Abstract
Aims
To characterize and contrast human sensory and vascular changes produced by topical application of the algesic chemical capsaicin to the glabrous lips and tongue.
Methods
Applications of 1% capsaicin or vehicle cream to the glabrous lips and tongue were randomized between two two-trial sessions. The capsaicin trial followed the vehicle trial for each session. Before and 5, 15, and 30 minutes after capsaicin or vehicle cream application, six parameters were recorded from the glabrous lips or the tongue dorsum: (1) burning pain intensity, as measured on a visual analog scale; (2) burning pain area, as indicated by subjects on an orofacial drawing; (3) mechanical sensitivity, as measured by a von Frey filament; (4) visual flare; (5) blood flow and temperature, as measured by laser-Doppler imaging and thermography, respectively; and (6) areas of increased temperature (hot spots), as calculated by a digital tracer from the thermographs. Data were analyzed by ANOVAs and Pearson’s correlations.
Results
Compared to vehicle application, capsaicin elicited burning pain, increases in blood flow and temperature, but no change in mechanical sensitivity in the glabrous lips or tongue. Greater increases in blood flow and temperature paralleled more intense burning pain and larger areas of perceived pain for the lips compared to the tongue. The location of distinct areas of increased temperature within the orofacial area differed between the capsaicin-lip and capsaicin-tongue trials.
Conclusion
The several differences between these responses to noxious stimulation of the glabrous lips and tongue may have implications for examinations of orofacial somatosensory functions.
Keywords: capsaicin, laser-Doppler imaging, orofacial pain, thermography
Capsaicin is an ingredient of hot peppers and binds to the vanilloid receptor (TRPV1)1,2 in nociceptive afferent endings and may sensitize both polymodal A-fibers3–7 and C-fibers.4,7,8 Numerous studies have revealed that topical or intradermal application of this algesic chemical to the skin of the forearm or hand produces burning pain, visual flare, mechanical and heat hyperalgesia, and vasodilatation.9–21 In comparison, few studies have simultaneously characterized both sensory and objective measures of vascular changes to noxious stimulation with capsaicin in the orofacial area in healthy humans.21,22 For example, Gazerani et al21 showed that capsaicin injection into the forehead produced similar but greater changes in vascular and sensory responses when compared to the hand and forearm, leading the authors to suggest that site-specific capsaicin effects may exist between spinal and trigeminal systems. There is also evidence that site-specific changes in sensory responses produced by noxious stimulation with capsaicin may also occur within the trigeminal system itself. Differences in capsaicin-induced burning pain between orofacial areas have previously been shown.22–23 Higher burning pain intensities were reported in the tongue tip compared to the buccal mucosa or the hard palate22 and the skin of the cheek compared to the tongue23 after topical capsaicin application. Additionally, thermal sensitivity has been shown to be more pronounced for the vermillion lip than for the tongue tip and also for the upper lip when compared to the lower lip and tongue.24
To date, it is unknown if there are site-specific changes in sensory and vascular responses produced by capsaicin application to the glabrous lips and tongue. Thus, the aims of this study were to characterize and contrast changes in sensory and vascular responses produced by topical application of capsaicin cream to the glabrous lips and tongue. It was hypothesized that application of capsaicin will induce changes in sensory and vascular responses in the glabrous lips and tongue and that these changes will differ between the glabrous lips and tongue.
Materials and Methods
Subjects
Thirteen healthy subjects participated in this experiment (10 male, 3 females, mean age 25.5 ± 3.7 years). Subjects with diets consisting of more than three spicy meals per week on a regular basis were excluded from the study. Subjects were requested not to eat spicy foods, such as chili peppers or garlic, for a minimum of 12 hours prior to an experimental session, as these foods may contain substances that can activate TRPV1 receptors1,2,25 and influence subjective burning pain ratings. Additionally, subjects were requested not to consume over-the-counter analgesics, such as Ibuprofen, since these have been shown to influence subjective burning pain ratings.26,27 Subjects were also requested not to apply lipsticks or lip balms to the lips, as these substances could have created a lipid barrier on the glabrous skin of the lips and, for example, interfered with capsaicin or vehicle cream absorption into the lips. Subjects were requested not to shave, for a minimum of 12 hours prior to an experimental session, since preliminary studies (in two subjects) revealed that shaving the facial region altered baseline blood flow and temperature in the shaved regions, possibly from small cuts or abrasions. Subjects with moustaches or beards were excluded from this study since the additional facial hair could interfere with the measurement devices. Subjects were also requested to abstain from coffee or other caffeine-related products 4 hours prior to an experimental session, as caffeine has been shown to influence subjective pain ratings.25,28 Each subject received a clear explanation of the procedures prior to participation and informed written consent was obtained according to the Declaration of Helsinki. This study was approved by the local ethics committee (2003-01-31).
Overview of Methodology
This study consisted of two randomized crossover experimental sessions, separated by a minimum of 24 hours, and each session consisted of a capsaicin and a vehicle trial. Subjects received 1 mL of topical application of 1% capsaicin or vehicle cream to the glabrous skin of the upper and lower lips or to the tongue dorsum for 5 minutes. Cross-contamination of the capsaicin cream was eliminated by performing the vehicle trial first and the capsaicin trial second. Thus, one of the two randomized sessions consisted of a vehicle-lip trial and a capsaicin-tongue trial, or a vehicle-tongue trial and a capsaicin-lip trial. In each session, a rest interval of 20 minutes occurred between the capsaicin and vehicle trial.
Subjects rested in a supine position on a comfortable bed for the duration of each trial, with the exception of the 20-minute rest interval before the onset of the second trial at which time they were able to sit or stand up. Before baseline measures were performed, subjects rested for approximately 3 minutes. For each trial, vascular changes were objectively measured by laser-Doppler imaging and thermography and were reported as changes in blood flow and changes in temperature, respectively. The area of capsaicin-induced flare was traced by the investigator onto acetate sheets. Mechanical sensitivity tests were performed by the investigator using a von Frey filament. Burning pain intensity was recorded on an electronic visual analog scale (VAS) and burning pain area was outlined by the subjects on drawings of the orofacial area that included the lips and tongue dorsum. All of the above measures were recorded immediately before and at 5, 15, and 30 minutes after capsaicin or vehicle cream application. For each capsaicin and vehicle trial, a modified version of the McGill Pain Questionnaire (MPQ)29 was completed immediately after the last measurement in order to determine common pain quality descriptors for each trial. The order of measurements was: (1) blood flow, (2) temperature, (3) mechanical sensitivity tests, (4) visual flare, if present, (5) completion of orofacial drawings, and (6) completion of the modified MPQ. For the capsaicin and vehicle trials, recordings of burning pain intensity began immediately before and continued until 30 minutes post-application.
Capsaicin and Vehicle Application to the Glabrous Lips and Tongue
The glabrous lips and tongue dorsum were dried with thin gauze bandages prior to application of capsaicin or vehicle cream (Pharmacy of Aalborg Hospital) in order to prevent spreading of the cream. Subjects were instructed to keep their lips closed when either capsaicin or vehicle cream was applied to the glabrous lips. Subjects were instructed not to move their tongue around the oral cavity during the capsaicin or vehicle tongue trials.
