Abstract
Neurotrophins regulate neuronal cell survival and synaptic plasticity through activation of Trk receptor tyrosine kinases. Binding of neurotrophins to Trk receptors results in receptor autophosphorylation and downstream phosphorylation cascades. Here, we describe an approach to use small molecule agonists to transactivate Trk neurotrophin receptors. Activation of TrkA receptors in PC12 cells and TrkB in hippocampal neurons was observed after treatment with adenosine, a neuromodulator that acts through G protein-coupled receptors. These effects were reproduced by using the adenosine agonist CGS 21680 and were counteracted with the antagonist ZM 241385, indicating that this transactivation event by adenosine involves adenosine 2A receptors. The increase in Trk activity could be inhibited by the use of the Src family-specific inhibitor, PP1, or K252a, an inhibitor of Trk receptors. In contrast to other G protein-coupled receptor transactivation events, adenosine used Trk receptor signaling with a longer time course. Moreover, adenosine activated phosphatidylinositol 3-kinase/Akt through a Trk-dependent mechanism that resulted in increased cell survival after nerve growth factor or brain-derived neurotrophic factor withdrawal. Therefore, adenosine acting through the A2A receptors exerts a trophic effect through the engagement of Trk receptors. These results provide an explanation for neuroprotective actions of adenosine through a unique signaling mechanism and raise the possibility that small molecules may be used to elicit neurotrophic effects for the treatment of neurodegenerative diseases.
Neurotrophins play a prominent role in the development of the vertebrate nervous system by influencing cell survival, differentiation, and cell death events (1, 2). Neurotrophins also exhibit acute regulatory effects on neurotransmitter release, synaptic strength, and connectivity (3, 4). In addition to promoting axonal and dendritic branching, neurotrophins serve as chemoattractants for extending growth cones in vitro (5). These actions are mediated by neurotrophin binding to two separate receptor classes, the Trk family of tyrosine kinase receptors and the p75 neurotrophin receptor, a member of the tumor necrosis factor receptor superfamily (6).
Mutations in Trk neurotrophin receptor function lead to deficits in survival, axonal and dendritic branching, long-term potentiation, and behavior (7–9). Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3, and neurotrophin-4 also bind to the p75 neurotrophin receptor, a potential cell death receptor whose actions are negated by Trk tyrosine kinase signaling (10, 11). Therefore, the ability to regulate Trk tyrosine kinase activity is critical for neuronal survival and differentiation.
Ligands for G protein-coupled receptors are capable of activating the mitogen-activated protein (MAP) kinase signaling pathway, in addition to classic G protein-dependent signaling pathways involving adenylyl cyclase and phospholipase C (12, 13). Induction of mitogenic receptor tyrosine kinase phosphorylation also occurs through signaling from several G protein-coupled receptors (14). In particular, receptors for epidermal growth factor, platelet-derived growth factor, and insulin-like growth factor 1 can be transactivated by G protein-coupled receptors (12, 15, 16). Whether transactivation of neurotrophic receptor tyrosine kinases occurs by means of G protein-coupled receptors has not been demonstrated to date.
We have tested the possibility that ligands of G protein-coupled receptors might activate neurotrophin receptors of the Trk tyrosine kinase subfamily. Here, we report that adenosine and adenosine agonists can activate Trk receptor phosphorylation, through a mechanism that requires the adenosine 2A (A2A) receptor. The activation does not require neurotrophin binding and is observed in PC12 cells, as well as primary cultures of hippocampal neurons. Unlike the results obtained with other tyrosine kinase receptors, increased Trk receptor activity provides increased cell survival over a prolonged time course that requires Akt, and not MAP kinase, signaling. These findings suggest alternative approaches of stimulating trophic functions in the nervous system by linking different receptor signaling pathways.
Materials and Methods
CGS 21680, CPA, A23187, and insulin-like growth factor-1 were purchased from Sigma-RBI. ZM 241385 was from Tocris Neurochemicals (Ballwin, MO), PP1 from Alexis Biochemicals (San Diego, CA), LY294002 from Biomol, K252a from Calbiochem, and PD98059 from New England Biolabs. NGF was obtained from Harlan Bioproducts (Indianapolis, IN) and BDNF from PeproTech (Rocky Hill, NJ). All other compounds were from Sigma. An anti-pan-Trk rabbit antiserum raised against the C-terminal region of the Trk receptor was from Barbara Hempstead (Cornell University); anti-NGF antibody was obtained from Chemicon. Anti-phosphotyrosine and anti-Akt antibodies were from Santa Cruz Biotechnologies. Anti-phospho-Akt, anti-MAP kinase, and anti-phospho-MAP kinase antibodies were from New England Biolabs.
Immunoprecipitation and Immunoblotting.
