Abstract
γδ T cells differ from αβ T cells in the antigens they recognize and their functions in immunity. While most αβ T cell receptors (TCR) recognize peptides presented by MHC class I or II, human γδ T cells expressing Vγ2Vδ2 TCRs recognize nonpeptide prenyl pyrophosphates. To define the molecular basis for this recognition, the effect of mutations in the TCR complementarity-determining regions (CDR) was assessed. Mutations in all CDR loops altered recognition and cover a large footprint. Unlike murine γδ TCR recognition of the MHC class Ib T22 protein, there was no CDR3δ motif required for recognition because only 1 residue is required. Instead, the length and sequence of CDR3γ was key. Although a potential prenyl pyrophosphate-binding site was defined by Lys109 in Jγ1.2 and Arg51 in CDR2δ, the area outlined by critical mutations is much larger. These results show that prenyl pyrophosphate recognition is primarily by germline-encoded regions of the γδ TCR, allowing a high proportion of Vγ2Vδ2 TCRs to respond. This underscores its parallels to innate immune receptors. Our results also provide strong evidence for the existence of an antigen-presenting molecule for prenyl pyrophosphates.
This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
Keywords: gamma-delta T cells, T cell antigen receptors, prenyl pyrophosphates, complementarity-determining region, isoprenoid metabolism
Introduction
T cells can be divided into two distinct subset, γδ and αβ, based on their expression of rearranging adaptive TCRs. γδ T cells, mucosal-associated invariant αβ T cells (1), and invariant natural killer αβ T cells (iNKTs) (2, 3) are innate lymphocytes that bridge innate and adaptive immunity through their recognition of unconventional ligands. The major subset of human γδ T cells express Vγ2Vδ2 TCR (also termed Vγ9Vδ2 or TRGV9TRDV2). Although a minor subset at birth (4), Vγ2Vδ2 T cells expand between the ages of 1–10 years old (5). This expansion is not due to genetic causes because identical twins can differ in their proportions of Vγ2Vδ2 T cells. Instead, a broad array of bacterial and protozoal infections expand Vγ2Vδ2 T cells to large numbers (>50% of blood T cells in some cases) (reviewed in Ref. 6).
The broad reactivity of Vγ2Vδ2 T cells to microbes and some tumors was explained by the finding that nonpeptide prenyl pyrophosphates, such as isopentenyl pyrophosphate (IPP) 3, act as antigens (Ags) for Vγ2Vδ2 T cells (7). Prenyl pyrophosphates are essential metabolites required by all organisms. The recognition of microbes by Vγ2Vδ2 T cells is due to the preferential recognition of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP) over IPP (HMBPP is 30,000-fold more active than IPP on a molar basis) (8, 9). HMBPP is a metabolite in the 2-C-methyl-d-erythritol-4 phosphate pathway for isoprenoid biosynthesis. This pathway is used by most Eubacteria (including all mycobacteria and Gram-negative rods) and Apicomplexan protozoa (the causative agents of malaria, toxoplasmosis, babesiosis, and cryptosporidiosis). Other stimulating compounds, such as bisphosphonates (10, 11) and alkylamines (12), act by increasing cellular IPP levels by blocking farnesyl pyrophosphate (FPP) synthase in the mevalonate pathway (13, 14).
Vγ2Vδ2 T cells function in both microbial and tumor immunity. The early expansion of Vγ2Vδ2 T cells to prenyl pyrophosphates results in conversion to memory cells (C. Jin and C. T. Morita, unpublished observations and 15) capable of mounting adaptive responses to Mycobacterium bovis bacillus Calmette-Guérin (BCG) (16) and other infections. As memory cells, Vγ2Vδ2 T cells can mount rapid responses to primary microbial infections with organisms using the 2-C-methyl-d-erythritol-4-phosphate pathway (9). Activation of Vγ2Vδ2 T cells leads them to release Th1 cytokines, including IFN-γ, TNF-α, and GM-CSF but not IL-2 (17, 18), and to secrete chemokines such as MIP-1α (CCL3), MIP-1β (CCL4), lymphotactin (XCL1), and RANTES (CCL5) (19, 20). Vγ2Vδ2 T cells are potent killer cells and can lyse infected cells via perforin and/or Fas-Fas ligand pathways (21) and kill released bacteria and protozoa through production of granulysin (21, 22) and the cathelicidin, LL-37 (23). Activated Vγ2Vδ2 T cells also kill tumor cells from a variety of tissue origins through both TCR- and natural killer receptor-mediated recognition (reviewed in Ref. 6). Treatment with bisphosphonates and IL-2 activates Vγ2Vδ2 T cells leading to stable disease or partial remissions in some patients with B cell malignancies (24) or with metastatic prostate cancer (25). There are presently several ongoing clinical trials examining immunotherapy with Vγ2Vδ2 T cells for a variety of cancers.
Recognition of prenyl pyrophosphates is mediated by the Vγ2Vδ2 TCR because transfection of the Vγ2Vδ2 TCR to β− Jurkat cells confers reactivity to prenyl pyrophosphates and to Daudi and RPMI-8226 tumor cell lines (26). We and others proposed that reactivity to the negatively charged prenyl pyrophosphates was dependent on positively charged residues in a potential prenyl pyrophosphate-binding site located in Vγ2Vδ2 TCR (27, 28). Consistent with this hypothesis, reactivity was dependent on N-encoded amino acid residues in the junctional segment of the Vγ2 chain (29) as well as lysine residues in the Jγ1.2 segment (also termed JγP), an arginine residue in CDR2δ, and an aliphatic amino acid residue in CDR3δ (30, 31).
Despite identification of a potential prenyl pyrophosphate-binding site, there was no evidence for prenyl pyrophosphate binding by the Vγ2Vδ2 TCR (28) or direct functional recognition (32). Instead, recognition of prenyl pyrophosphates requires cell-cell contact but neither Ag internalization nor processing (32, 33). This is similar to allergic CD4 and CD8 αβ T cell recognition of small nonpeptide drugs presented by MHC class I or class II molecules (34). In contrast to drug recognition, prenyl pyrophosphate Ag recognition is independent of classical MHC class I, MHC class II, β2M, MICA/MICB, and CD1 molecules (32). Moreover, photoaffinity analogs of prenyl pyrophosphates could form covalent bonds to a putative presenting molecule distinct from known Ag-presenting molecules (35) and tetramers of macaque Vγ2Vδ2 TCR could bind to the surface of human but not mouse cells only in the presence of HMBPP (36).
The available studies suggest, but do not prove, that a presenting molecule exists for prenyl pyrophosphates. To help clarify this issue, we have studied the effect of alanine mutations in residues in CDR1, CDR2, and CDR3 on prenyl pyrophosphate recognition and analyzed CDR3 sequences of reactive Vγ2Vδ2 T cell clones. We find that residues in all CDRs of the Vγ2Vδ2 TCR can affect recognition but no CDR3δ motif is required for recognition beyond the requirement for an aliphatic residue at position 97. Instead, recognition is critically dependent on the Vγ2Jγ1.2 chain which is either invariant or has limited diversity in sequence and length. Given the large footprint of the required residues, which surpasses the predicted prenyl pyrophosphate-binding site, it is likely that a presenting molecule is required for prenyl pyrophosphate Ags.
Material and Methods
Cell lines
The mutant Jurkat cell line, J.RT3-T3.5, was obtained from the American Type Culture Collection (ATCC, Rockville, MD) and is a TCR β-negative variant of Jurkat that lacks TCR cell surface expression (37). J.RT3-T3.5 and J.RT3-T3.5 transfectants were grown in RPMI 1640 supplemented with 10% fetal calf serum, 10 mM HEPES, 10 µg/ml gentamycin, 5×10−5 M β-ME and L-glutamine (Invitrogen, Carlsbad, CA). RPMI 8226 and Raji cells were obtained from ATCC and maintained in the medium described above. The Va2 human fibroblast cell line was obtained from Dr. Charles Stiles (Dana-Farber Cancer Institute) and maintained in DMEM with the additives listed above. Hygromycin B and G418 were obtained from Invitrogen.
mAbs and Ags
mAbs reactive with the human Vγ2Vδ2 TCR used in this paper were as follows: control mAb (P3), anti-pan TCRγδ (anti-TCRδ1), anti-Vγ2 (TiγA, 7A5, 360, and 4D7), and anti-Vδ2 (BB3, 4G6, 389, G1, and TiVδ2) and. The specificity of these mAbs was previously reviewed (38). Prenyl pyrophosphates (IPP, dimethylallyl pyrophosphate, and FPP) and alkylamines were purchased from Sigma-Aldrich (St. Louis, MO). Phosphoantigens, including mono-methyl-, mono-ethyl-, phenethyl-, and isoamyl-pyrophosphate and the nucleotide-conjugated compound, ethyl-dUTP, were synthesized as described (7, 38). Bromohydrin pyrophosphate (BrHPP), risedronate, and alendronate were provided by Eric Oldfield (University of Illinois, Urbana-Champagne). HMBPP was synthesized as previously described (39). KM20 and KM22 are extracts of E.coli strain lytB mutants that produce high levels of HMBPP (9, 40).
cDNA cloning and mutagenesis of human Vγ2Vδ2 TCR
RNA was isolated from the human T cell clone, DG.SF13 (Micro RNA isolation kit; Stratagene, La Jolla, CA) followed by cDNA synthesis using SuperScript II reverse transcriptase and random hexamers (SuperScript first-strand synthesis system for RT-PCR; Life Technologies, Gaithersburg, MD). PCR was done with Platinum Taq High Fidelity DNA polymerase (Life Technologies). PCR primers used to derive full-length Vγ2Cγ chain were as described previously (21). For the Vδ2Cδ chain the following primers were used to introduce an XhoI restriction site into the 5' region and a BamHI site into the 3' region of the Vδ2Cδ chain for cloning: 5'-gggctcgagCAGGCAGAAGGTGGTTGAGAG-3'; Vδ2 5' untranslated region; and 5'-gggggatccGGAGTGTAGCTTCCTCAT-3'; Vδ2 3' untranslated region. The Vγ2Jγ1.2Cγ1 PCR product was cloned into the pREP7 vector (Invitrogen) using the KpnI-XhoI sites. The Vδ2Cδ PCR product was cloned into the pREP9 vector (Invitrogen) using the XhoI-BamHI sites. For mutagenesis, TCR-γ and TCR-δ chain cDNAs mutated by a single amino acid residue were generated using QuikChange™ Site-Directed Mutagenesis Kit (Stratagene). To confirm the mutations, all Vγ2Cγ and Vδ2Cδ mutants were fully sequenced. Sequencing was done using an automated sequencer using the pREP forward and reverse primers along with the following reverse primers: Cγ 3'UT, 5'-ATGGCCTCCTTGTGCCACCG-3'; Cγ internal, 5'-TGTGTCGTTAGTCTTCATGG-3'; Cδ 3'UT, 5'-GGAGTGTAGCTTCCTCATGC-3'; and Cδ internal, 5'-GACAATAGCAGGATCAAACT-3'.
Derivation of Vγ2Vδ2 TCR transfectants
Human Vγ2Vδ2 TCR transfectants were derived by electroporation of the Jurkat mutant, J.RT3-T3.5, with unmutated or mutated DG.SF13 TCR-γ and -δ chain cDNAs, as described previously (41). Briefly, J.RT3-T3.5 cells were grown at low density (1–2 × 105 cells/ml) prior to use, centrifuged while in log phase growth, and resuspended at 3.33×107/ml in PBS containing 10 mM HEPES. A total of 0.3 ml resuspended cells was aliquoted into each electroporation cuvette. 20 µg of each plasmid (pREP7-TCR-γ and pREP9-TCR-δ) was then added to the cells followed by incubation at room temperature for 10 min. The cells were electroporated (960 µF, 250 V) using a Gene Pulser (Bio-Rad Laboratories, Inc., Burlingame, CA) and incubated at room temperature for an additional 10 min. The electroporated cells from each cuvette were washed twice in PBS, resuspended into 30 ml of complete media, plated in three 96-well round bottom plates, and cultured at 37°C. After 48 h, hygromycin B was added to 0.5 mg/ml. Transfectants surviving hygromycin B selection were then cultured in media containing both G418 (1 mg/ml) and hygromycin B (0.5 mg/ml). After 2–3 wk of culture, transfectants were screened for IL-2 release in response to anti-TCRδ1 stimulation. Transfectants specifically responsive to anti-TCR stimulation were expanded at low density and tested for their response to nonpeptide Ags. Multiple transfectants for each mutant that specifically released IL-2 to anti-TCR stimulation were derived from separate transfections and tested to confirm each result (Supplemental Figs. 1, 2). Because defects in Jurkat TCR signaling caused some TCR-expressing transfectants (despite high levels of TCR expression) to either not release IL-2 or to constitutively produce IL-2, only those mutants responding to anti-TCRδ1 stimulation were analyzed.
IL-2 release and assay
Stimulation of TCR transfectants for IL-2 release was performed as described (38, 42). Briefly, 1×105 transfectants were cultured with the indicated half-log dilution of the anti-TCRδ1 mAb, stimulatory compounds, or tumor cells in the presence of 1×105 glutaraldehyde-fixed Va-2 cells (except for tumor cells) and 10 ng/ml PMA. After 24 h, supernatants were harvested and frozen at −20°C. For IL-2 assays, the supernatants were thawed and used at a 1/8 dilution to stimulate the proliferation of the IL-2-dependent cell line, HT-2. The cultures were pulsed with 1 µCi of 3H–thymidine (2 Ci/mmol) at 18 h and harvested 6 h later.
