Abstract
Objective
Varus-valgus alignment has been linked to subsequent osteoarthritis progression within the mechanically stressed (medial for varus, lateral for valgus) tibiofemoral compartment. Cartilage data from the off-loaded compartment are sparse. We hypothesized: neutral and valgus (vs. varus) knees each have reduced odds of cartilage loss in medial subregions; and neutral and varus (vs. valgus) knees each have reduced odds of lateral subregional loss.
Methods
Participants with knee osteoarthritis underwent knee magnetic resonance imaging at baseline and two years. Mean cartilage thickness was quantified within five tibial and three femoral subregions. We used logistic regression with generalized estimating equations to analyze the relationship between baseline alignment and two-year subregional cartilage loss, adjusting for age, gender, body mass index, and disease severity.
Results
A reduced risk of cartilage loss in medial subregions was associated with neutral (vs. varus) alignment (external tibial, central femoral, external femoral) and valgus (vs. varus) alignment (central tibial, external tibial, central femoral, external femoral). A reduced risk of cartilage loss in lateral subregions, was associated with neutral (vs. valgus) alignment (central tibial, internal tibial, posterior tibial) and varus (vs. valgus) alignment (central tibial, external tibial, posterior tibial, external femoral).
Conclusion
Neutral and valgus alignment were each associated with a reduction in the risk of subsequent cartilage loss in certain medial subregions, and neutral and varus with a reduction in the risk of cartilage loss in certain lateral subregions. These results support load redistribution as an in vivo mechanism of long-term alignment effect on cartilage loss in knee osteoarthritis.
INTRODUCTION
Knee osteoarthritis (OA) is a complex and common condition characterized by joint pain and decreased mobility and function, for which there are few disease-modifying interventions. Advancement of knowledge regarding putative targets of intervention will aid development of novel approaches. Varus-valgus alignment has been associated with subsequent progression of knee OA (1–8) and represents a promising candidate as an intervention target.
Load distribution is not equal between the medial and lateral tibiofemoral compartments (9,10). Schipplein and Andriacchi predicted that 70% of total knee joint load passed through the medial compartment during normal gait in persons with healthy knees; the external adduction moment was the primary factor producing the higher medial joint reaction force (11). In varus-aligned knees, the proportion of load distributed to the medial compartment increases further, and the lateral compartment is relatively off-loaded (12). As valgus alignment increases, load distribution shifts from being greater medially to being more equally distributed and then, in knees with more severe valgus, to being greater in the lateral compartment; the medial compartment in valgus knees is relatively off-loaded (13–15).
However, radiographic assessment is flawed as a means of demonstrating any reduction of risk of progression in an off-loaded tibiofemoral compartment. A lack of lateral progression (i.e., progressive radiographic lateral joint space narrowing) in a varus knee could reflect a reduction in the rate of lateral cartilage loss. Alternatively, the lack of progressive lateral narrowing could be a manifestation of lateral joint space pseudowidening that can accompany the joint space narrowing of medial progression. A knee radiograph cannot distinguish between these two alternatives.
By providing visualization of articular cartilage, magnetic resonance imaging (MRI) is superior to radiography for examining the natural history of an off-loaded compartment. Previous reports from longitudinal studies using MRI show that varus and valgus alignment are each associated with an increased risk of subsequent cartilage loss within the compartment more stressed by the alignment direction, medial for varus knees, lateral for valgus knees (4,7,8). In contrast, longitudinal data concerning cartilage in the off-loaded compartment are sparse. In cross-sectional analyses, greater valgus alignment was associated with greater medial tibial and femoral cartilage volume (4) and lower frequency of medial cartilage defects (16). In a longitudinal report from our study (Mechanical Factors in Arthritis of the Knee), Eckstein et al found that medial:lateral ratios of cartilage loss depended upon alignment (8). This finding raises important questions relevant to development of interventions that seek to improve alignment or the associated distribution of forces, i.e., is non-varus (vs. varus) alignment associated with a reduced risk of cartilage loss in the medial compartment; and is non-valgus (vs. valgus) alignment associated with a reduced risk of lateral cartilage loss?
To better understand the mechanism of action of alignment in knee OA, we evaluated these hypotheses:
knees with neutral alignment and with valgus alignment each have reduced odds of cartilage loss in the medial tibial and femoral articular surface vs. knees with varus alignment (reference group); and
knees with neutral alignment and with varus alignment each have reduced odds of cartilage loss in the lateral tibial and femoral articular surface vs. knees with valgus alignment (reference group).
METHODS
Sample
Study participants are members of a cohort of a natural history study of knee OA, the MAK-2 Study (Mechanical Factors in Arthritis of the Knee-Study 2). MAK-2 participants were recruited from the community using advertising in periodicals targeting older persons, neighborhood organizations, letters to members of the registry of the Buehler Center on Aging, Health, and Society at Northwestern University, and via medical center referrals.
