Abstract
Background
The role of IL-17 producing cells in tumors is controversial. In the present study, we investigated the prognostic value of measuring tumor-infiltrating IL-17 producing cell levels in human esophageal squamous cell carcinoma (ESCC).
Methodology/Principal Findings
Immunohistochemical staining was performed to investigate the levels of IL-17+ tumor infiltrating lymphocytes (TILs), as well as CD8+ cytotoxic T lymphocytes (CTLs) and CD57+ natural killer (NK) cells from 181 ESCC patients. The prognostic value of measuring the densities of IL-17+TILs and the correlation with CTLs and NK was evaluated. IL-17 producing cells were detected in esophageal squamous cell carcinoma tissues. The IL-17 producing cells were major CD4 positive, but Foxp3 negative. The median level of IL-17+TILs was 3.90 cells/high power microscopic field (HPF). The density of IL-17 producing cells correlated negatively with T stage (P = 0.042). The higher densities of tumor infiltrating IL-17+ lymphocytes were associated with better overall survival (P = 0.031). Furthermore, we found that there were positive correlations between levels of IL-17 producing cells and the densities of CD8+cells, as well as CD57+cells (r = 0.198, P = 0.008 for CD8+ cells and r = 0.261, P<0.001 for CD57+ cells, respectively). The prognosis analysis also showed that the higher levels of CD8+ CTLs and CD57+ NK cells correlated with better overall survival of ESCC patients.
Conclusions
Our study suggests that tumor infiltrating IL-17 producing cells in ESCC patients may have protective roles in the tumor microenvironment and may be treated as a prognostic marker for ESCC patients.
Introduction
Substantial evidence indicates that the abundance of tumor-infiltrating lymphocytes in the microenvironment of certain tumor types is associated with the prognosis of cancer patients. Moreover, each subset of tumor-infiltrating lymphocytes has a unique role in the antitumor response [1]–[4]. The presence of tumor-infiltrating cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells correlates with improved survival and confers antitumor activity [5]. However, other tumor-infiltrating lymphocyte subsets exhibit bipolar roles: promoting tumor growth or inhibiting tumor progression [6]. These subsets include the newly identified tumor-infiltrating IL-17 producing cells. Interleukin-17 (IL-17), originally termed CTLA-8, plays an important role in inflammation and autoimmune diseases in both mice and humans [7]–[13]. Early research focused on the roles and mechanisms of IL-17 producing cells in inflammation and autoimmune diseases. Because chronic inflammation were correlated significantly to tumor invasion, migration and metastasis [14], [15], scientists have begun to pay more attention to the significance of IL-17 in tumor models. There is accumulating evidence that IL-17 producing cells are present in various cancers, including ovarian cancer, breast cancer, non-small cell lung cancer, hepatocellular carcinoma and gastric cancer [16]–[19]. Substantial evidence indicated that IL-17 was produced mainly by CD4+ T lymphocytes, and these cells were defined as T helper 17 (Th 17) cells [14], [15], [20]. However, in recent studies, it was found that other T cell subsets can also produce IL-17, such as NKT, gamma-delta T cells and Tregs, including mouse models and human beings [14], [20]–[26]. Although IL-17 producing cells have been detected in various tumors, their effect on tumor cell survival and exact physiological role in tumor immunity remain controversial. IL-17 producing cells could enhance tumor growth by promoting angiogenesis [14], [18]. Conversely, IL-17 producing cells might promote tumor regression by enhancing antitumor immunity [15], [27]–[30].
Esophageal squamous cell carcinoma (ESCC) is the major histological type of esophageal cancer in the "Esophageal Cancer Belt," which stretches westward from China through central Asia to northern Iran [31], [32]. ESCC is the eighth most common cancer worldwide [33], and ranks the sixth cancer mortality worldwide [34]. It was reported that the host immune response prompted by ESCC may influence patient prognosis; both adaptive and innate immunity play important roles in ESCC progression and regression [35]–[38]. Yasushi et al showed that the number of CD8+ T cells correlated with favorable outcomes in ESCC patients [39]. Hsia et al found that ESCC patient prognosis correlated positively with intratumoral NK cell infiltration [36]. Xue et al found that FOXP3 expression was associated with lymph node metastasis and pathological TNM staging, suggesting that regulatory T cells (Tregs) might promote tumor progression [38]. However, up until now, the presence and clinical significance of IL-17 producing cells have not been previously studied in ESCC. Thus, in this study, we evaluated the accumulation and clinicopathological significance of tumor-infiltrating IL-17 producing cells in tumor tissues from ESCC patients. The prognosis value of IL-17 producing cells was also evaluated. Furthermore, we detected CD8+ CTLs and CD57+ NK cells in the same tumor tissues and relationships between the number of IL-17 producing cells and the density of CD8+ CTLs or CD57+ NK cells were further evaluated.
