Abstract
While there is broad agreement that interactions of the human maternal immune system with the tissues and cells of the implanting embryo are likely to be critical contributors to pregnancy success, there remains a dearth of information which directly confirms this expectation. Although animal models of reproductive function often provide opportunities for confirming such hypotheses, progress in this area has been sporadic due to limitations of traditional laboratory or agricultural animal models, such as rodents, sheep, pigs and cattle. Many of these limitations derive from divergent modes of implantation and placentation across mammalian species. Over the past decade there has been progress in the development of the nonhuman primate as a model in which to address questions of pregnancy success in the area of immunology. The purpose of this review is to compare available model species, summarize current knowledge and recent progress with an emphasis on experimental in vivo manipulations, and suggest areas available for additional study and growth.
Keywords: placenta, HLA-G, NK cell, decidua
Introduction
The past several decades have seen a profound increase in our understanding of the ways in which the maternal immune system responds to pregnancy. Owing to the substantial diversity in placentation, interdigitation and intermixing of maternal and fetal tissues, and specialized populations of leukocytes in the maternal endometrium, there is undoubtedly a broad palette of mechanisms that can be invoked in order to address the need for maternal accommodation. These mechanisms include the control of maternal T cell accumulation and activation at the maternal-fetal interface by regulation of tryptophan availability, primarily by indoleamine-2,3-dioxygenase in the placenta (Mellor et. al, 2002), the balance of TH1 and TH2 type T cells in peripheral and decidual compartments (Wilczynski, 2006), the impact of immunoregulatory soluble factors and cytokines produced by the placenta in control of maternal leukocyte responses (Santoni et al., 2008; Yoshinaga, 2008), and the immunosuppressive effects of pregnancy hormones, including progesterone, as well as pro-implantation effects of chorionic gonadotropin (CG), particularly in the decidual response in primates (Fazleabas, 2007). These topics will not be specifically covered in this review and the reader is referred to other previous excellent reviews cited above or other contributions in this volume. In this review we will specifically discuss the unique attributes and challenges inherent in the nonhuman primate as a model for studying the function of placental major histocompatibility complex (MHC) class I molecules.
There are a number of characteristics of nonhuman primates that give them special relevance to the study of human reproduction and pregnancy. These include a menstrual cycle exceedingly similar to that of the human endometrium, very similar villous placental morphology, parallel endocrinology of pregnancy, including placental secretion of CG and somatomammotropins, progesterone and estradiol, formation of a decidual endometrium in response to implantation of the embryo, and with the focus of this review, expression of nonclassical placental MHC class I molecules, and unique populations of decidual leukocytes. The ability to conduct in vivo experiments, or obtain critical samples from embryos and early pregnancy (< 4 weeks gestation) is severely limited in human clinical research. Thus, we have focused on nonhuman primate models for further study in this area.
Characterization of the rhesus model: placental components
Maternal-fetal immune interactions and placental MHC class I expression
During pregnancy in mammals, the mother tolerates the presence of a fetus in which half of the MHC class I genes expressed are, under most circumstances, foreign to the mother, being of paternal origin. Yet, immunological tolerance of the fetus seems only rarely interrupted. Thus, the maternal immune system in some way recognizes and responds appropriately to the establishment of pregnancy. While multiple mechanisms likely contribute to this tolerance, in humans and nonhuman primates, placental MHC class I molecules are well-studied candidates for promoting maternal-fetal immune tolerance, and putting into motion local networks that direct an intrauterine environment supporting pregnancy success.
The products of the classical human MHC class I loci human leukocyte antigens (HLA-A, -B, -C) are highly polymorphic molecules which can bind peptides in a groove formed by the α1 and α2 helical domains in the extracellular region of the molecule. In the setting of a viral infection, these MHC class I molecules bind viral peptides and present them to cytotoxic T lymphocytes (CTLs), triggering destruction of the virus-infected cells. In humans hundreds of alleles of the HLA-A, -B and -C loci have been cloned and sequenced. The polymorphism of classical MHC class I molecules is critical to the surveillance function of an intact immune system. MHC class I genes have also been intensively studied in nonhuman primates (Watkins, 1995). Recently, the rhesus MHC has been sequenced in its entirety (Daza-Vamenta et al., 2004), and an individual rhesus monkey expresses the products of at least two HLA-A-like loci and as many as 28 HLA-B-like loci (termed Mamu-A and Mamu-B, for Macaca mulatta) (Daza-Vamenta et al., 2004). There is no MHC-C locus in the rhesus monkey (Daza-Vamenta et al., 2004). Please note that nucleic acid entities are designated in italics, proteins are in plain text.
In contrast to the highly polymorphic classical MHC class I molecules, the non-classical human MHC class I gene products (HLA-E, -F, -G) exhibit extremely limited polymorphism. Given that its expression is most clearly demonstrated in placental trophoblasts (Kovats et al., 1990; Hunt, 2006), HLA-G has been implicated in the regulation of maternal-fetal immune tolerance. Like classical MHC class I molecules, HLA-G is complexed with β2-microglobulin (Kovats et al., 1990), recognized by CD8+ cells (Sanders et al., 1991) and binds the same array of peptides as classical HLA molecules (Lee et al., 1995; Diehl et al., 1996). HLA-G protein is readily detected in the extravillous cytotrophoblasts that invade the maternal endometrium (Kovats et al., 1990; LeBouteiller, 2000; Loke et al., 1997; Hunt, 2006). There are novel features of HLA-G. In addition to mRNAs that encode a full-length membrane-bound molecule, alternatively spliced HLA-G mRNAs including transcripts which encode soluble HLA-G proteins have been described (Fujii et al., 1994; Ishitani et al., 1992). An antibody specific to the soluble isoform intron 4 peptide (16G1) has confirmed expression in the placenta (Ishitani et al., 2003; Chu et al., 1999). Several groups have reported assays for detecting soluble HLA-G in peripheral blood of pregnant women and other biological fluids (Hamai et al., 1999; Fournel et al., 1999; Rebmann et al., 1999; Hunt et al., 2000). Furthermore, potential relevance to pregnancy success was shown in additional studies which reported that soluble HLA-G in the culture medium from human in vitro fertilization (IVF)-derived embryos was significantly associated with subsequent pregnancy following transfer to recipients (Fuzzi et al., 2002; Yie et al., 2005; Sher et al., 2005; Desai et al., 2006).
Recombinant soluble HLA-G has been produced as a reagent to use with in vitro studies of target cell effects. Soluble HLA-G can induce apoptosis in peripheral CD8+ T cells via a fas-fasL mechanism (Fournel et al., 2000) and it was reported that soluble HLA-G incubation with a human macrophage-like cell line induces the expression of several cytokine mRNAs and mRNAs involved in programmed cell death (McIntire et al., 2004). There may be other direct targets of HLA-G in addition to leukocytes. In studies with cultured human umbilical vein or microvascular endothelial cells, soluble HLA-G was able to promote apoptosis in vitro through interaction with glycophosphatidylinositol (GPI)-anchored CD160 (Fons et al., 2006). Finally, associations have been made between circulating levels of soluble HLA-G and organ transplantation success (Rouas-Freiss et al., 2003), malignancy (Ugurel et al., 2002; Wiendl et al., 2002, Urosevic et al., 2003), or certain inflammatory diseases (Carosella et al., 2001). Thus, a role for HLA-G in regulation of tolerance has not been restricted to the maternal-fetal interface.
HLA-E is also expressed in the human placenta (Ishitani et al., 2003; King et al., 2000), as well as many other somatic cells. HLA-E binds peptides derived from the leader sequence of many MHC class I molecules and thus serves to convey a “summary” of MHC class I status of somatic cells in the body to natural killer (NK) cells and other cells of the innate immune system.
We have now seen nearly two decades of research on placental nonclassical MHC class I molecules. There has been admirable progress in its cell biology, patterns of expression, appearance in pathological situations, and molecular regulation. However, the main (and perhaps most important) area in which we lag is a precise understanding of the in vivo biological activity of HLA-G in human pregnancy. These include the potential clinical impact of placental MHC deficiency, its physiological effects on pregnancy success, implantation, and placental and fetal development, its significance for intrauterine programming and postnatal health, and the clinical impact of soluble MHC class I expression. To establish an experimental animal model to address these questions, we initiated studies to define MHC class I expression in the rhesus placenta.
Nonclassical MHC loci in nonhuman primates
The MHC-G locus has been demonstrated in other primates, including apes and macaques (Boyson et al., 1996; Arnaiz-Villena et al., 1999). Unexpectedly, we found in the rhesus that Mamu-G is a pseudogene (Boyson et al., 1996). However, we have defined the placental expression of a novel MHC class I locus, Mamu-AG, which shares a number of features of HLA-G (Golos, 2003; Boyson et al., 1997). These include alternative splicing (Boyson et al., 1997; Ryan et al., 2002), and the expression of a soluble isoform (Ryan et al., 2002) low polymorphism and a truncated cytoplasmic domain (Boyson et al., 1997), and a distinctive expression pattern in which the highest mRNA and protein levels are found in placental trophoblasts (Slukvin et al., 1998; Slukvin et al., 2000; Slukvin et al., 1999).