Inclusion criteria were: definite tibiofemoral osteophyte presence [Kellgren/Lawrence (K/L) radiographic grade ≥ 2] in one or both knees; and Likert category of at least “a little difficulty” for two or more items in the WOMAC physical function scale. Exclusion criteria were: corticosteroid injection within the previous three months; history of avascular necrosis, rheumatoid or other inflammatory arthritis, periarticular fracture, Paget’s disease, villonodular synovitis, joint infection, ochronosis, neuropathic arthropathy, acromegaly, hemachromatosis, gout, pseudogout, osteopetrosis, or meniscectomy; or exclusion criteria for MRI such as presence of a pacemaker, artificial heart valve, aneurysm clip or shunt, metallic stent, implanted device (e.g. pain control/nerve stimulator, defibrillator, insulin/drug pump, ear implant), or any metallic fragment in an eye.
Approval was obtained from the Institutional Review Boards of Northwestern University and Evanston Northwestern Healthcare. Written consent was obtained from all participants.
Measurement of Alignment
To assess alignment, a single anteroposterior radiograph of both lower extremities was obtained using a 51 × 14 inch graduated grid cassette to include the full limb of tall participants. By filtering the x-ray beam in a graduated fashion, this cassette accounted for the unique soft tissue characteristics of the hip and ankle. The tibial tubercle, a knee-adjacent site not distorted by OA, was used as a positioning landmark. Participants stood without footwear, with tibial tubercles facing forward. The x-ray beam was centered at the knee at a distance of 2.4 m. A setting of 100 to 300 mA/s and 80–90 kV was used, depending on limb size and tissue characteristics. All radiographs were obtained in the same unit by two trained technicians.
Alignment, i.e., the hip-knee-ankle angle, was measured as the angle formed by the intersection of the line connecting the centers of the femoral head and intercondylar notch with the line connecting the centers of the surface of the ankle talus and tips of the tibial spines. We previously reported excellent reliability for both varus and valgus knees [intraclass correlation coefficients (ICCs) 0.98–0.99] (1). Varus alignment was defined as ≥ 2° varus, valgus alignment was defined as ≥ 2° valgus, and neutral as between 2° varus and 2° valgus.
MRI Acquisition
All participants had MRI of both knees at baseline and two years later using a commercial knee coil and one of two whole-body scanners (1.5T or 3.0T, GE Healthcare, Waukesha, WI); all but 15 participants were scanned at 1.5T. Each participant was scanned and rescanned on the same machine and following the same protocol at the two time points (baseline and two-year follow-up). Quantitative measurements of tibial and femoral cartilage were obtained from double oblique coronal T1-weighted 3D spoiled gradient-echo (SPGR)/fast low angle shot (FLASH) sequences with water excitation. The acquisition parameters at 1.5T/3T were: repetition time (TR) = 17.2/18.5 ms; echo time (TE) = 9.7/5.7 ms; flip angle = 10°/15°; field of view = 16/16; matrix = 512/512; slice thickness - 1.5/1.5mm; and acquisition time = 8.8/9.0 minutes.
Quantification of Subregional Cartilage Thickness Loss on MR Images
Segmentation of the tibial and femoral cartilage involved manual tracing of the total subchondral bone area (tAB, using the standard nomenclature) and the cartilage surface area (AC) of the medial tibia, lateral tibia, central (weight-bearing) medial femoral condyle, and central (weight-bearing) lateral femoral condyle. Based on the boundaries of the cartilage plates, the algorithm described by Wirth et al (18) was applied using custom software (Chondrometrics GmbH, Ainring, Germany) (17,18) to select the region of interest, with demonstrated high reliability (18). Segmentation was performed on paired, i.e., baseline and follow-up, images displayed together, so that the number of slices and peripheral edges that were selected (and that defined the region analyzed) did not differ between the time points. There were ten readers with standardized training and expertise in knee cartilage segmentation; each reader segmented between 22 and 42 knees. Quality control of all segmentations was performed by one expert (F.E.). The readers and the quality control evaluator were blinded to the acquisition order of the paired images and to all other data.
Cartilage thickness (mean, considering denuded areas as “0”) was computed over the entire subchondral bone area and in five subregions (central, internal, external, anterior, posterior) of each (medial and lateral) tibial surface and three subregions (central, internal, external) of each central weightbearing femoral surface (18). The central (elliptical) subregion occupied 20% of the tAB around its center of gravity; as reported by Wirth et al, test-retest precision errors for subregional cartilage thickness measurement were 2.4% (RMS CV%) and 1.6% for the central subregion of the medial and lateral tibial surfaces, respectively (18). Planes running through the center of the tAB at a 45° angle with the plane connecting the center of gravity of the medial and lateral tibial surface, respectively, were used to define anterior, posterior, internal, and external subregions of the medial and lateral tibial surfaces. Precision errors ranged from 1.5% in external medial tibial subregion to 4.7% in the posterior lateral tibial subregion (18). Each of the three subregions of the weight-bearing femoral condyles occupied 33.3% of the tAB. Precision errors were 3.3% and 2.4% in the central medial and lateral femoral subregions, respectively, and ranged from 2.6% in the internal medial femoral subregion to 4.3% in the external lateral femoral subregion (18). For each subregion, cartilage thickness loss was defined as ≥ 5% decrease in cartilage thickness between baseline and two years, a threshold exceeding the precision error for each subregion (18).
