Abstract
Platelet aggregation in the blood stream is tightly associated with the secretory function of platelets based on several types of cytoplasmic secretory granules, each sequestering distinct chemical messenger species. Dense-body granules are one prominent type of secretory granules responsible for storing small molecule chemical messengers. Upon platelet activation, the timely and rapid release of these small molecules is critical in facilitating platelet aggregation. Therefore, techniques capable of measuring real-time granule content release are needed to understand the fundamental properties of platelet secretion and aggregation. Existing techniques lack adequate time resolution or require potentially toxic exogenous reagents for real-time measurement of granule content release. Herein, we demonstrate a label-free electrochemical method based on the endogenous electroactive chemical messenger serotonin (5-hydroxytryptamine or 5-HT) for the real-time measurement of dense-body granule secretion from platelet suspensions; fast-scan cyclic voltammetry (FSCV) using carbon-fiber microelectrodes was chosen based on its excellent temporal resolution, high sensitivity, and the ability to provide the electrochemical signature cyclic voltammograms for molecular identification. Real-time serotonin release from thrombin-stimulated human platelet suspensions was successfully measured and the amount and time course of the bulk serotonin release were found to agree well with data obtained from single platelet measurements, thus confirming accurate characterization of granular secretion. Furthermore, this electrochemical method was applied to study the stimulation-secretion coupling in platelets, serotonin storage and release dynamics with applied pharmacological agents, and chemical messenger storage deficiency in Hermansky-Pudlak Syndrome (HPS) platelets, and has clearly demonstrated the potential of this method to reveal secretion behavior in both normal and diseased platelets.
INTRODUCTION
Platelets in the circulatory system are charged with the critical task of maintaining the integrity of the vascular system. Upon vascular injury, these tiny anuclear cells sense vessel breakage and rapidly change shape and aggregate at the injury site to form a plug, and thus, assist in arrest of bleeding. To facilitate aggregation, platelets store and release a spectrum of chemical messengers ranging from ions and small molecules to adhesive proteins.1 These small and large chemical species are stored within two distinct secretory granule populations, dense-body granules (also called θ-granules) and α-granules, respectively. Following activation, platelets rapidly release the stored granule contents to the cell exterior within seconds to recruit additional platelets and facilitate the timely reseal of the injured blood vessel. Accordingly, characterization of the fundamental platelet secretion behavior and its critical function in platelet aggregation requires a technique capable of sub-second, quantitative measurement of platelet granule content release.
Traditionally employed methods for studies of platelet dense-body granule secretion include transmission electron microscopy (TEM) for microstructural analysis and a number of functional assays for content release monitoring and/or quantitation in platelet suspensions. While powerful in revealing occurrence and morphology of dense-body granules,2 TEM is limited to providing static images of the secretion process. In fact, due to the labile chemical nature of the dense-body granule content, capturing TEM images of dense bodies in the act of release has proven highly challenging. Conventional analytical tools including high-performance liquid chromatography (HPLC),3, 4 radiolabeling assays5 and ATP-luminescence assays6, 7 clearly provide a more dynamic picture of platelet dense-body granule release; however, some of these tools, such as the widely used separation-based HPLC technique and the current gold-standard release assay based on [14C]-serotonin radiolabeling, involve tedious periodic sampling from a platelet suspension, and thus, are clearly incapable of measuring real-time platelet release. To date, the most suitable analytical tools to measure this rapid release process are ATP luminescence assays and dye-based fluorescence assays. The ATP luminescence assay is a popular choice to measure real-time endogenous ATP release from platelet dense-body granules; however, this method is a multi-component assay that requires significant optimization to achieve satisfactory results.8 Fluorescence assays based on the release of the selectively accumulated exogenous dyes in the dense-body granules are useful to accurately monitor the time course of dense-body granule release;9 however these assays are incapable of providing quantitative information about endogenous chemical messenger storage and release, and, in fact, the loaded exogenous dye molecules may alter the native behavior of platelet dense-body granule release.10
Electrochemical methods provide an appealing alternative for quantitative and real-time measurement of content release from platelet dense-body granules based on the electroactive nature of the endogenous serotonin molecules. Since the pioneering electroanalytical work in brain chemistry by Adams and coworkers,11 electrochemical methods have been established as the tools of choice to investigate real-time release dynamics of several classes of important biological molecules. For example, electrochemical methods including fast-scan cyclic voltammetry, chronoamperometry, and constant-potential amperometry have been routinely used to measure dynamics of indolamine and catecholamine release and uptake in various preparations. Representative examples of such applications include measurements of dopamine dynamics in the brain,12 serotonin uptake kinetics in synaptosomes13 and cell suspensions,14 and catecholamine secretion from single adrenal chromaffin15 and PC12 cells.16 Previously, our group successfully implemented constant-potential amperometry at carbon-fiber microelectrodes to study the quantal secretion behavior of serotonin release from single rabbit platelets.17-19 While powerful in gaining insight into platelet quantal secretion behavior, single platelet measurement suffers from low throughput and difficulty in measuring secretion from single platelets with low serotonin content, such as human platelets. In this report, we further exploit serotonin electrochemistry at carbon-fiber microelectrodes, and demonstrate a fast-scan cyclic voltammetric method to reliably measure serotonin release from suspensions of human platelets with higher throughput. In addition, this electrochemical method has a sub-second (0.2 s) time resolution, two orders of magnitude improvement over a previously reported method based on differential pulse voltammetry.20 Compared to the aforementioned non-electrochemical based techniques, this electrochemical tool is superior in that it is a label-free method, introducing minimal platelet perturbation. More importantly, it is a simple and robust method capable of measuring real-time release of dense-body granule content based on endogenous chemical species, useful for examining pharmacologically or genetically induced alterations in chemical messenger storage and release.
