Abstract
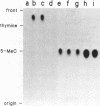
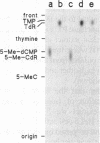
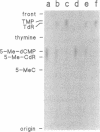
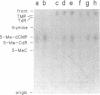
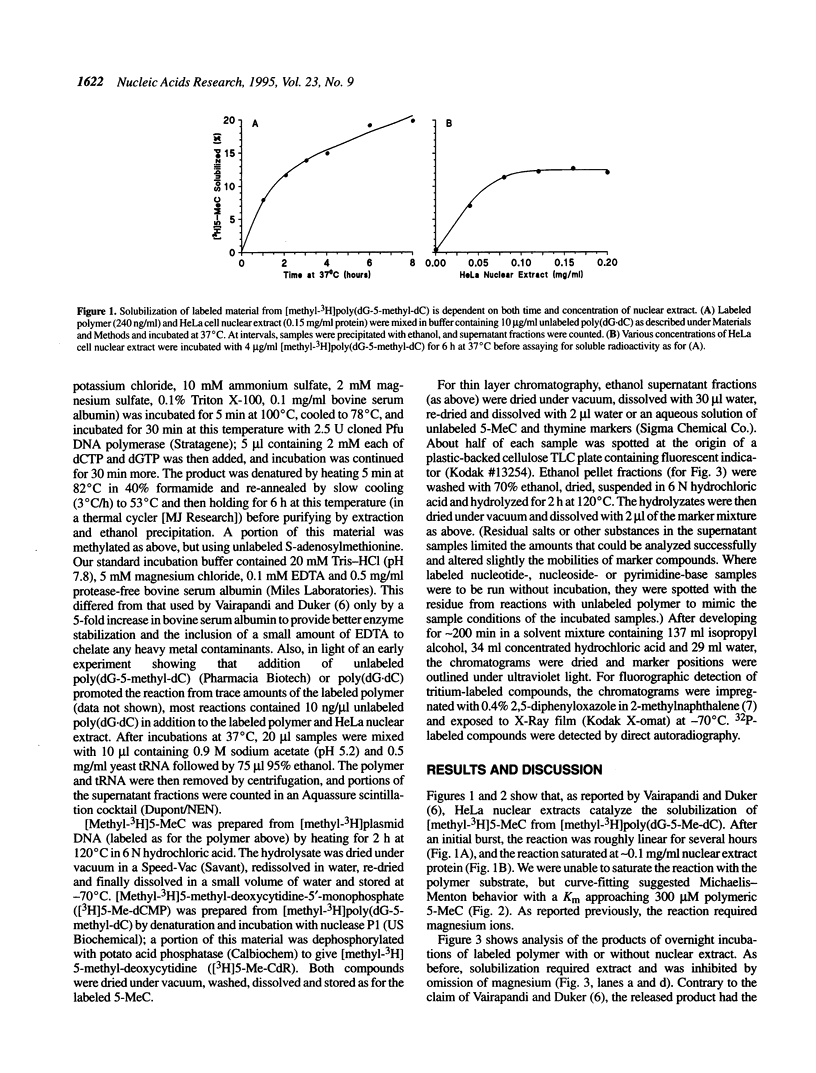
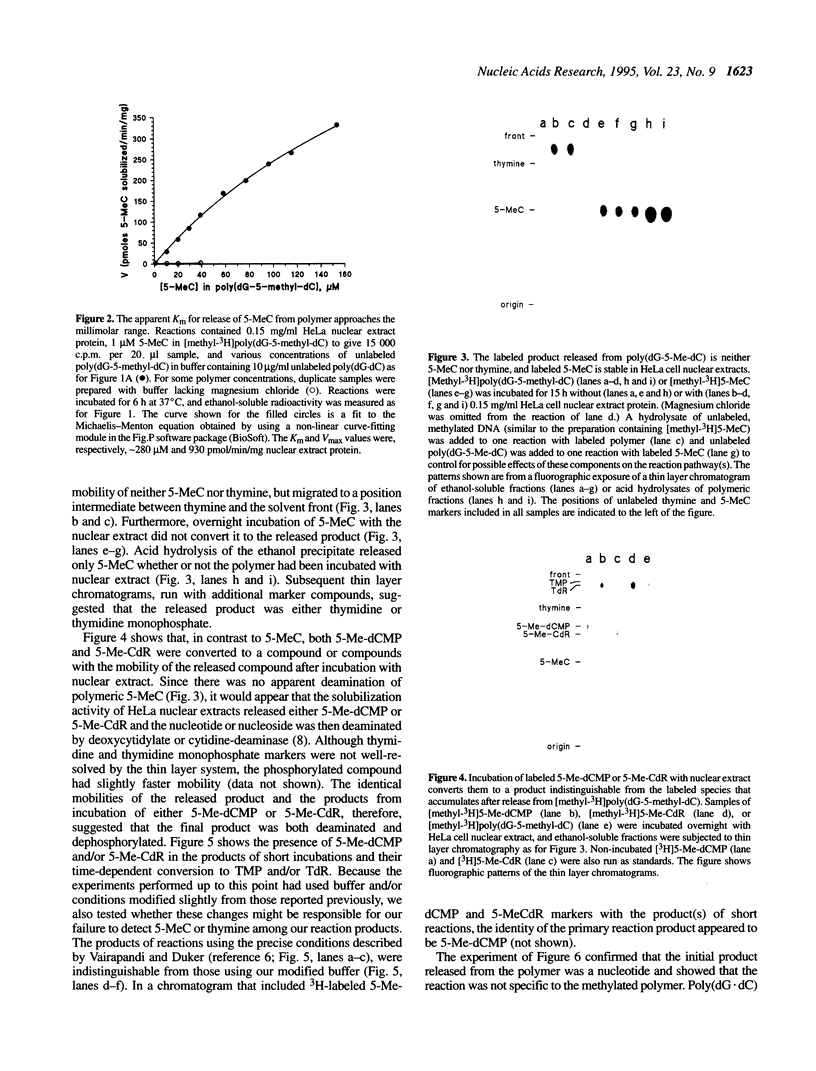
A recent report in this journal [Vairapandi, M. and Duker, N.J. (1993) Nucleic Acids Res. 21, 5323-5327) presented evidence of an activity in HeLa cell nuclear extracts that released radiolabeled material from a poly(dG.dC) polymer that had been methylated and simultaneously labeled on cytosine residues by incubation with a CpG-specific DNA methylase and [methyl-3H]S-adenosylmethionine. Based on chromatographic evidence that the released products were thymine and 5-methylcytosine and on f1p4olabeling data suggesting a concomitant increase in abasic sites, the authors concluded that the releasing activity was a 5-methylcytosine-specific glycosylase and that the solubilized 5-methylcytosine was converted to thymine by a nuclear deaminase. We have confirmed that HeLa nuclear extracts promote release of ethanol-soluble radioactivity from a methyl-labeled poly(dG-5-methyl-dC)polymer, but the products released were neither 5-methylcytosine nor thymine. Furthermore, free 5-methylcytosine was not deaminated by incubation with the nuclear extract. The labeled compound released initially from the polymer appeared to be 5-methyl-deoxycytidine monophosphate, which was converted to 5-methyl-deoxycytidine, thymidine monophosphate, and/or thymidine by further incubation with the nuclear extract. The activity responsible for the release, therefore, was a nuclease. Release of 32P-labeled nucleotides from a 32P-labeled poly(dG-dC) polymer suggested, furthermore, that the activity was not specific for methylated DNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Stedman J. D. Efficient fluorography of 3H and 14C on thin layers. Anal Biochem. 