PC12 cells or PC12 (615) cells (17), were maintained in DMEM containing 10% FBS supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine plus 200 μg/ml G418. Cells were placed in low-serum medium (1% FBS, 0.5% horse serum) overnight before experiments. Cell lysates from PC12, 615 cells, or hippocampal cells were incubated in lysis buffer (1% Nonidet P-40) for 4 h to overnight at 4°C with anti-pan-Trk polyclonal antibody followed by incubation with protein A-Sepharose beads. Equivalent amounts of protein were analyzed for each condition. The beads were washed five times with lysis buffer, and the immune complexes were boiled in SDS-sample buffer and loaded on SDS-PAGE gels for immunoblot analysis. The immunoreactive protein bands were detected by enhanced chemiluminescence (Amersham Pharmacia).
125I-NGF Binding Analysis.
For equilibrium binding studies, 125I-NGF was prepared as described previously (18). PC12 cells stably overexpressing TrkA (2 × 105 cells) and HEK 293 cells expressing TrkA (2 × 105) were incubated with 125I-NGF in the absence and presence of adenosine compounds for 30 min at 25°C. The cells were then washed twice with PBS, and 125I-NGF was stripped with an acid solution (0.2 M acetic acid, 0.5 M NaCl). Nonspecific binding was assessed by adding unlabeled NGF at a final concentration of 1000 ng/ml and represented <20% of total binding. Specific binding was defined as total binding minus nonspecific binding. All conditions were carried out in triplicate and SEM calculated.
Hippocampal Cell Cultures.
Dissociated primary cultures of hippocampal neurons from embryonic day 17 (E17) rats were prepared from timed-pregnant Sprague–Dawley rats as described previously (19). Fetuses were removed under sterile conditions and kept in PBS on ice for microscopic dissection of the hippocampus. The meninges were removed and the tissue was placed in Neurobasal media (GIBCO/BRL). The tissue was briefly minced with fine forceps and then triturated with a fire-polished pasteur pipet. Cells were counted and plated on culture wells coated with 0.01 mg/ml poly-d-lysine overnight. Hippocampal cells were maintained in Neurobasal media, containing B27 supplement and l-glutamine (0.5 mM). Experiments were conducted 7–10 days after plating.
Cell Death Assay.
PC12 cells were differentiated in DMEM supplemented with 0.33% FBS, 0.67% heat-inactivated horse serum, 2 mM l-glutamine, and NGF (50 ng/ml) for 7 days. Serum and NGF were then removed, and adenosine agonists or growth factors were added to the media. After 48 h, cell death was quantified by measuring lactate dehydrogenase (LDH) released from injured cells into the media by using the Cytox 96 Cytotoxicity Assay Kit (Promega). LDH values were normalized by subtracting the LDH released by cells maintained in NGF (50 ng/ml) and scaling to a full kill (=100%) reference induced by 5 min of treatment with 1% Triton, an exposure that consistently killed all PC12 cells.
Hippocampal neurons were maintained in Neurobasal media containing B27 supplement and 0.5 mM l-glutamine for 10 days. B27 was then removed, and adenosine agonists or BDNF (100 ng/ml) were added to the media. MK-801 (1 μM) was added to all conditions to decrease the contribution of N-methyl-d-aspartate-mediated cell death. After 48 h, cell death was assessed by measurement of LDH released into media. LDH values were normalized by subtracting the LDH released by cells maintained in BDNF (100 ng/ml) and scaling to full kill (=100%) reference induced by 24 h of treatment with A23187 (30 μM), a condition that resulted in complete cell death of all neurons (20).
Results
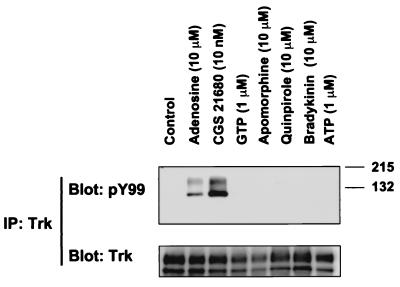
Transactivation of mitogenic tyrosine kinase receptors through G protein-coupled receptors has been described previously (12, 15, 16). To explore whether any G protein-coupled receptors exert an effect on neurotrophin receptor signaling, several ligands were tested for their ability to influence TrkA tyrosine kinase activity in PC12 cells. Receptors for each ligand are found on PC12 cells (21–24). TrkA receptors were immunoprecipitated from PC12 cell lysates and then probed with an anti-phosphotyrosine antibody. Activated TrkA receptors were observed with adenosine treatment (10 μM), but not with nucleotides such as ATP or GTP (Fig. 1). The TrkA doublet represents an underglycosylated form of 110 kDa and the fully glycosylated form of 140 kDa (17). Activation of TrkA receptors was not observed with other G protein-coupled ligands, including bradykinin and dopamine agonists, apomorphine and quinpirole (Fig. 1). The specificity of adenosine's effects was also confirmed by the use of CGS 21680, 2-[(4-(2-carboxyethyl)phenylethyl)] aminoadenosine-5′-N-ethylcarboxamide, a selective adenosine A2A agonist (25).