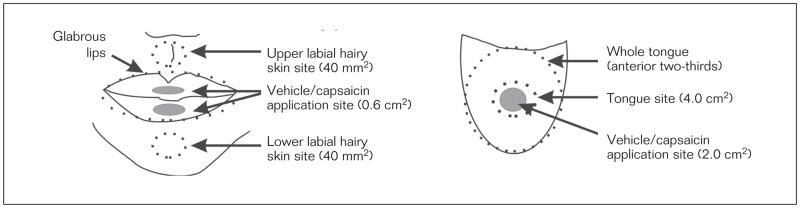
For the lip trials, the capsaicin or vehicle cream was applied with a flat edge applicator to a rectangular area of 0.6 cm2 (rectangular area of 1 cm × 2 mm for the upper glabrous lip and 1 cm × 4 mm for the lower glabrous lip; Fig 1). The different sizes of the application area for the upper and lower glabrous lips were chosen to account for the frequent differences in upper and lower glabrous lip sizes, and each application area was centered within the glabrous skin of the upper and lower lips. Additionally, the sizes of the application area for the upper and lower lips were chosen so that the application area was maximized within the vermillion border of the lips. For the tongue trials, capsaicin or vehicle cream was applied with a flat edge applicator to a standardized site on the tongue dorsum (circular area of 2 cm2) located 1.5 cm from the tongue tip (Fig 1). During capsaicin or vehicle cream application, the subjects were instructed to open their mouths and to press the tip of their tongue to the back of their lower front teeth such that the tongue was slightly arched. Subjects maintained this position until the investigator removed any unabsorbed capsaicin or vehicle cream 5 minutes post-application. Excess capsaicin or vehicle cream was removed to ensure that there were no barriers between the glabrous lip or tongue tissues and the measurement (eg, laser-Doppler imaging or thermography) instruments. During rest intervals (the time between measures), the subjects were instructed not to close their mouth, but they were able to relax their tongue. No additional drying of the lips or tongue dorsum occurred after the removal of the capsaicin or vehicle cream as measurements were taken in areas where saliva pooling would not occur.
Fig 1.
Illustration of the relative sizes, locations, and areas used for assessing changes in blood flow and temperature with respect to the capsaicin and vehicle application area (Drawing not to scale).
Sensory and Vascular Measurements
Assessment of Mechanical Sensitivity
Subjects were asked to rate the intensity of a von Frey filament (133g/mm2, 0.7 mm diameter, Somedic) applied by the investigator at the center of the capsaicin or vehicle cream application area immediately before and 5, 15, and 30 minutes after capsaicin or vehicle cream application. Subjects were asked to mark the intensity of the mechanical sensation produced by the von Frey filament on a sheet of paper that displayed a VAS. The VAS ranged from 0 to 10 cm, with 0 to 5 cm labeled as tactile and 6 to 10 cm labeled as painful. The 0 cm anchor on the VAS was labeled as no tactile sensation and the 10 cm anchor was labeled maximum pain imaginable. Pilot studies revealed that this von Frey filament produced a consistent and high level of mechanical sensation without visibly damaging or penetrating the glabrous lips or tongue dorsum. Furthermore, under baseline conditions (ie, before capsaicin or vehicle cream application), this von Frey filament did not elicit a painful (eg, pin-prick) sensation in the glabrous lips, tongue dorsum, or hairy skin. Immediately before and at 5, 15, and 30 minutes after capsaicin or vehicle application, application of the von Frey filament by the investigator started from the periphery and gradually proceeded toward the application area (in steps of 1 cm with intervals of 3 seconds) along eight 3-cm radiating lines oriented at 45-degree angles. The borders of any static mechanical allodynic area were identified when the subjects reported a clear transition from nonpainful mechanical sensation to a painful pin-prick sensation. After reaching the application area, the tip of the von Frey filament was cleaned with rubbing alcohol by the investigator to prevent spreading of any unabsorbed capsaicin or vehicle cream. The borders of static mechanical allodynia, if present, were traced by the investigator onto acetate paper. The area of static mechanical allodynia was calculated using a digitizer (ACECAD, model D9000 + digitizer).
Assessment of Burning Pain Intensity
Burning pain intensity was recorded using a hand-held electronic VAS (range 0 to 10 cm). The electronic VAS displayed a digital read-out, as reflected by a red light, which changed in magnitude when subjects adjusted a sliding bar on the VAS. Subjects adjusted the position of the sliding bar on the VAS such that the magnitude of the red light corresponded to their current burning pain intensity. The VAS was labeled with two anchor points (no burning pain and highest most imaginable burning pain), and these anchor points corresponded to the minimum level on the VAS (0 cm) and the maximum level on the VAS (10 cm), respectively. Before the onset of the experiment, subjects were also informed that every 3 minutes they would be reminded by the investigator to update the VAS. For the capsaicin and vehicle trials, recordings of burning pain intensity began immediately before and continued until 30 minutes post-application. A combined measure of burning pain intensity was recorded for the glabrous (upper and lower) lips. Peak burning pain intensity was defined as the maximum level on the VAS that occurred within the 30-minute recording period. The area under the curve (AUC) of the burning pain intensity curve was evaluated from the onset of capsaicin or vehicle cream application until 30 minutes post-application. Before the onset of the first trial, an outline of the upper and lower glabrous lips (in a closed position) was traced onto acetate paper by the investigator in order to quantify the glabrous skin area of the upper and lower lips for each subject. It was later determined if correlations existed between the glabrous lip area of each subject and their mean, peak, and AUC burning pain intensity. These correlations were not performed for the tongue dorsum as the whole tongue could not be accurately traced onto acetate paper.
Assessment of Pain Quality
In order to adhere to the experimental timeline, only pain quality descriptors were determined using a modified version of the MPQ. Each subject completed the modified version of the MPQ at the end of each trial by marking all the pain quality descriptors that best described the sensations that occurred during the trial. Pain quality descriptors, that were marked by three or more subjects, in the modified version of the MPQ for each capsaicin and vehicle trial, were considered common and are reported in the Results.