1978 Aug 15;89(1):247–256. doi: 10.1016/0003-2697(78)90747-9. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Jost Y. C. Transient DNA demethylation in differentiating mouse myoblasts correlates with higher activity of 5-methyldeoxycytidine excision repair. J Biol Chem. 1994 Apr 1;269(13):10040–10043. [PubMed] [Google Scholar]
- Jost J. P. Nuclear extracts of chicken embryos promote an active demethylation of DNA by excision repair of 5-methyldeoxycytidine. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4684–4688. doi: 10.1073/pnas.90.10.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E., Bestor T. H., Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992 Jun 12;69(6):915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Moore J. T., Silversmith R. E., Maley G. F., Maley F. T4-phage deoxycytidylate deaminase is a metalloprotein containing two zinc atoms per subunit. J Biol Chem. 1993 Feb 5;268(4):2288–2291. [PubMed] [Google Scholar]
- Razin A., Kafri T. DNA methylation from embryo to adult. Prog Nucleic Acid Res Mol Biol. 1994;48:53–81. doi: 10.1016/s0079-6603(08)60853-3. [DOI] [PubMed] [Google Scholar]
- Razin A., Szyf M., Kafri T., Roll M., Giloh H., Scarpa S., Carotti D., Cantoni G. L. Replacement of 5-methylcytosine by cytosine: a possible mechanism for transient DNA demethylation during differentiation. Proc Natl Acad Sci U S A. 1986 May;83(9):2827–2831. doi: 10.1073/pnas.83.9.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vairapandi M., Duker N. J. Enzymic removal of 5-methylcytosine from DNA by a human DNA-glycosylase. Nucleic Acids Res. 1993 Nov 25;21(23):5323–5327. doi: 10.1093/nar/21.23.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]