mAb 25D3 specifically recognizes Mamu-AG glycoproteins
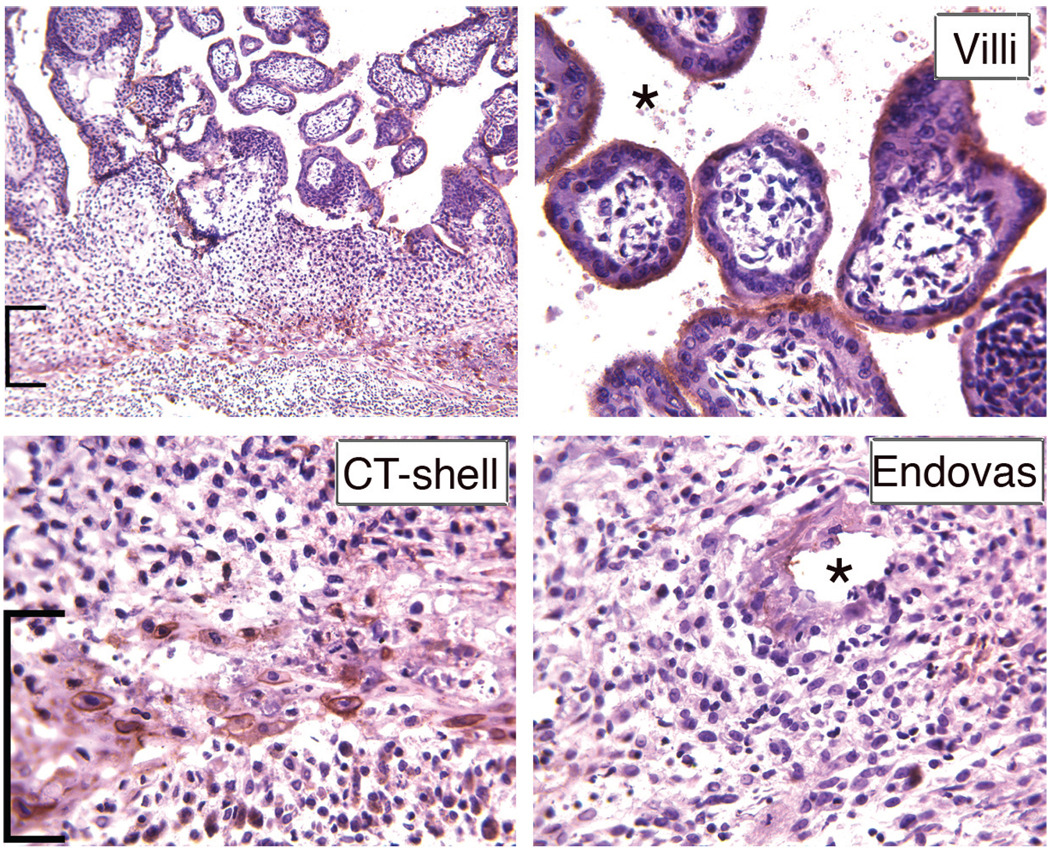
A Mamu-AG-specific mAb (25D3) was generated by subtractive immunization with the MHC class I-negative 721–221 B lymphoblastoid cell line, followed by immunization with the same cells stably transfected with a Mamu-AG expression plasmid (Slukvin et al., 2000). Flow cytometry showed that mAb 25D3 detects Mamu-AG trophoblasts and Mamu-AG transfectants, but not peripheral blood leukocyte (PBLs) (Slukvin et al., 2000). Immunohistochemical (IHC) analyses to localize Mamu-AG glycoprotein as early as 14 days of gestation (d14) or approximately 6–7 days after attachment of the blastocyst to the uterus demonstrated Mamu-AG expression in cytotrophoblasts at the fetal-maternal junction and endovascular cytotrophoblasts invading maternal arterioles. In the definitive villous placenta, Mamu-AG continues to be expressed in the trophoblastic shell at the border with the maternal decidua (Fig. 1) as well as in extravillous and villous syncytiotrophoblasts (Fig. 1). Thus, Mamu-AG expression is ideally situated for alerting decidual leukocytes, including numerous decidual CD56+ NK cells, to the presence of the placenta.
Fig. 1. Immunohistochemical localization of placental Mamu-AG at d50 of gestation.
Positive immunostaining is indicated by brick red color of trophoblasts (extravillous: brackets; villous or endovascular: asterisks). Higher magnification detail of villi, cytotrophoblastic (CT) shell, and endovascular trophoblasts are presented along with a low magnification overview of expression.
Mamu-E expression in the placenta
The MHC-E locus is remarkably well conserved during the evolution of primates, and a Mamu-E locus expressed in macaques, including in the placenta, was identified over a decade ago (Boyson et al., 1995). Progress in defining its cellular localization in the nonhuman primate placenta has been hampered by a lack of highly specific antibodies for immunohistochemical work, however, our most recent studies (Dambaeva et al., 2008) have identified an anti-HLA-E antibody (MEM-E/06, ExBio, Prague, Czech Republic) that recognizes Mamu-E in flow cytometry on peripheral blood leukocytes, and immunohistochemical and flow cytometry studies in the rhesus placenta. Like Mamu-AG, Mamu-E is expressed in the trophoblasts of the chorionic villi as well as extravillous trophoblasts. Interestingly, rather than primary expression in the syncytiotrophoblasts, protein was most highly expressed in the villous cytotrophoblasts in the first third of gestation, and at term was highly expressed in villous fetal endothelial cells (Dambaeva et al., 2008). We have also found that the anti-HLA-E antibody recognizes vervet and cynomolgus monkey trophoblasts and PBLs (Dambaeva, unpublished), and anticipate that it will also recognize Paan-E in the baboon. Table 1 summarizes results with these anti-MHC antibodies.
TABLE 1.
Characteristics of Placenta and Endometrium in Selected Primates
| Species | Placental MHC | Decidual leukocytes |
|---|---|---|
| Rhesus | Syncytiotrophoblasts: Mamu-AG | NK cells: CD56bright |
| (Macaca mulatta) | Villous cytotrophoblasts: Mamu-E | Macrophages: DC-SIGN+ |
| Extravillous trophoblasts: Mamu-AG, Mamu-E | ||
| cynomolgus | Syncytiotrophoblasts: Mamu-AG | NK cells: CD56bright |
| (Macaca fascicularis) | Extravillous trophoblasts: Mamu-AG, Mamu-E | Macrophages: DC-SIGN+ |
| vervet (African green) | Syncytiotrophoblasts: Mamu-AG | NK cells: CD56bright |
| (Chloroceus aethiops) | Extravillous trophoblasts: Mamu-AG, Mamu-E | Macrophages: DC-SIGN+ |
Soluble Mamu-AG isoforms
We evaluated whether the rhesus placenta expresses alternatively spliced Mamu-AG mRNAs homologous to soluble HLA-G mRNA (Ishitani et al., 1992) and demonstrated the expression of Mamu-AG mRNA with a retained 4th intron as seen with soluble HLA-G. Noting the close similarity between the Mamu-AG and HLA-G intron 4 peptides, we obtained the anti-intron 4 antibody 16G1 from Dan Geraghty (Ishitani et al., 2003). Immunostaining of the rhesus placenta (Ryan et al., 2002) gave a pattern of localization remarkably similar to that seen in the human placenta (Chu et al., 1999): there was soluble Mamu-AG identified in the extravillous trophoblasts (Ryan et al., 2002) as well as staining of the syncytiotrophoblasts and some villous cytotrophoblasts. These data underscore the importance of the rhesus monkey as a model to define function of Mamu-AG, and collectively support the concept that this molecule may be the functional homolog of HLA-G in macaques (Golos, 2003).
Placental MHC expression in other nonhuman primates
While it seems clear that there are substantial advantages to the use of nonhuman primates owing to their commonality with human reproductive patterns, several species are potential candidates for experimental investigation of the biology of MHC class I molecules in primate pregnancy. The first studies with placental MHC class I biology were reported in 1987 when Stern et al., demonstrated by immunohistochemistry and western blotting with the anti-MHC class I monoclonal antibody W6/32 that the baboon (Papio anubis) expressed MHC class I primarily on the syncytiotrophoblast membranes. No other studies were conducted in this area with the baboon until it was reported by Boyson et al. (1999) and Langat et al. (2002) that as in the rhesus, a novel locus was expressed in the baboon placenta, homologous with Mamu-AG, incorporating molecular components of HLA-G. Langat and co-workers (2002) subsequently developed a panel of monoclonal antibodies specific for exon-exon junctions, which allowed the further elucidation of protein isoforms arising from mRNA splice variants were expressed in vivo. These antibodies have demonstrated differential distribution of Paan-AG splice variants, much as has been done for HLA-G (Pace et al., 2006). These reagents and their use in baboon tissues have been recently reviewed (Langat et al., 2006) and will not be presented in further detail here. Specific advantages of the baboon as a model for human pathophysiology of pregnancy include a larger size than other species generally used in biomedical research, providing a greater volume of samples (e.g., endometrium from cycling females for cell culture and molecular studies), as well as careful study of the endometrial response to implantation (Fazleabas, 2007).
The maternal-fetal interface in cynomolgus and vervet monkeys
In recent years there have periodically been strategic limitations on the availability of female rhesus macaques, waxing and waning with the research in other areas of female reproductive physiology, health and disease (e.g., sexual transmission of immunodeficiency viruses). We have recently explored the potential of alternatives to the rhesus macaque for the study of placental MHC class I biology. Some results of these studies with the cynomolgus macaque (Macaca fasicularis) and the vervet or African green monkey (Chlorocebus aethiops) are summarized in Table 1. We have cloned Mamu-AG-like mRNAs in the cynomolgus and vervet monkeys (Bondarenko et al., 2009). The cynomolgus macaque, not surprisingly, expressed a mRNA that was virtually identical with the rhesus, demonstrating full-length as well as alternatively spliced mRNAs as seen in the rhesus (Boyson et al., 1997). Our first clue that the situation with the vervet monkey may be different was inferred from modest and somewhat patchy staining of vervet chorionic villi with W6/32, which strongly stains rhesus and cynomolgus villi. In addition, chorionic villi did not stain positively with 25D3 against Mamu-AG. Thus, immunohistochemical data was rather equivocal on the expression of a Chae-AG ortholog. Although work is still in progress, current studies also suggest that while an AG-like transcript is expressed in the vervet placenta, it is not as highly expressed as in the rhesus and cynomolgus, and while the α2 domains indicate close homology with Mamu-AG and Mafa-AG, the transmembrane and cytoplasmic domains lack the diagnostic stop codon shared by Mamu-AG, Mafa-AG, and HLA-G.
Decidual dNK cells and pregnancy success
Up to 75–80% of all lymphocytes in the human as well as the rhesus monkey decidua of early pregnancy are NK cells, with the balance primarily macrophages. We have shown in the rhesus decidua (Slukvin et al., 2001; Slukvin et al., 2004) that these cells of the innate immune system are located in close proximity to placental trophoblasts, as well as vessels with abundant trophoblast infiltration. The abundance of NK cells in the primate endometrium during pregnancy, in comparison with other nonlymphoid organs, is quite novel. It has long been known that human decidual (d)NK cells have a phenotype distinct from peripheral blood (p)NK cells, with their hallmark characteristics including bright CD56 expression (Moffett-King, 2002). Recent microarray studies further identified many genes differentially expressed between dNK cells and peripheral blood pNK cells (Koopman et al., 2003), including genes related to immune function, adhesion and migration, as well as immunomodulatory genes such as glycodelin and galectin-1 (Koopman et al., 2003). These observations have solidified the view that dNK cells have an important function distinct from peripheral NK (pNK) cells.
In comparison with the majority CD56dim cells, which have enhanced levels of cytotoxic activity and high levels of CD16, CD56bright cells are primarily immunoregulatory, producing cytokines and expressing low levels of CD16. Peripheral blood NK cells do not significantly change during the menstrual cycle and pregnancy. On the other hand, in the endometrium, the levels of NK cells increase from the follicular to the luteal phase, and continue to increase in early human and rhesus pregnancy (Breburda et al., 2006, see also below). The peripheral blood also contains a modest population (<10% of all NK cells) of CD56bright cells. It has been assumed that CD56bright pNK are the cells that traffic preferentially to the endometrium and that the increase in the numbers of dNK cells is likely to be contributed to by both enhanced trafficking as well as in situ proliferation. While culture data and studies with estrogen and progesterone receptor null mice have shown that sex steroids do not directly affect NK cell function, both recruitment of pNK cells to the endometrium and their proliferation may be supported by steroid effects on endometrial stromal cells (see Dosiou and Giudice (2005) for review).