Assessment of Blood Flow and Temperature
Blood flow in the glabrous lips, superior and inferior labial hairy skin sites, and tongue were recorded by a laser-Doppler imaging instrument (LDI, Moor Instruments). For data analysis purposes, the first of the three 48-second blood flow scans was used to ensure proper alignment of the scanned area and the second and third scans were used for data analysis (LDI Image Processing V3.08 software, LDI, Moor Instruments). Blood flow measures for the lip trials consisted of a scan area (10 cm × 6 cm) which included the glabrous lips (in a closed position) and superior and inferior labial hairy skin sites. Blood flow measures for the tongue trials consisted of a scan area (10 cm × 6 cm) which included the tongue dorsum and (as a result of the subjects opening the mouth) the glabrous and mucosal skin area of the upper and lower lips. The subjects were instructed to open their mouths and to press the tip of their tongues to the back of their lower front teeth such that the tongue was slightly arched. Preliminary tests (in two subjects) revealed that pressing the tongue tip to the back of the lower front teeth stabilized the tongue and reduced movement artifacts in the blood flow scans. Blood flow was measured in flux (arbitrary) units, which does not have a standard international (SI) equivalent and is based on calibrations provided by the manufacturer (Moor Instruments) of the laser-Doppler imaging instrument. The flux unit is defined as the product of the number of moving red blood cells in a given volume and the mean net velocity of their movement.30
Thermographs were measured by an infrared camera (Thermovision 900 Series, Agema Infrared Systems) that had a resolution of 0.1°C. For data analysis purposes, only one thermograph was required immediately before and at 5, 15, and 30 minutes post-application. Thermographs for the lip trials consisted of the whole face with the glabrous lips in a closed position (thus the tongue was not included in this thermograph). Thermographs for the tongue trials consisted of the whole face with the mouth in an open position such that the anterior two thirds of the tongue dorsum was exposed. The thermograph of the tongue dorsum also included glabrous and mucosal areas of the upper and lower lips; however, full view of the glabrous skin of the lips was not always possible due to the angle of the upper or lower lip relative to the infrared camera. For the tongue trials, subjects were instructed to gently press the tongue tip against the back of the lower front teeth in order to stabilize the tongue during the thermograph record. Skin temperature, as assessed by thermography, is an indirect measure of blood flow in the deep dermal vessels whereas blood flow, as measured by laser-Doppler flowmetry, is dependent on the blood flow of more superficial dermal capillaries.31–34 The combination of these two techniques allows for the measurement of vascular changes in both superficial and deeper skin layers and a simultaneous documentation of the magnitude and distribution of vasoactive reflex patterns.
Areas Used for Assessing Changes in Blood Flow and Temperature
For the lip trials, blood flow and temperature were measured at predefined sites. These sites were (1) the glabrous upper and lower lips (area was subject-dependent), (2) a superior labial hairy skin site (circular area = 40 mm2) located 0.7 cm from the edge of the upper lip, and (3) an inferior labial hairy skin site (circular area = 40 mm2) located 1.2 cm from the edge of the lower lip (Fig 1). For the tongue trials, blood flow and temperature were measured at the capsaicin and vehicle cream application area (tongue site, circular area = 4 cm2) and anterior two–thirds of the tongue dorsum (whole tongue) (Fig 1).
Assessment and Comparison of the Total Area of Increased Temperature, Visual Flare, and Burning Pain
After capsaicin or vehicle application, flare (the reddening of the glabrous lips or hairy skin around the lips), if present, was identified visually and traced by the investigator onto an acetate paper. The area of burning pain, if present, was outlined by the subject on drawings of the orofacial area that included the lips and tongue dorsum. The total area of increased temperature that occurred within each thermograph was also determined. A distinct area of increased temperature was defined as an area that reflected an increase in temperature greater than the standard error of the mean (SEM) obtained from the baseline thermograph. Distinct areas that increased 0.5°C from baseline were used to calculate the total area of increased temperature. Additionally, the locations of distinct areas of increased temperature (hot spots) that occurred within the orofacial area (eg, tongue dorsum, nose, cheeks, and forehead) were tabulated and are reported in the Results. A limitation of this study was that the total area of increased blood flow, as measured by laser-Doppler imaging, could not be assessed since the blood flow measures were only of the tongue dorsum, upper and lower lips, and the superior and inferior labial hairy skin sites (Fig 1). The quantified areas of burning pain, visual flare, blood flow, and temperature were transformed using a ratio factor (see below) in order to depict the life-sized areas of burning pain, visual flare, blood flow, and temperature. No transformation was required for the area of visual flare since this was traced directly from the face of each subject. Before the onset of the experiment, the investigator measured the horizontal length of the glabrous lips (from corner to corner) and the horizontal length of the tongue (from the left and right edge of the tongue, at 1.5 cm from the tip) from each subject in order to obtain the lip and tongue diameter. The diameter of lips and tongue were measured with a ruler (0 to 10 cm, 0.1-cm intervals) when the lips were in a relaxed position and when the tongue was pressed against the back of the subject’s lower front teeth such that the tongue was slightly arched. The areas of burning pain, blood flow, and increased temperature were first quantified using a digital tracer (ACECAD D9000). The quantified areas were then transformed using a ratio factor. The ratio factor consisted of each subject’s measured glabrous lip and tongue diameter versus the lip and tongue diameter measured from each data record (eg, burning pain drawing and thermograph).
Data Analysis and Statistics
Two-way repeated measures analysis of variance (two-way RM ANOVA) was used to determine differences between the capsaicin and vehicle trials and between the lip and tongue trials at 5, 15, or 30 minutes after capsaicin or vehicle application. One-way RM ANOVA was used to determine if baseline blood flow rates and temperature differed between the orofacial sites. Pearson’s correlations were determined for the increases in temperature and blood flow for the capsaicin-lip and capsaicin-tongue trials as well as the subject’s lip area and burning pain intensity for the capsaicin-lip trials. The significance of statistical analyses was set to P < .05 and post-hoc Tukey tests were used to further analyze differences between the vehicle and capsaicin trials and between the lip and tongue trials. All data in results are presented as mean ± SEM.
Results
Subjects
Six of the 13 subjects reported eating less than one spicy meal per week, three of the 13 subjects reported eating one spicy meal per week, and four of the 13 subjects reported eating two spicy meals per week. Eleven of the 13 subjects had no experience with capsaicin cream studies.