Flow cytometric analysis
TCR transfectants were analyzed by one- or two-color immunofluorescence after staining with the appropriate mAb as described (38). Cells were incubated with mouse mAbs on ice for 30 min, washed, and stained with PE-conjugated F(ab')2 goat anti-mouse Ig antisera (Biosource, Camarillo, CA) for an additional 30 min on ice. After washing, the cells were resuspended in FACS buffer containing propidium iodide and analyzed by flow cytometry. Flow cytometry was performed with a FACScan flow cytometer (BD Biosciences, Palo Alto, CA) and the data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Modeling the human Vγ2Vδ2 TCR
The DG.SF13 TCR is expressed by a synovial Vγ2Vδ2 T cell clone isolated from a patient with rheumatoid arthritis (43). This receptor was used for our previous transfection and mutagenesis experiments on the Vγ2Vδ2 TCR (26, 29), and its sequence has been published (26). The sequence of the DG.SF13 TCR varies from the G115 TCR that was crystallized (28) as it has slightly different CDR3 regions. For comparison with other studies, numbering of amino acid residues is the same as used for G115 TCR. Vγ2Vδ2 T cell clone sequences listed in Supplemental Table I are from Refs. (18, 27–29, 31, 38, 44–46). All figures were made with PyMOL X11 Hybrid (DeLano Scientific LLC, South San Francisco, CA). Electrostatic surface potential was calculated with the APBS PyMOL plugin (47). In silico mutations were made in PyMOL using the Mutagenesis Wizard. The contact residues for other TCRs for MHC/CD1-peptide/lipid complexes were assessed for unconventional TCRs (48, 49), for conventional αβ TCRs specific for MHC class I\Ib complexes (50–65), and for conventional αβ TCRs specific for MHC class II complexes (66–71). The TCR structures shown are taken from the TCR-ligand complex. The TCRs are identically scaled using a Pymol script kindly provided by Dr. DeLano but the diagonal orientations does not attempt to match the docking angles on their MHC/CD1 ligands. Contact residues were colored as follows; those in CDR1 α/δ are blue, CDR2 α/δ are magenta, CDR3 α/δ are yellow, CDR1 β/γ are red, CDR2 β/γ are orange, CDR3 β/γ are green, and HV4 β/γ are pink. Note that alternative terminology for human γ chain exists: Vγ2 is also termed Vγ9 (TRGV9); Jγ1.1 is also termed JγP1 (TRGJP1), Jγ1.2 is also termed JγP (TRGJP), Jγ1.3 is also termed Jγ1 (TRGJ1), Jγ2.1 is also termed JγP2 (TRGJP2), and Jγ2.3 is also termed Jγ2 (TRGJ2) (72). For discussions of murine γδ TCR, we use the nomenclature of Heilig and Tonegawa (73).
Results
Effects of CDR mutations in the Vγ2 and Vδ2 chains on the reactivity of anti-TCR Abs
Although mutations of CDR loops are less likely to affect the overall folding of the Vγ2Vδ2 TCR, we confirmed this by examining the reactivity of a panel of mAbs specific for the Vγ2 and Vδ2 regions to mutated Vγ2Vδ2 TCRs. None of the CDR mutations affected reactivity of the anti-TCRδ1 mAb directed to the Cδ region (Table I). Similarly, none of the mutation in CDR1γ affected reactivity by 4 anti-Vγ2 mAbs. Mutations in Arg59γ (for 4D7) and Lys60γ (for 4D7 and 7A5) of the CDR2γ did abolish reactivity for 2 of the mAbs while maintaining reactivity to the 2 others (Table I). None of the CDR1δ or CDR2δ mutations affected reactivity of 5 mAbs specific for the Vδ2 region (Table I). We were unable to express the Lys53Ala mutation in the Vδ2 region despite several attempts suggesting that this mutation does alter TCR folding or assembly. Thus, based on their continued reactivity with mAbs specific for the Vγ2 and Vδ2 regions, our CDR mutations generally did not result in major disruption in the conformation of the Vγ2Vδ2 TCR.
Table I.
Summary of Vγ2Vδ2 TCR mutations
| TCR region | Mutation | Anti-Vγ and anti-Vδ mAb staining | |||||
|---|---|---|---|---|---|---|---|
| Nucleic acid | Amino acid | ||||||
| Vγ2 chain | TiγA | 7A5 | 360 | 4D7 | |||
| CDR1γ | ACA → GCA | T29A | + | + | + | + | |
| ATT → GAG | I30E | + | + | + | + | ||
| ACA → GCA | T33A | + | + | + | + | ||
| TCT → GCC | S34A | + | + | + | + | ||
| CDR2γ | TAT → GCC | Y54A | + | + | + | + | |
| ACT → GCA | T57A | + | + | + | + | ||
| AGA → GCA | R59A | + | + | + | − | ||
| AAG → GCG | K60A | + | − | + | − | ||
| CDR3γ | AAA → GCA | K108A | + | + | + | + | |
| Vδ2 chain | BB3 | 4G6 | 389 | G1 | TiVδ2 | ||
| CDR1δ | GAA → GCA | E28A | + | ± | + | + | ± |
| ATC → GAG | I30E | NDa | ND | ND | ND | ND | |
| AAC → GCC | N32A | + | + | + | + | + | |
| CDR2δ | CGA → GCA | R51A | + | + | + | + | + |
| GAA → GCA | E52A | + | + | + | + | + | |
Not determined.
Essential residues in the CDR1γ and CDR2γ of the Vγ2 chain
Because the CDR mutations did not cause major disruptions in Vγ2Vδ2 TCR folding, we determined the effects of these CDR mutations on prenyl pyrophosphate Ag recognition. Four residues in CDR1γ and four residues in CDR2γ were mutated from polar hydrophilic amino acids to the non-polar, hydrophobic amino acid, alanine, except for Ile30Glu where the hydrophobic isoleucine residue was mutated to the negatively charged, hydrophilic amino acid, aspartic acid (Table I). For these studies, mutants and wild type transfectants were stimulated with HMBPP or with an anti-Cδ mAb to serve as a positive control in the presence of Va2 presenting cells. Multiple transfectants derived from separate transfections were tested for each mutant to confirm each result.
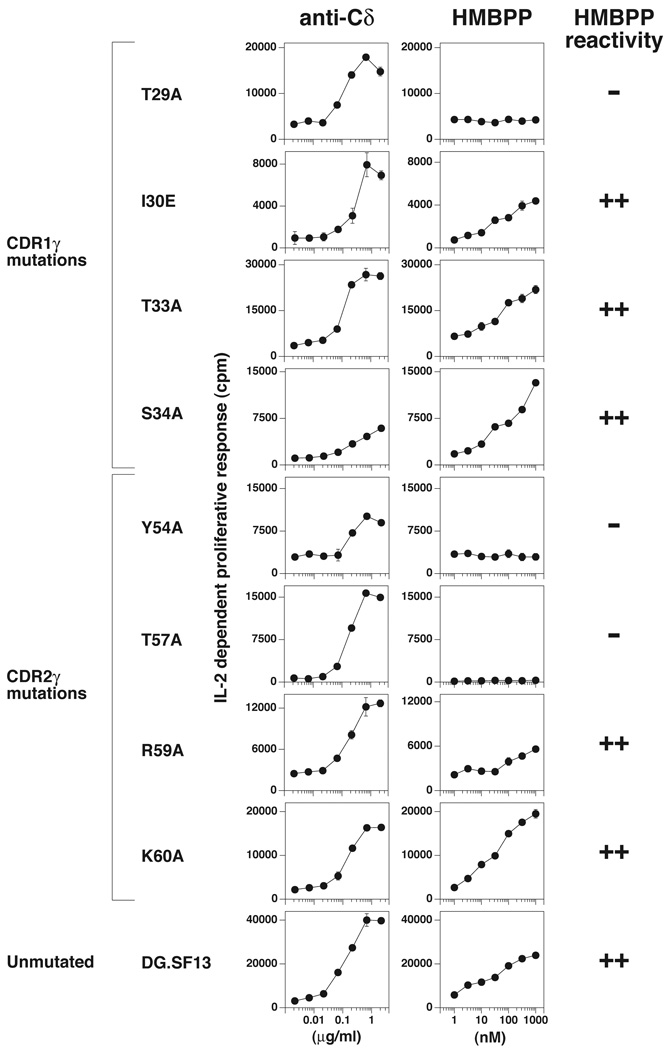
As expected, the native DG.SF13 Vγ2Vδ2 TCR maintained its responsiveness to HMBPP upon transfection into β− Jurkat cells. For CDR1γ mutations, mutation of the exposed residue, Thr29, to alanine abolished recognition of HMBPP (Fig. 1). In contrast, mutation of three other residues (Ile30Glu, Thr33Ala, and Ser34Ala) that are in areas not as readily accessible for binding did not alter recognition. Binding of Vγ2-specific mAbs was unaffected by these mutations (Table I). These results were verified by additional independently derived transfectants for each mutation (Supplemental Fig. 1). Additional residues (Ile28Val, Ala32Glu, and Val35Phe) that differ between human and rhesus monkey did not affect recognition because a chimeric TCR of rhesus monkey Vγ2 chain paired with the human Vδ2 chain still recognized HMBPP presented by human APC (38). The alanine to glutamic acid change at CDR1γ residue 32 is the only nonconservative change among the three residues.
FIGURE 1. Recognition of HMBPP by Vγ2Vδ2 TCR transfectants expressing mutant Vγ2 chains.
J.RT3-T3.5 β− Jurkat cells were transfected with unmutated or mutated DG.SF13 TCR-γ cDNAs together with the unmutated DG.SF13 TCR-δ chain cDNA. After drug selection, anti-Cδ responsive transfectants were identified and stimulated with HMBPP in the presence of Va2 APCs and 2.5 ng/ml PMA. The anti-Cδ mAb (anti-TCRδ1) and HMBPP were added to the cultures stating at 2.15 µg/ml and 1000 nM, respectively, and serially diluted by half-log intervals. After 24 h, the culture supernatants were harvested and assayed for IL-2 activity using the IL-2-dependent cell line, HT2. Results from one transfectant are shown for each mutation and are representative of the results obtained with two to four other independently derived transfectants (Supplemental Fig. 1). Values shown are mean ± SEM of duplicate or triplicate samples. HMBPP reactivity was considered (++) if the maximum HMBPP response was >40% of the control anti-Cδ response, (+) if between 20–40% of the control response, and (−) if <10% of the control response.
The CDR2γ residues that were mutated include two (Tyr54 and Thr57) on the solvent-exposed portion of the CDR2γ loop near the CDR1γ loop. Mutation of either of these two residues to alanine (Tyr54Ala and Thr57Ala) abolished recognition of HMBPP (Fig. 1). In contrast, mutation of two basic residues (Arg59 and Lys60) did not. These residues are located at the end of the binding groove and form a positively charged region that could potentially bind to the negatively charged pyrophosphate moiety of prenyl pyrophosphates. Because mutation of these residues to the neutral amino acid, alanine, did not affect HMBPP recognition, we find no evidence for involvement by this region in Ag recognition (Fig. 1). However, these mutations did alter mAb binding with both Arg59Ala and Lys60Ala mutations abolishing 4D7 mAb binding and with Lys60Ala also abolishing 7A5 mAb binding. Binding by the TiγA and 360 mAbs was unaffected (Table I). Differences between human and rhesus monkey at residues Ser53Phe and Arg59Lys are conservative changes and did not affect recognition. The serine to phenylalanine mutation is located in a recessed area of the CDR2γ loop.
Thus, all of the mutations in CDR1γ and CDR2γ that alter HMBPP recognition are located in highly accessible, solvent exposed areas of the CDR loops of the Vγ2Vδ2 TCR (28). In contrast, mutations that did not affect HMBPP recognition were located in recessed, less solvent exposed areas. Importantly, all three mutations that affected HMBPP response were distant (16–23 Å) from the proposed prenyl pyrophosphate-binding area that is composed mainly of residues from CDR3γ and CDR2δ.
Essential residues in the CDR1δ and CDR2δ region of the Vδ2 chain
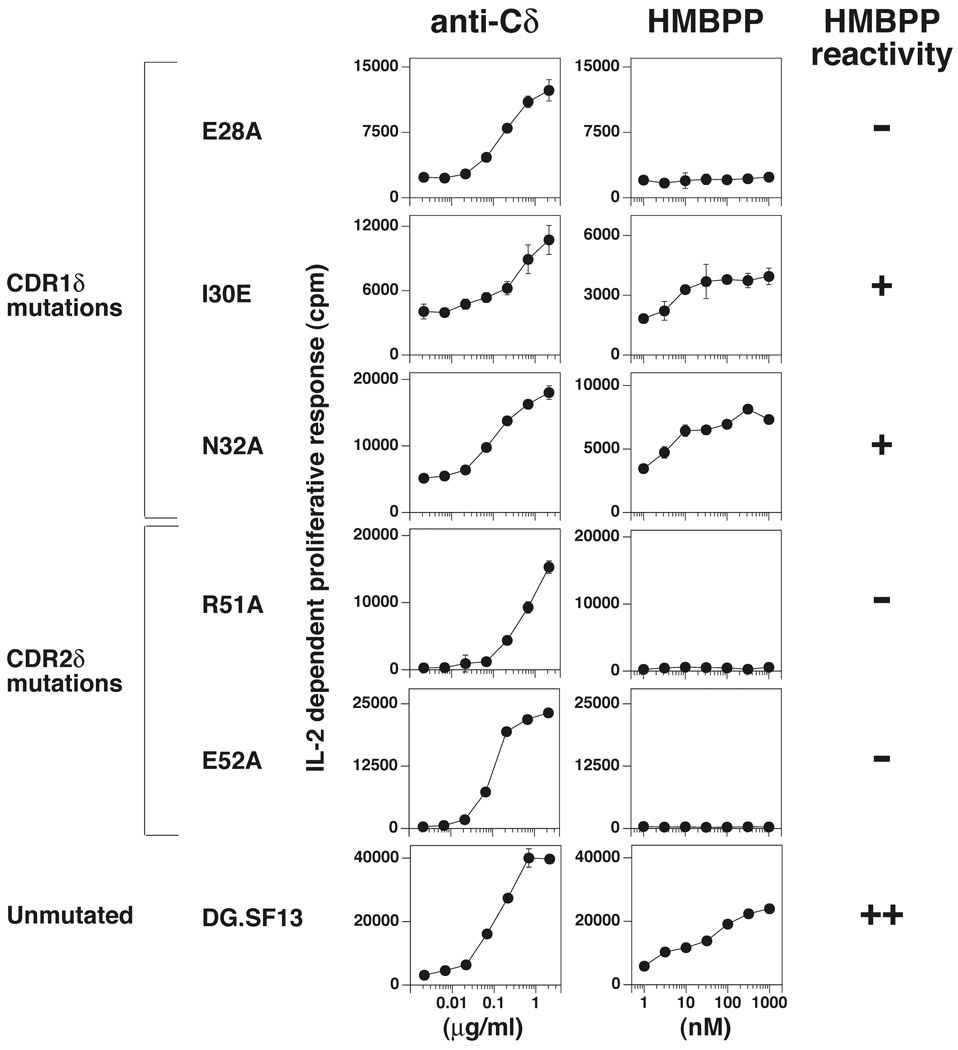
To investigate the role of Vδ chain residues in non-peptide Ag recognition, three residues in CDR1δ and two residues in CDR2δ were selected for mutation. One of the three mutations in CDR1δ affected HMBPP recognition. The residue mutated, Glu28, is highly accessible but spaced 19–22 Å away from the proposed prenyl pyrophosphate-binding area. This mutation was non-conservative with an alanine being substituted for a negatively charged glutamic acid residue (Fig. 2). The other mutations, Ile30Glu and Asn32Ala, did not affect recognition (Fig. 2).