Several potential roles of dNK cells in human pregnancy have been proposed. Since CD56bright pNK and dNK cells are proposed to be primarily immunoregulatory rather than cytotoxic, their secretion of factors such as various colony-stimulating factors (CSFs), transforming growth factors (TGF-β), and other growth factors may promote trophoblast growth and differentiation. Expression of dNK immunosuppressive factors such as glycodelin and galectin-1 may promote an appropriate implantation environment. While there is little evidence for a role of pNK cells in recurrent pregnancy loss or other aspects of pregnancy failure, some authors have suggested that dNK cells may play a role in implantation defects or recurrent spontaneous abortion (Lachapelle et al., 1996). However, careful analysis of cause vs. effect in the evaluation of dNK cells from specimens collected during pregnancy loss is difficult. Studies with the highly relevant primate experimental model in which the dNK population (or other endometrial cell populations) can be manipulated in a controlled setting would be a valuable advance in this field.
Characterization of the rhesus model: maternal components
Additional characterization of rhesus monkey decidual leukocytes
When we initiated our studies virtually nothing was known about rhesus decidual NK cells. We carried out a phenotypic and functional characterization of leukocytes isolated from the maternal decidua of the pregnant rhesus monkey. A majority (50–80% of CD45+/Lin+) of these cells were CD56bright+, CD3−, had typical large granular lymphocyte/uterine NK cell morphology and contained numerous cytoplasmic granules (Slukvin et al., 2001). Extensive flow cytometric evaluation of decidual leukocytes showed that rhesus CD56bright+ cells shared other phenotypic features of human uterine NK cells, expressed low levels of CD16, but did not express monocyte/macrophage markers. In contrast, most rhesus CD16+ peripheral blood cells NK cells were CD56−. As described for human dNK cells, rhesus decidual lymphocytes had relatively lower lytic activity against K562 or Raji cells, and had similar activity to pNK against 721.221 targets in cytotoxicity assays (Slukvin et al., 2001). Together, these results suggest that as in the human uterus, rhesus decidual CD56bright+ cells represent a distinct lymphocyte subset that belongs to the NK cell lineage, albeit with reduced cytotoxicity.
Immunohistochemical characterization of rhesus leukocytes
Using immunohistochemistry (IHC) we examined the distribution of macrophages, NK cells, and T cells in the nonpregnant proliferative endometrium and in the endometrium at day 14, 19 and 36 of pregnancy in the rhesus monkey. CD68+ macrophages, CD56+ lymphocytes and CD3+ T cells were present in the proliferative endometrium. The number of macrophages and CD56+ lymphocytes increased at implantation and continued to be high in early pregnancy decidua (Slukvin et al., 2004; Fig. 2). Accumulation of CD68+ macrophages was evident in endometrium immediately adjacent to the implantation site (Fig 3), and in close association with arteries invaded by cytotrophoblasts, whereas CD56+ lymphocytes were more evenly distributed throughout the decidua. Few CD3+ T cells were seen in the decidua. Note that as in the human (Proll et al., 1996), rhesus endovascular trophoblasts also express CD56 (Fig. 3).
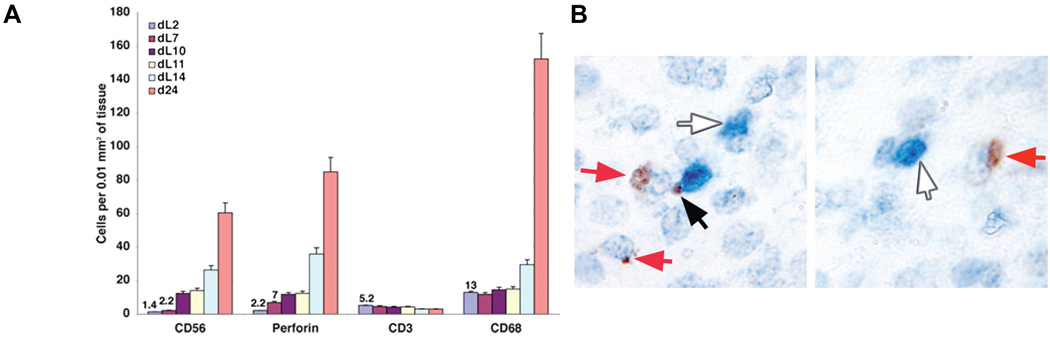
Fig. 2.
(A) Comparison of the number of CD56, perforin, CD3 and CD68-postiive cells in the rhesus endometrium through the luteal (L) phase (day 2, d7, d10, d11, d14) in comparison with d24 of pregnancy. The means of 5 fields are shown, with values indicated for low numbers. (B) Double immunohistochemistry for CD3 (white arrows) and perforin (red arrows). The black arrow points to a possible NK-T cell synapse.
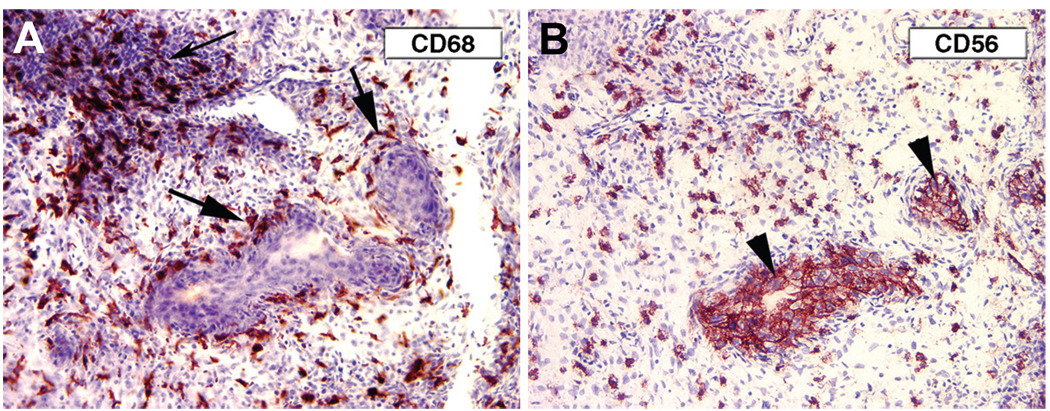
Fig. 3. CD68+ and CD56+ cells in decidua, d14 of pregnancy.
(A) CD68+ macrophages at the decidua-trophoblast interface are particularly evident surrounding a vessel filled with trophoblasts (large arrows). (B) Serial section: CD56+ NK cells are numerous but more widely distributed. Endovascular trophoblasts (arrowheads) also express CD56, as in the human (Proll et al., 1996).
To more accurately phenotype the perivascular CD68+ cells, we evaluated expression of a macrophage/dendritic cell marker, DC-SIGN, in early (1 week postimplantation) pregnancy (Breburda et al., 2006). Whereas CD68+ cells were distributed throughout the endometrium, DC-SIGN+ cells were noted only in the region directly adjacent to the implanting trophoblasts. In addition, DC-SIGN+ cells had a distinct, highly dendritic morphology. Since DC-SIGN is often a dendritic cell (DC) marker, we evaluated other DC markers. The endometrium was consistently negative for mature dendritic cell markers (CD83, DEC-205, CD86, or CD1a (Breburda et al., 2006), suggesting that DC-SIGN+ cells in the decidua represent a distinct subset of pregnancy-specific macrophages. Evaluation of the nonpregnant uterus during the menstrual cycle demonstrated that DC-SIGN is not seen except in pregnancy, and may be a marker for recognition of the presence of the placenta at implantation by immune cells in the rhesus monkey uterus.
Decidual leukocytes in cynomolgus and vervet monkeys
The close similarity in the morphological organization of the maternal-fetal interface has been previously described in excellent detail in the rhesus and cynomolgus macaques by Allen Enders and colleagues (e.g., Enders, 1993). We surveyed the immunohistochemical expression of accepted leukocyte markers in the decidua for NK cells, macrophages, and T cells and found that the distribution of these cells was remarkably similar to that seen for the rhesus as we have extensively described (Slukvin et al., 2004; Breburda et al., 2006).
The vervet implantation site has not previously been characterized in any immunohistochemical manner, although its scant pattern of menstruation in nonpregnant cycles has recently been described (Carroll et al., 2007). We have delineated the distribution of NK cells and macrophages in the decidua (Table 1). Remarkably, our previous observation that the appearance of decidual DC-SIGN+ macrophage-like cells is generally limited to the zone underneath the implantation site was seen, as expected, in the cynomolgus macaque, but also in the more distantly related vervet monkey (Dambaeva et al., in preparation). While the precise function of these cells remains uncertain, their conservation in Asian and African monkeys, as well as in the human implantation site, suggests a commonality of function, which nonetheless remains incompletely understood.
Phenotype analysis of rhesus decidual NK cells
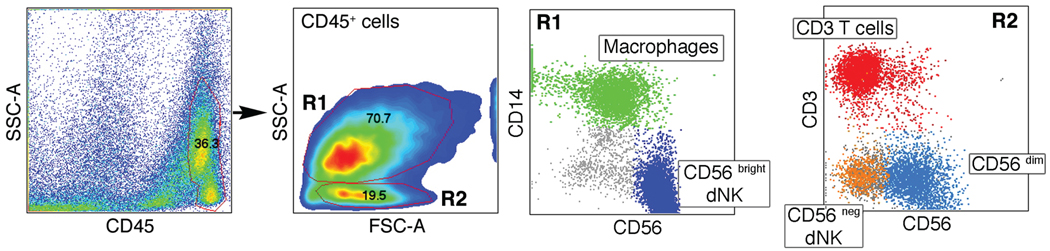
A current area of interest and debate concerns the relative contributions of trafficking into decidua of pNK cells and the proliferation of endometrial NK cells from a resident stem population to give rise to the dNK cells. Flow cytometry analysis of rhesus decidual cells from d36 of pregnancy was performed with Ficoll gradient separated mononuclear cells. Three main leukocyte populations are present in the rhesus decidua: NK cells, macrophages and T cells (Fig. 4). NK cells are the most prevalent population, and the majority (>80%) of dNK cells are CD56bright cells (Fig. 4). CD56bright cells are large granular lymphocytes characterized by high light-scattering properties more similar to macrophages than classical small lymphocytes (Fig. 4). There are also minor populations of CD56dim and CD56neg dNK cells, which are detected within the small lymphocyte gate, as are CD3+ T cells. Because pNK cells are predominantly CD56neg, the question arises if these minor decidual cells are blood contaminants, but they are substantially different from pNK cells. First, the expression of CD56 is not detected on rhesus pNK cells. Moreover, the expression of CD16 was found on only a minor population (~ 20%) of CD56dim dNK, while pNK cells are ~94% CD16+ (Slukvin et al., 2001). The expression of NCR p30 and p46 on CD56dim dNK cells also was found to be lower in comparison with pNK cells (not shown). CD56neg decidual NK cells are rare (about 2% of leukocytes), and are similar to pNK cells with a CD16+8+ phenotype. However, a non-typical low level of NCR p46 and p30 expression (not shown) again indicates that these cells are distinct from pNK cells and more likely true tissue residents. The heterogeneity of rhesus dNK cells presented here may likely reflect developmental stages of the dNK cell population.