Quality and Intensity of Burning Pain and Area of Burning Pain
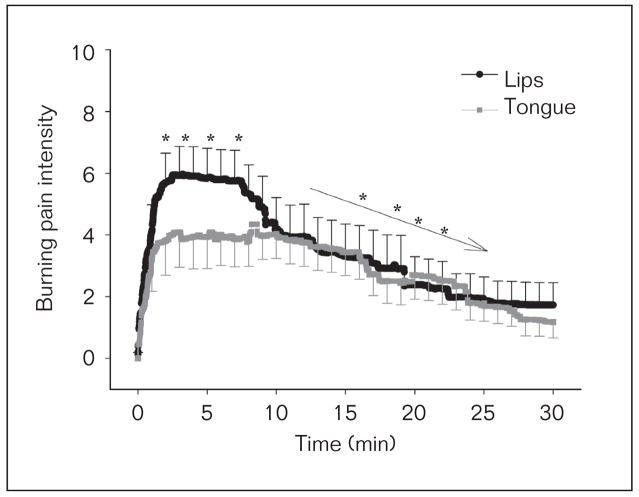
The results from the modified MPQ revealed that the subjects regarded the vehicle-lip trials as pricking, itchy, tender, and annoying, and capsaicin-lip trials as pulsing, drilling, sharp, pinching, burning, itchy, hurting, taut, annoying, intense, penetrating, and continuous. The results from the modified MPQ revealed that the subjects considered the vehicle-tongue trials as pricking, annoying, and cool, and the capsaicin-tongue trials as pricking, sharp, burning, annoying, and spreading. After capsaicin but not vehicle cream application to the lips or tongue dorsum, all subjects reported a burning pain sensation in the lips or tongue, respectively. The burning pain intensity of all subjects in the capsaicin-lip and capsaicin-tongue trials (Fig 2) was 3.5 ± 1.5 and 3.0 ± 1.0 cm, respectively. The peak burning pain intensity of all subjects during the capsaicin-lip and capsaicin-tongue trials occurred within the first 5 minutes and decreased thereafter (two-way RM ANOVA, P < .001, post-hoc Tukey P < .05). The burning pain intensity (as measured from 0 to 8 minutes) in lips for capsaicin-lip trials (5.2 ± 1.0) was greater than the tongue for the capsaicin-tongue trials (3.7 ± 3.0, two-way RM ANOVA, P = .006, post-hoc Tukey P < .05, Fig 2). There were no reports of a burning pain area with respect to time in the vehicle-lip or vehicle-tongue trials and the area of burning pain with respect to time was greater for the capsaicin-lip compared to the capsaicin-tongue trials (two-way RM ANOVA, P = .012, post-hoc Tukey P < .05). There were no correlations between peak, mean, or AUC burning pain intensity and the glabrous lip areas (6.5 ± 0.53 cm2, range 3.0 to 9.1 cm2) of all subjects (Pearson’s correlations, P > .05). Tests for correlations between peak, mean, or AUC burning pain intensity and the whole tongue area were not performed, as previously noted in Materials and Methods.
Fig 2.
The burning pain intensity of the glabrous lips and tongue of all subjects for the capsaicin-lip and capsaicin-tongue trials, respectively. No burning pain intensity was reported for the vehicle-lip and vehicle-tongue trials (data not shown). *Represents significant difference between burning pain intensity for the lip and tongue trials. → Represents significant decrease in burning pain intensity with respect to time. All data are presented as mean (± SEM).
Mechanical Sensitivity and Area of Static Mechanical Allodynia
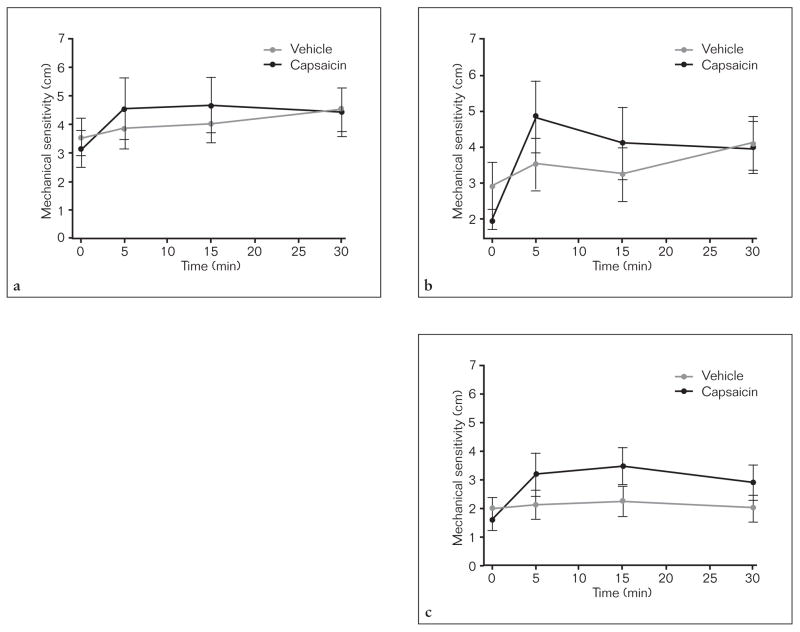
The mechanical sensitivity, as measured before capsaicin or vehicle application, was consistently higher in the upper glabrous lip (2.4 ± 0.48 cm) compared to the tongue site (1.24 ± 0.28 cm, one-way RM ANOVA, P = 0.022, post-hoc Tukey P < .05). Compared to the vehicle-lip and vehicle-tongue trials, there were no differences in the ratings of mechanical sensitivity in the upper or lower glabrous lips in the capsaicin-lip trials or in the tongue dorsum in the capsaicin-tongue trials, respectively (two-way RM ANOVA, P > .73, Fig 3). Static mechanical allodynia specifically occurred at the vermillion border of the upper and lower glabrous lips (area enclosed by the vermillion border of the lips = 5.3 ± 1.9 cm2) in the capsaicin-lip trials. No static mechanical allodynia was found in the capsaicin-tongue trials.
Fig 3.
The mechanical sensitivity of the upper (a) and lower (b) lips and tongue site (c) before, 5, 15, and 30 minutes after capsaicin or vehicle application. All data are presented as mean (± SEM).
Blood Flow, Temperature, and Visual Flare
There was no difference in baseline blood flow, as measured before capsaicin or vehicle application, between the glabrous lips, whole tongue, and tongue site (one-way RM ANOVA, P > .05), but the baseline blood flow was higher in these areas when compared to the superior and inferior labial hairy skin sites (one-way RM ANOVA, P < .02). There was no difference in the baseline temperature, as measured before capsaicin or vehicle application, between the glabrous lips, whole tongue, tongue site, superior and inferior labial hairy skin sites (one-way RM ANOVA, P = .165).