Radiographic Acquisition and Reading
All participants underwent bilateral, anteroposterior, weightbearing knee radiographs at baseline in the semi-flexed position with fluoroscopic confirmation of superimposition of the anterior and posterior tibial plateau lines and centering of the tibial spines within the femoral notch (full protocol in 19).
To describe radiographic OA status, the K/L global radiographic score was used (0 = normal; 1 = possible osteophytes; 2 = definite osteophytes without definite joint space narrowing; 3 = definite joint space narrowing, some sclerosis, and possible attrition; and 4 = large osteophytes, marked narrowing, severe sclerosis and definite attrition). Intratester reliability for radiographic grading for the single x-ray reader was high (kappa coefficient 0.86).
Statistical Analysis
All persons had radiographic knee OA (i.e., K/L grade ≥ 2) in one or both knees. Data were summarized descriptively for varus, neutral, and valgus knees using means and standard deviations (SDs) for continuous variables and percentages for dichotomous variables. All analyses were knee-based. We used logistic regression analysis with generalized estimating equations (GEE, to account for the potential correlation between measurements, e.g. of the right and left knees, within a person) to assess the association between baseline knee alignment and baseline-to-two-year subregional cartilage thickness loss. The dependent (outcome) variable for each subregion analysis was an indicator variable, defined as one if cartilage thickness loss was ≥ 5% vs. zero otherwise. Using the logistic models, we first examined the relationships for knees with neutral and valgus alignment vs. varus knees (reference group) and cartilage thickness loss in each of the medial subregions. Next, we examined the relationships for neutral and varus knees vs. valgus knees (reference group) and cartilage thickness loss in each of the lateral subregions. All logistic regression analyses and results were adjusted for age, gender, body mass index (BMI), and K/L grade. Results are reported as adjusted odds ratios (ORs) and associated 95% confidence intervals (CIs). Statistical significance is defined using a two-sided alpha level = 0.05. A significant protective effect for a specific alignment category relative to the reference group was declared if the adjusted OR was less than one and the associated 95% CI included only values less than 1.0. Analyses were done using SAS statistical software version 9.2 (SAS Institute Inc. Cary, NC).
RESULTS
Of the initial 202 participants with knee OA in one or both knees who completed the evaluation at baseline, 20 did not return for the two-year follow-up evaluation due to the following reasons (in equal proportions): deceased, bilateral total knee replacement, moved away, or new MRI contraindication. Among the 302 knees from the remaining 182 participants, 30 knees were excluded due to missing (or of insufficient quality) MRI data at baseline or at two-year follow-up, and 11 knees were excluded for having cartilage thickness of zero in at least one subregion at baseline. The resulting analysis sample was comprised of 261 knees from 159 persons. These participants had a mean age of 66.1 years (± 11.1, SD), mean BMI of 30.1 kg/m2 (± 5.9), and 120 of 159 (75%) were women. Persons without longitudinal data did not differ in mean age (66.6 years ± 11.5, SD) or gender (77% women) but had a higher mean BMI (31.9 kg/m2 ± 6.2, SD). Of the 261 knees in the analysis sample, 99 knees were varus (38%), 81 knees were valgus (31%), and 81 knees were neutral (31%) (Table 1). The distribution of varus ranged from 2° to 19° varus with 27% of varus knees greater than 5° varus. Similarly, for valgus knees, 28% were greater than 5° valgus with a range from 2° to 13° valgus.
Table 1. Characteristics of Knees.