Fig. 4. Analysis of rhesus decidual CD45+ leukocytes.
Two distinct populations (R1,R2) were further analyzed by flow cytometry for CD14, CD56, and CD3 staining. Individual cell populations in the R1 and R2 groups delineated by forward scatter and side scatter are named in small boxes and depicted by individual colors.
Perforin IHC of decidual leukocytes has proven to be very reliable in our lab. The possibility exists that perforin+ cells represent both NK cells and cytotoxic T cells. To clarify this situation, we performed perforin/CD3 double-staining (Fig. 2). Perforin+/CD3-NK cells (red arrow) and CD3+/perforin-T cells (blue arrow) are easily seen, whereas CD3+/perforin+ T cells were rare; while the number of CD3+ cells declines dramatically. Thus, less than 5% of the perforin+ cells in pregnancy represent T cells (note the possible NK cell: T cell synapse in Fig. 2).
Receptors for placental MHC class I recognition
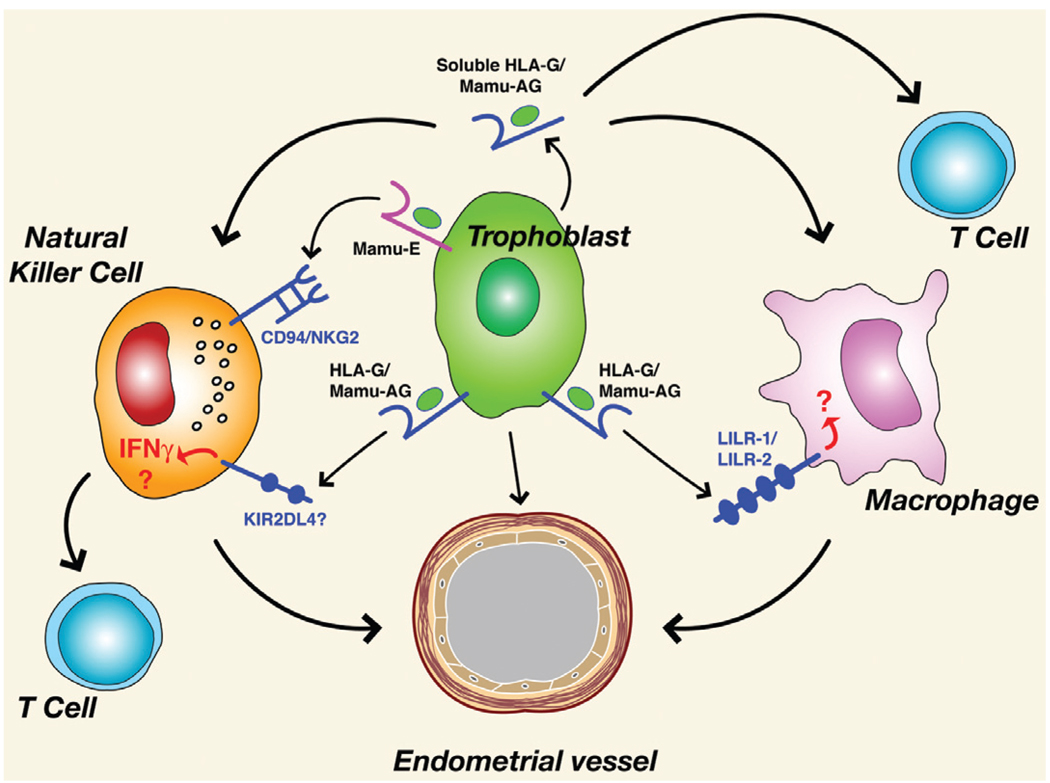
Interaction of NK cells with placental MHC class I molecules may play a key role in fetal-maternal interactions. With respect to HLA-G, several putative Ig-domain superfamily receptors have been identified which include leukocyte immunoglobulin-like receptor B/immunoglobulin-like transcript/leukocyte inhibitory receptor (LILRB1/ILT2/LIR-1 (Navarro et al., 1999), LILRB2/ILT4/LIR-2 (Allan et al., 1999; Shiroishi et al., 2003; Gonen-Gross, et al., 2003), and killer inhibitory preceptor (KIR)2DL4 (Cantoni et al., 1998; Ponte et al., 1999; Rajagopalan et al., 1999; Rajagopalan et al., 2006). These molecules are expressed on NK cells and macrophages in the decidua. Although an early prevalent hypothesis held that the primary biological role for HLA-G was to prevent an anti-fetal response by the maternal immune system, actions other than simple inhibition of lysis through KIR ligation (King et. al., 2000; Rajagopalan et al., 2001; Hanna et al., 2006) are likely for HLA-G or other MHC class I molecules in the placenta. For example, engagement of NK cells in the decidua by trophoblasts could simultaneously modify cytokine expression (HLA-G) and inhibit cytolytic activation (HLA-E) (Hanna et al., 2006). Decidual NK cells may also be significant sources of pro-angiogenic factors such as vascular endothelial growth/permeability factor (VEGF) (Li et al., 2001). The observation that cytokines such as interferon-γ secreted from NK cells or macrophages promote decidual differentiation, vascular development and implantation in mouse models (Ashkar et al., 2000) indicates a more complex role of decidual leukocytes and suggests that actions of trophoblast MHC molecules independent of lysis inhibition may be equally if not more important at the maternal-fetal interface. A summary of potential interactions between rhesus placental MHC Class I molecules is shown in Fig. 5.
Fig. 5. Summary of potential trophoblast major histocompatibility complex (MHC)-leukocyte interactions and leukocyte effects on maternal blood vessels in the rhesus monkey.
See text for details.
We have shown that homologs of these receptors for HLA-G are expressed in rhesus decidual leukocytes (Kravitz, et al., 2001; Grendell et al., 2001). In addition, the rhesus also expresses orthologs of CD94 and NKG2A, B, C, D, and E, some of which form heteodimeric receptors for HLA-E (Kravitz et al., 2001; Labonte et al., 2000). Additional crucial reagents are required in order to move the field of experimental reproductive immunology forward with nonhuman primate models. Sorely needed are monospecific antibodies against the receptors for nonhuman primate MHC class I molecules. Several NK cell receptors are recognized by anti-human reagents (Table 2). Somewhat surprisingly, a variety of anti-human KIR or LILR antibodies against human CD85d, j, k (LILRB1/ILT-2/LIR1, LILRB2/ILT4/LILR, and LILRB4/ILT-3/LIR5, respectively) or CD158a, b, e, i (KIR2DLI, KIR2DL3, KIR3DL1, KIR2DS4, respectively) have thus far failed to recognized nonhuman primate molecules (Dambaeva, unpublished). A comprehensive list of reactivity and nonreactivity of antibodies against multiple nonhuman primate species is available at http://nhpreagents.bidmc.harvard.edu/NHP/ReagentList.aspx. Concerted efforts to derive such important antibodies, and to apply them in vitro to define specific receptor-MHC class I ligand-receptor interactions are critically important. With success in derivation of such antibodies, further studies to determine their importance in pregnancy will logically follow with in vivo paradigms.
TABLE 2.
Anti-Natural Killer (NK) Cell Receptor Antibody Reactivity in the Rhesus Monkey
| Receptor | Source/clone | Comment |
|---|---|---|
| NKG2A | Beckman Coulter, Z199 | Reactive against rhesus, cynomolgus, vervet PBLs |
| (CD159a) | ||
| NKG2D | Beckman Coulter, ON72 | Reactive against rhesus, cynomolgus, vervet PBLs |
| (314) | Miltenyi, BAT221 | |
| NKp46 | Beckman Coulter, BAB281 | Reactive against rhesus, cynomolgus, vervet PBLs |
| (CD335) | ||
| NKp30 | Beckman Coulter, Z35 | Reactive against rhesus, cynomolgus, vervet PBLs |
| (CD337) | Miltenyi, AF29-4D12 |
In vivo experiments with the rhesus monkey
Passive immunization in nonhuman primates
There is a significant literature on passive immunization regimens with nonhuman primates, particularly with rhesus and cynomolgus monkeys. Passive immunization to neutralize circulating hormones or other soluble factors has achieved some significant success; e.g., in investigating hormonal feedback mechanisms in the control of follicle-stimulating hormone (FSH) secretion (Medhamurthy et al., 1990; Medhamurthy et al., 1991), the role of estrogen in pituitary function during the menstrual cycle (Zeleznik et al., 1987), and the neutralization of gonadotropin releasing hormone (GnRH) as a viable contraceptive approach (Hodges et al., 1977). Immunodepletion paradigms targeting cell-surface antigens have also been successful. Passive immunization studies with macaques in a renal allograft setting, against endothelial and lymphocyte intercellular adhesion molecule (ICAM)-1 (Cosimi et al., 1990) or against MHC class II molecules in experimental allergic encephalomyelitis showed no apparent deleterious effects of murine monoclonal antibody (mAb) treatment (Jonker et al., 1988).
Effect of anti-Mamu-AG passive immunization on rhesus monkey placental development
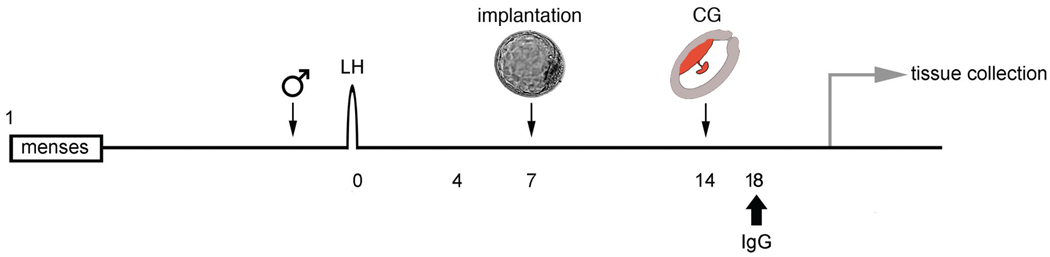
We conducted studies to gain insight into in vivo function of anti-Mamu-AG through passive immunization in early pregnancy. Rhesus monkeys were bred“and the day of the ovulatory luteinizing hormone (LH) surge was determined by radioimmunoassay (RIA). Beginning 10 days after the LH peak, daily blood samples were drawn and assayed for monkey chorionic gonadotropin (mCG) secretion to confirm pregnancy (Hodgen et al., 1974). We prepared 3 treatment groups: 4 rhesus monkeys from normal pregnancy (untreated control), 4 monkeys treated in vivo with 4 mg/day non-specific (NS) purified F(ab)’2 fragments given iv in sterile saline (NS treated), and 4 monkeys treated in vivo with 4 mg anti-Mamu-AG F(ab)’2 (25D3-treated). Animals were given antibody in a bolus injection administered slowly over 1–2 minutes between 8 AM and 9 AM each day from day 18 through day 24 of gestation, and intact uteroplacental units were obtained from animals following perfusion on day 24 with 2% paraformaldehyde/PBS (Bondarenko et al., 2007). A general schematic diagram of the experiment is shown in Fig. 6. Immunostaining of placental tissues with biotinylated horse anti-mouse IgG was’identical to Mamu-AG distribution, confirming adequate 25D3 mAb penetration and binding to endogenous Mamu-AG. Uteroplacental tissues were stained for cytokeratin, smooth muscle actin, KDR, FLT1, Von Willebrand factor (VWF), Ki67, CD3, CD56, CD68, DC-SIGN, or VEGF expression, and morphological data were collected on villous development, decidual histology, and decidual leukocyte number and distribution.