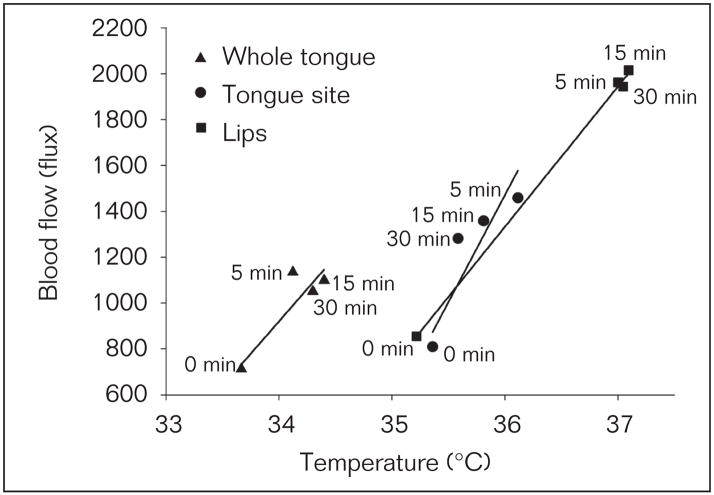
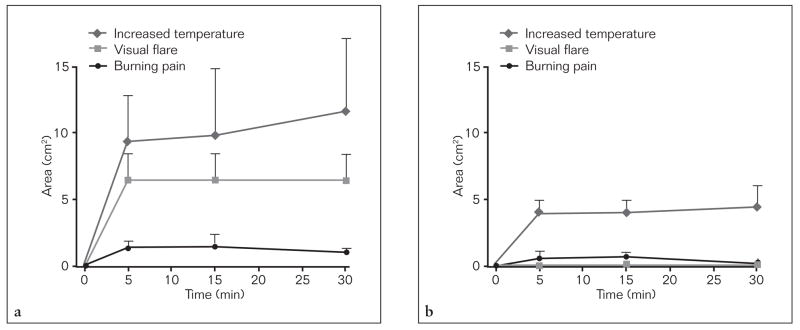
Compared to the vehicle trials, an increase in blood flow in the glabrous lips, superior and inferior labial hairy skin sites occurred in the capsaicin-lip trials (Table 1, two-way RM ANOVA, P < .011; post-hoc Tukey, P < .05) and an increase in blood flow occurred in the tongue site and whole tongue in the capsaicin-tongue trials (Table 1, two-way RM ANOVA, P < .001; post-hoc Tukey, P < .05). Compared to the vehicle-lip and vehicle-tongue trials, an increase in temperature only occurred in the glabrous lips in the capsaicin-lip trials (Table 2, two-way RM ANOVA, P = .027; post-hoc Tukey, P < .05) and an increase in temperature only occurred in the tongue site in the capsaicin-tongue trials (Table 2, two-way RM ANOVA, P < .001, post-hoc Tukey, P < .05), respectively. The relative percent increase in blood flow in the glabrous lips was greater than in the inferior labial hairy skin site in the capsaicin-lip trials and whole tongue and tongue site in the capsaicin-tongue trials (two-way RM ANOVA P < .001, post-hoc Tukey P < .05). Similarly, the relative percent increase in temperature in the glabrous lips in the capsaicin-lip trials was greater than the whole tongue and tongue site in the capsaicin-tongue trials (two-way RM ANOVA P < .001, post-hoc Tukey P < .05). Correlated increases in blood flow and temperature occurred in the glabrous lips in the capsaicin-lip trials (Pearson’s correlation 0.50, P = .003, Fig 4) and in the tongue site in the capsaicin-tongue trials (Pearson’s correlation 0.39, P = .003), but not the whole tongue (Pearson’s correlation 0.18, P = .24, Fig 4). In the capsaicin-lip trials visual flare occurred within the glabrous lips (Fig 5), whereas distinct areas of increased temperature (hot spots) occurred in areas distant to the site of capsaicin application (Table 3). No visible flare was observed in the tongue dorsum in the capsaicin-tongue trials. There was no difference in the total area of increased temperature (ie, the sum of the distinct areas of increased temperature that increased 0.5°C from baseline) with respect to time between the capsaicin-lip and capsaicin-tongue trials (two-way RM ANOVA, P = .68, Fig 5) but the locations of these distinct areas of increased temperature differed (Table 3).
Table 1.
Blood Flow in the Glabrous Lips, Superior and Inferior Labial Hairy Skin Sites, and Tongue at Baseline, 5, 15, and 30 Minutes After Capsaicin or Vehicle Cream Application
| Site/application | Baseline | 5 min | 15 min | 30 min |
|---|---|---|---|---|
| Lips | ||||
| Vehicle | 1028.4 (74.9) | 1115.2 (91.8) | 1132.4 (93.0) | 1197.2 (121.2) |
| Capsaicin | 855.0 (108.5) | 2016.0 (145.8)* | 1943.6 (144.9)* | 1963.1 (127.4)* |
| Superior skin | ||||
| Vehicle | 480.2 (69.6) | 484.9 (53.2) | 544.0 (75.9) | 543.1 (76.3) |
| Capsaicin | 392.1 (57.7) | 769.7 (121.2)* | 770.1 (126.6)* | 659.2 (78.5)* |
| Inferior skin | ||||
| Vehicle | 518.8 (90.9) | 581.7 (121.3) | 630.3 (115.1) | 605.1 (101.1) |
| Capsaicin | 464.8 (64.5) | 664.6 (76.2) | 725.6 (107.9) | 662.5 (79.7) |
| Whole tongue | ||||
| Vehicle | 703.1 (77.5) | 759.5 (88.6) | 736.3 (83.1) | 773.0 (83.6) |
| Capsaicin | 712.3 (60.9) | 1134.4 (67.1)* | 1099.0 (48.1)* | 1050.7 (78.6)* |
| Tongue site | ||||
| Vehicle | 836.9 (86.7) | 860.1 (84.8) | 864.9 (95.2) | 910.4 (94.9) |
| Capsaicin | 809.2 (74.4) | 1458.8 (79.7)* | 1358.0 (76.0)* | 1282.0 (107.7)* |
Represents a significant difference in blood flow between the capsaicin and vehicle sessions.
Table 2.
Temperature in the Glabrous Lips, Superior and Inferior Labial Hairy Skin Sites, and Tongue at Baseline, 5, 15, and 30 Minutes After Capsaicin or Vehicle Cream Application
| Site/application | Baseline | 5 min | 15 min | 30 min |
|---|---|---|---|---|
| Lips | ||||
| Vehicle | 35.0 (0.4) | 36.1 (0.2) | 36.2 (0.1) | 36.2 (0.2) |
| Capsaicin | 35.2 (0.3) | 37.1 (0.1)* | 37.0 (0.1)* | 37.0 (0.1)* |
| Superior skin | ||||
| Vehicle | 35.1 (0.5) | 35.9 (0.1) | 36.0 (0.2) | 36.0 (0.2) |
| Capsaicin | 35.3 (0.3) | 36.6 (0.1) | 36.5 (0.1) | 36.5 (0.1) |
| Inferior skin | ||||
| Vehicle | 34.7 (0.6) | 35.7 (0.2) | 36.0 (0.2) | 35.9 (0.2) |
| Capsaicin | 35.6 (0.2) | 36.4 (0.1) | 36.4 (0.1) | 36.5 (0.1) |
| Whole tongue | ||||
| Vehicle | 35.5 (0.2) | 36.0 (0.2) | 36.1 (0.2) | 36.2 (0.2) |
| Capsaicin | 35.6 (0.2) | 36.0 (0.2) | 35.9 (0.2) | 35.7 (0.2) |
| Tongue site | ||||
| Vehicle | 35.0 (0.2) | 34.7 (0.3) | 35.0 (0.3) | 35.1 (0.3) |
| Capsaicin | 35.4 (0.2) | 36.1 (0.2)* | 35.8 (0.2)* | 35.6 (0.2)* |
Represents significant difference in temperature between the capsaicin and vehicle sessions.
Fig 4.
Correlation between blood flow and temperature with respect to time for the whole tongue (Pearson’s correlation = 0.18, P = .24), tongue site (Pearson’s correlation = 0.39, P = .003), and glabrous lips (Pearson’s correlation = 0.50, P = .003). All data are presented as mean (± SEM).
Fig 5.
The life-sized areas of increased temperature, visible flare, and burning pain within the orofacial area before, 5, 15, and 30 minutes after capsaicin application to the (a) glabrous lips and (b) tongue dorsum. All data are presented as mean (± SEM).
Table 3.