Figure 1.
Activation of TrkA receptors with G protein-coupled receptor ligands. Stably transfected PC12 cells expressing high levels of TrkA (615) were treated with the indicated compounds for 2 h. The cells were subsequently harvested in lysis buffer as described in Materials and Methods. Lysates were immunoprecipitated with anti-pan Trk rabbit antiserum. Immunocomplexes were analyzed by immunoblotting with anti-phosphotyrosine antibody (PY99). Immunoprecipitation of TrkA receptors was then confirmed by immunoblotting of the immunocomplex with anti-pan Trk antiserum.
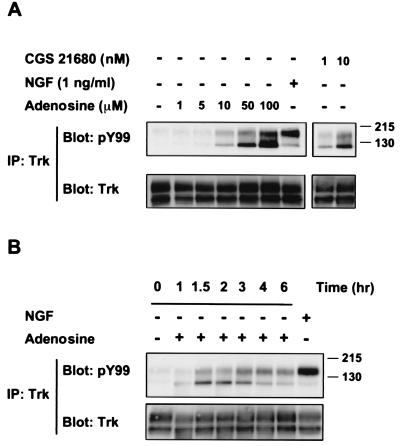
The effect of adenosine on TrkA receptor activity occurred in a low concentration range (Fig. 2A). This response was verified by the use of 1 nM CGS 21680. A time course of adenosine action showed that the increase in TrkA activation was slow and required at least 90 min (Fig. 2B), which is delayed compared with NGF treatment. This increase was inhibited by K252a, a known inhibitor of Trk tyrosine kinases (see below). It is formally possible that adenosine treatment leads to the production of NGF by PC12 cells that could act in an autocrine fashion to stimulate TrkA receptors. This possibility was discounted by the absence of neurite outgrowth activity of supernatants taken from PC12 cells treated with adenosine and by a lack of effect of anti-NGF antibody on adenosine's action (data not shown).
Figure 2.
Time course and dose of adenosine activation of TrkA receptors. (A) Different concentrations of adenosine were administered to PC12 615 cells for 2 h or NGF (1 ng/ml) for 10 min. Cells were also treated with CGS 21680 at the indicated doses for 2 h. (B) PC12 cells (615) were treated with adenosine (10 μM) for various times or with 5 ng/ml NGF for 10 min. Phosphorylated TrkA receptors were detected by immunoblot analysis using PY99 anti-phosphotyrosine antibodies. The amount of Trk receptors in each condition was verified by immunoblotting.
Adenosine interacts with four different G protein-coupled receptors, designated A1, A2A, A2B, and A3 receptors (26). The A2 class of adenosine receptors are expressed in PC12 cells and have been detected by radioligand binding (23). Adenosine does not bind to the TrkA receptor. There was no displacement of 125I-NGF binding with an excess of adenosine (1 mM) or CGS 21680 (1 μM) in PC12 cells overexpressing TrkA (Table 1). As PC12 cells express the p75 neurotrophin receptor, which also binds 125I-NGF, similar experiments were carried out in HEK 293 cells after transfection with TrkA. Again, excess concentrations of adenosine or CGS 21680 did not displace 125I-NGF binding to HEK 293 cells that expressed TrkA (Table 1). The concentrations of adenosine and CGS 21680 were approximately 100-fold greater than those normally used in A2A receptor binding and signaling studies (27).
Table 1.
No effect of adenosine on 125I-NGF binding
| Condition | Specific binding |
|---|---|
| PC12 | |
| Control | 14316 ± 350 |
| Adenosine (1 mM) | 14403 ± 888 |
| CGS 21680 (1 μM) | 14237 ± 1055 |
| 293/TrkA | |
| Control | 39078 ± 2885 |
| Adenosine (1 mM) | 36618 ± 4185 |
| CGS 21680 (1 μM) | 39568 ± 2032 |
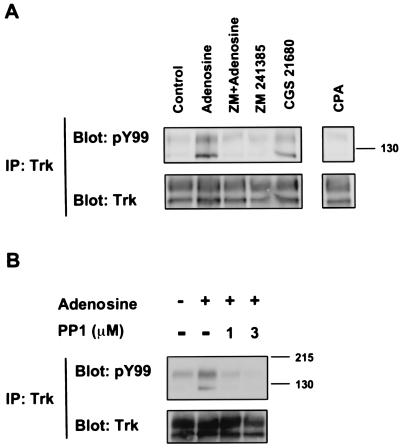
To verify adenosine interacted specifically with the A2A receptor, several adenosine analogs were used. A low concentration (10 nM) of CGS 21680 gave a similar increase in phosphorylated TrkA receptors with the same time course as adenosine (Figs. 2A and 3A). In contrast, a selective A1 receptor agonist, CPA, N(6)-cyclopentyladenosine, had no effect (Fig. 3A). Incubation of PC12 cells with the A2A antagonist, ZM 241385, 4- (2-[7-amino-2-(2-furyl)-1,2,4-triazolo[1,5-a] (1, 3, 5)triazin-5-ylamino]ethyl] phenol, that binds the A2A receptor with high affinity (28) antagonized the effects of adenosine on the phosphorylation of TrkA receptors (Fig. 3A). These results are consistent with the involvement of adenosine A2A receptors in mediating the increase in Trk receptor activity.