FIGURE 2. Recognition of HMBPP by Vγ2Vδ2 TCR transfectants expressing mutant Vδ2 chains.
J.RT3-T3.5 β− Jurkat cells were transfected with unmutated or mutated DG.SF13 TCR-δ cDNAs together with the unmutated DG.SF13 TCR-γ chain cDNA. Culture conditions and the IL-2 assay were as in Fig. 1. Results from one transfectant are shown for each mutation and are representative of the results obtained with one to four other independently derived transfectants (Supplemental Fig. 2).
Two mutations in the CDR2δ, Arg51Ala and Glu52Ala, both affected recognition of HMBPP. The Arg51 and Glu52 residues are located within the proposed prenyl pyrophosphate-binding site (within 4–10 Å of Lys109 for Arg51 and 10–13 Å for Glu52). The loss of reactivity by mutating Arg51 confirms an earlier study where mutation of Arg51 to either alanine or glutamic acid abolished recognition (30). Changing one residue in CDR2δ outside of this area, Asp54, to glycine did not affect recognition because glycine is used at this position in the rhesus monkey Vδ2 chain and a chimeric receptor of rhesus monkey Vδ2 paired with human Vγ2 still recognized prenyl pyrophosphates (38). Note that this residue is located 11–15 Å away from the proposed binding site. These findings demonstrate that part but not all of CDR2δ contributes to prenyl pyrophosphate recognition.
Abrogation of Ag and tumor recognition by mutation of K108 in CDR3γ
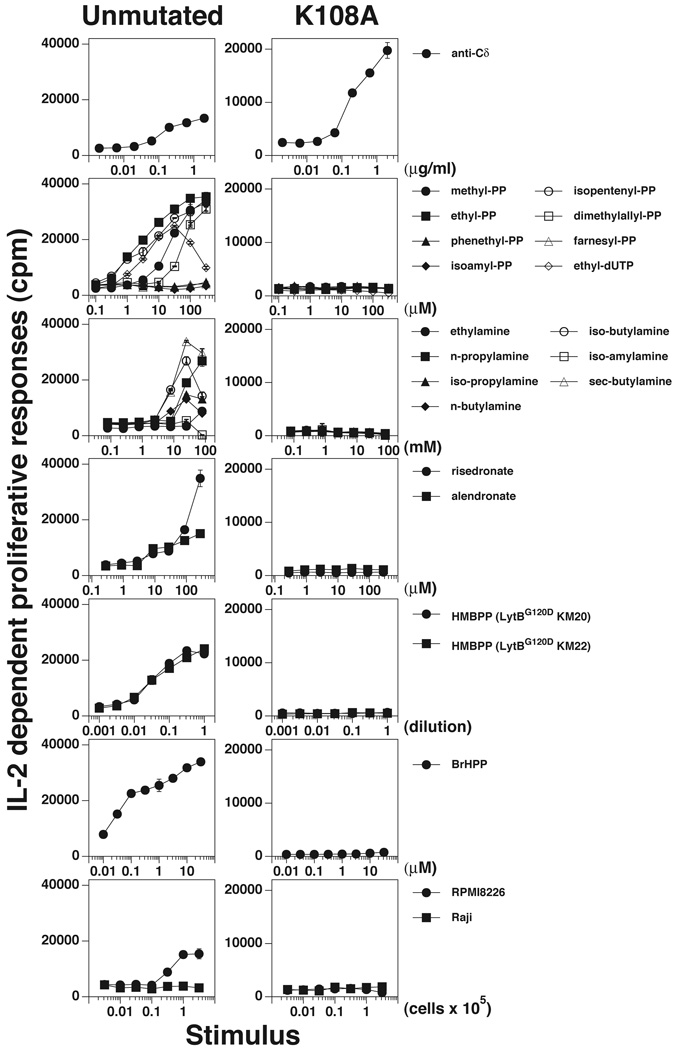
We had earlier established the importance of the CDR3γ in prenyl pyrophosphate recognition by altering reactivity though mutation of its junctional region (29). We and others have proposed that lysine residues in the Jγ region of CDR3γ combined with Arg51 in CDR2δ constitute a potential binding site for the pyrophosphate residues of IPP and HMBPP because the positive charges on the amino groups are available for ionic bonding (27, 28, 30). In addition to prenyl pyrophosphates, there have been reports that the mitochondrial protein, F1-ATPase, expressed on tumor cells can be recognized by the Vγ2Vδ2 TCR (74). To determine the importance of this proposed site in Ag and tumor recognition, we mutated one of these residues, Lys108, in the Jγ1.2 segment of CDR3γ to alanine.
To study tumor reactivity reportedly due to F1-ATPase (74), we determined reactivity of the wild type Vγ2Vδ2 TCR and the Lys108Ala TCR mutant for the stimulatory plasmacytoma, RPMI 8226. Whereas the unmutated TCR transfectant responded to the RPMI 8226, but not to the control Raji cell line, mutation of Lys108 abrogated reactivity to RPMI 8226 (Fig. 3, right bottom panel).
FIGURE 3. Critical role of K108 in the CDR3 of Vγ2 in the recognition of nonpeptide Ags and tumor cells.
J.RT3-T3.5 β− Jurkat cells were transfected with the DG.SF13 TCR-γ chain cDNA with a lysine (K) to alanine (A) mutation at position 108 in the CDR3γ region and the unmutated DG.SF13 TCR-δ cDNA. The transfectant obtained was named K108A and compared with an unmutated DG.SF13 TCR transfectant for stimulation by anti-Cδ, prenyl pyrophosphates, alkylamines, bisphosphonates (risedronate and alendronate), HMBPP (in supernatants of the E.coli lytB mutants, KM20, and KM22), bromohydrin pyrophosphate, and lymphoma cell lines (RPMI 8226 and Raji). Culture conditions and the IL-2 assay were as in Fig. 1.
Similarly, mutation of Lys108 completely abrogated recognition of HMBPP (Fig. 3) while preserving stimulation by an anti-Cδ mAb. This mutation also abrogated direct stimulation by seven other prenyl pyrophosphates (Fig. 3). Other stimulatory compounds, such as bisphosphonates and alkylamines, stimulate Vγ2Vδ2 T cells indirectly by blocking the enzyme FPP synthase causing increases in cellular IPP levels (13, 14). Consistent with IPP being the direct stimulating Ag, the Lys108Ala mutation also abrogated stimulation by six alkylamines and two bisphosphonates (Fig. 3). These results confirm an earlier report where Lys108Ala, Lys108Glu, and Lys109Glu mutations (but not Lys109Ala) abolished recognition of prenyl pyrophosphate, bisphosphonate and alkylamine stimulatory compounds (30, 31).
The loss of reactivity to the stimulatory compounds and to the tumor cell line by the Lys108Ala mutation suggests that there are shared structural features for these Ags (including the Ag expressed on the RPMI 8226 plasmacytoma).
Narrow length distribution of CDR3γ in Vγ2Jγ1.2 chains but not in Vγ2Jγ1.3/2.3 chains
To determine if there were any length restrictions or common motifs in the CDR3γ junctional region of reactive Vγ2Vδ2 T cells, we examined reported sequences of 107 reactive and non-reactive Vγ2Vδ2 T cell clones (Supplemental Table I). The Jγ1.2 segment was highly favored being used by 84 out of 90 (93%) reactive clones. The length of CDR3γ in Vγ2Jγ1.2 chains was also highly restricted, with 98% of T cell clones with CDR3γ lengths of ±2 residues. There were no major differences in CDR3γ length between reactive and non-reactive clones (Supplemental Fig. 3). However, because TCR differences between reactive and non-reactive clones could be mapped to the CDR3δ region in some cases (Table II), at least some of the Vγ2 chains expressed by nonreactive clones would be reactive if paired with a reactive Vδ2 chain.
Table II.
Diversity of CDR3γ or CDR3δ sequences in Vγ2Vδ2 T cells expressing an identical Vγ2 or Vδ2 chaina
| Clone/Transfectant | Vγ2 | N | Jγ | Jγ | Vδ2 | (aa97) | N/D/N | Jδ | Jδ | CDR3δ Length |
Reactivity | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AC2b | LWEV | QELGKKIK | Jγ1.2 | CDT | T | GG | SWDTRQM | Jδ3 | 13 | + | |||
| AC8b | LWEV | QELGKKIK | Jγ1.2 | CDT | W | G | S | SWDTRQM | Jδ3 | 13 | + | ||
| G1c | LWEV | QELGKKIK | Jγ1.2 | CD | R | V | PP | STGD | TDKL | Jδ1 | 14 | + | |
| G2c | LWEV | QELGKKIK | Jγ1.2 | CDT | V | NADAEN | DKL | Jδ1 | 13 | + | |||
| G3c | LWEV | QELGKKIK | Jγ1.2 | CD | A | V | LGDTSRP | DKL | Jδ1 | 14 | + | ||
| G4c | LWEV | QELGKKIK | Jγ1.2 | CD | L | V | LGVNTG | WDTRQM | Jδ3 | 16 | + | ||
| M3c | LWEV | QELGKKIK | Jγ1.2 | CD | W | L | LGDTV | TDKL | Jδ1 | 13 | + | ||
| G5c | LWEV | QELGKKIK | Jγ1.2 | CDT | G | GYAN | WDTRQM | Jδ3 | 14 | + | |||
| G6c | LWEV | QELGKKIK | Jγ1.2 | CDT | V | EA | LTAQL | Jδ2 | 11 | + | |||
| M4c | LWEV | QELGKKIK | Jγ1.2 | CD | N | W | GDKM | TAQL | Jδ2 | 12 | + | ||
| T2c | LWEV | QELGKKIK | Jγ1.2 | CD | N | T | GGYY | SWDTRQM | Jδ3 | 15 | + | ||
| T3c | LWEV | QELGKKIK | Jγ1.2 | CDT | V | LGDS | SWDTRQM | Jδ3 | 15 | + | |||
| T4c | LWEV | QELGKKIK | Jγ1.2 | CD | I | L | GDAA | LTAQ | Jδ2 | 12 | + | ||
| T5c | LWEV | QELGKKIK | Jγ1.2 | CDT | I | LGDT | WDTRQM | Jδ3 | 14 | + | |||
| T6c | LWEV | QELGKKIK | Jγ1.2 | CDT | L | PLGAKGY | DKL | Jδ1 | 14 | + | |||
| T7c | LWEV | QELGKKIK | Jγ1.2 | CDT | V | SGDTH | SWDTRQM | Jδ3 | 16 | + | |||
| M12c | LWEV | ELGKKIK | Jγ1.2 | CD | S | L | LTSQLSLGDH | TDKL | Jδ1 | 18 | + | ||
| M6c | LWEV | ELGKKIK | Jγ1.2 | CD | T | G | FRGGKDT | WDTRQM | Jδ3 | 17 | + | ||
| T22c | LWEV | ELGKKIK | Jγ1.2 | CD | K | S | AGNP | SWDTRQM | Jδ3 | 15 | − | ||
| C.15 | LWE | A | QELGKKIK | Jγ1.2 | CDT | L | GSGGSAER | TDKL | Jδ1 | 12 | + | ||
| I.7 | LWE | A | QELGKKIK | Jγ1.2 | CDT | P | GIGYT | WDTRQM | Jδ3 | 15 | − | ||
| γ001/δ263d | LWEV | QELGKKIK | Jγ1.2 | CDT | I | LGD | TDKL | Jδ1 | 10 | + | |||
| γ001/δ016d | LWEV | QELGKKIK | Jγ1.2 | CDT | V | EGMRRNLLGERGSY | TDKL | Jδ1 | 22 | + | |||
| γ001/δ255d | LWEV | QELGKKIK | Jγ1.2 | CDT | P | MRAPDWGTLGN | TDKL | Jδ1 | 19 | − | |||
| PBLγ/DGδf | LWE | GN | YKK | Jγ1.3 | CDT | L | VS | TDKL | Jδ1 | 10 | + | ||
| mutDGγ/DGδf | LWE | GN | ELGKKIK | Jγ1.2 | CDT | L | VS | TDKL | Jδ1 | 10 | − | ||
| DG.SF13 | LWE | W | ELGKKIK | Jγ1.2 | CDT | L | VS | TDKL | Jδ1 | 10 | + | ||
| DGγ/Monkeyδe | LWE | W | ELGKKIK | Jγ1.2 | SD | H | I | LEGGIRG | TDKL | Jδ1 | 13 | + | |
| Monkeyγe/DGδ | LWEV | Q | QFGRKVK | Jγ1.2 | CDT | L | VS | TDKL | Jδ1 | 10 | + | ||
| G115/G9g | LWE | AQ | QELGKKIK | Jγ1.2 | CD | T | L | GMGGEY | TDKL | Jδ1 | 13 | + | |
Horizontal dividers separate groups of TCRs that share either identical Vγ2 or Vδ2 chains but that differ in the other chain and, in some cases, in prenyl pyrophosphate reactivity. Position 97 in the Vδ2 chains is bolded and its position indicated in the column title. Likely sequence differences causing loss of reactivity are underlined. Note that alternative names for Vγ2, Jγ1.2, and Jγ1.3 are Vγ9 (TRGV9), JγP (TRGJP), and Jγ1 (TRGJ1).
Sequences from T cell clones reported in Ref (18).
Sequences from T cell clones, reactivity reported in Ref (44).
Sequences from T cell transfectants, reactivity reported in Ref (31).
Sequences from T cell transfectants, reactivity reported in Ref (38).
Sequences from T cell transfectants, reactivity reported in Ref (29).