Results
Immunohistochemical staining of IL-17 producing cells and their associations with clinicopathological characteristics
The representative photomicrographs of tissue sections immunostained for IL-17 are shown in Figure 1. IL-17 producing cells were detected in esophageal squamous cell carcinoma tissue. In order to clarify which set of T cells could produce IL-17, double immunohistochemical staining was performed in the same tissue section. Immunofiuorescence results showed that IL-17 producing cells were major CD4 positive, but FOXP3 negative in ESCC tissues (Figure 1). The median value of IL-17 producing cells was 3.90 cells/HPF (range: 0.00–21.40 cells/HPF).
Figure 1. Representative immunohistochemical staining photomicrographs of IL-17 producing cells in tumor tissues of ESCC.
Positive staining of IL-17 was detected in cellular cytoplasm of tumor tissues. (A), (B), (E) and (F): Low density of IL-17+ TILs. (C), (D), (G) and (H): High density of IL-17+ TILs. (J) and (K): Double stainings of CD4 (green, on the menbrane) and IL-17 (red, in the cytoplasm), IL-17 (green, in the cytoplasm) and FOXP3 (red, nuclei) in paraffin-embedded specimens were analyzed by immunofiuorescence. Original magnification: A–D×200; E–K×400.
The associations between the levels of IL-17 producing cells and clinicopathological factors of the ESCC patients are summarized in Table 1. According to previous studies, patients were divided into two groups based on the median of IL-17 producing cells (high level group vs. low level group) [4], [18]. There was a significant inverse correlation between the densities of IL-17+ TILs and T (depth of primary tumor invasion, P = 0.042).
Table 1. Relationship between IL-17 producing cell density and clinical-pathologic factors.
| Variables | Number of Patients | IL-17 producing cell density | P value | |
| Low level group, n = 90 | High level group, n = 91 | |||
| Age(years) | 0.506 | |||
| <60 | 105 | 50 | 55 | |
| ≥60 | 76 | 40 | 36 | |
| Gender | 0.080 | |||
| Male | 141 | 75 | 66 | |
| Female | 40 | 15 | 25 | |
| Tumor length (cm) | 0.110 | |||
| <5 | 75 | 32 | 43 | |
| ≥5 | 106 | 58 | 48 | |
| Differentiation | 0.199 | |||
| G1 | 45 | 26 | 19 | |
| G2 | 85 | 39 | 46 | |
| G3 | 51 | 25 | 26 | |
| Location | 0.952 | |||
| Upper third | 13 | 6 | 7 | |
| Middle third | 113 | 56 | 57 | |
| Lower third | 55 | 28 | 27 | |
| T | 0.042* | |||
| T1+T2 | 57 | 22 | 35 | |
| T3+T4 | 124 | 68 | 56 | |
| N | 0.335 | |||
| No | 101 | 47 | 54 | |
| Yes | 80 | 43 | 37 | |
| M | 0.444 | |||
| M0 | 175 | 86 | 89 | |
| M1 | 6 | 4 | 2 | |
| TNM staging | 0.177 | |||
| Stage I-II | 117 | 53 | 64 | |
| Stage III-IV | 64 | 37 | 27 | |
G1, well differentiated; G2, moderately differentiated; G3, poorly differentiated; T, depth of primary tumor invasion; N, regional lymph nodes; M, distant metastasis; TNM, tumor-lymph node-metastasis classification.
* Statistically significant (P<0.05).
Correlation between the densities of IL-17 producing cells and patients' survival
The median survival time of the 181 ESCC patients was 44 months (range 1–87 months). The five-year survival rate was 49.9%. The overall survival curves of the patients in this study are depicted in Figure 2. The statistical analysis demonstrated a positive correlation between overall survival and the density of IL-17+ TILs (Figure 2, long-rank test: P = 0.031).
Figure 2. Kaplan–Meier survival curves of ESCC patients (n = 181) after surgical resection.