Figure 3.
Adenosine activation of TrkA by A2A receptors. (A) PC12 cells (615) were treated with the A2A agonist CGS 21680 (10 nM) and an A1 agonist CPA (10 nM) for 2 h. ZM 241385 (10 nM), an A2A antagonist, was incubated with the cells for 15 min before treatment with adenosine (10 μM) for 2 h. (B) PC12 cells (615) were incubated with the indicated concentrations of PP1, a Src family kinase inhibitor (30), for 30 min, and then treated with adenosine (10 μM) for 2 h. Activation of TrkA was assessed by immunoprecipitation and immunoblot analysis using PY99 anti-phosphotyrosine antibody.
The mechanism by which G protein-coupled receptors are linked to the activation of receptor tyrosine kinases is not well understood. Src family kinases have been implicated as mediators of mitogenic receptor tyrosine kinase transactivation by several G protein-coupled receptor agonists, such as lysophosphatidic acid, angiotensin II, thrombin, and bradykinin (14, 29). To test whether a Src family member is involved in the activation of Trk receptors by adenosine, the inhibitor PP1 (30) was used. Treatment of PC12 cells with 1 μM PP1 resulted in a marked decrease in the level of tyrosine phosphorylated TrkA receptors elicited by adenosine (Fig. 3B). Increasing concentrations of PP1 produced a progressively stronger inhibition. These results suggest that the regulation of TrkA activity by adenosine may be mediated by a Src family member. An involvement of Src was previously implicated in NGF signaling downstream of its receptor (31). However, it is conceivable that members of the Src tyrosine kinase activity may be activated by G proteins. This has been demonstrated for Lck, which acts in thymocytes downstream of the β-adrenergic receptor and whose activity can be increased in vitro by Gs (32).
Hippocampal Neurons.
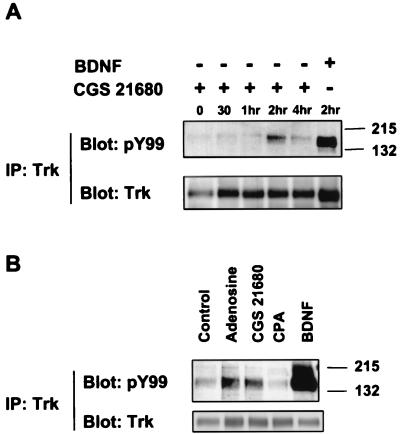
To extend the generality of adenosine effects on Trk receptors, we established primary hippocampal neuronal cultures from E17 rat embryos. Hippocampal neurons predominately express the TrkB receptor and respond to BDNF (Fig. 4). These neurons also express and A1 and A2A receptors (33), but not TrkA receptors. Treatment with 10 μM adenosine or 10 nM CGS 21680 for 2 h gave rise to phosphorylated TrkB receptors in hippocampal neurons (Fig. 4 A and B), similar to the activation of TrkA receptors by adenosine. An A1-specific agonist, CPA, however, did not activate TrkB receptors (Fig. 4B), confirming the specificity of this effect to the A2A receptor. These results not only extend the effects of adenosine to hippocampal neurons, but also demonstrate that TrkB can also be activated by signaling through the A2A receptors.
Figure 4.
Adenosine activation of TrkB receptors in hippocampal neurons. Primary cultures of E17 hippocampal neurons were prepared as described in Materials and Methods and treated with (A) CGS 21680 (10 nM) or BDNF (1 ng/ml) for various times and (B) adenosine (10 μM), CGS 21680 (10 nM), CPA (10 nM), or BDNF (10 ng/ml) for 2 h. Activation of TrkB receptors was assessed by immunoprecipitation and Western blotting with anti-phosphotyrosine antibody.
Downstream Signal Transduction.
To characterize the signaling pathways activated by adenosine, further experiments were carried out. Pretreatment with 100 nM K252a abolished adenosine's activation of TrkA tyrosine kinase activity (Fig. 5). This concentration of K252a has been used to block NGF activation of TrkA receptors (34) and the subsequent biological effects of neurotrophins.