Sequences from G115/G9 T cell clone used for crystal structure, reactivity reported in Ref (28)
Many clones (18 out of 84, 21%) used the invariant Vγ2Jγ1.2 amino acid sequence (75) either due to germline gene rearrangement or the addition of junctional residues with trimming of the germline Vγ2 and Jγ1.2 segments (Supplemental Table I). This frequency of invariant Vγ2Jγ1.2 chains is consistent with that reported for peripheral blood Vγ2Vδ2 T cells (75). In most of the non-invariant Vγ2Jγ1.2 chains, there were only one to two junctional residues (with a maximum of four) with corresponding losses in the germline Vγ2 and Jγ1.2 segments to preserve CDR3γ length. Thus, all of the Vγ2Jγ1.2 chains had germline amino acid sequences for much or all of the CDR3γ region. When junctional residues were added, they favored basic lysine and arginine residues with 25 out of 66 (38%) reactive Vγ2Jγ1.2 chains having at least 1 basic residue added. Also, none of the TCRs had deletion of the Lys108 or Lys109 residues. The glutamic acid located at position 105 is not required for reactivity since it is absent in some human Vγ2Jγ1.2 chains (Supplemental Table I) and in rhesus monkey Vγ2 which is able to recognize HMBPP presented by human APC when paired with human Vδ2 (Table II) (38).
In contrast to Vγ2Jγ1.2 chains, a high proportion of Vγ2Vδ2 T cell clones using Vγ2Jγ1.3/2.3 chains were nonreactive to prenyl pyrophosphates (46% nonreactive versus 14% nonreactive for Vγ2Jγ1.2 chains). Moreover, the Vγ2Jγ1.3/2.3 chains had shorter chain lengths (6–14 residues versus 11–14 residues for Vγ2Jγ1.2 chains) with higher variability in length. This shorter length may be optimal for reactivity since a short Vγ2Jγ1.3 chain (eight residues) using a GN junctional sequence paired with the DG.SF13 Vδ2 chain was responsive to HMBPP but a longer mutant Vγ2Jγ1.2 (12 residues) chain, where the GN residues were substituted for a W residue, was unresponsive when paired with the same DG.SF13 Vδ2 chain (Table II).
Conserved hydrophobic residue at position 97 but variable CDR3δ length and sequence
CDR3δ regions are the most variable junctional region in mammals due to the availability of multiple V, D, and J gene segments for rearrangement and due to the ability of multiple D segments to rearrange in tandem and in any reading frame (76). In mice, the CDR3δ region has been shown to be critical for γδ T cell recognition of T10 and T22 MHC class Ib molecules. A 9 aa conserved loop motif in the CDR3δ region inserts into a cavity on the T10 molecule and provides most of contact residues and 67% of the interface. To determine if a similar motif could be identified in the CDR3δ region of Vγ2Vδ2 TCR, we examined the sequences of 107 reactive and non-reactive Vγ2Vδ2 T cell clones (Supplemental Table I).
Unlike its Vγ2 chain partner, the length of the CDR3 of the Vδ2 chain varied widely, ranging from 10 to 18 aas, while retaining responsiveness to prenyl pyrophosphates (Supplemental Fig. 3, Supplemental Table I). Moreover, unlike Vγ2 chains in which there are critical residues in the Jγ segment and a preference for Jγ1.2, the Vδ2 chain can use Jδ1, Jδ2, or Jδ3 and retain responsiveness despite their significantly different sequences and lengths. The longer and more variable length of the CDR3δ segment is a general characteristic of δ chains (77).
Although the length of CDR3δ is variable, there is a strong preference for a hydrophobic residue at position 97. Ninety-two percent of Vδ2 chains (61 out of 71) from reactive T cell clones used a hydrophobic residue at this position versus 53% (8 out of 15) of Vδ2 chains from non-reactive T cell clones. No reactive clone used proline, lysine, or arginine at this position (Supplemental Table I). There are examples of clones with identical Vγ2 chains but different Vδ2 chains in which loss of reactivity can be correlated with a nonhydrophobic residue at position 97 (Table II). In these cases, the nonreactive Vγ2Vδ2 T cell clone had either a polar serine amino acid at this position (for the nonreactive T22 clone versus the reactive M6/M12 clones) or the kinked proline amino acid (for the nonreactive I.7 clone compared with the reactive C.15 clone). Similarly, transfection of a Vγ2 chain (γ001) paired with a Vδ2 chain (δ255) with a proline at this position did not confer reactivity to prenyl pyrophosphates although two other Vδ2 chains (δ263 and δ016) with hydrophobic residues did (31). Mutation of leucine97 to either alanine or serine abolished prenyl reactivity (31), confirming its importance.
Whereas position 97 was mainly restricted to hydrophobic amino acids, the residue prior to and after 97 were quite diverse, including both positively and negatively charged amino acids as well as proline (Supplemental Table I). Moreover, there were no other clearly defined motifs in the N-, Dδ-, or Jδ-encoded portions of CDR3δ that are required for reactivity. Thus, beyond a strong preference for a hydrophobic residue at position 97, no other motif can be found within CDR3δ that correlates with reactivity. Taken together, the diverse length and amino acid composition of CDR3δ from reactive Vγ2Vδ2 T cell clones suggests that optimal prenyl pyrophosphate reactivity requires a hydrophobic residue at position 97 but is tolerant to a wide variety of sequences in and lengths of the N, D, and J regions.
Model of Vγ2Vδ2 TCR contact residues in relation to the proposed prenyl pyrophosphate-binding site
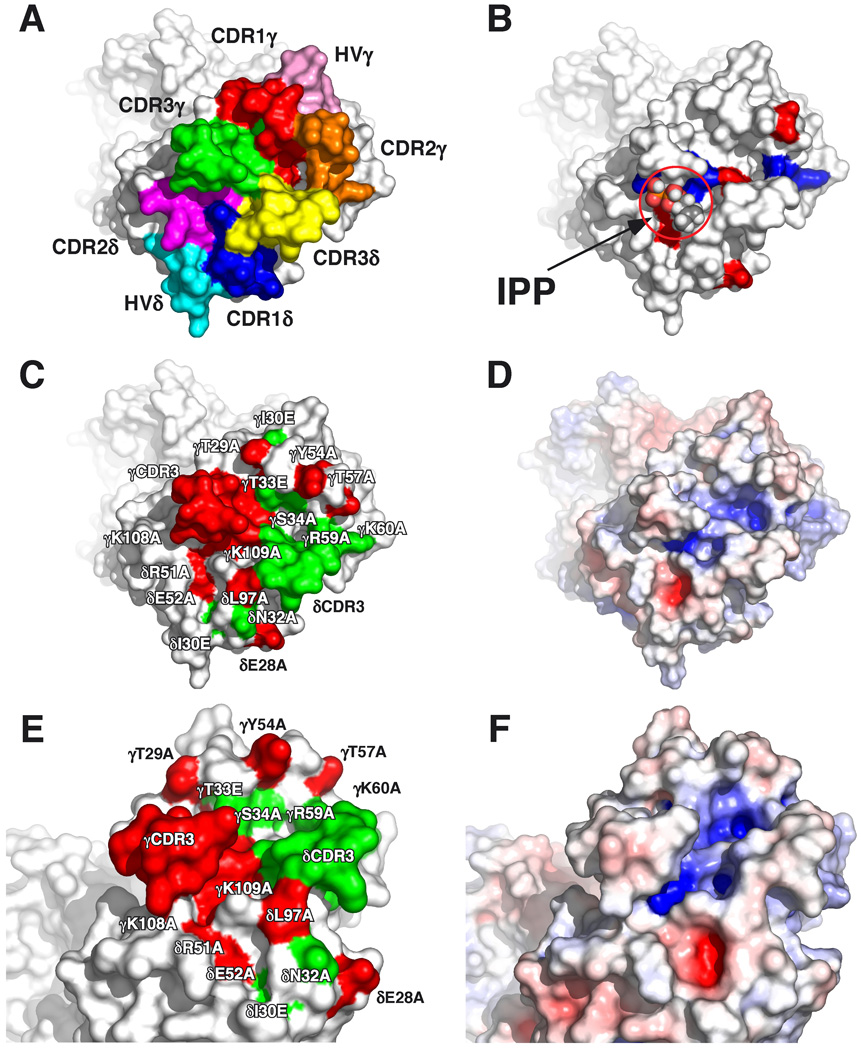
The structures of human G115 Vγ2Vδ2 TCR (Fig. 4) and murine G8 Vγ4Vα/δ11.3 TCR show more protrusions and clefts in the binding face of the TCR (48, 78) compared with the flatter surfaces of αβ TCRs specific for peptide-MHC class I/II complexes. The CDRs of the Vγ2Vδ2 TCR protrude (Fig. 4A). CDR3γ and CDR3δ are especially prominent creating a cleft between the two CDRs. There are two positively charged regions in this cleft (shaded blue) (Fig. 4D, 4F); one positioned near the base of CDR3δ formed by Lys109γ from Jγ1.2 and Arg51δ from CDR2δ, and a second located on the other side of the cleft formed by Arg59γ and Lys60γ from CDR2γ.
FIGURE 4. Recognition of prenyl pyrophosphates by human Vγ2Vδ2 TCR is dependent on all CDRs.
A, Top-down view of the Vγ2Vδ2 TCR with complementarity determining regions for the γ and δ chains labeled. CDR residues are colored such that those in: CDR1δ are blue, CDR1δ are magenta, CDR3δ are yellow, HV4δ are cyan, CDR1γ are red, CDR2γ are orange, CDR3γ are green, and HV4γ are pink. B, Top-down view of the Vγ2Vδ2 TCR with basic amino acids colored blue and acidic amino acids colored red. IPP is shown in the potential prenyl pyrophosphate-binding site for size comparison. C, Top-down view of critical amino acid residues that disrupt prenyl pyrophosphate recognition are colored red on the Vγ2Vδ2 TCR whereas non-critical amino acid residues are colored green. D, Top-down view of the surface potential of the Vγ2Vδ2 TCR (colored from red [−8 kT] to blue [+8 kT]) reveals two positively charged potential binding sites. E, Side view of critical amino acid residues that disrupt prenyl pyrophosphate recognition are colored red on the Vγ2Vδ2 TCR whereas non-critical amino acid residues are colored green. F, Side view of the surface potential of the Vγ2Vδ2 TCR (colored as in D).
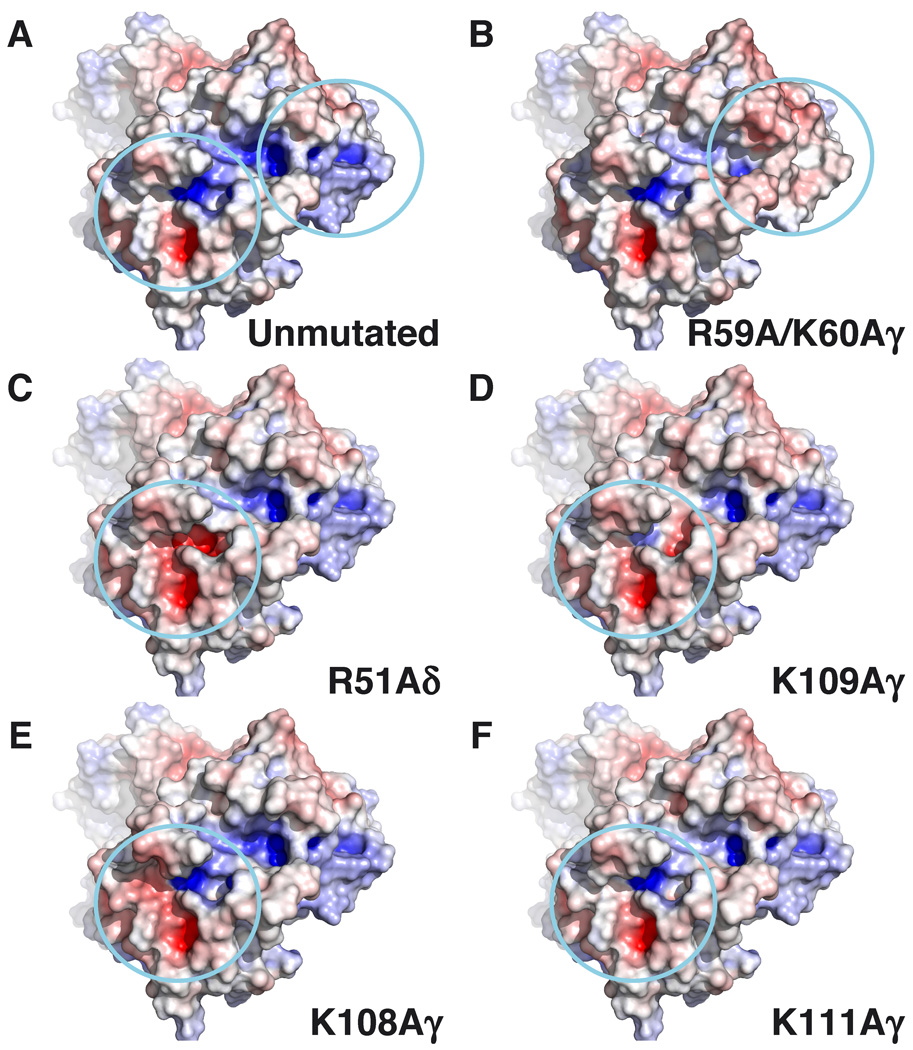
Mutation of residues in the second positively charged region formed by Arg59γ and Lys60γ from CDR2γ, although abolishing binding by some anti-Vγ2 mAbs (Table I), did not affect prenyl pyrophosphate recognition (Table III), arguing against the involvement of this second positively charged region. In contrast, alanine mutation of Lys108 (Fig. 3) and Lys109 (30) that is located in the first positively charged region closest to the Leu97 residue in CDR3δ, completely abrogated recognition of all prenyl pyrophosphates studied. Alanine mutation of Lys108 also abolished bisphosphonate and alkylamine recognition. Importantly, recognition of the plasmacytoma, RPMI 8226, was also lost. This result suggests that the Ag recognized by Vγ2Vδ2 T cells in RPMI 8226 is IPP or another prenyl pyrophosphate and not F1-ATPase since these two Ags have grossly different structures and would be expected to use different contact residues. However, F1-ATPase could function as a presenting molecule although we have found no evidence for this (data not shown).
Table III.