(n = 261 knees from 159 persons)
| Varus knees | Valgus knees | Neutral knees | |
|---|---|---|---|
| Number of knees | 99 | 81 | 81 |
| Alignment (°) (mean ± SD) |
4.6* ± 3.1 | 4.5** ± 2.6 | 0.06*** ± 0.7 |
| K/L grade number of knees (column %), 0 1 2 3 4 |
12 (12.1%) 22 (22.2%) 29 (29.3%) 26 (26.3%) 10 (10.1%) |
10 (12.3%) 9 (11.1%) 35 (43.2%) 20 (24.7%) 7 (8.6%) |
17 (21.0%) 18 (22.2%) 36 (44.4%) 8 (9.9%) 2 (2.5%) |
mean varus alignment in varus knees (i.e., knees ≥ 2° varus)
mean valgus alignment in valgus knees (i.e., knees ≥ 2° valgus)
mean alignment in neutral knees (i.e., between 2° varus and 2° valgus), with positive value here reflecting varus direction
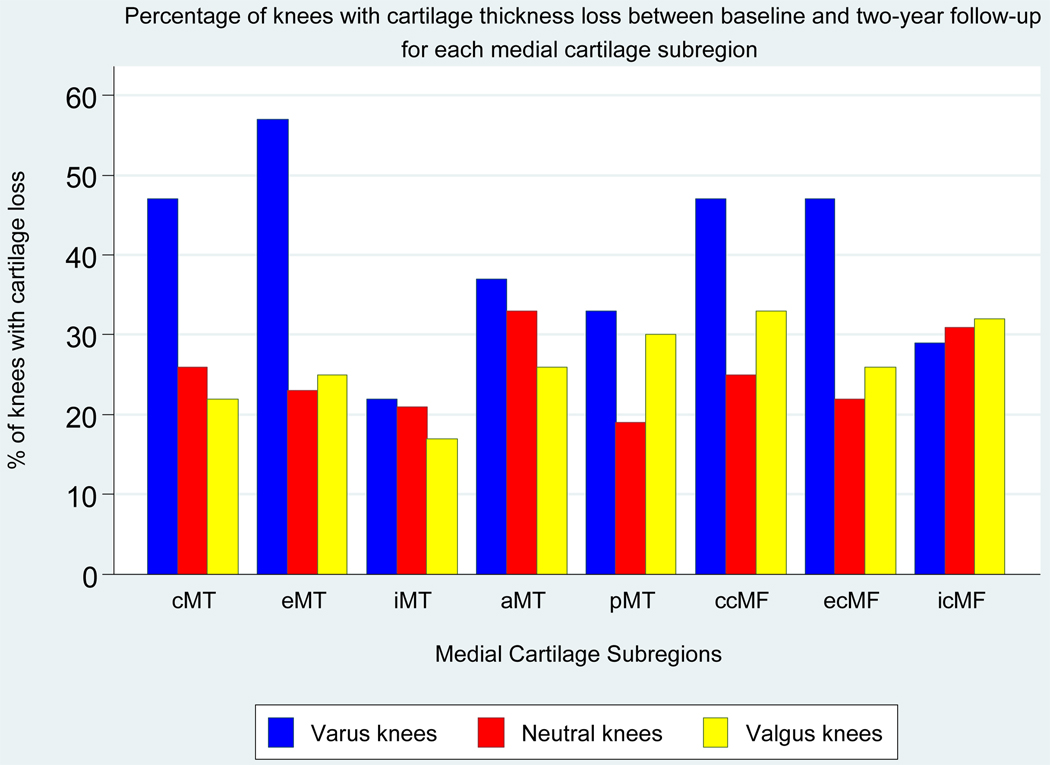
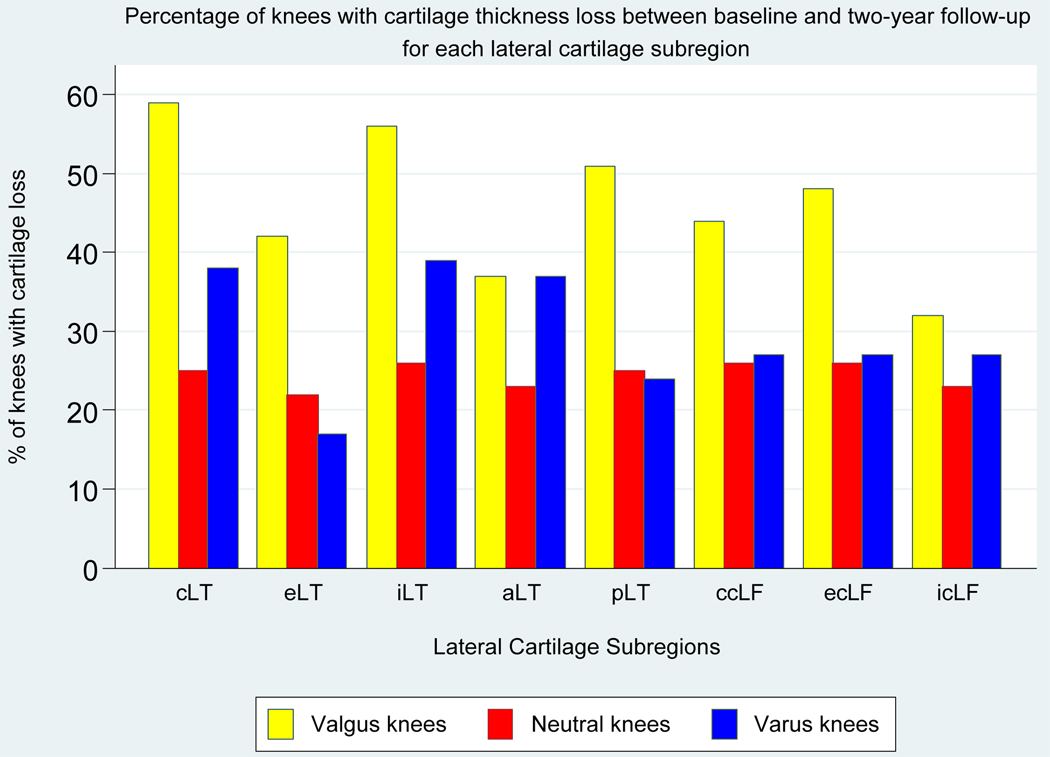
Figure 1 shows the percentage of knees with baseline-to-two-year cartilage thickness loss in each medial subregion within alignment groups; varus knees had the highest percentage of knees with medial cartilage loss. In varus knees, cartilage loss was most frequent in these medial subregions: central tibial (47% of varus knees), external tibial (57%), central weightbearing femoral (47%), and external weightbearing femoral (47%) (Figure 1). The percentage of knees with cartilage loss in each lateral subregion within the alignment groups is shown in Figure 2; valgus knees had the highest percentage of knees with lateral cartilage loss. In valgus knees, cartilage loss was most frequent in these lateral subregions: central tibial (59% of valgus knees), internal tibial (56%), posterior tibial (51%), and external weightbearing femoral (48%) (Figure 2).