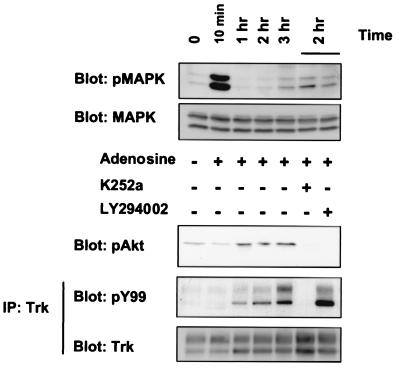
Figure 5.
Effects of adenosine on MAP kinase and Akt activation. PC12 cells (615) were treated with adenosine (10 μM) for various times in the presence or absence of K252a (100 nM) or LY294002 (10 μM). The cells were subsequently harvested in lysis buffer; lysates and immunoprecipitated samples were subsequently immunoblotted with anti-phospho-MAP kinase, anti-phospho-Akt, and anti-phosphotyrosine. Reprobing with anti-MAP kinase and anti-pan-Trk antibodies was carried out to ensure equal protein loading.
Many G protein-coupled receptors activate the MAP kinase pathway. Indeed, within 10 min of adenosine treatment, a marked increased in phosphorylated MAP kinase was detected in PC12 cells (Fig. 5), consistent with previous observations (35–37). After 10 min, the levels of activated MAP kinase declined to a baseline level. Activation of MAP kinases can be achieved either by A2A receptors, or through Trk receptor signaling. To distinguish between these alternatives, PC12 cells were treated with adenosine in the presence and absence of K252a. Using a concentration of K252a that specifically blocks TrkA signaling (34), we found that MAP kinase activity was not altered (Fig. 5). Thus, MAP kinase induction occurred quickly, whereas Trk activation by adenosine followed a slower time course and did not influence MAP kinase activity. This result is in contrast to other examples of G protein-coupled receptor transactivation, in which MAP kinase activities are directly stimulated downstream of the tyrosine kinase receptor (14).
Another pathway activated by receptor tyrosine kinases is phosphatidylinositol 3′-kinase (PI3-kinase)/Akt. Interestingly, adenosine (Fig. 5) or CGS 21680 treatment (data not shown) of PC12 cells was also able to activate Akt as detected by a phospho-specific antibody. This response has not been previously associated with adenosine action. The time course of Akt activation was very similar to Trk autophosphorylation induced by adenosine. This effect was eliminated either by pretreatment with K252a (100 nM) or LY2494002 (10 μM), a PI3-kinase inhibitor. These results indicate that Akt activation by adenosine is Trk- and PI3-kinase-dependent.
Trophic Effects.
To test the functional consequences of adenosine-activated Trk receptor activity, we assessed the ability of adenosine to maintain survival of differentiated PC12 cells after withdrawal of NGF. After culture for 48 h in the absence of NGF, cell survival was assessed by measuring LDH release. Whereas cells grown without NGF underwent rapid cell death, a one-time treatment with CGS 21680 effectively rescued nearly 50% of the cells (Fig. 6A). The action of CGS 21680 required TrkA receptors, because K252a (100 nM) eliminated the positive effects of CGS 21680 under similar conditions that blocked the activation of TrkA receptors (Fig. 5). Likewise, a similar dose of K252a reversed the survival effects of NGF, but not of insulin-like growth factor 1, in this deprivation assay.
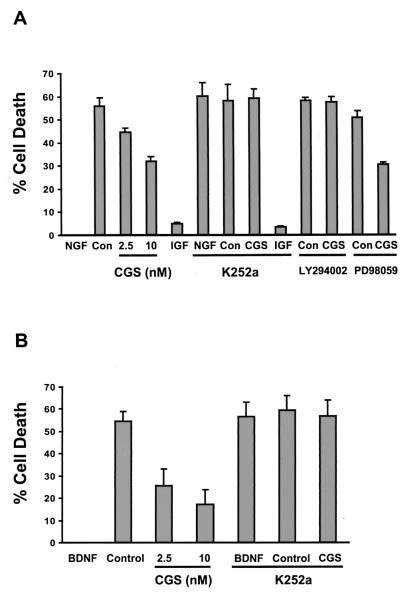
Figure 6.
Trophic effects of adenosine agonist in PC12 and hippocampal cells deprived of neurotrophins. (A) NGF-differentiated PC12 cells were prepared, and then NGF and serum were withdrawn for 48 h as described in Materials and Methods. On NGF withdrawal, various concentrations of CGS 21680 (CGS) were added to the media. CON, no addition. NGF (50 ng/ml), insulin-like growth factor 1 (=IGF-1) at 100 ng/ml, and CGS 21680 (10 nM) were added together with K252a (100 nM), LY294002 (10 μM), or PD98059 (25 μM) upon NGF withdrawal. (B) Hippocampal neurons were prepared and B27 was withdrawn for 48 h as described in Materials and Methods. Upon B27 withdrawal, various concentrations of CGS 21680 (CGS) were added to the media. CGS 21680 (10 nM) and BDNF (100 ng/ml) were added together with K252a (100 nM) on B27 withdrawal. All LDH levels were quantitated and % cell death calculated as described in Materials and Methods. All bars depict mean + SEM from three independent experiments.