Distinct Areas of Increased Temperature Within the Orofacial Area, as Measured 15 Minutes After Capsaicin Application, for the Capsaicin-lip and Capsaicin-tongue Trials (n = 13)
| Trials | Location of the Areas of Increased Mean Temperature | |||||||
|---|---|---|---|---|---|---|---|---|
| Upper lip | Lower lip | Tongue | Nose | Nasal side walls | Cheek | Chin | Forehead | |
| Lips | 13 | 13 | NA* | 3 | 8 | 4 | 4 | 4 |
| Tongue | 2 | 8 | 13 | 1 | 3 | 0 | 2 | 1 |
Not available, as thermographs were taken with the mouth closed.
Discussion
Overall, this study has shown that there are some similarities but several differences between the responses evoked by noxious stimulation of the glabrous lips with capsaicin compared to those evoked from the tongue. The main findings were that the burning pain and increases in blood flow and temperature occurred after 5 minutes of capsaicin application to the glabrous lips and tongue, and greater increases in blood flow and temperature accompanied more intense burning pain and larger areas of perceived pain for the lips compared to the tongue. Additionally, the location of distinct areas of increased temperature differed between the capsaicin-lip and capsaicin-tongue trials. Lastly, capsaicin application to the glabrous lips and tongue did not alter mechanical sensitivity in these tissues. The several differences between these responses to noxious stimulation of the glabrous lips and tongue may have implications for examinations of orofacial somatosensory functions.
Burning Pain and Mechanical Sensitivity in the Lips and Tongue
Capsaicin has been shown to excite a subpopulation of nociceptors that express the capsaicin-specific vanilloid receptor (TRPV1)1,2 and these receptors have been found in the circumvallate, foliate, and fungiforum papillae of the tongue.35 In addition, TRPV1 receptors in the fungiforum papillae have been shown to be co-localized with substance P, and a subpopulation of these receptors was also positive for CGRP.35 To date, TRPV1 receptors have not been specifically identified in the glabrous lips; however, injections of capsaicin into the rat’s upper and lower lip, tongue, and lateral facial skin are associated with nociceptive behavior and the expression of extracellular signal-regulated kinase (ERK) phosphorylation in nociceptive brainstem relay regions of the trigeminal somatosensory system.36,37 The results from this present study further support the presence of capsaicin-sensitive nociceptive afferents in the glabrous lips.
Burning pain occurred in the glabrous lips and tongue after capsaicin application to the glabrous lips and tongue dorsum, respectively. Interestingly, even though the area of capsaicin application was smaller for the lips than for the tongue, the burning pain intensity was far more intense for the lips than for the tongue. One explanation for this difference could be a higher concentration of capsaicin in the lips compared to the tongue. However, since the excess cream was removed 5 minutes post-application, differences in the concentration of diffused capsaicin cream between the lip and tongue application sites might conceivably have occurred if the diffusion rate of capsaicin into these tissues differed, but, to our knowledge, there have been no reports of differences in the diffusion rates of capsaicin into these orofacial tissues. Additionally, it has been shown that intradermal injection of two differing concentrations of capsaicin to the same area in the forehead resulted in similar peak burning pain intensities; however, the duration and size of the burning pain area differed.21 Furthermore, our findings that burning pain intensity was greater for the lips than for the tongue is consistent with several other studies which have characterized differences in somatosensation within the orofacial area. For example, thermal sensitivity was more pronounced for the vermilion lip than for the tongue tip38 and was higher in the upper lip than the lower lip.24 Additionally, differences in capsaicin-related burning pain intensity between orofacial tissues have been shown.22–23 For example, higher burning pain intensities have been reported for the tongue tip compared to the buccal mucosa or hard palate22 and the skin of the cheek compared to the tongue23 after topical capsaicin application. In addition to differences in capsaicin-related burning pain intensity between orofacial tissues, Gazerani et al21 showed that capsaicin injection into the forehead produced similar but greater changes in vascular and sensory responses when compared to the hand and forearm, leading the authors to suggest that site-specific capsaicin effects may exist between spinal and trigeminal systems.
The present study found no difference in mechanical sensitivity, as measured before capsaicin or vehicle cream application, for the upper and lower glabrous lips but the mechanical sensitivity was more intense for the upper glabrous lip (center) than the tongue (1.5 cm posterior to the tongue tip). When compared to vehicle, the application of capsaicin to the glabrous lips and tongue did not alter the mechanical sensitivity in these tissues. This finding suggests that nociceptive (or non-nociceptive) mechanoreceptors in the glabrous lips and tongue were not sensitized by the application of topical capsaicin. However, static mechanical allodynia specifically occurred at the vermillion border (the transition area from glabrous to hairy skin) following capsaicin application to the glabrous lips. The development of static mechanical allodynia has also been shown to occur after intradermal injection of capsaicin into the hairy skin of the forehead21,39,40 and after topical application of capsaicin to the hairy maxillary skin of awake monkeys and humans.41 However, no mechanical allodynia (static or dynamic) or hyperalgesia developed in the alveolar mucosa following topical application of capsaicin to the gingiva.42 In contrast, topical application of capsaicin to the glabrous area of the palm was associated with mechanical hyperalgesia.43 Collectively, these studies suggest that the development of static mechanical allodynia and possibly hyperalgesia, as elicited by (topical or intradermal) capsaicin, may not occur in glabrous or mucosal tissues within the orofacial region. It is also possible that additional differences to noxious stimulation with capsaicin exist between the glabrous areas of the hand and face, but future studies are required to test for such possible site-specific differences.
Increased Blood Flow and Temperature in the Lips and Tongue
Temperature, as evaluated by thermography, is an indirect measure of blood flow in the deep dermal vessels whereas blood flow, as measured by laser-Doppler imaging, is reported to be dependent on the blood flow of superficial dermal capillaries.31–34 Based on these measurement techniques, the present study found that 5 minutes of capsaicin application resulted in relatively immediate and sustained (minimum of 30 minutes) changes in superficial and deep dermal blood flow in the glabrous lips and tongue. In the present study, the increases in blood flow but not temperature in the superior and labial hairy skin sites also suggest that only the superficial dermal capillaries may have been affected in these areas. These findings may also indicate that laser-Doppler flowmetry may be more sensitive to changes in vascular responses when compared to thermography. A disadvantage of laser-Doppler flowmetry, in general, is that the sensitivity and accuracy of the blood flow measurement is highly dependent upon scanning frequency and, consequently, limits the size of the skin area that can be assessed in a short period of time. The inclusion of thermography, however, allowed for a rapid and simultaneous measurement of the temperature distribution in the entire face, and as a result revealed distinct areas of increased temperature (hot spots). Notably, these hot spots occurred distant to the capsaicin application site, as well as the burning pain and visual flare areas. These findings indicate that thermography may be useful for a relatively quick assessment of changes in the vascular responses in areas distant to the site of capsaicin application. Furthermore, the inclusion of laser-Doppler flowmetry and thermography measures revealed that the subjective psychophysical assessments (eg, burning pain area) and area of visual flare provide a limited view of the vascular responses produced by the application of capsaicin to a particular (orofacial) site. Nonetheless, the thermography measures in this study showed that the total area of increased temperature (ie, the sum of distinct areas of increased temperature) was similar in the capsaicin-lip and capsaicin-tongue trials and that the location of these areas (hot spots) differed. In rats, the appearance of hot spots, as measured by thermography, has also been shown to occur outside the receptive field of the nerve fibers stimulated by capsaicin and that these hot spots corresponded with large-diameter perforating vessels, as verified anatomically.15 The majority of the hot spots that occurred in the present study also corresponded to the anatomical locations of the major perforating blood vessels supplying the glabrous lips and tongue (eg, the superior or inferior labial artery in the capsaicin-lip trials and the inferior labial or lingual artery in the capsaicin-tongue trials44). Kemppainen et al45 have proposed that vascular responses resulting from noxious stimulation may differ within the orofacial area due to separate vasoactive reflex mechanisms for different orofacial sites. In two separate studies, noxious tooth stimulation has been shown to induce long-lasting blood flow increases in the upper and lower glabrous lips but no change of blood flow in the contralateral cheek,45 and vasoconstriction in the nose.46 In the present study, the distribution of hot spots is consistent with the notion that vascular responses may vary within the orofacial area and extend well beyond the site of capsaicin application.