Effect of Vγ2Vδ2 TCR mutations and rhesus monkey polymorphisms on prenyl pyrophosphate reactivity
| TCR region | Polymorphism between Human and Rhesus Monkeya |
Mutation of Human Vγ2Vδ2 TCR |
Prenyl Pyrophosphate Reactivity |
Reference |
|---|---|---|---|---|
| Vγ2 chain | ||||
| CDR1γ | I28V | + | (38) | |
| T29A | − | Current paper | ||
| I30E | + | Current paper | ||
| A32E | + | (38) | ||
| T33A | + | Current paper | ||
| S34A | + | Current paper | ||
| V35I | + | (38) | ||
| CDR2γ | S53F | + | (38) | |
| Y54A | − | Current paper | ||
| T57A | − | Current paper | ||
| R59K | + | (38) | ||
| R59A | + | Current paper | ||
| K60A | + | Current paper | ||
| CDR3γ | W104GN | − | (29) | |
| E105Q | + | (38) | ||
| F106L | + | (38) | ||
| K108R | + | (38) | ||
| K108A | − | Current paper and (31) | ||
| K108E | − | (30) | ||
| K109A | + | (31) | ||
| K109E | − | (30) | ||
| Vδ2 chain | ||||
| CDR1δ | E28A | − | Current paper | |
| I30E | + | Current paper | ||
| G31S | + | (38) | ||
| CDR2δ | R51A | − | Current paper and (31) | |
| R51E | − | (31) | ||
| E52A | − | Current paper | ||
| D54G | + | (38) | ||
| CDR3δ | C94S | + | (38) | |
| L97A | − | (31) | ||
| L97S | − | (31) |
Reactivity of rhesus monkey V gene segments was assessed by pairing with the corresponding partner human V gene segments and transfecting into J.RT3-T3.5 (β− Jurkat) followed by stimulation with antigens presented by human APCs as described (38).
This first positively charged region is positioned immediately adjacent to the conserved hydrophobic amino acid at position 97 of CDR3δ and has potential contributions from Lys108γ, Lys109γ, and Lys111γ from Jγ1.2 and Arg51δ from CDR2δ (Fig. 4D, 4F). However, in silico mutagenesis and calculation of the surface potential suggests that most of the positive potential in the region is from Arg51δ with some contribution from Lys109γ but little or no contribution from Lys108γ and Lys111γ (Fig. 5). Consistent with this analysis, mutation of Lys109γ to alanine reduced but did not abolish prenyl pyrophosphate recognition (31) whereas mutation of Arg51δ to alanine completely abolished recognition. We now show that the acidic amino acid, Glu52δ, from CDR2δ that is near but outside the binding pocket is also required for recognition. The area is a potential prenyl pyrophosphate-binding site since the positively charged amino acids can form ionic bonds to the negatively charged pyrophosphate moiety on IPP or HMBPP. The alkenyl chain could be positioned over the hydrophobic amino acid at position 97. Most proteins that bind phosphate(s) use either this mechanism of binding or else use negatively charged amino acids to form an ionic bond to one of the positive charges on a divalent cation leaving the second free to bind to the negative charges on the phosphate group. However, there is no evidence for direct high affinity binding of prenyl pyrophosphates to the Vγ2Vδ2 TCR either by equilibrium dialysis (C. T. Morita, unpublished results) or by soaking Vγ2Vδ2 TCR crystals in IPP or HMBPP (28).
FIGURE 5. Surface potential effect of in silico alanine mutations of basic residues in the Vγ2Vδ2 TCR.
Basic residues were mutated to alanine in silico using Pymol (DeLano Scientific), and the surface potential of the mutated TCRs were calculated using the APBS plugin in Pymol. The TCRs are oriented with the γ chains on the top left and the δ chains on the bottom right of the panels. Top down views of the surface potential of the Vγ2Vδ2 TCR are shown (colored from red (−8 kT) to blue (+8 kT). The two positively charged regions are circled. A, Surface potential for the unmutated Vγ2Vδ2 TCR. B–F, Effect on surface potential of alanine replacement of R59A and K60Aγ (B), R51Aδ (C), K109Aγ (D), K108Aγ (E), and K111Aγ (F).
When the mutated CDR residues are localized on the Vγ2Vδ2 TCR structure (critical residues colored red and noncritical residues colored green, Table III), a potential binding footprint of the TCR to its ligand is delineated (Fig. 4C, 4E). This area is much larger than the size of IPP or HMBPP (IPP and HMBPP are 10.3 Å and 10.9 Å in extended conformation, respectively) and encompasses more that just the prenyl pyrophosphate-binding site (Fig. 4B, 4D, 4F). Moreover, although both abolish recognition, Thr29 in CDR1γ is 34 Å from Glu28 in CDR1δ. Similarly, Lys109 in CDR3γ is 15.3 Å from Thr29 in CDR1γ and 20.1 Å from Glu28 in CDR1δ; again, much larger distances than the size of a prenyl pyrophosphate. Thus, the footprint of Vγ2Vδ2 TCR residues required for prenyl pyrophosphate recognition is significantly larger than the proposed prenyl pyrophosphate-binding site.
Discussion
In this study, we show that mutations in all CDRs of the Vγ2Vδ2 TCR can affect recognition of prenyl pyrophosphates. Mutation of highly accessible amino acids in CDR1γ, CDR2γ, and CDR1δ loops all can abolish recognition whereas mutations localized on the sides of the loops do not. Unlike TCR recognition by the two other unconventional innate T cells in which the contact residues are defined, there is no evidence that an extended Dα/δ or Jα/δ amino acid motif is required for recognition. Instead, there is a preference for a single hydrophobic, aliphatic amino acid at position 97 of CDR3δ as previously reported (31, 79) but no restriction on 1) the type of amino acid immediately preceding or following position 97; 2) the overall length of the CDR3δ loop; or 3) other sequences in the D/N or J portions of the CDR3δ loop. In contrast, the length of CDR3γ shows low variability, basic amino acids are preferred in its N region, and the Jγ1.2 segment is preferentially used. Basic amino acids (Lys108 and Lys109) in the Jγ segment, the basic Arg51 and acidic Glu52 residues in the CDR2δ, and the aliphatic amino acid at position 97 in CDR3δ are all important for recognition and are located in or near a proposed prenyl pyrophosphate-binding site. Taken together, these results show that a large portion of the Vγ2Vδ2 TCR is required for prenyl pyrophosphate recognition.
How well do alanine mutations predict residues in T cell Ag receptors that contact Ag or their presenting molecule? In three studies, αβ TCR contact residues for peptide-MHC class I ligands were determined from crystal structures. CDR contact and non-contact residues were then mutated to alanine and the mutant TCRs tested for binding to their respective peptide-MHC class I ligands. Mutation of contact residues to alanine reduced MHC/peptide ligand binding for 81% (17 out of 21) of the contact residues in the LC13 TCR (80), for 70% (7 out of 10) in the 2C TCR (81), and for 59% (13 out of 22) in the JM22 TCR (82). Noncontact residues in CDRs containing contact residues also decreased binding albeit, as might be expected, at lower rates (39%, 7 out of 18 for the LC13 TCR and 60%, 9 out of 15 for the 2C TCR). Importantly, none of 12 mutations in the α and β hypervariable four-regions of the 2C TCR affected binding (none were contact residues). Most mutations that grossly affected structure were not expressed (only 1 of 40 in LC13 (80) 9 out of 51 in 2C (81), and 0 out of 12 in JM22 (82)). Thus, most mutations (70% overall) in CDR contact residues greatly reduce binding whereas mutations in HV4 loops that did not contain contact residues had no effect. From these results, we would predict that mutations that abolish recognition likely identify either contact residues for the Ag/presenting molecule or non-contact residues in loops that contain contact residues. Our finding that residues well outside the proposed prenyl pyrophosphate-binding site affect recognition suggest that a larger molecule binds and presents these small molecules.
Unconventional innate versus conventional peptide-MHC TCR recognition by T cells
Although expressing rearranging TCRs, innate T cells, such as γδ T cells and iNKT αβ T cells, likely play roles that more closely resemble those of innate myeloid cells. In keeping with their unique roles in the immune system, innate T cells commonly express TCR that use germline-encoded portions of the TCR to either recognize nonpeptide Ags presented by MHC class Ib or CD1 molecules or to directly recognize MHC class I-like molecules. Many γδ T cell populations, such as murine dendritic epidermal γδ T cells (83), express TCR with invariant receptors. αβ iNKT cells that recognize self and foreign glycolipids presented by CD1d also express a semi-invariant TCR composed of a conserved VαJα chain paired with a limited set of Vβ chains (84).
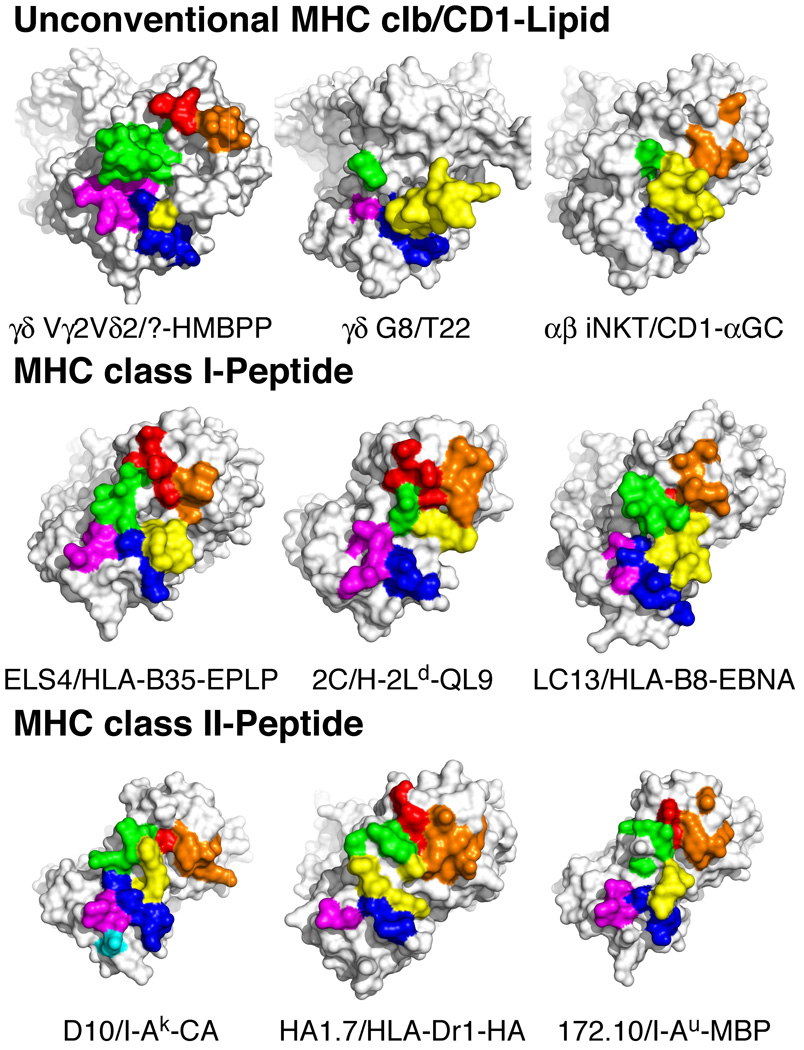
There are only two examples of unconventional TCR recognition by innate T cells that have been characterized at the molecular level to compare with Vγ2Vδ2 TCR recognition of prenyl pyrophosphates. The only example for γδ T cells is the structure of a murine γδ TCR, G8, bound to the MHC class Ib molecule, T22. In this study, a W…EGYEL motif in an extended CDR3δ loop (85) contacts a disordered region at the end of the α2 helix of the T22 molecule from the side (48). The contact residues of the G8 TCR are shown in Fig. 6 (top middle panel). Eighty nine percent of the γδ TCR interface with T22 is contributed by the Vδ chain with 67% contributed by the CDR3δ loop. Underscoring the importance of the CDR3δ loop, swapping of this region for the CDR3α of an αβ TCR is sufficient to confer recognition of T22 (86). All of the γδ T cells that recognized T22 have the W…EGYEL sequence motif in CDR3δ. Importantly, this motif is commonly derived from the germline-encoded Dδ2 gene segment and is present on ~0.85% of nonselected murine Vδ2 chains; this may account for the high frequency of T22-reactive γδ T cells (1/50-1/1000) in unimmunized mice (85).
FIGURE 6. Potential contact residues of the Vγ2Vδ2 TCR for a putative antigen presenting molecule-prenyl pyrophosphate complex differs from other unconventional TCRs and from conventional TCRs specific for MHC-peptide complexes.
The TCRs are oriented with the β/γ chains on the top left and the α/δ chains on the bottom right of the panels. Contact residues are colored such that those in: CDR1α/δ are blue, CDR2α/δ are magenta, CDR3α/δ are yellow, HV4α/δ are cyan, CDR1β/γ are red, CDR2β/γ are orange, and CDR3β/γ are green. The diagonal orientation does not attempt to match the docking angle on the MHC/CD1 ligand. Top panels, Putative contact residue "footprint" of the Vγ2Vδ2 TCR based on the effects of point mutations on human Vγ2Vδ2 T cell reactivity to nonpeptide Ags (top left panel). Note the potential large contribution of germline-encoded regions of CDR3γ compared with only a minor contribution by L97 in CDR3δ. In contrast, the G8 γδ TCR (top middle panel), that is specific for the T22 MHC class Ib molecule, predominantly uses CDR3δ for recognition. The iNKT αβ TCR (top left panel) binding to the CD1d-α-GalCer complex also predominantly uses the CDR3α region (which corresponds to CDR3δ). Middle panels, Conventional αβ TCRs specific for MHC class I-foreign peptide complexes generally use both CDR3α and CDR3β for MHC-peptide recognition in addition to CDR1 and CDR2. Bottom panels, Conventional αβ TCRs specific for MHC class II/peptide complexes are similar to MHC class I/peptide-specific αβ TCRs and involve extensive CDR3α and CDR3β contacts in addition to CDR1 and CDR2 contacts. Additional examples of αβ TCR recognition of MHC-peptide complexes are shown in Supplemental Figs. 4 and 5.