Similar survival results with CGS 21680 were obtained in hippocampal neurons grown in the absence of BDNF (Fig. 6B). The action of CGS 21680 was again dose-dependent and K-252a-sensitive. Treatment with CGS 21680 effectively rescued >60% of the cells (Fig. 6B). Hence, a potent adenosine agonist at nanomolar concentrations was able to reverse cell death in both PC12 cells and hippocampal neurons initiated by withdrawal of trophic support by neurotrophins.
The ability of K252a to block adenosine's trophic effect as well as induction of Trk receptor activity suggested that Trk receptor downstream signaling was involved in this process. This was confirmed by the ability of LY294002 to eliminate the trophic effect of CGS 21680 after NGF withdrawal (Fig. 6), indicating that the PI3-kinase/Akt pathway was involved in the survival effects of adenosine. Consistent with the lack of a MAP kinase response, we did not find that the mitogen-activated kinase kinase inhibitor PD98059 had any effect on survival imparted by CGS 21680.
Discussion
Adenosine receptor activation leads to many modulatory effects on neuropeptide and neurotransmitter systems (38). Here, we report a property of adenosine in neuronal cells that affects neurotrophin signaling. Through crosstalk with Trk receptor tyrosine kinases, adenosine is capable of activating the PI3-kinase/Akt cascade, resulting in a survival response in PC12 and hippocampal cells. This response is similar to the effect of NGF and BDNF on their Trk receptors, but differs in the longer time course.
Neurotrophin receptors and A2A receptors have considerable overlap in their central and peripheral nervous system distribution. In the central nervous system, A2A receptors are expressed in striatum, amygdala, and olfactory tubercles, and in cerebral cortex, hippocampus, and cerebellum (39). All of these regions express TrkB receptors. In the peripheral nervous system, A2A receptor expression has been localized primarily to dorsal root ganglion and superior cervical ganglion (40), two regions that express TrkA receptors. Interestingly, mice deficient in the adenosine A2A receptor display decreased sensitivity to thermal stimulation (41). It is noteworthy that mice with mutations in NGF or TrkA also display hypoalgesia to thermal and mechanical stimuli.
What are the in vivo consequences of these events observed in culture? During hypoxia or ischemic conditions, adenosine is released in large amounts and can mediate cellular protection. A2A receptor agonists, such as CGS 21680, have been shown to be neuroprotective against ischemia (42, 43) and kainate-induced neuronal damage (44) in animals. However, A2A antagonists have been also reported to reduce hypoxic-ischemic neuronal injury (43, 45). The differential effects of A2A receptor ligands may reflect short term versus long term effects by adenosine receptors (46). Acute effects of adenosine analogs may lead to opposite effects on neuroprotection than chronic treatment. Engagement of receptor tyrosine kinases such as the Trk subfamily may account for differences in the functional consequences of adenosine action. A distinctive feature of adenosine's transactivation of Trk is the longer time course of Trk mediated signaling, which is similar to neurotrophin-induced signaling.
Adenosine has been proposed as a potential treatment for a wide number of neurological disorders, including cerebral ischemia, sleep disorders, hyperalgesia, Parkinson's disease, and other neurodegenerative conditions (47). Previous effects on growth arrest and enzyme induction by adenosine in PC12 cells (48, 49) might well be explained by transactivation of TrkA receptors. Our findings on adenosine delineate a pathway for activating the neurotrophin signaling system in the absence of neurotrophins. In contrast to other transactivation events involving receptor tyrosine kinases that lead to transient increases in MAP kinase activity, G protein-coupled receptor signaling to neurotrophin receptors leads to selective activation of the PI3-kinase/Akt pathway over a prolonged time course.
These findings provide a mechanism for the neuroprotective actions of adenosine involving engagement of the A2A receptor, transactivation of Trk tyrosine kinase receptors, and selective induction of the PI3-kinase/Akt pathway. A number of approaches have been taken to use neurotrophins to treat Alzheimer's dementia, amyotrophic lateral sclerosis, and peripheral sensory neuropathy (50, 51). However, there are considerable hurdles in the use of neurotrophic molecules that are related to difficulties in their delivery and pharmcokinetics and unanticipated side effects (51). The selective and sustained effects of adenosine on survival signaling pathways suggest that small molecules may be used to target populations of neurons that express both adenosine and Trk receptors. The identification of small ligands in the G protein-coupled receptor family that regulate tyrosine kinase activity in neural cells offers a strategy for promoting trophic effects during normal and neurodegenerative conditions.