Fig. 6. Generalized schematic diagram of passive immunization protocol to study rhesus monkey pregnancy.
The timing of introduction to the male, blastocyst implantation, and detection of placental chorionic gonadotropin (CG) secretion are indicated. Antibody treatment can be initiated after or before confirmation of pregnancy. Blood sampling can be done for hormone detection as well as analysis of peripheral blood cell populations.
Table 3 presents a general summary of the results for analysis of the placental and decidual parameters. Treatment with mAb 25D3 caused dramatic changes in placental villi as well as in the villous vasculature. These data suggest that the overall development of the placental villi in animals passively immunized against Mamu-AG is retarded in comparison to untreated or NS mAb-treated controls, and that the critical vascularization of the placenta was also delayed in animals passively immunized against Mamu-AG. Other IHC suggested that VEGF and KDR expression but not FLT1 or epidermal growth factor receptor (EGFR) were reduced or more irregular in the STB, villous CTB, and endothelial cells of 25D3-treated placentas. Thus, a delay in vascularization may be associated with reduced VEGF signaling.
TABLE 3.
Summary of Placental and Decidual Outcomes in Rhesus Monkeys Treated with Anti-Mamu-Ag Passive Immunization on Days 18–24 of Gestation
| Parameter | Effect of 25D3 treatment |
|---|---|
| mean number of villous cross sections per mm2 | Reduced in 25D3-treated monkeys, compared with untreated or nonspecific-treated controls |
| length of stem villi | Reduced |
| diameter of all villi | Reduced |
| thickness of the villous trophoblast layer (Cytokeratin IHC) | Reduced |
| stromal/trophoblast area ratio | Reduced |
| trophoblast proliferation (Ki67 IHC) | Reduced |
| number of vessels per villus (CD31 IHC) | Reduced |
| diameter of large villous vessels (CD31 IHC) | Reduced |
| VEGF expression | Reduced/patchy in trophoblasts |
| KDR expression | Reduced/patchy in trophoblasts |
| EGFR expression | No change |
| Flt1 expression | No change |
| Decidualization of endometrial stroma | Delayed/inconsistent |
| Loss of epithelial plaque response | Delayed/inconsistent |
| Endometrial gland dilation (cytokeratin IHC) | Delayed/inconsistent |
| Number of CD56+ NK cells | No change |
| Number of CD68+ macrophages | No change |
| Number of DC-SIGN+ macrophages | Reduced |
| Number of CD3+ T cell aggregates | Increased around vessels |
All parameters were assessed in H&E stained sections or by specific IHC as indicated. Villous parameters were quantified within defined categories of chorionic villi (stem, floating, and branching types I and II), by using an ImageJ program for measuring photomicrograph image features. The statistical significance of group differences was assessed using ANOVA and Tukey’s honestly significant difference test. See Bondarenko et al.,2007) for further details of all categories.
Trophoblast invasion and vessel modification
We examined trophoblast invasion which occurs very quickly after implantation in the rhesus monkey (Slukvin et al., 2000, Enders, 1993). Maternal spiral arterioles directly beneath the implantation site were completely occluded by cytokeratin-positive extravillous trophoblasts (EVT) in all groups of animals, suggesting that cytotrophoblastic migration to maternal vessels was not impaired by mAb treatment. In addition, the arterial VWF-positive endothelial layer in the vessels is dramatically reduced or absent in all treatment groups, thus, endothelial removal was not altered by Mamu-AG immunization. EVT invasion into the smooth muscle actin (SMA)-positive tunica media appears through gaps in the smooth muscle layer in control placentas and results in smooth muscle loss. By contrast, the SMA-positive vascular smooth muscle layer of spiral arterioles in 25D3-treated placentas remained intact. Overall decidual vascularization, as defined by IHC for CD31 or indoleamine 2,3 dioxygenase, which is an excellent marker for decidual endothelium (Bondarenko et al., 2007), was reduced in 25D3-treated monkeys. Thus, decidual vascular remodeling and growth are disrupted by anti-Mamu-AG.
Effect of passive immunization against Mamu-AG on decidual tissues
Overall changes in the uterus with 25D3 treatment included inconsistent decidualization of endometrial stromal cells, epithelial plaque resolution, and delayed endometrial gland dilation (Table 3). Because of the importance of MHC-leukocyte interactions, we evaluated the distribution of decidual leukocytes in all animals. CD68-positive macrophages and CD56-positive NK cells abundantly infiltrated the decidua in all groups of animals, however DC-SIGN-positive cell density in the decidua was significantly lower in anti-Mamu-AG passive immunized monkeys (Table 3; Bondarenko et al., 2007). Since this is one of the earliest responses to embryo attachment and placenta formation noted among decidual immune cells, the data suggest a role for placental Mamu-AG in the DC-SIGN response. In untreated control and NS treated groups there were a few isolated CD3-positive cells, whereas in 25D3-treated animals, CD3+ cells formed a dense infiltrate around some of the non-invaded vessels (Bondarenko et al., 2007). This is uncharacteristic of normal pregnancy, and links altered placental development with aberrant decidual leukocyte disposition. Since IHC for indoleamine 2,3 dioxygenase (IDO) (Bondarenko et al., 2007) illustrated that the overall amount of IDO in the placental bed would be reduced due to the reduced vascular compartment, this may compromise capacity to regulate T cell proliferation (Mellor et al., 2002), which could be related to the changes in T cell distribution.
Significance of these outcomes
These results are very exciting in that they constitute in vivo evidence for a role of placental MHC Class I on decidual development and responses to the placenta in primate pregnancy. There is in vitro evidence for HLA-G interactions with NK cells, T cells and monocyte-derived cells. The failure to up-regulate DC-SIGN expression and the increase in T cell aggregates in 25D3-treated monkeys may be consistent with an interruption by passive immunization of Mamu-AG signaling directly with these target cells. Alternatively, it may be that Mamu-AG directly interacts with NK cells in the decidua, and immunoneutralization of Mamu-AG results in a change in NK cell phenotype (e.g., cytokine and growth factor secretion) that alters T cell migration or survival, as well as macrophage DC-SIGN expression. Changes in these leukocytes may then impact directly on placental growth (e.g., through growth factor secretion), or secondarily through effects on decidual stroma or decidual vessels. The next challenge will be to design further studies to differentiate between these alternative interpretations.
Further studies with passive immunization approaches
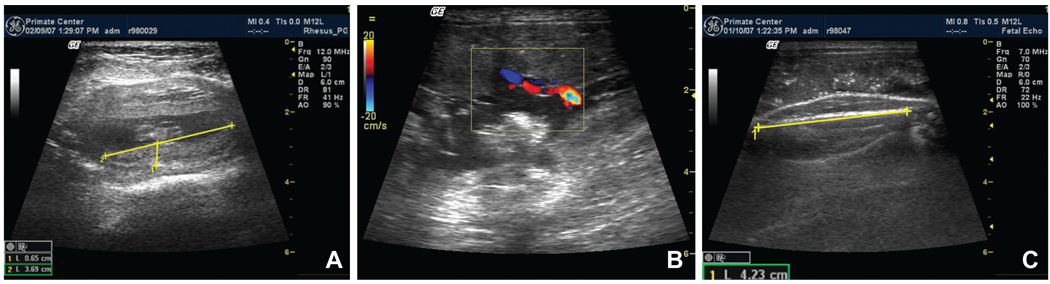
Now that we have demonstrated that there are significant acute effects of anti-Mamu-AG passive immunization on a broad spectrum of parameters of placental and decidual development, the question arises whether these acute effects have a long-term impact on pregnancy outcome. Compromised vasculogenesis within the placenta and the decidua would be expected to have both maternal and fetal impact. Human pregnancy characterized by these changes would have an increased risk for preeclampsia, fetal growth restriction, and pregnancy loss, including spontaneous abortion and miscarriage (Mayhew et al., 2004a; Mayhew et al., 2004b; Kwak-Kim et al., 2004; Wilczynski, et al., 2006). Long-term effects for pregnancies proceeding to term may include renal and cardiovascular deficits in the offspring (Woods et al., 2004; Eriksson et al., 2001). We hope in future studies to passively immunize pregnant rhesus monkeys during weeks 2 and 3 of gestation and examine the effects on pregnancy maintenance and fetal outcome. We anticipate that an early insult will enhance the opportunity for establishing a primate model of placental insufficiency. It will be important to carefully monitor intrauterine fetal outcomes during gestation in such treated animals. We provide below representative ultrasound images of fetal rhesus monkeys documenting that it will be possible to obtain quantitative information on parameters of fetal development and well-being, including placental diameter and thickness for volume estimation, umbilical blood flow, femur length (Fig. 7), as well as biparietal diameter, and fetal heart and kidney measurements (not shown). Longitudinal measurement of these parameters will be a valuable step forward for refinement of the primate model.
Fig. 7. Representative fetal ultrasound.
(A) d36 placenta, (B) ~d133 umbilical blood flow and (C) fetal femur.
NK cell immunodepletion studies
There have been successful applications of monoclonal antibodies to rhesus monkeys to immunodeplete both circulating as well as tissue (lymph node) CD8+ T lymphocytes and pNK cells (Schmitz et al., 1999), as well as CD16 immunodepletion of pNK cells (Choi et al., 2008). Anti-CD16 treatment should also target most decidual CD56bright NK cells. The minor CD56dim dNK cell subpopulation could remain unaffected, but because the ratio of CD56bright/CD56dim is about 5/1, the vast majority of dNK cells will be depleted by anti-CD16 treatment. Anti-CD16 treatment will not cause decidual T cell depletion, and thus CD16+ NK cell depletion effects can be contrasted with CD8+ NK cell depletion, which will also deplete the few CD8+ T cells in the decidua. The success of these studies is particularly relevant to our model, since the enormous number of T cells both in the peripheral circulation (80% of PBLs) as well as the lymphoid organs suggest that depletion of decidual and peripheral NK cells (~10% of all PBLs) is a feasible goal, at least based on target quantity. The immunodepletion of peripheral blood NK cells could provide an incredible tool to disrupt trafficking to the endometrium and decidua, and probe the importance of uterine NK cells in primate pregnancy.