The other example of unconventional innate TCR recognition is human and murine iNKT αβ TCR recognition of glycolipids presented by CD1d (49, 87). iNKT TCRs bind CD1d/α– GalCer in a top down orientation paralleling the α helixes of CD1d. Again, CDR3α, which is the equivalent of CDR3δ, plays an important role contributing 52% of the buried surface area (BSA) (Fig. 6, top left panel). Only the Jα18 region of CDR3α contacts the CD1d–lipid complex explaining the use of this J region by the invariant Vα24 chain of the iNKT TCR. Like the G8 γδ TCR in which CDR3γ contains only two contact residues, CDR3β contains only one contact residue that contribute only 6% of the BSA. Instead, the germline-encoded CDR2β contributes 28% of the BSA. When the contact residues in the iNKT TCR were mutated to alanine, four out of seven CDR3α loop residues and two out of four CDR2β abolished recognition whereas three other residues in other CDRs had no or little effect (88). Thus, despite large differences in the docking orientation of the iNKT αβ TCR and the G8 γδ TCR for their ligands, unconventional recognition by both involves primarily germline-encoded regions in CDR3α/δ with critical contributions from the germline-encoded CDR2β in iNKT TCR.
In contrast to these examples, our study shows a different mode of recognition for the Vγ2Vδ2 TCR that does not rely as heavily on the CDR3α/δ region. Instead, CDR3γ appears to be the critical region for prenyl pyrophosphate recognition. Mutation of either Lys108 or Lys109 in the conserved Jγ1.2 region abolishes recognition as does altering the CDR3γ length/sequence (29). Moreover, 20–30% of adult Vγ2Vδ2 T cells express an invariant Vγ2Jγ1.2 sequence with the rest exhibiting little differences in CDR3γ length while favoring basic residues in the junctional region. Conservation of CDR3γ length may be required to maintain the correct positioning of the Jγ1.2 region in the putative prenyl pyrophosphate-binding site (Fig. 4B, 5). We find that the Arg51δ and Glu52δ residues in CDR2δ are also required for recognition and confirm that there is a strong preference for an aliphatic hydrophobic amino acid (generally leucine, isoleucine, or valine but not proline) at position 97 in CDR3δ but find few restrictions on CDR3δ length or sequence. All of these critical residues cluster in or around an area of positive surface potential due to Arg51δ and, to a lesser extent, Lys109γ, as determined by in silico mutation (Fig. 5).
In addition to this site, we now show that critical residues are also present in exposed areas of CDR1γ and CDR2γ as well as CDR1δ. These residues are distant (15.3 Å for Thr29 in CDR1γ, 15.2 Å for Tyr54 in CDR2γ, and 20.1 Å for Glu28 in CDR1δ) from the proposed binding site making it unlikely that they would directly influence prenyl pyrophosphate binding. Instead, we would propose that these residues contact a presenting molecule for the prenyl pyrophosphates similar to αβ TCRs contacting peptide-MHC class I or class II complexes.
The potential footprint of the Vγ2Vδ2 TCR on the putative Ag presenting molecule shows more similarities than differences with the footprints of conventional αβ TCR that bind to MHC class I or class II/foreign peptide complexes (89). Like the Vγ2Vδ2 TCR, most antimicrobial αβ TCRs contact their ligands using all six CDRs although their relative energetic contributions to binding vary (contact residues of TCRs specific for peptide-MHC class I and class II are shown in Fig. 6, Supplemental Figs. 4, 5) The main exceptions are A6 and B7 TCRs specific for HLA-A2-TAX (57) (Supplemental Fig. 4).
Generally, CDR1α and CDR2α are centered over the MHC α2 helix and CDR1β and CDR2β are centered over the MHC α1 helix in a diagonal orientation. The CDR3α region is centered on the N-terminal end of the peptide and the CDR3β region is centered on the C-terminal end but both regions can also contact MHC. Centering the diverse CDR3 regions over the peptide allows for the recognition of an array of sequences. Autoimmune recognition of self-peptides represent a major exception to the diagonal orientation of αβ TCR docking to their ligands because autoimmune TCRs may bind with unusual topologies (90) and may shift to docking over the extreme N-terminus of the peptide (91). Although the docking orientation of the Vγ2Vδ2 TCR and the structure of the putative presenting molecule is unknown, the similarities between the footprints of antimicrobial αβ TCRs and the footprint of the Vγ2Vδ2 TCR would suggest that a presenting molecule is required.
Vγ2Vδ2 TCR recognition also differs from antimicrobial αβ TCR recognition in important ways. In contrast to antimicrobial αβ TCRs in which most have multiple contact residues in CDR3α (Fig. 6, Supplemental Figs. 4, 5), the CDR3δ region (equivalent to CDR3α) makes a limited contribution to recognition because the only preference found was for an aliphatic residue at position 97. Moreover, Vγ2Vδ2 recognition is primarily though the germline-encoded gene segments, CDR1γ, CDR2γ, CDR1δ, and CDR2δ regions, coupled with Vγ2Jγ1.2 chains which are either invariant (lacking N nucleotide additions) or with limited diversity in sequence and length.
Given the large footprint for Vγ2Vδ2 TCR, we speculate that there is a presenting molecule for prenyl pyrophosphate Ags. Because there is no evidence for genetic restriction in Vγ2Vδ2 TCR recognition, we would predict that the putative presenting molecule has little or no polymorphisms in contact regions for the TCR. Additionally, since human tumor cells of diverse origin and since different hematopoietic APC present Ag, the putative presenting molecule would need to be widely expressed much like MHC class I molecules. Although prenyl pyrophosphate reactivity is preserved in both New World and Old World monkeys (92, 93), rodent and guinea pig γδ T cells (94) are unable to respond to prenyl pyrophosphate Ags nor can rodent, hamster, or other species APCs present prenyl pyrophosphate Ags to human Vγ2Vδ2 T cells (35, 95). There may exist ruminant γδ T cells with prenyl pyrophosphate reactivity although this has not been definitively established (96). Despite the reactivity of monkeys to prenyl pyrophosphates, both Vero and COS-7 cell lines (95) (derived from African green monkeys) are unable to present to human Vγ2Vδ2 T cells. Thus, there may be xenogeneic differences between African green monkeys and humans in the putative presenting molecules. Finally, we would predict that the putative presenting molecule has a structure homologous to MHC class I or class II like all other non-classical presenting molecules and ligands (CD1, MR1, and MICA/MICB) so far discovered although none of the known MHC class Ib molecules are viable candidates. It also is possible that the presentation molecule belongs to a new class of presenting molecules, such as an enzyme, a heat shock protein, or an immunoglobulin superfamily protein, that is not related in structure to MHC class I/II molecules.
Presentation of prenyl pyrophosphates by a presenting molecule is also supported by functional and TCR binding studies. Recognition of prenyl pyrophosphates requires cell-cell contact for Vγ2Vδ2 T cell activation as assessed by intracellular calcium flux (32) and TNF-α release (33) that is identical to the contact requirement for αβ T cell recognition of MHC/peptide complexes but distinct from ligand recognition by hormone and neurotransmitter receptors. Also, there is no evidence for direct recognition of prenyl pyrophosphates since attempts to soak IPP and HMBPP into Vγ2Vδ2 TCR crystals failed (28) and since no binding of prenyl pyrophosphates to soluble Vγ2Vδ2 TCRs was noted in equilibrium dialysis or microcalorimetry experiments (C. T. Morita, unpublished observations). Similarly, a monkey Vγ2Vδ2 TCR tetramer does not directly bind HMBPP but will bind to APC when the APC are incubated with HMBPP or infected with Mycobacterium bovis bacillus Calmette-Guérin (36). This binding was observed with human and monkey but not mouse, rat, or pig cells demonstrating specificity for primate APCs identical to the results on presentation of prenyl pyrophosphates (33, 97), bisphosphonates (95), alkylamines (97), and photoaffinity prenyl pyrophosphate analogs (35). Binding was abolished by protease treatment of the cell surface strongly suggesting that the surface binding molecule was a protein (36). Finally, photoaffinity analogs of prenyl pyrophosphates are able to covalently crosslink to protein(s) on the APC surface for presentation and this binding could be specifically inhibited by an inactive prenyl pyrophosphate analog (35). Taken together, these findings provide additional evidence for the existence of a cell surface presenting molecule for prenyl pyrophosphate Ags.
As discussed above, recognition of α-GalCer/CD1d by the iNKT TCR is primarily through germline-encoded invariant Vα24Jα 18 CDR3α and CDR2β regions (49). However, for other lipids, iNKT recognition also requires residues in CDR1α in addition to the CDR3α and CDR2β regions. Again, these residues are encoded by germline gene segments (98). Thus, the basic orientation and framework for binding of both human and mouse iNKTs to their respective CD1d molecules and to different lipids is conserved such that others have proposed that the iNKT receptor can be considered to have characteristics of a pattern-recognition receptor (98). However, the fine specificity and binding affinity of iNKT TCR can be affected by the Vβ-chain partner and its CDR3β sequence (87, 99, 100) allowing iNKT TCR to distinguish between a number of different lipids. Similar differences in the CDR3 sequences of the Vγ2Vδ2 TCR also likely affect prenyl pyrophosphate recognition.
Although Vγ2Vδ2 TCRs appear to function like pattern-recognition receptors (101) as proposed for iNKT TCRs (98), this is not to assert that there is no selection on Vγ2Vδ2 TCRs during development or during Ag stimulation, which is a hallmark of adaptive immune recognition. Vγ2Vδ2 TCR selection clearly occurs because Vγ2Vδ2 T cells using the Jγ1.2 region and having an aliphatic amino acid at position 97 are preferentially expanded in vitro (31, 102) and overrepresented in adult Vγ2Vδ2 T cells as compared with fetal/thymic Vγ2Vδ2 T cells (79). However, even in fetuses and neonates, there is a very high proportion of Vγ2Vδ2 T cells that respond to prenyl pyrophosphates with two out of two fetal liver and at least three out of eight cord blood Vγ2Vδ2 T cell clones responding (9; data not shown). Also, Davadou et al. (44) found that 22 out of 25 (88%) PHA-derived thymic Vγ2Vδ2 T cells using the Vγ2Jγ1.2 chain responded to prenyl pyrophosphates and to Daudi. Overall, 30 out of 38 (79%) of thymic Vγ2Vδ2 T cell clones responded. In all of these cases, selection by exogenous Ags is unlikely. Therefore, although peripheral selection is likely responsible for the near universal reactivity of Vγ2Vδ2 T cells that is found in adults, a high proportion of Vγ2Vδ2 T cells start out reactive probably due to constraints in gene rearrangement (103).
Based on our results, we would predict that most of the recognition is mediated by germline-encoded elements of the Vγ2Vδ2 TCR and that there is a presenting molecule for prenyl pyrophosphate Ags. Although only three examples of unconventional recognition have now been molecularly defined, all share the use of certain germline elements of the TCR for recognition, the invariant Vα14Jα24 gene segments for lipid/CD1d recognition (49), a Dδ motif for T10/T22 (48, 85), and, as noted by others (79, 103), the Vγ2Jγ1.2 chain and the Vδ2 gene segment for prenyl pyrophosphates. We speculate that unconventional innate γδ and αβ TCRs containing invariant V chains, highly biased V gene usage, or conserved CDR3 motifs, are likely to bind their ligands using the invariant or biased portion of the TCR. Moreover, the portions of invariant chains, biased V genes, or conserved motifs of innate TCRs that contact the ligands seem to favor germline-encoded segments as also suggested by Born and O'Brien (104). Thus, innate TCRs with a single reactivity may have diverse CDR3 regions like Vγ2Vδ2 T cells but would contact their ligands through either germline-encoded CDR1/2 regions in one or both chains or through germline-encoded D and/or J regions in the CDR3s. However, unlike T10-specific γδ TCR and iNKT TCR, in situations in which both chains are invariant (like the murine dendritic epidermal Vγ5Vδ1 TCR and the murine Vγ6Vδ1 TCR), both CDR3 regions would likely be involved in binding. This type of recognition allows the majority of a given population of γδ T cells to react to their Ag either because they express monoclonal TCRs or because the diversity in the CDR3 regions is of less importance.
Supplementary Material
Acknowledgments
We thank K. Ness, G. Workalemahu, and C. Jin for critical review of this manuscript.
Footnotes
This work was supported by grants from the NIH National Institute of Arthritis and Musculoskeletal and Skin Disease (AR045504), the National Institute of Allergy and Infectious Diseases (Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research, AI057160), and the National Cancer Institute (CA113874) to C.T.M.
Abbreviations used in this paper: BCG, Mycobacterium bovis Bacille Calmette-Guérin; CDR, complementarity-determining region; HMBPP, (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate; iNKT, invariant natural killer αβ T cells; IPP, isopentenyl pyrophosphate; MAIT, mucosal-associated invariant αβ T cells; MEP, methyl-d-erythritol-4 phosphate
Disclosures
The authors have no conflicting financial interests.
Online supplemental materials
Supplemental Table I shows the sequences of Vγ2Vδ2 TCRs from Vγ2Vδ2 T cell clones.
Supplemental Fig. 1 shows additional Vγ2 TCR mutants. Supplemental Fig. 2 shows additional Vδ2 TCR mutants. Supplemental Fig. 3 shows the CDR3 length variation of the Vγ2Vδ2 TCR. Supplemental Fig. 4 shows the contact residues of additional αβ TCRs specific for MHC classI/peptide complexes. Supplemental Fig. 5 shows the contact residues of additional αβ TCRs specific for MHC classII/peptide complexes.