Figure 1. Percentage of Knees with Baseline-to-two-year Cartilage Thickness Loss in Each Medial Subregion, Within Alignment Groups.
The figure shows the percentage of knees with cartilage loss in each subregion within each alignment group.
Subregion abbreviations are: cMT, central medial tibia; eMT, external medial tibia; iMT, internal medial tibia; aMT, anterior medial tibia; pMT, posterior medial tibia; ccMF, central weightbearing medial femur; ecMF, external weightbearing medial femur; icMF, internal weightbearing medial femur.
Figure 2. Percentage of Knees with Baseline-to-two-year Cartilage Thickness Loss in Each Lateral Subregion, Within Alignment Groups.
The figure shows the percentage of knees with cartilage loss in each subregion by alignment group.
Subregion abbreviations are: cLT, central lateral tibia; eLT, external lateral tibia; iLT, internal medial tibia; aLT, anterior medial tibia; pLT, posterior medial tibia; ccLF, central weightbearing medial femur; ecLF, external weightbearing medial femur; icLF, internal weightbearing medial femur.
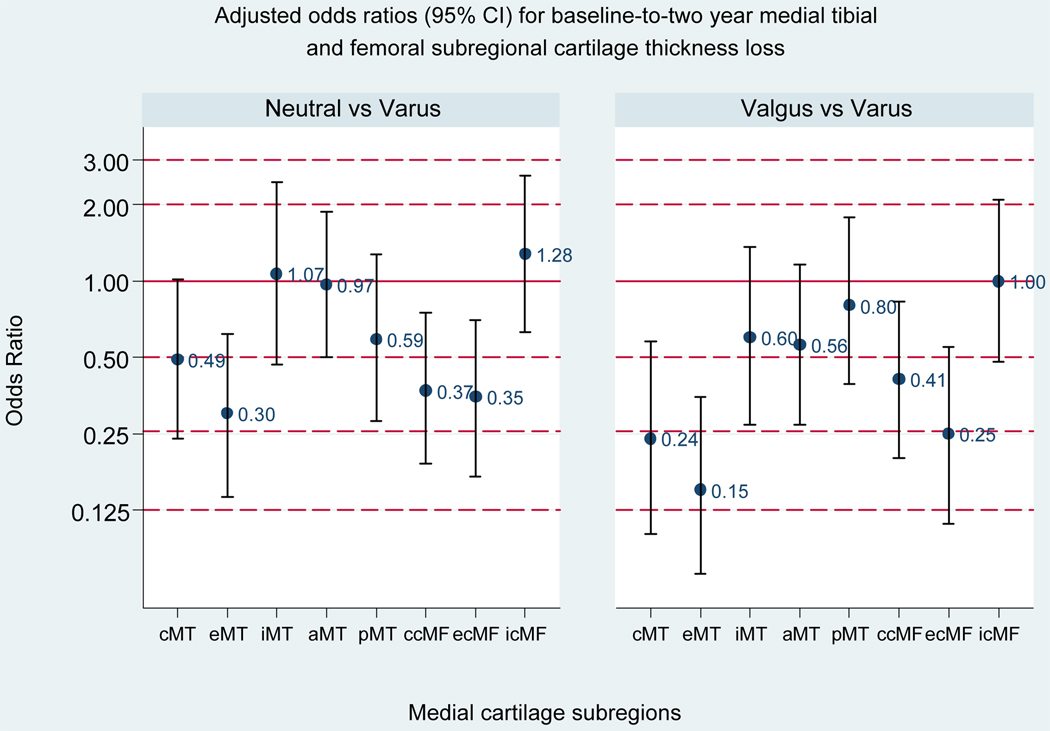
As shown in Figure 3, neutral (vs. varus) alignment was associated with a significant reduction in the risk of cartilage loss in the external medial tibial, central weightbearing medial femoral, and external weightbearing medial femoral subregions, and valgus (vs. varus) alignment with a reduction in the risk of loss in the central medial tibial, external medial tibial, central weightbearing medial femoral, and external weightbearing medial femoral subregions.
Figure 3. Adjusted Odds Ratios for Baseline-to-two-year Medial Tibial and Femoral Subregional Cartilage Thickness Loss (vs. varus, reference group).