Promising opportunities for research in reproductive immunology
We are excited to have progressed from our initial demonstration of the molecular and biochemical homology of Mamu-AG with HLA-G, to the in vivo demonstration of a biological role for Mamu-AG and potentially for NK cells as well. These results confirm and extend in vitro studies from the HLA-G field, and the opportunity afforded by passive immunization/immunodepletion paradigms in vivo in the rhesus will allow us to further specifically probe placental MHC function and Mamu-AG interactions with selected decidual leukocytes, and the impact of these interactions on implantation success, fetal development, and pregnancy outcome. The central advantage of the rhesus monkey is the available of large numbers of animals, the largest background information of currently used primate species, and the availability of well-characterized reagents for study in this species. These include species-specific microarrays (Affymetrix), species-validated antibodies (e.g., http://nhpreagents.bidmc.harvard.edu/NHP), multiplex cytokine assay platforms (Giavedoni, 2005), recombinant molecules, and reagent resources. Thus, we will close by briefly describing novel new approaches with the nonhuman primate model.
Adoptive lymphocyte transfer
Specific populations of cynomolgus macaques have been identified that are MHC identical. Adoptive transfer of leukocytes between individuals has been shown to be feasible, at least for T lymphocytes (Greene et al., 2008). Adoptively transferred cells are identifiable by fluorescent staining administered ex vivo, or by molecular tools such as microsatellite markers. This approach may allow the design of paradigms for studying trafficking of peripheral cells, including NK cells and monocytes, to the endometrium during pregnancy.
Rhesus monkeys and the mechanisms of preterm labor
Recent exciting studies have made use of the nonhuman primate in studies of the mechanisms underlying premature labor, and premature rupture of the membranes. In an experimental model of reproductive tract bacterial infection, it has been shown that intraamniotic bacterial inoculation resulted in reliable premature membrane rupture, accompanied by elevations in proinflammatory cytokines, as well as prostaglandins, and matrix metalloproteinases. In recent exciting studies using this model, IL-1β and TNF-α but not IL-6 or IL-8 infusion into the amniotic fluid induces the onset of preterm labor in pregnant rhesus monkeys (Sadowsky et. al, 2006). This effect mimics that seen with infusion of group B Streptococcus. A similar model was used to show that treatment with a toll-like receptor 4 (TLR-4) antagonist inhibited LPS-induced uterine activity and was associated with significantly lower amniotic fluid IL-8, TNF-α, and prostaglandins (Waldorf et al., 2008). Similar paradigms may be important further steps for understanding the interplay of immune modulators in early pregnancy establishment. suggesting not only a potential clinically useful therapeutic approach, but also potentially powerful tools for further explorations of the cytokines and chemokines expressed at the maternal-fetal interface, as well as their functions in early gestation.
Nonreproductive biology of HLA-G
There are many hypotheses as to the potential roles of HLA-G outside the setting of pregnancy. However, the safety and efficacy of HLA-G as a therapeutic tool/reagent is unknown, especially given its association with pathophysiological settings in some cases (Carosella et al., 2008). The production and testing of recombinant Mamu-AG or Mamu-E reagents will allow direct examination of the in vivo effectiveness of these reagents on leukocyte function, decidual maturation, pregnancy success, and additionally on potential “off-target” tissues where HLA-G/Mamu-AG may not be expected to be expressed in normal physiology, but where therapeutic treatment may expose lymphoid targets to unphysiological nonclassical MHC molecules.
Closing Comments
The many questions which remain unanswered regarding the immunological mechanisms which contribute to human pregnancy success make it imperative that a variety of models be employed for addressing the questions to which each is peculiarly suited. The rhesus monkey and other nonhuman primates benefit from close homology with the human maternal-fetal interface, and one advantage of the rhesus monkey is that it is widely used in infectious disease research, making available a broad palette of investigative tools. Interfacing reproductive biology with a diversity of approaches from other fields will be one key to making progress in this important and stimulating area of research.
Acknowledgements
We are grateful for the assistance of the animals services and veterinary staff for their excellent contributions to all of our studies with nonhuman primates. We thank the David Watkins lab at the University of Wisconsin-Madison for collaborations in the initial phases of this work, and Judith Peterson for assistance with preparation of this manuscript. This research was supported by NIH grants HD37120 and HD34215 to T.G.G., and P51 RR000167 to the Wisconsin National Primate Research Center, University of Wisconsin-Madison. This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01. This publication’s contents are solely the responsibility of the authors and does not necessarily represent the official views of NCRR or NIH.
Abbreviations used in this paper
- CG
chorionic gonadotropin
- HLA
human leukocyte antigen
- Mamu
Macaca mulatta
- MHC
major histocompatibility complex
- NK
natural killer
References
- Adams Waldorf KM, Persing D, Novy MJ, Sadowsky DW, Gravett MG. Pretreatment with toll-like receptor 4 antagonist inhibits lipopolysaccharid-induced preterm uterine contractability, cytokines, and prostaglandins in rhesus monkeys. Reprod. Sci. 2008;15:121–127. doi: 10.1177/1933719107310992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan DS, Colonna M, Lanier LL, Churakova TD, Abrams JS, Ellis SA, Mcmichael AJ, Braud VM. Tetrameric complexes of human histocompatibility leukocyte antigen (HLA)-G bind to peripheral blood myelomonocytic cells. J. Exp. Med. 1999;189:1149–1156. doi: 10.1084/jem.189.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaiz-Villena A, Morales P, Gomez-Casado E, Castro MJ, Varela P, Rojo-Amigo R, Martinez-Laso J. Evolution of MHC-G in primates: a different kind of molecule for each group of species. J. Reprod. Immunol. 1999;43:111–125. doi: 10.1016/s0165-0378(99)00026-1. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, Disanto JP, Croy BA. Interferon gamma Contributes to Initiation of Uterine Vascular Modification, Decidual Integrity, and Uterine Natural Killer Cell Maturation during Normal Murine Pregnancy. J. Exp. Med. 2000;192:259–269. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko GM, Durning M, Burleigh D, Breburda E, Grendell R, Golos TG. Passive immunization against the MHC Class I molecule disrupts rhesus placental development and endometrial responses. J. Immunol. 2007;179:8042–8050. doi: 10.4049/jimmunol.179.12.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko GI, Dambaeva SV, Grendell RL, Hughes AL, Durning M, Garthwaite MA, Golos TG. Characterization of cynomolgus and vervet monkey placental MHC class I expression: Diversity of the nonhuman primate AG locus. Immunogenetics. 2009;61:431–442. doi: 10.1007/s00251-009-0376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson JE, Mcadam SN, Gallimore A, Golos TG, Liu X, Gotch FM, Hughes AL, Watkins DI. The MHC E locus in macaques is polymorphic and is conserved between macaques and humans. Immunogenetics. 1995;41:59–68. doi: 10.1007/BF00182314. [DOI] [PubMed] [Google Scholar]
- Boyson JE, Iwanaga KK, Golos TG, Watkins DI. Identification of the rhesus monkey HLA-G ortholog - Mamu-G is a pseudogene. J. Immunol. 1996;157:5428–5437. [PubMed] [Google Scholar]
- Boyson JE, Iwanaga KK, Golos TG, Watkins DI. Identification of a novel MHC class I gene, Mamu-AG in the placenta of primate with an inactivated G locus. J. Immunol. 1997;159:3311–3321. [PubMed] [Google Scholar]
- Breburda EE, Dambaeva SV, Slukvin II, Golos TG. Selective distribution and pregnancy specific expression of DC-SIGN at the maternal-fetal interface in the rhesus macaque: DC-SIGN is a putative marker of the recognition of pregnancy. Placenta. 2006;27:11–21. doi: 10.1016/j.placenta.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Cantoni C, Verdiani S, Falco M, Pessino A, Cilli R, Conte R, Pende D, Ponte M, Mikaelsson MS, Moretta L, Biassoni R. A putative HLA class I-specific inhibitory receptor belonging to the immunoglobulin superfamily. Eur. J. Immunol. 1998;28:1980–1990. doi: 10.1002/(SICI)1521-4141(199806)28:06<1980::AID-IMMU1980>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Carosella ED, Favier B, Rouas-Freiss N, Moreau P, Lemaoult J. Beyond the increasing complexity of the immunomodulatory HLA-G molecule. Blood. 2008;111:4862–4870. doi: 10.1182/blood-2007-12-127662. [DOI] [PubMed] [Google Scholar]
- Carosella ED, Moreau P, Aractingi S, Rouas-Freiss N. HLA-G: a shield against inflammatory aggression. Trends. Immunol. 2001;22:553–555. doi: 10.1016/s1471-4906(01)02007-5. [DOI] [PubMed] [Google Scholar]
- Carroll RL, Mah K, Fanton JW, Maginnis GN, Brenner RM, Slayden OD. Assessment of menstruation in the vervet (Cercopithecus aethiops) Am. J. Primatol. 2007;69:901–916. doi: 10.1002/ajp.20396. [DOI] [PubMed] [Google Scholar]
- Choi EI, Wang R, Peterson L, Letvin NL, Reimann KA. Use of an anti-CD16 antibody for in vivo depletion of natural killer cells in rhesus macaques. Immunology. 2008;124:215–222. doi: 10.1111/j.1365-2567.2007.02757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu W, Yang Y, Geraghty DE, Hunt JS. Interferons enhance HLA-G nRNA and protein in transfected mouse fibroblasts. J. Repro. Immunol. 1999;42:1–15. doi: 10.1016/s0165-0378(98)00077-1. [DOI] [PubMed] [Google Scholar]