Increased IL-17-producing cells correlate with improved patient survival. Patients in high IL-17-producing cell density group exhibited significantly better survival than the low IL-17-producing cell density group (log-rank test: P = 0.031).
The univariate analysis demonstrated that IL-17 producing cell density (P = 0.006), differentiation (P = 0.006), T (depth of tumor invasion, P = 0.002), N (lymph node metastases, P<0.001) and TNM staging (P<0.001) were significantly associated with overall survival (Table 2). The subsequent multivariate analysis, however, indicated that only differentiation was an independent predictor for overall survival in ESCC (P = 0.030, Table 2).
Table 2. Univariate and multivariate analyses of variables associated with overall Survival.
| Variables | Univariate analysis | Multivariate analysis | ||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| IL-17+TIL (high vs. low) | 0.633 | 0.416–0.964 | 0.033* | 0.662 | 0.431–1.015 | 0.058 |
| Age, (≥60 vs. <60) | 1.141 | 0.752–1.731 | 0.535 | |||
| Gender (female vs. male) | 0.813 | 0.490–1.351 | 0.425 | |||
| Location(lower/middle/upper) | 1.093 | 0.754–1.585 | 0.638 | |||
| Length, (≥5 vs. <5) | 1.226 | 0.801–1.876 | 0.348 | |||
| Differentiation (G3/G2/G1) | 1.510 | 1.123–2.032 | 0.006* | 1.384 | 1.033–1.856 | 0.030* |
| T (T3+T4 vs. T1+T2) | 2.233 | 1.330–3.750 | 0.002 | 1.605 | 0.875–2.942 | 0.126 |
| N (Yes vs. No) | 3.235 | 2.103–4.979 | <0.001* | 2.176 | 1.001–4.728 | 0.050 |
| M (M1 vs. M0) | 2.328 | 0.944–5.741 | 0.067 | |||
| TNM (III+IV vs. I+II) | 3.477 | 2.282–5.298 | <0.001* | 1.433 | 0.632–3.251 | 0.389 |
* Statistically significant (P<0.05).
Relationship between the levels of IL-17+ TILs and CD8+ CTLs cells as well as CD57+ NK cells in tumor microenvironment
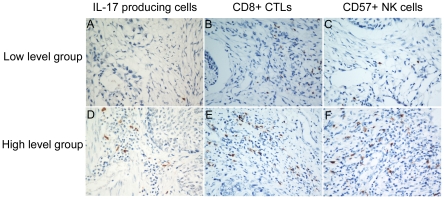
The levels of CD8+ CTLs and CD57+ NK cells in tumor tissue in ESCC patients were evaluated. In addition, the Pearson correlation coefficient was calculated, and linear regression analysis was applied to assess the relationships between the IL-17 producing cells and CD8+ CTLs or CD57+ NK cells in the tumor microenvironment. Infiltrating CD8+ CTLs and CD57+ NK cells were detected in tumor tissues by immunohistochemical staining (Figure 3), and statistical analysis showed that the levels of IL-17 producing cells positively correlated with levels of CD8+ CTLs in tumor tissues (r = 0.198, P = 0.008, Figure 4A). A significant association was also identified between the densities of IL-17+ TILs and CD57+ NK cells (r = 0.261, P<0.001, Figure 4B). From prognosis analysis, we found that higher levels of infiltrating CD8+ CTLs or CD57+NK cells correlated with a better overall survival of ESCC patients (P<0.001 and P = 0.002, respectively, determined by long-rank test, Figure 5).
Figure 3. Representative photomicrographs showing immunohistochemical staining of IL-17, CD8 and CD57 in the same ESCC tissues.
(A) Low density of IL-17 producing cells. (B) Low density of CD8+ CTLs cells. (C) Low density of CD57+ NK cells. (D) High density of IL-17 producing cells. (E) High density of CD8+ CTLs cells. (F) High density of CD57+ NK cells (original magnification ×400).
Figure 4. IL-17 producing cells correlated positively with the densities of CD8+ CTLs and CD57+ NK cells.
The correlation between the densities of IL-17 producing cells and CD8+T lymphocytes (A), CD57+NK cells (B). The samples were divided into two groups based on the median value of IL-17 producing cells.
Figure 5. Kaplan–Meier survival curves of ESCC patients (n = 181) after surgical resection.
