar;7(3):195–200. doi: 10.1002/rcm.1290070306. [DOI] [PubMed] [Google Scholar]
- Li S., Haces A., Stupar L., Gebeyehu G., Pless R. C. Elimination of band compression in sequencing gels by the use of N4-methyl-2'-deoxycytidine 5'-triphosphate. Nucleic Acids Res. 1993 Jun 11;21(11):2709–2714. doi: 10.1093/nar/21.11.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordhoff E., Cramer R., Karas M., Hillenkamp F., Kirpekar F., Kristiansen K., Roepstorff P. Ion stability of nucleic acids in infrared matrix-assisted laser desorption/ionization mass spectrometry. Nucleic Acids Res. 1993 Jul 25;21(15):3347–3357. doi: 10.1093/nar/21.15.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordhoff E., Ingendoh A., Cramer R., Overberg A., Stahl B., Karas M., Hillenkamp F., Crain P. F. Matrix-assisted laser desorption/ionization mass spectrometry of nucleic acids with wavelengths in the ultraviolet and infrared. Rapid Commun Mass Spectrom. 1992 Dec;6(12):771–776. doi: 10.1002/rcm.1290061212. [DOI] [PubMed] [Google Scholar]
- Pieles U., Zürcher W., Schär M., Moser H. E. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry: a powerful tool for the mass and sequence analysis of natural and modified oligonucleotides. Nucleic Acids Res. 1993 Jul 11;21(14):3191–3196. doi: 10.1093/nar/21.14.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieltz D. M., Chou C. W., Luo C. W., Thomas R. M., Williams P. Mass spectrometry of DNA mixtures by laser ablation from frozen aqueous solution. Rapid Commun Mass Spectrom. 1992 Oct;6(10):631–636. doi: 10.1002/rcm.1290061009. [DOI] [PubMed] [Google Scholar]
- Smith L. M. The future of DNA sequencing. Science. 1993 Oct 22;262(5133):530–532. doi: 10.1126/science.8211178. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Yoshida S., Saneyoshi M., Yamaguchi T. Utilization of 5-fluoro-2'-deoxyuridine triphosphate and 5-fluoro-2'-deoxycytidine triphosphate in DNA synthesis by DNA polymerases alpha and beta from calf thymus. Cancer Res. 1981 Oct;41(10):4132–4135. [PubMed] [Google Scholar]
- Tang K., Allman S. L., Chen C. H. Matrix-assisted laser desorption ionization of oligonucleotides with various matrices. Rapid Commun Mass Spectrom. 1993 Oct;7(10):943–948. doi: 10.1002/rcm.1290071016. [DOI] [PubMed] [Google Scholar]
- Wang B. H., Biemann K. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of chemically modified oligonucleotides. Anal Chem. 1994 Jun 1;66(11):1918–1924. doi: 10.1021/ac00083a023. [DOI] [PubMed] [Google Scholar]
- Wang R., Chait B. T. High-accuracy mass measurement as a tool for studying proteins. Curr Opin Biotechnol. 1994 Feb;5(1):77–84. doi: 10.1016/s0958-1669(05)80074-6. [DOI] [PubMed] [Google Scholar]
- Wu K. J., Shaler T. A., Becker C. H. Time-of-flight mass spectrometry of underivatized single-stranded DNA oligomers by matrix-assisted laser desorption. Anal Chem. 1994 May 15;66(10):1637–1645. doi: 10.1021/ac00082a007. [DOI] [PubMed] [Google Scholar]
- Zoltewicz J. A., Clark D. F., Sharpless T. W., Grahe G. Kinetics and mechanism of the acid-catalyzed hydrolysis of some purine nucleosides. J Am Chem Soc. 1970 Mar 25;92(6):1741–1749. doi: 10.1021/ja00709a055. [DOI] [PubMed] [Google Scholar]