Bars represent 95% confidence intervals (CIs); a 95% CI excluding one is significant. For neutral vs. varus, the adjusted ORs (95% CI) are: 0.49 (0.24, 1.01) for cMT; 0.30 (0.14, 0.62) for eMT; 1.07 (0.47, 2.45) for iMT; 0.97 (0.50, 1.88) for aMT; 0.59 (0.28, 1.28) for pMT; 0.37 (0.19, 0.74) for ccMF; 0.35 (0.17, 0.70) for ecMF; 1.28 (0.63, 2.61) for icMF. For valgus vs. varus, the adjusted OR (95% CI) are: 0.24 (0.10, 0.58) for cMT; 0.15 (0.07, 0.35) for eMT; 0.60 (0.27, 1.36) for iMT; 0.56 (0.27, 1.16) for aMT; 0.80 (0.36, 1.78) for pMT; 0.41 (0.20, 0.83) for ccMF; 0.25 (0.11, 0.55) for ecMF; 1.00 (0.48, 2.09) for icMF.
Subregion abbreviations are: cMT, central medial tibia; eMT, external medial tibia; iMT, internal medial tibia; aMT, anterior medial tibia; pMT, posterior medial tibia; ccMF, central weightbearing medial femur; ecMF, external weightbearing medial femur; icMF, internal weightbearing medial femur.
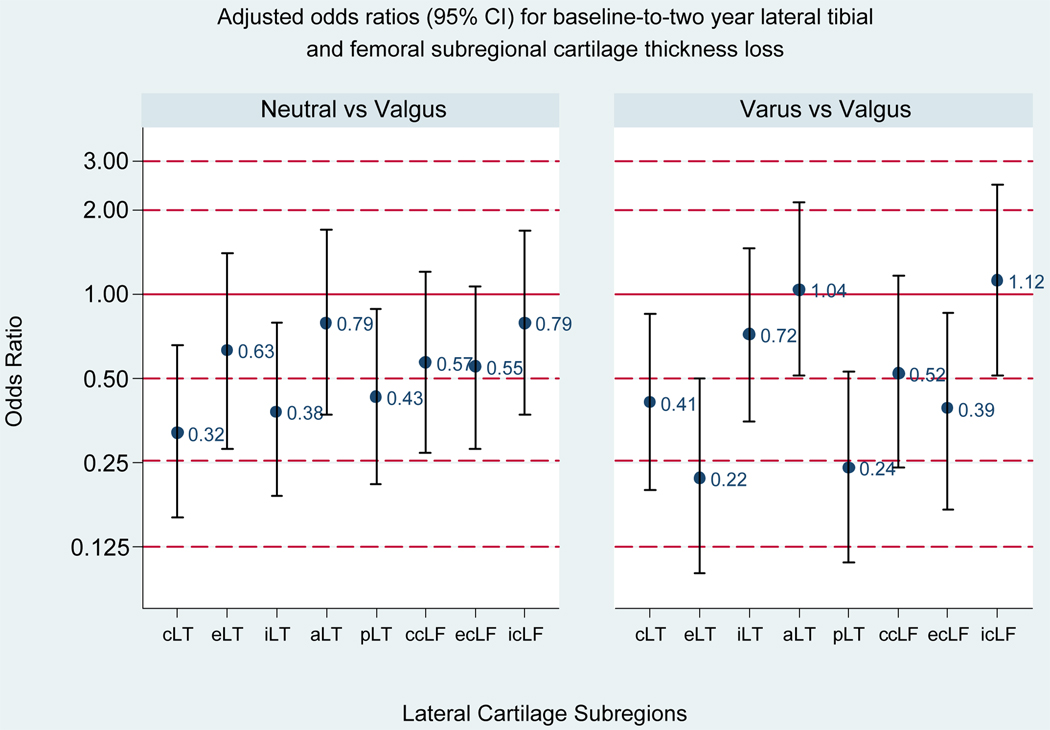
As shown in Figure 4, neutral (vs. valgus) alignment was associated with a significant reduction in the risk of cartilage loss in the central lateral tibial, internal lateral tibial, and posterior lateral tibial subregions, and varus (vs. valgus) alignment with a reduction in the risk of loss in the central lateral tibial, external lateral tibial, posterior lateral tibial, and external weightbearing lateral femoral subregions.
Figure 4. Adjusted Odds Ratios for Baseline-to-two-year Lateral Tibial and Femoral Subregional Cartilage Thickness Loss (vs. valgus, reference group).