References
- 1.Gapin L. Where do MAIT cells fit in the family of unconventional T cells? PLoS Biol. 2009;7:435–438. doi: 10.1371/journal.pbio.1000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat. Rev. Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 3.Kronenberg M, Engel I. On the road: progress in finding the unique pathway of invariant NKT cell differentiation. Curr. Opin. Immunol. 2007;19:186–193. doi: 10.1016/j.coi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Morita CT, Parker CM, Brenner MB, Band H. T cell receptor usage and functional capabilities of human γδ T cells at birth. J. Immunol. 1994;153:3979–3988. [PubMed] [Google Scholar]
- 5.Parker CM, Groh V, Band H, Porcelli SA, Morita C, Fabbi M, Glass D, Strominger JL, Brenner MB. Evidence for extrathymic changes in the T cell receptor γ/δ repertoire. J. Exp. Med. 1990;171:1597–1612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol. Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 8.Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas A-K, Beck E, Wiesner J, Eberl M, Jomaa H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. FEBS Lett. 2001;509:317–322. doi: 10.1016/s0014-5793(01)03191-x. [DOI] [PubMed] [Google Scholar]
- 9.Puan K-J, Jin C, Wang H, Sarikonda G, Raker AM, Lee HK, Samuelson MI, Märker-Hermann E, Pasa-Tolic L, Nieves E, Giner J-L, Kuzuyama T, Morita CT. Preferential recognition of a microbial metabolite by human Vγ2Vδ2 T cells. Int. Immunol. 2007;19:657–673. doi: 10.1093/intimm/dxm031. [DOI] [PubMed] [Google Scholar]
- 10.Kunzmann V, Bauer E, Wilhelm M. γ/δ T-cell stimulation by pamidronate. N. Engl. J. Med. 1999;340:737–738. doi: 10.1056/NEJM199903043400914. [DOI] [PubMed] [Google Scholar]
- 11.Sanders JM, Ghosh S, Chan JMW, Meints G, Wang H, Raker AM, Song Y, Colantino A, Burzynska A, Kafarski P, Morita CT, Oldfield E. Quantitative structure-activity relationships for γδ T cell activation by bisphosphonates. J. Med. Chem. 2004;47:375–384. doi: 10.1021/jm0303709. [DOI] [PubMed] [Google Scholar]
- 12.Bukowski JF, Morita CT, Brenner MB. Human γδ T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 13.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson K, Rojas-Navea J, Rogers MJ. Alkylamines cause Vγ9Vδ2 T-cell activation and proliferation by inhibiting the mevalonate pathway. Blood. 2006;107:651–654. doi: 10.1182/blood-2005-03-1025. [DOI] [PubMed] [Google Scholar]
- 15.De Rosa SC, Andrus JP, Perfetto SP, Mantovani JJ, Herzenberg LA, Roederer M. Ontogeny of γδ T cells in humans. J. Immunol. 2004;172:1637–1645. doi: 10.4049/jimmunol.172.3.1637. [DOI] [PubMed] [Google Scholar]
- 16.Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L, Kou Z, Wang Q, Jiang L, Estep J, Hunt R, Clagett M, Sehgal PK, Li Y, Zeng X, Morita CT, Brenner MB, Letvin NL, Chen ZW. Adaptive immune response of Vγ2Vδ2+ T cells during mycobacterial infections. Science. 2002;295:2255–2258. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García VE, Sieling PA, Gong J-H, Barnes PF, Tanaka Y, Bloom BR, Morita CT, Modlin RL. Single cell cytokine analysis of γδ T cell responses to nonpeptide mycobacterial antigens. J. Immunol. 1997;159:1328–1335. [PubMed] [Google Scholar]
- 18.Morita CT, Verma S, Aparicio P, Martinez-A. C, Spits H, Brenner MB. Functionally distinct subsets of human γ/δ T cells. Eur. J. Immunol. 1991;21:2999–3007. doi: 10.1002/eji.1830211215. [DOI] [PubMed] [Google Scholar]
- 19.Cipriani B, Borsellino G, Poccia F, Placido R, Tramonti D, Bach S, Battistini L, Brosnan CF. Activation of C-C β-chemokines in human peripheral blood γδ T cells by isopentenyl pyrophosphate and regulation by cytokines. Blood. 2000;95:39–47. [PubMed] [Google Scholar]
- 20.Tikhonov I, Deetz CO, Paca R, Berg S, Lukyanenko V, Lim JK, Pauza CD. Human Vγ2Vδ2 T cells contain cytoplasmic RANTES. Int. Immunol. 2006;18:1243–1251. doi: 10.1093/intimm/dxl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spada FM, Grant EP, Peters PJ, Sugita M, Melián A, Leslie DS, Lee HK, van Donselaar E, Hanson DA, Krensky AM, Majdic O, Porcelli SA, Morita CT, Brenner MB. Self recognition of CD1 by γ/δ T cells: Implications for innate immunity. J. Exp. Med. 2000;191:937–948. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dieli F, Troye-Blomberg M, Ivanyi J, Fournié JJ, Krensky AM, Bonneville M, Peyrat MA, Caccamo N, Sireci G, Salerno A. Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vγ9/Vδ2 T lymphocytes. J. Infect. Dis. 2001;184:1082–1085. doi: 10.1086/323600. [DOI] [PubMed] [Google Scholar]
- 23.Dudal S, Turriere C, Bessoles S, Fontes P, Sanchez F, Liautard J, Liautard JP, Lafont V. Release of LL-37 by activated human Vγ9Vδ2 T cells: a microbicidal weapon against Brucella suis. J. Immunol. 2006;177:5533–5539. doi: 10.4049/jimmunol.177.8.5533. [DOI] [PubMed] [Google Scholar]
- 24.Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony H-P. γδ T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–206. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 25.Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, Roberts A, Buccheri S, D'Asaro M, Gebbia N, Salerno A, Eberl M, Hayday AC. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bukowski JF, Morita CT, Tanaka Y, Bloom BR, Brenner MB, Band H. Vγ2Vδ2 TCR-dependent recognition of non-peptide antigens and Daudi cells analyzed by TCR gene transfer. J. Immunol. 1995;154:998–1006. [PubMed] [Google Scholar]
- 27.Morita CT, Lee HK, Wang H, Li H, Mariuzza RA, Tanaka Y. Structural features of nonpeptide prenyl pyrophosphates that determine their antigenicity for human γδ T cells. J. Immunol. 2001;167:36–41. doi: 10.4049/jimmunol.167.1.36. [DOI] [PubMed] [Google Scholar]
- 28.Allison TJ, Winter CC, Fournié JJ, Bonneville M, Garboczi DN. Structure of a human γδ T-cell antigen receptor. Nature. 2001;411:820–824. doi: 10.1038/35081115. [DOI] [PubMed] [Google Scholar]
- 29.Bukowski JF, Morita CT, Band H, Brenner MB. Crucial role of TCRγ chain junctional region in prenyl pyrophosphate antigen recognition by γδ T cells. J. Immunol. 1998;161:286–293. [PubMed] [Google Scholar]
- 30.Miyagawa F, Tanaka Y, Yamashita S, Mikami B, Danno K, Uehara M, Minato N. Essential contribution of germline-encoded lysine residues in Jγ1.2 segment to the recognition of nonpeptide antigens by human γδ T cells. J. Immunol. 2001;167:6773–6779. doi: 10.4049/jimmunol.167.12.6773. [DOI] [PubMed] [Google Scholar]
- 31.Yamashita S, Tanaka Y, Harazaki M, Mikami B, Minato N. Recognition mechanism of non-peptide antigens by human γδ T cells. Int. Immunol. 2003;15:1301–1307. doi: 10.1093/intimm/dxg129. [DOI] [PubMed] [Google Scholar]
- 32.Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR, Golan DE, Brenner MB. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human γδ T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 33.Lang F, Peyrat MA, Constant P, Davodeau F, David-Ameline J, Poquet Y, Vié H, Fournié JJ, Bonneville M. Early activation of human Vγ9Vδ2 T cell broad cytotoxicity and TNF production by nonpeptidic mycobacterial ligands. J. Immunol. 1995;154:5986–5994. [PubMed] [Google Scholar]
- 34.Pichler WJ, Beeler A, Keller M, Lerch M, Posadas S, Schmid D, Spanou Z, Zawodniak A, Gerber B. Pharmacological interaction of drugs with immune receptors: the p-i concept. Allergol Int. 2006;55:17–25. doi: 10.2332/allergolint.55.17. [DOI] [PubMed] [Google Scholar]
- 35.Sarikonda G, Wang H, Puan K-J, Liu X-H, Lee HK, Song Y, Distefano MD, Oldfield E, Prestwich GD, Morita CT. Photoaffinity antigens for human γδ T cells. J. Immunol. 2008;181:7738–7750. doi: 10.4049/jimmunol.181.11.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei H, Huang D, Lai X, Chen M, Zhong W, Wang R, Chen ZW. Definition of APC presentation of phosphoantigen (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate to Vγ2Vδ2 TCR. J. Immunol. 2008;181:4798–4806. doi: 10.4049/jimmunol.181.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito T, Weiss A, Miller J, Norcross MA, Germain RN. Specific antigen-Ia activation of transfected human T cells expressing murine Ti αβ-human T3 receptor complexes. Nature. 1987;325:125–130. doi: 10.1038/325125a0. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Lee HK, Bukowski JF, Li H, Mariuzza RA, Chen ZW, Nam K-H, Morita CT. Conservation of nonpeptide antigen recognition by rhesus monkey Vγ2Vδ2 T cells. J. Immunol. 2003;170:3696–3706. doi: 10.4049/jimmunol.170.7.3696. [DOI] [PubMed] [Google Scholar]
- 39.Giner J-L. A convenient synthesis of (E)-4-hydroxy-3-methyl-2-butenyl pyrophosphate and its [4-13C]-labeled form. Tetrahedron Lett. 2002;43:5457–5459. [Google Scholar]
- 40.Puan K-J, Wang H, Dairi T, Kuzuyama T, Morita CT. fldA is an essential gene required in the 2-C-methyl-D-erythritol 4-phosphate pathway for isoprenoid biosynthesis. FEBS Lett. 2005;579:3802–3806. doi: 10.1016/j.febslet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 41.Grant EP, Degano M, Rosat JP, Stenger S, Modlin RL, Wilson IA, Porcelli SA, Brenner MB. Molecular recognition of lipid antigens by T cell receptors. J. Exp. Med. 1999;189:195–205. doi: 10.1084/jem.189.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morita CT, Li H, Lamphear JG, Rich RR, Fraser JD, Mariuzza RA, Lee HK. Superantigen recognition by γδ T cells: SEA recognition site for human Vγ2 T cell receptors. Immunity. 2001;14:331–344. doi: 10.1016/s1074-7613(01)00113-3. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka Y, Sano S, Nieves E, De Libero G, Roca D, Modlin RL, Brenner MB, Bloom BR, Morita CT. Nonpeptide ligands for human γδ T cells. Proc. Natl. Acad. Sci. USA. 1994;91:8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davodeau F, Peyrat M-A, Hallet M-M, Gaschet J, Houde I, Vivien R, Vie H, Bonneville M. Close correlation between Daudi and mycobacterial antigen recognition by human γδ T cells and expression of V9JPC1γ/V2DJCδ-encoded T cell receptors. J. Immunol. 1993;151:1214–1223. [PubMed] [Google Scholar]
- 45.De Libero G, Casorati G, Giachino C, Carbonara C, Migone N, Matzinger P, Lanzavecchia A. Selection by two powerful antigens may account for the presence of the major population of human peripheral γ/δ T cells. J. Exp. Med. 1991;173:1311–1322. doi: 10.1084/jem.173.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bender A, Heckl-Östreicher B, Grondal EJM, Kabelitz D. Clonal specificity of human γδ T cells: Vγ9+ T-cell clones frequently recognize Plasmodium falciparum merozoites, Mycobacterium tuberculosis, and group-A streptococci. Int. Arch. Allergy Immunol. 1993;100:12–18. doi: 10.1159/000236381. [DOI] [PubMed] [Google Scholar]
- 47.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adams EJ, Chien YH, Garcia KC. Structure of a γδ T cell receptor in complex with the nonclassical MHC T22. Science. 2005;308:227–231. doi: 10.1126/science.1106885. [DOI] [PubMed] [Google Scholar]
- 49.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J. CD1d–lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 50.Tynan FE, Reid HH, Kjer-Nielsen L, Miles JJ, Wilce MC, Kostenko L, Borg NA, Williamson NA, Beddoe T, Purcell AW, Burrows SR, McCluskey J, Rossjohn J. A T cell receptor flattens a bulged antigenic peptide presented by a major histocompatibility complex class I molecule. Nat. Immunol. 2007;8:268–276. doi: 10.1038/ni1432. [DOI] [PubMed] [Google Scholar]
- 51.Gagnon SJ, Borbulevych OY, Davis-Harrison RL, Turner RV, Damirjian M, Wojnarowicz A, Biddison WE, Baker BM. T cell receptor recognition via cooperative conformational plasticity. J. Mol. Biol. 2006;363:228–243. doi: 10.1016/j.jmb.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 52.Garboczi DN, Ghosh P, Utz U, Fan QR, Biddison WE, Wiley DC. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- 53.Kjer-Nielsen L, Clements CS, Purcell AW, Brooks AG, Whisstock JC, Burrows SR, McCluskey J, Rossjohn J. A structural basis for the selection of dominant αβ T cell receptors in antiviral immunity. Immunity. 2003;18:53–64. doi: 10.1016/s1074-7613(02)00513-7. [DOI] [PubMed] [Google Scholar]
- 54.Garcia KC, Degano M, Pease LR, Huang M, Peterson PA, Teyton L, Wilson IA. Structural basis of plasticity in T cell receptor recognition of a self peptide-MHC antigen. Science. 1998;279:1166–1172. doi: 10.1126/science.279.5354.1166. [DOI] [PubMed] [Google Scholar]
- 55.Colf LA, Bankovich AJ, Hanick NA, Bowerman NA, Jones LL, Kranz DM, Garcia KC. How a single T cell receptor recognizes both self and foreign MHC. Cell. 2007;129:135–146. doi: 10.1016/j.cell.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 56.Buslepp J, Wang H, Biddison WE, Appella E, Collins EJ. A correlation between TCR Vα docking on MHC and CD8 dependence: implications for T cell selection. Immunity. 2003;19:595–606. doi: 10.1016/s1074-7613(03)00269-3. [DOI] [PubMed] [Google Scholar]
- 57.Ding YH, Smith KJ, Garboczi DN, Utz U, Biddison WE, Wiley DC. Two human T cell receptors bind in a similar diagonal mode to the HLA- A2/Tax peptide complex using different TCR amino acids. Immunity. 1998;8:403–411. doi: 10.1016/s1074-7613(00)80546-4. [DOI] [PubMed] [Google Scholar]