Acknowledgments
We thank B. Hempstead and A. Kim for advice and R. Rajagopal and L. Aibel for assistance. F.S.L. was supported by the DeWitt–Wallace Fund in the New York Community Trust and an American Psychiatric Association Program for Minority Research Training in Psychiatry Fellowship, and M.V.C. was supported by grants from the National Institutes of Health.
Abbreviations
- NGF
nerve growth factor
- BDNF
brain-derived neurotrophic factor
- A2A
adenosine 2A
- PI3-kinase
phosphatidylinositol 3′-kinase
- MAP
mitogen-activated protein
- LDH
lactate dehydrogenase
- En
embryonic day n
References
- 1.Levi-Montalcini R. Science. 1987;237:1154–1164. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 2.Lewin G R, Barde Y-A. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 3.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 4.Bonhoeffer T. Curr Opin Neurobiol. 1996;6:119–126. doi: 10.1016/s0959-4388(96)80017-1. [DOI] [PubMed] [Google Scholar]
- 5.Gallo G, Lefcort F, Letourneau P. J Neurosci. 1997;17:5445–5454. doi: 10.1523/JNEUROSCI.17-14-05445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao M V, Hempstead B L. Trends Neurosci. 1995;19:321–326. [PubMed] [Google Scholar]
- 7.McAllister A, Katz L, Lo D. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- 8.Minichiello L, Korte M, Wolfer D, Kuhn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp H P, Bonhoeffer T, Klein R. Neuron. 1999;24:401–414. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- 9.Lyons W E, Mamounas L A, Ricaurte G A, Coppola V, Reid S W, Bora S H, Wihler C, Koliatsos V E, Tessarollo L. Proc Natl Acad Sci USA. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobrowsky R T, Jenkins G M, Hannun Y A. J Biol Chem. 1995;270:22135–22142. doi: 10.1074/jbc.270.38.22135. [DOI] [PubMed] [Google Scholar]
- 11.Yoon S O, Carter B D, Casaccia-Bonnefil P, Chao M V. J Neurosci. 1998;18:3273–3281. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daub H, Weiss F U, Wallasch C, Ullrich A. Nature (London) 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 13.Castellino A M, Chao M V. Cytokine Growth Factor Rev. 1997;7:297–302. doi: 10.1016/s1359-6101(96)00038-x. [DOI] [PubMed] [Google Scholar]
- 14.Luttrell L, Daaka Y, Lefkowitz R. Curr Opin Cell Biol. 1999;11:177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- 15.Linseman D A, Benjamin C W, Jones D A. J Biol Chem. 1995;270:12563–12568. doi: 10.1074/jbc.270.21.12563. [DOI] [PubMed] [Google Scholar]
- 16.Rao G, Delafontaine P, Runge M. J Biol Chem. 1995;270:27871–27875. doi: 10.1074/jbc.270.46.27871. [DOI] [PubMed] [Google Scholar]
- 17.Hempstead B L, Rabin S J, Kaplan L, Reid S, Parada L F, Kaplan D R. Neuron. 1992;9:883–896. doi: 10.1016/0896-6273(92)90241-5. [DOI] [PubMed] [Google Scholar]
- 18.Hempstead B L, Schleifer L, Chao M V. Science. 1989;243:373–375. doi: 10.1126/science.2536190. [DOI] [PubMed] [Google Scholar]
- 19.Aibel L, Martin-Zanca D, Perez P, Chao M. J Neurosci Res. 1998;54:424–431. doi: 10.1002/(SICI)1097-4547(19981101)54:3<424::AID-JNR13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Kim A, Sheline C, Tian M, Higashi T, McMahon R, Cousins R, Choi D. Brain Res. 2000;886:99–107. doi: 10.1016/s0006-8993(00)02944-9. [DOI] [PubMed] [Google Scholar]
- 21.Etschied B, Ko P, Villereal M. Br J Phamacol. 1991;103:1347–1350. doi: 10.1111/j.1476-5381.1991.tb09791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue K, Nakazawa K, Watano T, Ohara-Imaizumi M, Fujimori K, Takanaka A. Eur J Pharmacol. 1992;215:321–324. doi: 10.1016/0014-2999(92)90049-a. [DOI] [PubMed] [Google Scholar]
- 23.Williams M, Abreu M, Jarvis M, Noronha-Blob L. J Neurochem. 1987;48:498–502. doi: 10.1111/j.1471-4159.1987.tb04120.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim W, Rabin R. J Biol Chem. 1994;269:6471–6477. [PubMed] [Google Scholar]