The present study also found that greater increases in blood flow and temperature accompanied more intense burning pain and larger areas of perceived pain. Gazerani et al21 also showed that intradermal injection of capsaicin into the forehead compared to the forearm not only resulted in higher burning pain intensities, longer duration of burning pain, and larger visual flare areas, but also paralleled greater increases in blood flow and temperature. Overall, these present findings support the notion that differences in somatosensory and vascular responses exist within the orofacial area in response to noxious stimulation and such site-specific differences need to be taken into account in studies of orofacial somatosensory functions.
Acknowledgments
This study was supported by the Danish Research Council, CIHR STP 53877 CellSignals, and CIHR grant MOP-4918.
Contributor Information
Shellie A. Boudreau, Doctoral Student, Faculty of Dentistry, University of Toronto, Canada, Orofacial Pain Laboratory, Centre for Sensory-Motor Interaction, Aalborg University, Denmark.
Kelun Wang, Associate Professor, Orofacial Pain Laboratory, Centre for Sensory-Motor Interaction, Aalborg University, Department of Oral and Maxillofacial Surgery, Aalborg Hospital, Aalborg, Denmark.
Peter Svensson, Professor and Chairman, Department of Clinical Oral Physiology, School of Dentistry, Adjunct Professor, Orofacial Pain Laboratory, Centre for Sensory-Motor Interaction, Aalborg University, Aalborg, Denmark.
Barry J. Sessle, Professor, Faculty of Dentistry, University of Toronto, Canada, Honorary Fellow, Orofacial Pain Laboratory, Centre for Sensory-Motor Interaction, Aalborg University, Denmark.
Lars Arendt-Nielsen, Professor, Centre for Sensory-Motor Interaction, Aalborg University, Denmark.
References
- 1.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 2.Caterina MJ, Julius D. The vanilloid receptor: A molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 3.Kenins P. Responses of single nerve fibres to capsaicin applied to the skin. Neurosci Lett. 1982;29:83–88. doi: 10.1016/0304-3940(82)90369-x. [DOI] [PubMed] [Google Scholar]
- 4.LaMotte RH, Lundberg LE, Torebjork HE. Pain, hyperalgesia, and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol. 1992;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beydoun A, Dyke DB, Morrow TJ, Casey KL. Topical capsaicin selectively attenuates heat pain and A delta fiber-mediated laser-evoked potentials. Pain. 1996;65:189–196. doi: 10.1016/0304-3959(95)00161-1. [DOI] [PubMed] [Google Scholar]
- 6.Magerl W, Fuchs PN, Meyer RA, Treede RD. Roles of capsaicin-insensitive nociceptors in cutaneous pain and secondary hyperalgesia. Brain. 2001;124:1754–1764. doi: 10.1093/brain/124.9.1754. [DOI] [PubMed] [Google Scholar]
- 7.Rau KK, Jiang N, Johnson RD, Cooper BY. Heat sensitization in skin and muscle nociceptors expressing distinct combinations of TRPV1 and TRPV2 protein. J Neurophysiol. 2007;97:2651–2662. doi: 10.1152/jn.00840.2006. [DOI] [PubMed] [Google Scholar]
- 8.Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: The search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991;66:212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- 9.Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- 10.Simone DA, Ochoa J. Early and late effects of prolonged topical capsaicin on cutaneous sensibility and neurogenic vasodilatation in humans. Pain. 1991;47:285–294. doi: 10.1016/0304-3959(91)90217-L. [DOI] [PubMed] [Google Scholar]
- 11.Serra J, Campero M, Ochoa J. Flare and hyperalgesia after intradermal capsaicin injection in human skin. J Neurophysiol. 1998;80:2801–2810. doi: 10.1152/jn.1998.80.6.2801. [DOI] [PubMed] [Google Scholar]
- 12.Liu M, Max MB, Robinovitz E, Gracely RH, Bennett GJ. The human capsaicin model of allodynia and hyperalgesia: Sources of variability and methods for reduction. J Pain Symptom Manage. 1998;16:10–20. doi: 10.1016/s0885-3924(98)00026-8. [DOI] [PubMed] [Google Scholar]
- 13.Mohammadian P, Andersen OK, Arendt-Nielsen L. Correlation between local vascular and sensory changes following tissue inflammation induced by repetitive application of topical capsaicin. Brain Res. 1998;792:1–9. doi: 10.1016/s0006-8993(97)01478-9. [DOI] [PubMed] [Google Scholar]
- 14.Wasner G, Baron R, Janig W. Dynamic mechanical allodynia in humans is not mediated by a central presynaptic interaction of A beta-mechanoreceptive and nociceptive C-afferents. Pain. 1999;79:113–119. doi: 10.1016/s0304-3959(98)00159-6. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi Y, Murata A, Nakajima Y. Dilatation of subcutaneous perforating blood vessels associated with capsaicin-induced cutaneous axon reflex: Demonstration with subtraction thermography. J Auton Nerv Syst. 1999;75:87–92. doi: 10.1016/s0165-1838(98)00172-6. [DOI] [PubMed] [Google Scholar]
- 16.Baron R, Wasner G, Borgstedt R, et al. Effect of sympathetic activity on capsaicin-evoked pain, hyperalgesia, and vasodilatation. Neurology. 1999;52:923–932. doi: 10.1212/wnl.52.5.923. [DOI] [PubMed] [Google Scholar]
- 17.Andrews K, Baranowski A, Kinnman E. Sensory threshold changes without initial pain or alterations in cutaneous blood flow, in the area of secondary hyperalgesia caused by topical application of capsaicin in humans. Neurosci Lett. 1999;266:45–48. doi: 10.1016/s0304-3940(99)00248-7. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs PN, Campbell JN, Meyer RA. Secondary hyperalgesia persists in capsaicin desensitized skin. Pain. 2000;84:141–149. doi: 10.1016/s0304-3959(99)00194-3. [DOI] [PubMed] [Google Scholar]
- 19.Sumikura H, Andersen OK, Drewes AM, Arendt-Nielsen L. Spatial and temporal profiles of flare and hyperalgesia after intradermal capsaicin. Pain. 2003;105:285–291. doi: 10.1016/s0304-3959(03)00243-4. [DOI] [PubMed] [Google Scholar]