Increased tumor-infiltrating CD8+ CTLs and CD57+ NK cells predict improved patient survival. (A) The survival rate for patients in the high CD8+ CTLs density group was significantly better than that for patients in the low density group (log rank test, P<0.001). (B) Kaplan–Meier survival curves for high CD57+ NK cell density group versus low CD57+ NK cell density group showed a highly significant separation (log-rank test: P = 0.002).
Discussion
Accumulating evidence suggests that IL-17 producing cells play a significant role in tumor immunity. However, IL-17 may play pro-tumor or anti-tumor effects in different tumor contexts [14], [18], [27], [28], [40]. Zhang et al found that IL-17 producing cells could promote tumor growth by stimulating angiogenesis in hepatocellular carcinoma patients [18]. Conversely, Kryczek et al demonstrated that IL-17 promoted antitumor activity in ovarian cancer patients [28]. The explanation for this discrepancy remains unknown.
We first detected tumor-infiltrating-IL-17 producing cells in human esophageal squamous cell carcinoma (ESCC) and observed that the levels of IL-17 producing cells correlated inversely with T (depth of primary tumor invasion, P = 0.042), indicating that enriched IL-17 producing cells in the tumor microenvironment may inhibit tumor invasion. Kaplan-Meier analysis revealed that increased levels of IL-17 producing cells were linked to better overall survival in ESCC patients (Figure 2, P = 0.031), indicating that IL-17 producing cell levels could potentially serve as a prognostic marker for ESCC. Our results are consistent with those of Kryczek et al, who demonstrated that the presence of IL-17+TILs correlated with favorable outcome in and enhanced survival of ovarian cancer patients [28]. Thus, in ESCC, our results suggest that IL-17 producing cells may mediate antitumor immunity.
We also detected CD8+ CTLs and CD57+ NK cells in tumor tissues from ESCC patients using serial tissue sections. Our study found that infiltrating CD8+ CTLs cells and CD57+ NK cells were present in ESCC tumors, and the abundance of CD8+ T or CD57+ cells correlated positively with the number of IL-17 producing cells. Martin-Orozco et al reported that Th17 cells can induce a strong antitumor CD8 response by eliciting the priming and recruitment of CD8+ T cells in a mouse model of lung melanoma [30]. Benchetrit et al found that IL-17 could inhibit tumor growth by inducing tumor-specific cytotoxic T lymphocyte (CTL) activity in hematopoietic tumors in immunocompetent mice [27]. In addition, Kryczek et al reported that both natural killer cell-mediated innate immunity and tumor-specific T-cell immunity were weakened in IL-17 deficient mice bearing MC38 tumors [29]; they also demonstrated that the abundance of IL-17 producing cells correlated positively with CD8+ T and NK cells in the same tumor microenvironment [28]. Our results are consistent with these studies, and indicate that IL-17 producing cells in ESCC might exert antitumor effects by enhancing cytotoxic T lymphocytes and NK cell responses.
In conclusion, our data show that IL-17 producing TILs were detected in ESCC, and the density of IL-17+ TILs was associated with better overall survival. Furthermore, the number of IL-17 producing cells correlated positively with the numbers of CD8+ CTLs and CD57 + NK cells. Thus, tumor infiltrating IL-17 producing cells may constitute a novel prognosis marker for ESCC and may play an antitumor role by activating innate and adaptive immunity.
Materials and Methods
Patients and tissue samples
Paraffin-embedded samples were obtained from 181 ESCC patients who underwent surgery at the Sun Yat-sen University Cancer Center between 2002 and 2003. There were 141 male and 40 female patients with a median age of 56 years (range, 33–79 years). Patients with autoimmune diseases and other esophageal cancers (e.g., adenocarcinoma) were excluded. None of the patients had received anticancer treatment prior to surgery. The follow-up data from the ESCC patients in this study are available and complete. There were 117 cases of stage I–II and 64 cases of stage III–IV cancer according to the American Joint Committee on Cancer (AJCC, 2002) TNM staging system. Each lesion was graded histologically according to the WHO classification criteria. Overall survival (OS) was defined as the interval between the date of surgery and date of death or the last known follow-up. The study was approved by the Ethics Committee of Sun Yat-sen University Cancer Center, and informed consent was obtained from each patient.