- 25.Jarvis M, Schulz R, Hutchinson A, Do U, Sills M, Williams M. J Pharmacol Exp Ther. 1989;251:888–893. [PubMed] [Google Scholar]
- 26.Neary J, Rathbone M, Cattabeni F, Abbracchio M, Burnstock G. Trends Neurosci. 1996;19:1996. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- 27.Ralevic V, Burnstock G. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 28.Poucher S M, Keddie J R, Singh P, Stoggall S M, Caulkett P W, Jones G, Coll M G. Br J Pharmacol. 1995;115:1096–1102. doi: 10.1111/j.1476-5381.1995.tb15923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daub H, Wallash C, Lankenau A, Herrlich A, Ullrich A. EMBO J. 1997;16:7032–7044. doi: 10.1093/emboj/16.23.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanke J H, Gardner J P, Dow R L, Changelian P S, Brissette W H, Weringer E J, Pollok B A, Connelly P A. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 31.D'Arcangelo G, Halegoua S. Mol Cell Biol. 1993;13:3146–3155. doi: 10.1128/mcb.13.6.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu C, Ma Y-C, Benjamin J, Littman D, Chao M, Huang X-Y. J Biol Chem. 2000;275:20726–20733. doi: 10.1074/jbc.M000152200. [DOI] [PubMed] [Google Scholar]
- 33.Dixon A, Gabitz A, Sirinathsinghji D, Richardson P, Freeman T. Br J Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berg M M, Sternberg D, Parada L F, Chao M V. J Biol Chem. 1992;267:13–16. [PubMed] [Google Scholar]
- 35.Sexl V, Mancusi G, Holler C, Gloria-Maercker E, Schutz W, Freissmuth M. J Biol Chem. 1997;272:5792–5799. doi: 10.1074/jbc.272.9.5792. [DOI] [PubMed] [Google Scholar]
- 36.Gao Z, Chen T, Weber M, Linden J. J Biol Chem. 1999;274:5972–5980. doi: 10.1074/jbc.274.9.5972. [DOI] [PubMed] [Google Scholar]
- 37.Seidel M, Klinger M, Freissmuth M, Holler C. J Biol Chem. 1999;274:25833–25841. doi: 10.1074/jbc.274.36.25833. [DOI] [PubMed] [Google Scholar]
- 38.Sebastiao A, Ribeiro J. Trends Pharmacol Sci. 2000;21:341–346. doi: 10.1016/s0165-6147(00)01517-0. [DOI] [PubMed] [Google Scholar]
- 39.Rosin D, Robeva A, Woodard R, Guyenet P, Linden J. J Comp Neurol. 1998;401:163–186. [PubMed] [Google Scholar]
- 40.Kaelin-Lang A, Lauterburg T, Burgunder J-M. Neurosci Lett. 1998;246:21–24. doi: 10.1016/s0304-3940(98)00216-x. [DOI] [PubMed] [Google Scholar]
- 41.Ledent C, Vaugeois J-M, Schiffmann S N, Pedrazzini T, El Yacoubi M, Vanderhaeghen J-J, Costentin J, Heath J K, Vassart G, Parmentier M. Nature (London) 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- 42.Scheardown M, Knutsen L. Drug Dev Res. 1996;39:108–114. [Google Scholar]
- 43.von Lubitz D, Lin R, Jacobson K. Eur J Pharmacol. 1995;287:295–302. doi: 10.1016/0014-2999(95)00498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones P, Smith R, Stone T. Neuroscience. 1998;85:229–237. doi: 10.1016/s0306-4522(97)00613-1. [DOI] [PubMed] [Google Scholar]
- 45.Phillis J. Brain Res. 1995;705:79–84. doi: 10.1016/0006-8993(95)01153-6. [DOI] [PubMed] [Google Scholar]
- 46.Jacobson K, von Lubitz D, Daly J, Fredholm B. Trends Pharmacol Sci. 1996;17:108–113. doi: 10.1016/0165-6147(96)10002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moreau J-L, Huber G. Brain Res Rev. 1999;31:65–82. doi: 10.1016/s0165-0173(99)00059-4. [DOI] [PubMed] [Google Scholar]
- 48.Guroff G, Dickens G, End D, Londos C. J Neurochem. 1981;37:1431–1439. doi: 10.1111/j.1471-4159.1981.tb06312.x. [DOI] [PubMed] [Google Scholar]
- 49.Huffaker T, Corcoran T, Wagner J A. J Cell Physiol. 1984;120:188–196. doi: 10.1002/jcp.1041200212. [DOI] [PubMed] [Google Scholar]
- 50.Hefti F. J Neurobiol. 1994;25:1418–1435. doi: 10.1002/neu.480251109. [DOI] [PubMed] [Google Scholar]
- 51.Thoenen H. In: Axonal Regeneration in the Central Nervous System. Ingoglia N, Murray M, editors. New York: Dekker; 2001. pp. 675–697. [Google Scholar]