- Cosimi AB, Conti D, Delmonico FL, Preffer FI, Wee S-L, Rothlein R, Faanes R, Colvin RB. In vivo effects of monoclonal antibody to ICAM-1 (CD54) in nonhuman primates with renal allografts. J. Immunol. 1990;144:4604–4612. [PubMed] [Google Scholar]
- Dambaeva SV, Bondarenko GI, Grendell RL, Kravitz RH, Durning M, Golos TG. Nonclassical MHC-E (Mamu-E) expression in the rhesus monkey placenta. Placenta. 2008;29:58–70. doi: 10.1016/j.placenta.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daza-Vamenta R, Glusman G, Rowen L, Guthrie B, Geraghty DE. Genetic divergence of the rhesus macaque major histocompatibility complex. Genome Res. 2004;14:1501–1515. doi: 10.1101/gr.2134504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai N, Filipovits J, Goldfarb J. Secretion of soluble HLA-G by day 3 human embryos associated with higher pregnancy and implantation rates: assay of culture media using a new ELISA kit. Reprod. Biomed. Online. 2006;13:272–277. doi: 10.1016/s1472-6483(10)60626-8. [DOI] [PubMed] [Google Scholar]
- Diehl M, Munz C, Keilholz W, Stevanovic S, Holmes N, Loke YW, Rammensee HG. Nonclassical HLA-G molecules are classical peptide presenters. Curr. Biol. 1996;6:305–314. doi: 10.1016/s0960-9822(02)00481-5. [DOI] [PubMed] [Google Scholar]
- Dosiou C, Giudice LC. Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocrine Reviews. 2005;26:44–62. doi: 10.1210/er.2003-0021. [DOI] [PubMed] [Google Scholar]
- Enders AC. Overview of the morphology of implantation in primates. In: Wolf DP, Stouffer RL, Brenner RM, editors. In vitro fertilization and embryo transfer in primates. New York: Springer-Verlag; 1993. pp. 145–157. [Google Scholar]
- Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJP. Early growth and coronary hearth disease in later life: longitudinal study. Brit. Med. J. 2001;233:949–953. doi: 10.1136/bmj.322.7292.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazleabas AT. Physiology and pathology of implantation in the human and nonhuman primate. Semin Reprod Med. 2007;25:405–409. doi: 10.1055/s-2007-991037. [DOI] [PubMed] [Google Scholar]
- Fons P, Chabot S, Cartwright JE, Lenfant F, L’faqihi F, Giustiniani J, Herault JP, Gueguen G, Bono F, Savi P, Aguerre-Girr M, Fournel S, Malecaze F, Bensussan A, Plouët J, Le Bouteiller P. Soluble HLA-G1 inhibits angiogenesis through an apoptotic pathway and by direct binding to CD160 receptor expressed by endothelial cells. Blood. 2006;108:2608–2615. doi: 10.1182/blood-2005-12-019919. [DOI] [PubMed] [Google Scholar]
- Fournel S, Aguerre-Girr M, Campan A, Salauze L, Berrebi A, Lone Y-C, Lenfant F, Le Bouteiller P. Soluble HLA-G: Purification from eukaryotic transfected cells and detection by a specific ELISA. Am. J. Reprod. Immunol. 1999;42:22s–29s. doi: 10.1111/j.1600-0897.1999.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Fournel S, Aguerre-Girr M, Huc X, Lenfant F, Alam A, Toubert A, Bensussan A, Le Bouteiller P. Cutting Edge: Soluble HLA-G1 triggers CD95/CD95 Ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J. Immunol. 2000;164:6100–6104. doi: 10.4049/jimmunol.164.12.6100. [DOI] [PubMed] [Google Scholar]
- Fujii T, Ishitani A, Geraghty DE. A soluble form of the HLA-G antigen is encoded by a messenger ribonucleic acid containing intron 4. J. Immunol. 1994;153:5516–5524. [PubMed] [Google Scholar]
- Fuzzi B, Rizzo R, Criscuoli L, Noci I, Melchiorri L, Scarselli B, Bencini E, Menicucci A, Baricordi OR. HLA-G expression in early embryos is a fundamental prerequisite for the obtainment of pregnancy. Eur. J. Immunol. 2002;32:311–315. doi: 10.1002/1521-4141(200202)32:2<311::AID-IMMU311>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Giavedoni LD. Simultaneous detection of multiple cytokines and chemokines from nonhuman primates using luminex technology. J. Immunol. Methods. 2005;301:89–101. doi: 10.1016/j.jim.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Golos TG. Nonhuman primate placental MHC expression: a model for exploring mechanisms of human Maternal-Fetal immune tolerance. Hum Immunol. 2003;64:1102–1109. doi: 10.1016/j.humimm.2003.08.349. [DOI] [PubMed] [Google Scholar]
- Gonen-Gross T, Achdout H, Gazt R, Hanna J, Mizrahi S, Markel G, Goldman-Wohl D, Yagel S, Horejsi V, Levy O, Baniyash M, Mandelboim O. Complexes of HLA-G protein on the cell surface are important for leukocyte Ig-like receptor-1 function. J Immunol. 2003;171:1343–1351. doi: 10.4049/jimmunol.171.3.1343. [DOI] [PubMed] [Google Scholar]
- Greene JM, Burwitz BJ, Blasky AJ, Mattila TL, Hong JJ, Rakasz EG, Wiseman RW, Hasenkrug KJ, Skinner PJ, O’connor SL, O’connor DH. Allogeneic lymphocytes persist and traffic in feral MHC-matched mauritian cynomolgus macaques. PLoS ONE. 2008;3:e2384. doi: 10.1371/journal.pone.0002384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grendell RL, Hughes AL, Golos TG. Cloning of rhesus monkey killer-cell Ig-like receptors (KIRs) from early pregnancy decidua. Tissue Antigens. 2001;58:329–334. doi: 10.1034/j.1399-0039.2001.580507.x. [DOI] [PubMed] [Google Scholar]
- Hamai Y, Fujii T, Miki A, Geraghty DE, Harada I, Takai Y, Kozuma S, Tsutsumi O, Taketani Y. Quantitative assessment of human leukocyte antigen-G protein in amniotic fluid by a double-determinant enzyme-linked immunosorbent assay using anti-human leukocyte antigen-G-specific antibody «87G». Am. J. Reprod. Immunol. 1999;41:293–295. doi: 10.1111/j.1600-0897.1999.tb00441.x. [DOI] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- Hodgen GD, Tullner WW, Vaitukaitis JL, Ward DN, Ross GT. Specific radioimmunoassay of chorionic gonadotropin during implantation in rhesus monkeys. J. Clin. Endocrinol. Metab. 1974;39:457–464. doi: 10.1210/jcem-39-3-457. [DOI] [PubMed] [Google Scholar]
- Hodges JK, Hearn JP. Effects of immunisation against luteinising hormone releasing hormone on reproduction of the marmoset monkey Callithrixjacchus. Nature. 1977;265:746–748. doi: 10.1038/265746b0. [DOI] [PubMed] [Google Scholar]
- Hunt JS. Stranger in a strange land. Immunol. Rev. 2006;213:36–47. doi: 10.1111/j.1600-065X.2006.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt JS, Jadhav L, Chu W, Geraghty DE, Ober C. Soluble HLA-G circulates in maternal blood during pregnancy. Am. J. Obstet. Gynecol. 2000;183:682–688. doi: 10.1067/mob.2000.106762. [DOI] [PubMed] [Google Scholar]
- Ishitani A, Geraghty DE. Alternative splicing of HLA-G transcripts yields proteins with primary structures resembling both class I and class II antigens. Proc. Natl. Acad. Sci. USA. 1992;89:2669–2673. doi: 10.1073/pnas.89.9.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani A, Sageshima N, Lee N, Dorofeeva N, Hatake K, Marquardt H, Geraghty DE. Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, F, and G in maternal-placental immune recognition. J. Immunol. 2003;171:1376–1384. doi: 10.4049/jimmunol.171.3.1376. [DOI] [PubMed] [Google Scholar]
- Jonker M, Van Lambalgen R, Mitchell DJ, Durham SK, Steinman L. Successful treatment of EAE in rhesus monkeys with MHC class II specific monoclonal antibodies. J. Autoimmun. 1988;1:399–414. doi: 10.1016/0896-8411(88)90064-9. [DOI] [PubMed] [Google Scholar]
- King A, Allan DS, Bowen M, Powis SJ, Joseph S, Verma S, Hiby SE, Mcmichael AJ, Loke YW, Braudt VM. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur J Immunol. 2000;30:1623–1631. doi: 10.1002/1521-4141(200006)30:6<1623::AID-IMMU1623>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, Strominger JL. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J. Exp. Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, Demars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248:220–223. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- Kravitz RH, Grendell RL, Slukvin II, Golos TG. Selective expression of NKG2-A and NKG2-C mRNAs and novel alternative splicing of 5' exons in rhesus monkey decidua. Immunogenetics. 2001;53:69–73. doi: 10.1007/s002510000289. [DOI] [PubMed] [Google Scholar]
- Kwak-Kim J, Kim JW, Gilman-Sachs A. Immunology and pregnancy losses: HLA, autoantibodies and cellular immunity. In: Mor G, editor. Immunology of Pregnancy. Georgetown, TX: Landes Bioscience/Eurekah.com; 2004. pp. 122–134. [Google Scholar]
- Labonte ML, Levy DB, Letvin NL. Characterization of rhesus monkey CD94/NKG2 family members and identification of novel transmembrane-deleted forms of NKG2-A, B, C, and D. Immunogenetics. 2000;51:496–499. doi: 10.1007/s002510050650. [DOI] [PubMed] [Google Scholar]
- Lachapelle MH, Miron P, Hemmings R, Roy DC. Endometrial T, B, and NK cells in patients with recurrent spontaneous abortion. Altered profile and pregnancy outcome. J. Immunol. 1996;156:4027–4034. [PubMed] [Google Scholar]
- Langat DK, Fazleabas AT, Hunt JS. Methods for evaluating histocompatibility antigen gene expression in the baboon. In: Soares MJ, Hunt JS, editors. Placenta and Trophoblast: Methods and Protocols (Vol. 2) New Jersey: Humana Press; 2006. pp. 165–180. [DOI] [PubMed] [Google Scholar]
- Lebouteiller P. HLA-G in the human placenta: expression and potential functions. Biochem. Soc. Trans. 2000;28:208–212. doi: 10.1042/bst0280208. [DOI] [PubMed] [Google Scholar]
- Lee N, Malacko AR, Ishitani A, Chen MC, Bajorath J, Marquardt H, Geraghty DE. The membrane-bound and soluble forms of HLA-G bind identical sets of endogenous peptides but differ with respect to TAP association. Immunity. 1995;3:591–600. doi: 10.1016/1074-7613(95)90130-2. [DOI] [PubMed] [Google Scholar]
- Li XF, Charnock-Jones DS, Zhang E, Hiby S, Malik S, Day K, Licence D, Bowen JM, Gardner L, King A, Loke YW, Smith SK. Angiogenic growth factor messenger ribonucleic acids in uterine natural killer cells. J. Clin. Endocrinol. Metab. 2001;86:1823–1834. doi: 10.1210/jcem.86.4.7418. [DOI] [PubMed] [Google Scholar]