Immunohistochemistry and immunofiuorescence
Formalin-fixed, paraffin-embedded samples were cut at a thickness of 2 µm. Each tissue section was deparaffinized and rehydrated through graded ethanol. For antigen retrieval, the slides were boiled in EDTA (1 mM, pH 8.0) for 15 min in a microwave oven. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide solution for 10 min at room temperature. After rinsing with PBS, the slides were incubated overnight at 4°C with primary monoclonal antibodies, including goat anti-human IL-17 (R&D systems; dilution 1/300), mouse anti-CD8 (Zhong shan Golden Bridge Biotech., Beijing, China; dilution 1/100), rabbit anti-CD57 (Zhongshan Golden Bridge Biotech., Beijing, China; dilution 1/100). After three washes in PBS, sections were incubated with biotinylated secondary antibody (Zhongshan Golden Bridge Biotech., Beijing, China) for 30 min at room temperature. Finally, the visualization signal was developed with 3, 3′-diaminobenzidine tetrahydrochloride (DAB), and all slides were counterstained with hematoxylin. Some paraffin-embedded specimens were simultaneously incubated with goat anti-IL-17 (R&D systems; dilution 1/100) and rabbit anti-CD4 (Zhongshan Golden Bridge Biotech., Beijing, China; dilution 1/50), or with goat anti-IL-17 (R&D systems; dilution 1/100) and mouse anti-FOXP3 (abcam; dilution 1/100), followed by Phycoerythrin-conjugated Affinipure donkey anti-goat IgG(H+L) (Proteintech Group; dilution 1/50) and Fluorescein (FITC)-conjugated Affinipure donkey anti-rabbit IgG(H+L) (Proteintech Group; dilution 1/50), or by Alexa Fluor 488 donkey anti-goat IgG (H+L) (Molecular Probes; concentration 10 μg/ml) and Alexa Fluor 594 donkey anti-mouse IgG (H+L) (Molecular Probes; concentration 10 μg/ml).
Data were obtained by manually counting positively stained cells in ten separate fields under 400× high power magnification. The density of stained cells was determined by computing the mean number of positively stained cells per high power microscopic field (HPF).
Statistical analysis
Quantitative values were expressed as means ± SD or median (range). Patients were divided into two groups based on the median of various immunohistochemical variables in our data (high level group vs. low level group). The Chi-square test or Fisher exact test was used to assess the relationships between the levels of IL-17 producing cells and clinicopathological features. The overall survival curves were calculated using the Kaplan-Meier method and analyzed using the long-rank test. Prognostic factors were examined by univariate and multivariate analyses using the Cox proportional hazards model. The correlation between the density of IL-17 producing cells and CD8+ CTLs or CD57+ NK cells were determined by Pearson correlation coefficient and linear regression analyses. A two-tailed P-value<0.05 was considered statistically significant. All statistical analyses were performed with SPSS software (version 16.0; SPSS Inc., Chicago, IL, USA).
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the National Natural Science Foundation of China (u0772002, 30700985, 30973398) and Guangdong Natural Science Foundation (925100890). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Couzin J. Cancer. T cells a boon for colon cancer prognosis. Science. 2006;313:1868–1869. doi: 10.1126/science.313.5795.1868b. [DOI] [PubMed] [Google Scholar]
- 2.Dunn GP, Dunn IF, Curry WT. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immun. 2007;7:12. [PMC free article] [PubMed] [Google Scholar]
- 3.Leffers N, Gooden MJ, de Jong RA, Hoogeboom BN, ten Hoor KA, et al. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother. 2009;58:449–459. doi: 10.1007/s00262-008-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang YL, Li J, Mo HY, Qiu F, Zheng LM, et al. Different subsets of tumor infiltrating lymphocytes correlate with NPC progression in different ways. Mol Cancer. 2010;9:4. doi: 10.1186/1476-4598-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 6.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 9.Dong G, Ye R, Shi W, Liu S, Wang T, et al. IL-17 induces autoantibody overproduction and peripheral blood mononuclear cell overexpression of IL-6 in lupus nephritis patients. Chin Med J (Engl) 2003;116:543–548. [PubMed] [Google Scholar]
- 10.Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol. 2007;148:32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Traves SL, Donnelly LE. Th17 cells in airway diseases. Curr Mol Med. 2008;8:416–426. doi: 10.2174/156652408785160998. [DOI] [PubMed] [Google Scholar]