Bars represent 95% confidence intervals (CIs); a 95% CI excluding one is significant. For neutral vs. valgus, the adjusted ORs (95% CI) are: 0.32 (0.16, 0.66) for cLT; 0.63 (0.28, 1.40) for eLT; 0.38 (0.19, 0.79) for iLT; 0.79 (0.37, 1.70) for aLT; 0.43 (0.21, 0.89) for pLT; 0.57 (0.27, 1.20) for ccLF; 0.55 (0.28, 1.06) for ecLF; 0.79 (0.37, 1.68) for icLF. For varus vs. valgus, the adjusted OR (95% CI) are: 0.41 (0.20, 0.85) for cLT; 0.22 (0.10, 0.50) for eLT; 0.72 (0.35, 1.46) for iLT; 1.04 (0.51, 2.12) for aLT; 0.24 (0.11, 0.53) for pLT; 0.52 (0.24, 1.16) for ccLF; 0.39 (0.17, 0.86) for ecLF; 1.12 (0.51, 2.47) for icLF.
Subregion abbreviations are: cLT, central lateral tibia; eLT, external lateral tibia; iLT, internal medial tibia; aLT, anterior medial tibia; pLT, posterior medial tibia; ccLF, central weightbearing medial femur; ecLF, external weightbearing medial femur; icLF, internal weightbearing medial femur.
DISCUSSION
In summary, neutral alignment (vs. varus) at baseline was associated with a reduced risk of baseline-to-two-year medial cartilage thickness loss in one tibial and two weightbearing femoral subregions, and valgus alignment (vs. varus) with a reduced risk in these same three subregions and one additional tibial subregion. Neutral alignment (vs. valgus) at baseline was associated with a reduced risk of baseline-to-two-year lateral cartilage thickness loss in three tibial subregions, and varus (vs. valgus) with a reduced risk in three tibial subregions and one femoral subregion.
Previous longitudinal studies of alignment and MRI-based outcomes, including that of Cicuttini et al (4) and our previous report (7) have emphasized investigation of the alignment effect on cartilage loss in the mechanically stressed tibiofemoral compartment. We previously examined the relationship of local factors including alignment on cartilage loss in stressed cartilage regions, in analyses in which alignment was handled as a continuous variable and alternative cartilage loss outcome measures were compared (7). The current manuscript explicitly tests hypotheses to extend this work in three ways: by evaluating categorical alignment groups of knees; by evaluating the relationship between knee group and risk of cartilage loss in the compartment benefiting from the alignment; and by examining outcome within articular surface subregions.
Results from the study by Eckstein et al of rates of change in cartilage parameters within knee alignment groups included the finding of a medial:lateral ratio of femorotibial cartilage loss of 1.4:1.0 in neutral knees, 3.7:1.0 in varus knees, and 1.0:6.0 in valgus knees (8), introducing the hypotheses we evaluate in the current manuscript, whether there is a significant reduction in risk of cartilage loss in the compartment benefitting from the alignment direction, adjusting for potential confounders. We examined persons with knee OA; it would also be interesting to examine these questions in persons without knee OA.
We defined varus and valgus knees as the respective reference group, because they represent potential targets in a disease-modifying clinical trial. The questions posed were designed to inform intervention development and evaluation, e.g. is there evidence that neutral alignment and valgus alignment are each associated with a reduced risk of medial compartment cartilage thickness loss vs. varus alignment, and is there evidence that neutral and varus alignment are each associated with a reduced risk of lateral cartilage thickness loss vs. valgus alignment. Of note, a reduction in risk was detected even with neutral alignment vs. the compartment-stressing alignment in certain subregions. In three subregions, the central medial tibial, the external lateral tibial, and the external weightbearing lateral femoral, alignment in the off-loading direction conferred additional benefit; in these subregions, neutral alignment was not associated with a reduced risk of cartilage loss.
Our results add support to the theory that an important mechanism of action of alignment on the natural course of knee OA relates to its influence on load distribution between the medial and lateral tibiofemoral compartments. Varus-valgus alignment influences force distribution between the tibiofemoral compartments. The significance of this mechanical effect has been demonstrated in previous studies showing the relationship between alignment and subsequent disease progression in the compartment stressed by the alignment (1–5,7,8). The current study provides in vivo longitudinal support of a benefit to the “off-loaded” compartment, and, thereby, the need for development and testing of noninvasive interventions to improve malalignment that is not as yet rigid or tibiofemoral force distribution in rigid malalignment, a field in early stages at present.
This study has limitations. It is likely that the study is underpowered to more definitively examine differences between neutral and valgus alignment in their relationship with medial loss and neutral and varus alignment in their relationship with lateral loss. We used widely applied cut-points to define alignment groups; our study was not powered for exploration of alternative cut-points. The amount of cartilage thickness loss that may be considered a meaningful loss has not been established. We relied upon a threshold of 5% or more because this magnitude exceeds the previously reported precision error of measurement of thickness loss of each subregion (18). Typical of knee OA cohorts in the U.S., the mean BMI of our cohort falls in the obese range. It would be of interest to evaluate whether results are consistent in a sample with healthier BMI. The larger percentage of women is not unexpected; with this gender breakdown, it is not clear if these results can be generalized to men. Some knees without follow-up MRI data came from persons whose BMI was greater than the persons with follow-up data; we do not believe that the difference was in a direction or of a sufficient magnitude to alter our findings. While we relied on the gold standard approach to measure alignment, standing alignment is nevertheless a static measure; a measure of frontal plane alignment during activity may be more strongly related to a lower risk of cartilage loss in the off-loaded compartment.