- Loke YW, King A, Burrows T, Gardner L, Bowen M, Hiby S, Howlett S, Holmes N, Jacobs D. Evaluation of trophoblast HLA-G antigen with specific monoclonal antibody. Tissue Antigens. 1997;50:135–146. doi: 10.1111/j.1399-0039.1997.tb02852.x. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta. 2004a;25:127–139. doi: 10.1016/j.placenta.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Wijesekara J, Baker PN, Ong SS. Morphometric evidence that villous development and fetoplacental angiogenesis are compromised by intrauterine growth restriction but not by pre-eclampsia. Placenta. 2004b;25:829–833. doi: 10.1016/j.placenta.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Mcintire RH, Morales PJ, Petroff MG, Colonna M, Hunt JS. Recombinant HLA-G5 and –G6 drive U937 myelomonocytic cell production of TGF-b1. J Leukocyte Biol. 2004;76:1220–1223. doi: 10.1189/jlb.0604337. [DOI] [PubMed] [Google Scholar]
- Medhamurthy R, Abeyawardene SA, Culler MD, Negro-Vilar A, Plant TM. Immunoneutralization of circulating inhibin in the hypophysiotropically clamped male rhesus monkey (Macaca mulatta) results in a selective hypersecretion of follicle-stimulating hormone. Endocrinol. 1990;126:2116–2124. doi: 10.1210/endo-126-4-2116. [DOI] [PubMed] [Google Scholar]
- Medhamurthy R, Culler MD, Gay VL, Negro-Vilar A, Plant TM. Evidence that inhibit plays a major role in the regulation of follicle-stimulating hormone secretion in the fully adult male rhesus monkey (Macaca mulatta) Endocrinol. 1991;129:389–395. doi: 10.1210/endo-129-1-389. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Chandler P, Lee GK, Johnson T, Keskin DB, Lee J, Munn DH. Indoleamine 2,3-dioxygenase, immunosuppression and pregnancy. J. Repro. Immunol. 2002;57:143–150. doi: 10.1016/s0165-0378(02)00040-2. [DOI] [PubMed] [Google Scholar]
- Moffett-King A. Natural Killer cells and pregnancy. Nat. Rev. Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- Navarro F, Llano M, Bellon T, Colonna M, Geraghty DE, Lopez-Botet M. The ILT2(LIR1) and CD94/NKG2A NK cell receptors respectively recognize HLA-G1 and HLA-E molecules co-expressed on target cells. Eur. J. Immunol. 1999;29:277–283. doi: 10.1002/(SICI)1521-4141(199901)29:01<277::AID-IMMU277>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Pace JL, Morales PJ, Phillips TA, Hunt JS. Analysis of the soluble isoforms of HLA-G: mRNAs and Proteins. In: Soares MJ, Hunt JS, editors. Placenta and Trophoblast: Methods and Protocols (Vol. 2) New Jersey: Humana Press; 2006. pp. 181–203. [DOI] [PubMed] [Google Scholar]
- Ponte M, Cantoni C, Biassoni R, Tradori-Cappai A, Bentivoglio G, Vitale C, Bertone S, Moretta A, Mingari MC. Inhibitory receptors sensing HLA-G1 molecules in pregnancy: decidua-associated natural killer cells express LIR-1 and CD94/NKG2A and acquire p49, an HLA-G1-specific receptor. Proc. Natl. Acad. Sci. USA. 1999;96:5343–5345. doi: 10.1073/pnas.96.10.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proll J, Blaschitz A, Hartmann M, Thalhamer J, Do Hr G. Human first-trimester placenta intra-arterial trophoblast cells express the neural cell adhesion molecule. Early Pregnancy. 1996;2:271–275. [PubMed] [Google Scholar]
- Rajagopalan S, Long EO. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J. Exp. Med. 1999;189:1093–1099. doi: 10.1084/jem.189.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Long EO. Activation of Resting NK Cells by the Killer-Cell IG-Like Receptor KIR2DL4. Amer. J. of Repro. Immunol. 2001;45:347–348. doi: 10.4049/jimmunol.167.4.1877. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Bryceson YT, Kuppusamy SP, Geraghty DE, Van Der Meer A, Joosten I, Long EO. Activation of NK cells by an endocytosed receptor for soluble HLA-G. PloS Biol. 2006;4:70–86. doi: 10.1371/journal.pbio.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebmann V, Pfeiffer K, Passler M, Ferrone S, Maier S, Weiss E, Grosse-Wilde H. Detection of soluble HLA-G molecules in plasma and amniotic fluid. Tissue Antigens. 1999;53:14–22. doi: 10.1034/j.1399-0039.1999.530102.x. [DOI] [PubMed] [Google Scholar]
- Rouas-Freiss N, Lemaoult J, Moreau P, Dausset J, Carosella ED. HLA-G in transplantation: a relevant molecule for inhibition of grant rejection. Am. J. Transplant. 2003;3:11–16. doi: 10.1034/j.1600-6143.2003.30103.x. [DOI] [PubMed] [Google Scholar]
- Ryan AF, Grendell RG, Geraghty DE, Golos TG. A soluble isoform of the rhesus monkey nonclassical MHC class I molecule Mamu-AG is expressed in the placenta and the testis. J. Immunol. 2002;169:673–683. doi: 10.4049/jimmunol.169.2.673. [DOI] [PubMed] [Google Scholar]
- Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195:1578–1589. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Giblin PA, Kavathas P. Cell-cell adhesion mediated by CD8 and human histocompatibility leukocyte antigen G, a nonclassical major histocompatibility complex class I molecule on cytotrophoblasts. J. Exp. Med. 1991;174:737–740. doi: 10.1084/jem.174.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni A, Carlino C, Stabile H, Gismondi A. Mechanisms underlying recruitment and accumulation of decidual NK cells in uterus during pregnancy. Am. J. Reprod. Immunol. 2008;59:417–424. doi: 10.1111/j.1600-0897.2008.00598.x. [DOI] [PubMed] [Google Scholar]
- Schmitz JE, Simon MA, Kuroda MJ, Lifton MA, Ollert MW, Vogel CW, Racz P, Tenner-Racz K, Scallon BJ, Dalesandro C, Ghrayeb J, Rieber EP, Sasseville VG, Reimann KA. A nonhuman primate model for selective elimination of CD8+ lymphocytes using a mouse-human chimeric monoclonal antibody. Am. J. Pathol. 1999;154:1923–1932. doi: 10.1016/S0002-9440(10)65450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher G, Keskintepe L, Fisch JD, Acacio BA, Ahlering P, Batzofin J, Ginsburg M. Soluble human leukocyte antigen G expression in phase I culture media at 46 hours after fertilization predicts pregnancy and implantation from day 3 embryo transfer. Fertil. Steril. 2005;83:1410–1413. doi: 10.1016/j.fertnstert.2004.11.061. [DOI] [PubMed] [Google Scholar]
- Shiroishi M, Tsumoto K, Amano K, Shirakihara Y, Colonna M, Braud VM, Allan DS, Makadzange A, Rowland-Jones S, Willcox B, Jones EY, Van Der Merwe PA, Kumagai I, Maenaka K. Human inhibitory receptors Ig-like transcript (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci USA. 2003;100:8856–8861. doi: 10.1073/pnas.1431057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slukvin I, Breburda EE, Golos TG. Dynamic changes in primate endometrial leukocyte populations: differential distribution of macrophages and natural killer cells at the rhesus monkey implantation site and in early pregnancy. Placenta. 2004;25:297–307. doi: 10.1016/j.placenta.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Slukvin II, Watkins DI, Golos TG. Tissue distribution of the mRNA for a rhesus monkey class Ib molecule, Mamu-AG. Tissue Antigens. 1999;53:282–291. doi: 10.1034/j.1399-0039.1999.530309.x. [DOI] [PubMed] [Google Scholar]
- Slukvin II, Watkins DI, Golos TG. Phenotypic and functional characterization of rhesus monkey decidual lymphocytes: rhesus decidual large granular lymphocytes express CD56 and have cytolytic activity. J. Reprod. Immunol. 2001;50:57–79. doi: 10.1016/s0165-0378(00)00090-5. [DOI] [PubMed] [Google Scholar]
- Slukvin II, Lunn DP, Watkins DI, Golos TG. Placental expression of the nonclassical MHC class I molecule Mamu-AG at implantation in the rhesus monkey. Proc. Natl. Acad. Sci. USA. 2000;97:9104–9109. doi: 10.1073/pnas.97.16.9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slukvin II, Boyson JE, Watkins DI, Golos TG. The rhesus monkey analog of HLA-G is expressed primarily in villous syncytiotrophoblasts. Biol. Reprod. 1998;58:728–738. doi: 10.1095/biolreprod58.3.728. [DOI] [PubMed] [Google Scholar]
- Stern PL, Beresford N, Friedman CI, Stevens VC, Risk JM, Johnson PM. Class I-like MHC molecules expressed by baboon placental syncytiotrophoblast. J. Immunol. 1987;138:1088–1091. [PubMed] [Google Scholar]
- Ugurel S, Reinhold U, Tilgen W. HLA-G in melanoma: A new strategy to escape from immunosurveillance? Onkologie. 2002;25:129–134. doi: 10.1159/000055222. [DOI] [PubMed] [Google Scholar]
- Urosevic M, Dummer R. HLA-G in skin cancer: a wolf in sheep’s clothing? Human Immunol. 2003;64:1073–1080. doi: 10.1016/j.humimm.2003.08.351. [DOI] [PubMed] [Google Scholar]
- Watkins DI. The evolution of major histocompatibility class I genes in primates. Crit. Rev. Immunol. 1995;15:1–29. doi: 10.1615/critrevimmunol.v15.i1.10. [DOI] [PubMed] [Google Scholar]
- Wiendl H, Mitsdoerffer M, Hofmeister V, Wischhusen J, Bornemann A, Meyermann R, Weiss EH, Melms A, Weller M. A functional role of HLA-G expression in human gliomas: an alternative strategy of immune escape. J. Immunol. 2002;168:4772–4780. doi: 10.4049/jimmunol.168.9.4772. [DOI] [PubMed] [Google Scholar]
- Wilczynski JR. Immunological analogy between allograft rejection, recurrent abortion and pre-eclampsia – the same basic mechanism? Human Immunol. 2006;67:492–511. doi: 10.1016/j.humimm.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int. 2004;65:1339–1348. doi: 10.1111/j.1523-1755.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- Yie SM, Balakier H, Motamedi G, Librach CL. Secretion of human leukocyte antigen-G by human embryos is associated with a higher in vitro fertilization pregnancy rate. Fertil. Steril. 2005;83:30–36. doi: 10.1016/j.fertnstert.2004.06.059. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K. Review of factors essential for blastocyst implantation for their modulating effects on the maternal immune system. Semin. Cell Dev. Biol. 2008;19:161–169. doi: 10.1016/j.semcdb.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ, Hutchinson JS, Schuler HM. Passive immunization with anti-oestradiol antibodies during the luteal phase of the menstrual cycle potentiates the perimenstrual rise in serum gonadotrophin concentrations and stimulates follicular growth in the cynomolgus monkey (Macaca fascicularis) J. Reprod. Fertil. 1987;80:403–410. doi: 10.1530/jrf.0.0800403. [DOI] [PubMed] [Google Scholar]