- 20.Sumikura H, Andersen OK, Drewes AM, Arendt-Nielsen L. A comparison of hyperalgesia and neurogenic inflammation induced by melittin and capsaicin in humans. Neurosci Lett. 2003;337:147–150. doi: 10.1016/s0304-3940(02)01325-3. [DOI] [PubMed] [Google Scholar]
- 21.Gazerani P, Andersen OK, Arendt-Nielsen L. Site-specific, dose-dependent, and sex-related responses to the experimental pain model induced by intradermal injection of capsaicin to the foreheads and forearms of healthy humans. J Orofac Pain. 2007;21:289–302. [PubMed] [Google Scholar]
- 22.Lawless HT, Stevens DA. Responses by humans to oral chemical irritants as a function of locus of stimulation. Percept Psychophys. 1988;43:72–78. doi: 10.3758/bf03208975. [DOI] [PubMed] [Google Scholar]
- 23.Green BG. Capsaicin desensitization and stimulus-induced recovery on facial compared to lingual skin. Physiol Behav. 1998;65:517–523. doi: 10.1016/s0031-9384(98)00202-9. [DOI] [PubMed] [Google Scholar]
- 24.Said YS, Ellrich J. Quantitative sensory testing in the orofacial region. Acta Physiol. 2007;189(suppl 653):P20-L1-05. [Google Scholar]
- 25.Bautista DM, Movahed P, Hinman A, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hargreaves KM, Keiser K. Development of new pain management strategies. J Dent Educ. 2002;66:113–121. [PubMed] [Google Scholar]
- 27.List T, Axelsson S, Leijon G. Pharmacologic interventions in the treatment of temporomandibular disorders, atypical facial pain, and burning mouth syndrome. A qualitative systematic review. J Orofac Pain. 2003;17:301–310. [PubMed] [Google Scholar]
- 28.Motl RW, O’Connor PJ, Dishman RK. Effect of caffeine on perceptions of leg muscle pain during moderate intensity cycling exercise. J Pain. 2003;4:316–321. doi: 10.1016/s1526-5900(03)00635-7. [DOI] [PubMed] [Google Scholar]
- 29.Melzack R. The McGill pain questionnaire: Major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 30.Heden P, Jurell G, Arnander C. Prediction of skin flap necrosis: A comparative study between laser Doppler flowmetry and fluorescein test in a rat model. Ann Plast Surg. 1986;17:485–488. doi: 10.1097/00000637-198612000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Carpentier PH. New techniques for clinical assessment of the peripheral microcirculation. Drugs. 1999;59:17–22. [PubMed] [Google Scholar]
- 32.Choi CM, Bennett RG. Laser dopplers to determine cutaneous blood flow. Dermatol Surg. 2003;29:272–280. doi: 10.1046/j.1524-4725.2003.29042.x. [DOI] [PubMed] [Google Scholar]
- 33.Herrick AL, Hutchinson C. Vascular imaging. Best Pract Res Clin Rheumatol. 2004;18:957–979. doi: 10.1016/j.berh.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Wright CI, Kroner CI, Draijer R. Non-invasive methods and stimuli for evaluating the skin’s microcirculation. J Pharmacol Toxicol Methods. 2006;54:1–25. doi: 10.1016/j.vascn.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Ishida Y, Ugawa S, Ueda T, Murakami S, Shimada S. Vanilloid receptor subtype-1 (VR1) is specifically localized to taste papillae. Brain Res Mol Brain Res. 2002;107:17–22. doi: 10.1016/s0169-328x(02)00441-2. [DOI] [PubMed] [Google Scholar]
- 36.Honda K, Kitagawa J, Sessle BJ, et al. Mechanisms involved in an increment of multimodal excitability of medullary and upper cervical dorsal horn neurons following cutaneous capsaicin treatment. Mol Pain. 2008;4:59. doi: 10.1186/1744-8069-4-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noma N, Tsuboi Y, Kondo M, et al. Organization of pERK-immunoreactive cells in trigeminal spinal nucleus caudalis and upper cervical cord following capsaicin injection into oral and craniofacial regions in rats. J Comp Neurol. 2008;507:1428–1440. doi: 10.1002/cne.21620. [DOI] [PubMed] [Google Scholar]
- 38.Green BG, Gelhard B. Perception of temperature on oral and facial skin. Somatosens Res. 1987;4:191–200. doi: 10.3109/07367228709144606. [DOI] [PubMed] [Google Scholar]
- 39.Gazerani P, Andersen OK, Arendt-Nielsen L. A human experimental capsaicin model for trigeminal sensitization: Gender-specific differences. Pain. 2005;118:155–163. doi: 10.1016/j.pain.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Gazerani P, Staahl C, Drewes AM, Arendt-Nielsen L. The effects of botulinum toxin type A on capsaicin-evoked pain, flare, and secondary hyperalgesia in an experimental human model of trigeminal sensitization. Pain. 2006;122:315–325. doi: 10.1016/j.pain.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Kupers RC, Chen CC, Bushnell MC. A model of transient hyperalgesia in the behaving monkey induced by topical application of capsaicin. Pain. 1997;72:269–275. doi: 10.1016/s0304-3959(97)00052-3. [DOI] [PubMed] [Google Scholar]
- 42.Baad-Hansen L, Jensen TS, Svensson P. A human model of intraoral pain and heat hyperalgesia. J Orofac Pain. 2003;17:333–340. [PubMed] [Google Scholar]
- 43.Culp WJ, Ochoa J, Cline M, Dotson R. Heat and mechanical hyperalgesia induced by capsaicin: Cross modality threshold modulation in human C nociceptors. Brain. 1989;112:1317–1331. doi: 10.1093/brain/112.5.1317. [DOI] [PubMed] [Google Scholar]
- 44.Crouzet C, Fournier H, Papon X, Hentati N, Cronier P, Mercier P. Anatomy of the arterial vascularization of the lips. Surg Radiol Anat. 1998;20:273–278. doi: 10.1007/BF01628490. [DOI] [PubMed] [Google Scholar]
- 45.Kemppainen P, Leppanen H, Jyvasjarvi E, Pertovaara A. Blood flow increase in the orofacial area of humans induced by painful stimulation. Brain Res Bull. 1994;33:655–662. doi: 10.1016/0361-9230(94)90229-1. [DOI] [PubMed] [Google Scholar]
- 46.Kemppainen P, Forster C, Handwerker HO. The importance of stimulus site and intensity in differences of pain-induced vascular reflexes in human orofacial regions. Pain. 2001;91:331–338. doi: 10.1016/S0304-3959(00)00462-0. [DOI] [PubMed] [Google Scholar]