- 12.Yamada H. [Th17 cells in human rheumatoid arthritis]. Nihon Rinsho Meneki Gakkai Kaishi. 2009;32:249–255. doi: 10.2177/jsci.32.249. [DOI] [PubMed] [Google Scholar]
- 13.Oukka M. Th17 cells in immunity and autoimmunity. Ann Rheum Dis. 2008;67(Suppl 3):iii26–29. doi: 10.1136/ard.2008.098004. [DOI] [PubMed] [Google Scholar]
- 14.Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol. 2009;183:4169–4175. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- 15.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato T, Furumoto H, Ogura T, Onishi Y, Irahara M, et al. Expression of IL-17 mRNA in ovarian cancer. Biochem Biophys Res Commun. 2001;282:735–738. doi: 10.1006/bbrc.2001.4618. [DOI] [PubMed] [Google Scholar]
- 17.Zhu X, Mulcahy LA, Mohammed RA, Lee AH, Franks HA, et al. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008;10:R95. doi: 10.1186/bcr2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 19.Zhang B, Rong G, Wei H, Zhang M, Bi J, et al. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008;374:533–537. doi: 10.1016/j.bbrc.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 20.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–333. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 21.O'Brien RL, Roark CL, Born WK. IL-17-producing gammadelta T cells. Eur J Immunol. 2009;39:662–666. doi: 10.1002/eji.200839120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mills KH. Induction, function and regulation of IL-17-producing T cells. Eur J Immunol. 2008;38:2636–2649. doi: 10.1002/eji.200838535. [DOI] [PubMed] [Google Scholar]
- 23.Simonian PL, Roark CL, Born WK, O'Brien RL, Fontenot AP. Gammadelta T cells and Th17 cytokines in hypersensitivity pneumonitis and lung fibrosis. Transl Res. 2009;154:222–227. doi: 10.1016/j.trsl.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Brien RL, Taylor MA, Hartley J, Nuhsbaum T, Dugan S, et al. Protective role of gammadelta T cells in spontaneous ocular inflammation. Invest Ophthalmol Vis Sci. 2009;50:3266–3274. doi: 10.1167/iovs.08-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michel ML, Mendes-da-Cruz D, Keller AC, Lochner M, Schneider E, et al. Critical role of ROR-gammat in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation. Proc Natl Acad Sci U S A. 2008;105:19845–19850. doi: 10.1073/pnas.0806472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JJ, Chang YL, Lai WL, Ko JY, Kuo MY, et al. Increased prevalence of interleukin-17-producing CD4(+) tumor infiltrating lymphocytes in human oral squamous cell carcinoma. Head Neck. 2010 doi: 10.1002/hed.21607. [DOI] [PubMed] [Google Scholar]
- 27.Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 28.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Islami F, Kamangar F, Aghcheli K, Fahimi S, Semnani S, et al. Epidemiologic features of upper gastrointestinal tract cancers in Northeastern Iran. Br J Cancer. 2004;90:1402–1406. doi: 10.1038/sj.bjc.6601737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taghavi N, Biramijamal F, Sotoudeh M, Khademi H, Malekzadeh R, et al. p16INK4a hypermethylation and p53, p16 and MDM2 protein expression in Esophageal Squamous Cell Carcinoma. BMC Cancer. 2010;10:138. doi: 10.1186/1471-2407-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 34.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 35.Cho Y, Miyamoto M, Kato K, Fukunaga A, Shichinohe T, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63:1555–1559. [PubMed] [Google Scholar]
- 36.Hsia JY, Chen JT, Chen CY, Hsu CP, Miaw J, et al. Prognostic significance of intratumoral natural killer cells in primary resected esophageal squamous cell carcinoma. Chang Gung Med J. 2005;28:335–340. [PubMed] [Google Scholar]
- 37.Liu J, Lu G, Li Z, Tang F, Liu Y, et al. Distinct compartmental distribution of mature and immature dendritic cells in esophageal squamous cell carcinoma. Pathol Res Pract. 2010 doi: 10.1016/j.prp.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 38.Xue L, Lu HQ, He J, Zhao XW, Zhong L, et al. Expression of FOXP3 in esophageal squamous cell carcinoma relating to the clinical data. Dis Esophagus. 2010;23:340–346. doi: 10.1111/j.1442-2050.2009.01013.x. [DOI] [PubMed] [Google Scholar]
- 39.Yoshioka T, Miyamoto M, Cho Y, Ishikawa K, Tsuchikawa T, et al. Infiltrating regulatory T cell numbers is not a factor to predict patient's survival in oesophageal squamous cell carcinoma. Br J Cancer. 2008;98:1258–1263. doi: 10.1038/sj.bjc.6604294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]