In conclusion, in persons with knee OA, neutral and valgus alignment (vs. varus) at baseline were each associated with a reduced risk of baseline-to-two-year cartilage thickness loss in medial subregions, and neutral and varus alignment (vs. valgus) were each associated with a reduced risk of cartilage thickness loss in lateral subregions. These results support load redistribution as an in vivo mechanism of long-term alignment effect on cartilage loss in tibiofemoral OA.
Acknowledgments
Support: NIH/NIAMS R01 AR48216, R01 AR48748, P60 AR48098
REFERENCES
- 1.Sharma L, Song J, Felson DT, et al. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188–195. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 2.Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 2002;61:617–622. doi: 10.1136/ard.61.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felson DT, McLaughlin S, Goggins J, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139:330–336. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 4.Cicuttini F, Wluka A, Hankin J, et al. Longitudinal study of the relationship between knee angle and tibiofemoral cartilage volume in subjects with knee osteoarthritis. Rheumatology (Oxford) 2004;43:321–324. doi: 10.1093/rheumatology/keh017. [DOI] [PubMed] [Google Scholar]
- 5.Felson DT, Gale DR, Elon Gale M, Niu J, Hunter DJ, Goggins J, et al. Osteophytes and progression of knee osteoarthritis. Rheumatology (Oxford) 2005;44:100–104. doi: 10.1093/rheumatology/keh411. [DOI] [PubMed] [Google Scholar]
- 6.Brouwer GM, van Tol AW, Bergink AP, et al. Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis Rheum. 2007;56:1204–1211. doi: 10.1002/art.22515. [DOI] [PubMed] [Google Scholar]
- 7.Sharma L, Eckstein F, Song J, et al. The relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58:1716–1726. doi: 10.1002/art.23462. [DOI] [PubMed] [Google Scholar]
- 8.Eckstein F, Wirth W, Hudelmaier M, Stein V, Lengfelder V, Cahue S, Marshall M, Prasad P, Sharma L. Patterns of femorotibial cartilage loss in knees with neutral, varus, and valgus alignment. Arthritis Rheum. 2008;59:1563–1570. doi: 10.1002/art.24208. [DOI] [PubMed] [Google Scholar]
- 9.Andriacchi TP. Dynamics of knee malalignment. Orthop Clin North Am. 1994;25:395–403. [PubMed] [Google Scholar]
- 10.Morrison JB. The mechanics of the knee joint in relation to normal walking. J Biomech. 1970;3:51–61. doi: 10.1016/0021-9290(70)90050-3. [DOI] [PubMed] [Google Scholar]
- 11.Schipplein OD, Andriacchi TP. Interaction between active and passive stabilizers during level walking. J Orthop Res. 1991;9:113–119. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- 12.Hsu RWW, Himeno S, Coventry MB, et al. Normal axial alignment of the lower extremity and load-bearing distribution at the knee. Clin Orthop. 1990;255:215–227. [PubMed] [Google Scholar]
- 13.Bruns J, Volkmer M, Luessenhop S. Pressure distribution at the knee joint. Influence of varus and valgus deviation without and with ligament dissection. Arch Orthop Trauma Surg. 1993;133:12–19. doi: 10.1007/BF00440588. [DOI] [PubMed] [Google Scholar]
- 14.Johnson F, Leitl S, Waugh W. The distribution of load across the knee. A comparison of static and dynamic measurements. J Bone Joint Surg. 1980;62-B:346–349. doi: 10.1302/0301-620X.62B3.7410467. [DOI] [PubMed] [Google Scholar]
- 15.Harrington IJ. Static and dynamic loading patterns in knee joints with deformities. J Bone Joint Surg. 1983;65-A:247–259. doi: 10.2106/00004623-198365020-00016. [DOI] [PubMed] [Google Scholar]
- 16.Janakiramanan N, Teichtahl A, Wluka A, Ding C, Jones G, Davis S, et al. Static knee alignment is associated with the risk of unicompartmental knee cartilage defects. J Orthop Res. 2008;26:225–230. doi: 10.1002/jor.20465. [DOI] [PubMed] [Google Scholar]
- 17.Eckstein F, Charles HC, Buck RJ, et al. Accuracy and precision of quantitative assessment of cartilage morphology by magnetic resonance imaging at 3.0T. Arthritis Rheum. 2005;52(10):3132–3136. doi: 10.1002/art.21348. [DOI] [PubMed] [Google Scholar]
- 18.Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging. 2008;27(6):737–744. doi: 10.1109/TMI.2007.907323. [DOI] [PubMed] [Google Scholar]
- 19.Buckland-Wright C. Protocols for precise radio-anatomical positioning of the tibiofemoral and patellofemoral compartments of the knee. Osteoarthritis Cartilage. 1995;3 Suppl A:71–80. [PubMed] [Google Scholar]