- 58.Stewart-Jones GB, McMichael AJ, Bell JI, Stuart DI, Jones EY. A structural basis for immunodominant human T cell receptor recognition. Nat. Immunol. 2003;4:657–663. doi: 10.1038/ni942. [DOI] [PubMed] [Google Scholar]
- 59.Reiser JB, Grégoire C, Darnault C, Mosser T, Guimezanes A, Schmitt-Verhulst AM, Fontecilla-Camps JC, Mazza G, Malissen B, Housset D. A T cell receptor CDR3β loop undergoes conformational changes of unprecedented magnitude upon binding to a peptide/MHC class I complex. Immunity. 2002;16:345–354. doi: 10.1016/s1074-7613(02)00288-1. [DOI] [PubMed] [Google Scholar]
- 60.Hoare HL, Sullivan LC, Pietra G, Clements CS, Lee EJ, Ely LK, Beddoe T, Falco M, Kjer-Nielsen L, Reid HH, McCluskey J, Moretta L, Rossjohn J, Brooks AG. Structural basis for a major histocompatibility complex class Ib-restricted T cell response. Nat. Immunol. 2006;7:256–264. doi: 10.1038/ni1312. [DOI] [PubMed] [Google Scholar]
- 61.Tynan FE, Burrows SR, Buckle AM, Clements CS, Borg NA, Miles JJ, Beddoe T, Whisstock JC, Wilce MC, Silins SL, Burrows JM, Kjer-Nielsen L, Kostenko L, Purcell AW, McCluskey J, Rossjohn J. T cell receptor recognition of a 'super-bulged' major histocompatibility complex class I-bound peptide. Nat. Immunol. 2005;6:1114–1122. doi: 10.1038/ni1257. [DOI] [PubMed] [Google Scholar]
- 62.Reiser JB, Darnault C, Grégoire C, Mosser T, Mazza G, Kearney A, van der Merwe PA, Fontecilla-Camps JC, Housset D, Malissen B. CDR3 loop flexibility contributes to the degeneracy of TCR recognition. Nat. Immunol. 2003;4:241–247. doi: 10.1038/ni891. [DOI] [PubMed] [Google Scholar]
- 63.Reiser JB, Darnault C, Guimezanes A, Grégoire C, Mosser T, Schmitt-Verhulst AM, Fontecilla-Camps JC, Malissen B, Housset D, Mazza G. Crystal structure of a T cell receptor bound to an allogeneic MHC molecule. Nat. Immunol. 2000;1:291–297. doi: 10.1038/79728. [DOI] [PubMed] [Google Scholar]
- 64.Mazza C, Auphan-Anezin N, Gregoire C, Guimezanes A, Kellenberger C, Roussel A, Kearney A, van der Merwe PA, Schmitt-Verhulst AM, Malissen B. How much can a T-cell antigen receptor adapt to structurally distinct antigenic peptides? EMBO J. 2007;26:1972–1983. doi: 10.1038/sj.emboj.7601605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gras S, Burrows SR, Kjer-Nielsen L, Clements CS, Liu YC, Sullivan LC, Bell MJ, Brooks AG, Purcell AW, McCluskey J, Rossjohn J. The shaping of T cell receptor recognition by self-tolerance. Immunity. 2009;30:193–203. doi: 10.1016/j.immuni.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 66.Reinherz EL, Tan K, Tang L, Kern P, Liu J, Xiong Y, Hussey RE, Smolyar A, Hare B, Zhang R, Joachimiak A, Chang HC, Wagner G, Wang J. The crystal structure of a T cell receptor in complex with peptide and MHC class II. Science. 1999;286:1913–1921. doi: 10.1126/science.286.5446.1913. [DOI] [PubMed] [Google Scholar]
- 67.Hennecke J, Carfi A, Wiley DC. Structure of a covalently stabilized complex of a human αβ T-cell receptor, influenza HA peptide and MHC class II molecule, HLA-DR1. EMBO J. 2000;19:5611–5624. doi: 10.1093/emboj/19.21.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maynard J, Petersson K, Wilson DH, Adams EJ, Blondelle SE, Boulanger MJ, Wilson DB, Garcia KC. Structure of an autoimmune T cell receptor complexed with class II peptide-MHC: insights into MHC bias and antigen specificity. Immunity. 2005;22:81–92. doi: 10.1016/j.immuni.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 69.Li Y, Huang Y, Lue J, Quandt JA, Martin R, Mariuzza RA. Structure of a human autoimmune TCR bound to a myelin basic protein self-peptide and a multiple sclerosis-associated MHC class II molecule. EMBO J. 2005;24:2968–2979. doi: 10.1038/sj.emboj.7600771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deng L, Langley RJ, Brown PH, Xu G, Teng L, Wang Q, Gonzales MI, Callender GG, Nishimura MI, Topalian SL, Mariuzza RA. Structural basis for the recognition of mutant self by a tumor-specific, MHC class II-restricted T cell receptor. Nat. Immunol. 2007;8:398–408. doi: 10.1038/ni1447. [DOI] [PubMed] [Google Scholar]
- 71.Dai S, Huseby ES, Rubtsova K, Scott-Browne J, Crawford F, Macdonald WA, Marrack P, Kappler JW. Crossreactive T Cells spotlight the germline rules for αβ T cell-receptor interactions with MHC molecules. Immunity. 2008;28:324–334. doi: 10.1016/j.immuni.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brenner MB, Strominger JL, Krangel MS. The γδ T cell receptor. Adv. Immunol. 1988;43:133–192. [PubMed] [Google Scholar]
- 73.Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature. 1986;322:836–840. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
- 74.Scotet E, Martinez LO, Grant E, Barbaras R, Jenö P, Guiraud M, Monsarrat B, Saulquin X, Maillet S, Estève JP, Lopez F, Perret B, Collet X, Bonneville M, Champagne E. Tumor recognition following Vγ9Vδ2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A–I. Immunity. 2005;22:71–80. doi: 10.1016/j.immuni.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 75.Delfau M-H, Hance AJ, Lecossier D, Vilmer E, Grandchamp B. Restricted diversity of Vγ9-JP rearrangements in unstimulated human γ/δ T lymphocytes. Eur. J. Immunol. 1992;22:2437–2443. doi: 10.1002/eji.1830220937. [DOI] [PubMed] [Google Scholar]
- 76.Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 77.Rock EP, Sibbald PR, Davis MM, Chien Y-h. CDR3 length in antigen-specific immune receptors. J. Exp. Med. 1994;179:323–328. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Allison TJ, Garboczi DN. Structure of γδ T cell receptors and their recognition of non-peptide antigens. Mol. Immunol. 2002;38:1051–1061. doi: 10.1016/s0161-5890(02)00034-2. [DOI] [PubMed] [Google Scholar]
- 79.Davodeau F, Peyrat MA, Hallet MM, Houde I, Vie H, Bonneville M. Peripheral selection of antigen receptor junctional features in a major human γδ subset. Eur. J. Immunol. 1993;23:804–808. doi: 10.1002/eji.1830230405. [DOI] [PubMed] [Google Scholar]
- 80.Borg NA, Ely LK, Beddoe T, Macdonald WA, Reid HH, Clements CS, Purcell AW, Kjer-Nielsen L, Miles JJ, Burrows SR, McCluskey J, Rossjohn J. The CDR3 regions of an immunodominant T cell receptor dictate the 'energetic landscape' of peptide-MHC recognition. Nat. Immunol. 2005;6:171–180. doi: 10.1038/ni1155. [DOI] [PubMed] [Google Scholar]
- 81.Manning TC, Schlueter CJ, Brodnicki TC, Parke EA, Speir JA, Garcia KC, Teyton L, Wilson IA, Kranz DM. Alanine scanning mutagenesis of an αβ T cell receptor: mapping the energy of antigen recognition. Immunity. 1998;8:413–425. doi: 10.1016/s1074-7613(00)80547-6. [DOI] [PubMed] [Google Scholar]
- 82.Ishizuka J, Stewart-Jones GBE, van der Merwe A, Bell JI, McMichael AJ, Jones EY. The structural dynamics and energetics of an immunodominant T cell receptor are programmed by its Vβ domain. Immunity. 2008;28:171–182. doi: 10.1016/j.immuni.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 83.Havran WL, Chien Y-h, Allison JP. Recogntion of self antigens by skin-derived T cells with invariant γδ receptors. Science. 1991;252:1430–1432. doi: 10.1126/science.1828619. [DOI] [PubMed] [Google Scholar]
- 84.Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD48 α/β T cells demonstrates preferential use of several Vβ genes and an invariant TCR α chain. J. Exp. Med. 1993;178:1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shin S, El-Diwany R, Schaffert S, Adams EJ, Garcia KC, Pereira P, Chien Y-H. Antigen recognition determinants of γδ T cell receptors. Science. 2005;308:252–255. doi: 10.1126/science.1106480. [DOI] [PubMed] [Google Scholar]
- 86.Adams EJ, Strop P, Shin S, Chien YH, Garcia KC. An autonomous CDR3δ is sufficient for recognition of the nonclassical MHC class I molecules T10 and T22 by γδ T cells. Nat. Immunol. 2008;9:777–784. doi: 10.1038/ni.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pellicci DG, Patel O, Kjer-Nielsen L, Pang SS, Sullivan LC, Kyparissoudis K, Brooks AG, Reid HH, Gras S, Lucet IS, Koh R, Smyth MJ, Mallevaey T, Matsuda JL, Gapin L, McCluskey J, Godfrey DI, Rossjohn J. Differential recognition of CD1d-α-galactosyl ceramide by the Vβ8.2 and Vβ7 semi-invariant NKT T cell receptors. Immunity. 2009;31:47–59. doi: 10.1016/j.immuni.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wun KS, Borg NA, Kjer-Nielsen L, Beddoe T, Koh R, Richardson SK, Thakur M, Howell AR, Scott-Browne JP, Gapin L, Godfrey DI, McCluskey J, Rossjohn J. A minimal binding footprint on CD1d–glycolipid is a basis for selection of the unique human NKT TCR. J. Exp. Med. 2008;205:939–949. doi: 10.1084/jem.20072141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Godfrey DI, Rossjohn J, McCluskey J. The fidelity, occasional promiscuity, and versatility of T cell receptor recognition. Immunity. 2008;28:304–314. doi: 10.1016/j.immuni.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 90.Hahn M, Nicholson MJ, Pyrdol J, Wucherpfennig KW. Unconventional topology of self peptide-major histocompatibility complex binding by a human autoimmune T cell receptor. Nat. Immunol. 2005;6:490–496. doi: 10.1038/ni1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deng L, Mariuzza RA. Recognition of self-peptide-MHC complexes by autoimmune T-cell receptors. Trends Biochem. Sci. 2007;32:500–508. doi: 10.1016/j.tibs.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sicard H, Ingoure S, Luciani B, Serraz C, Fournié J-J, Bonneville M, Tiollier J, Romagné F. In vivo immunomanipulation of Vγ9Vδ2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J. Immunol. 2005;175:5471–5480. doi: 10.4049/jimmunol.175.8.5471. [DOI] [PubMed] [Google Scholar]
- 93.Daubenberger CA, Salomon M, Vecino W, Hubner B, Troll H, Rodriques R, Patarroyo ME, Pluschke G. Functional and structural similarity of Vγ9Vδ2 T cells in humans and Aotus monkeys, a primate infection model for Plasmodium falciparum malaria. Journal of Immunology. 2001;167:6421–6430. doi: 10.4049/jimmunol.167.11.6421. [DOI] [PubMed] [Google Scholar]
- 94.Xiong X, Morita CT, Bukowski JF, Brenner MB, Dascher CC. Identification of guinea pig γδ T cells and characterization during pulmonary tuberculosis. Vet. Immunol. Immunopathol. 2004;102:33–44. doi: 10.1016/j.vetimm.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 95.Kato Y, Tanaka Y, Tanaka H, Yamashita S, Minato N. Requirement of species-specific interactions for the activation of human γδ T cells by pamidronate. J. Immunol. 2003;170:3608–3613. doi: 10.4049/jimmunol.170.7.3608. [DOI] [PubMed] [Google Scholar]
- 96.Welsh MD, Kennedy HE, Smyth AJ, Girvin RM, Andersen P, Pollock JM. Responses of bovine WC1+ γδ T cells to protein and nonprotein antigens of Mycobacterium bovis. Infect. Immun. 2002;70:6114–6120. doi: 10.1128/IAI.70.11.6114-6120.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Green AE, Lissina A, Hutchinson SL, Hewitt RE, Temple B, James D, Boulter JM, Price DA, Sewell AK. Recognition of nonpeptide antigens by human Vγ9Vδ2 T cells requires contact with cells of human origin. Clin. Exp. Immunol. 2004;136:472–482. doi: 10.1111/j.1365-2249.2004.02472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scott-Browne JP, Matsuda JL, Mallevaey T, White J, Borg NA, McCluskey J, Rossjohn J, Kappler J, Marrack P, Gapin L. Germline-encoded recognition of diverse glycolipids by natural killer T cells. Nat. Immunol. 2007;8:1105–1113. doi: 10.1038/ni1510. [DOI] [PubMed] [Google Scholar]
- 99.Mallevaey T, Scott-Browne JP, Matsuda JL, Young MH, Pellicci DG, Patel O, Thakur M, Kjer-Nielsen L, Richardson SK, Cerundolo V, Howell AR, McCluskey J, Godfrey DI, Rossjohn J, Marrack P, Gapin L. T cell receptor CDR2β and CDR3β loops collaborate functionally to shape the iNKT cell repertoire. Immunity. 2009;31:60–71. doi: 10.1016/j.immuni.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Florence WC, Xia C, Gordy LE, Chen W, Zhang Y, Scott-Browne J, Kinjo Y, Yu KO, Keshipeddy S, Pellicci DG, Patel O, Kjer-Nielsen L, McCluskey J, Godfrey DI, Rossjohn J, Richardson SK, Porcelli SA, Howell AR, Hayakawa K, Gapin L, Zajonc DM, Wang PG, Joyce S. Adaptability of the semi-invariant natural killer T-cell receptor towards structurally diverse CD1d–restricted ligands. EMBO J. 2009;28:3579–3590. doi: 10.1038/emboj.2009.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morita CT, Mariuzza RA, Brenner MB. Antigen recognition by human γδ T cells: pattern recognition by the adaptive immune system. Springer Semin. Immunopathol. 2000;22:191–218. doi: 10.1007/s002810000042. [DOI] [PubMed] [Google Scholar]
- 102.Evans PS, Enders PJ, Yin C, Ruckwardt TJ, Malkovsky M, Pauza CD. In vitro stimulation with a non-peptidic alkylphosphate expands cells expressing Vγ2-Jγ1.2/Vδ2 T-cell receptors. Immunology. 2001;104:19–27. doi: 10.1046/j.0019-2805.2001.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chien Y-H, Bonneville M. Gamma delta T cell receptors. Cell. Mol. Life Sci. 2006;63:2089–2094. doi: 10.1007/s00018-006-6020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Born WK, O'Brien RL. Antigen-restricted γδ T-cell receptors? Arch. Immunol. Ther. Exp. (Warsz) 2009;57:129–135. doi: 10.1007/s00005-009-0017-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.