EXPERIMENTAL SECTION
Preparation of Washed Human Platelets
ACD-anticoagulated whole blood samples (~9 ml per vial) from random donors and in-dated platelet-rich plasma (PRP) used for transfusion (~1.5 × 109 platelets/mL, 60 mL) were obtained from the Memorial Blood Centers (St. Paul, MN). HPS blood samples were obtained from known HPS patients under approved IRB protocol #0309M51992. The whole blood sample was centrifuged at 250 g for 14 min to obtain PRP. Two thirds of the upper PRP was transferred, mixed with half volume of ACD, and centrifuged at 2200 g for 16 min to pellet the platelets. The resulting pellet was resuspended in PGI2-containing (Prostaglandin I2, 0.5 μM, stock 1.0 mM prepared in 50 mM Tris buffer, pH 8.8) and Ca2+-free Tyrode’s buffer (NaCl 137 mM, KCl, 2.6 mM, MgCl2, 1.0 mM, D-glucose, 5.6 mM, HEPES 5.0, and NaHCO3, 12.1 mM with pH adjusted to 7.4), and washed twice at 1900g for 8 min. Finally, the platelets were resuspended in Ca2+-free Tyrode’s buffer (2-4 × 108 platelets/mL) and stored at room temperature with occasional agitation to prevent platelet sedimentation. Platelets were counted manually using a standard hemocytometer on a microscope equipped with phase-contrast optics.
Fabrication of Carbon-Fiber Microelectrodes
The fabrication procedures for disk-shaped and cylindrical carbon-fiber microelectrodes followed the previously published protocols.18, 21 Briefly, a 5-μm-diameter carbon-fiber was aspirated into a glass capillary which was then heated and pulled to generate two conical-shaped electrodes. For the disk-shaped microelectrodes used in single platelet measurements, the exposed carbon-fiber at the electrode tips was trimmed as short as possible with a scalpel under a microscope, and then insulated by dipping electrodes in epoxy solution. After epoxy hardening by baking at 100 °C and 150 °C for 4 and 12 hours, respectively, the electrodes were polished to a 45° angle on a beveler to expose the carbon-fiber surface. For the cylindrical microelectrodes used in platelet suspension measurements, the exposed carbon fibers were trimmed to have ~20 μm protruding length beyond the glass encasement. To re-expose the cylindrical carbon-fiber surface after epoxy dipping, the electrodes were immediately washed three times in acetone (~1 s per wash) to remove excess epoxy. Then, these electrodes were heated to cure the epoxy at 100 °C and 150 °C for 4 and 2 hours, respectively. For both types of microelectrodes, a minimum 10 min soaking in isopropanol was used to activate the carbon surface prior to serotonin release measurements.
Measurements of Serotonin Release from Human Platelet Suspension
Measurements of serotonin release from platelet suspension were performed in siliconized glass vials (Chrono-log Inc.) commonly used for platelet aggregation experiments. The schematic for the experimental setup is shown in Figure 1A. All experiments were performed either at 37 ± 1 °C or at room temperature. Typically, 0.5 mL washed platelet suspension (adjusted to 1.0 × 107 platelets/mL if not specified) was first added to the vial, to which a siliconized stir bar (Chrono-log Inc.) was also added. Then, a cylindrical carbon-fiber microelectrode and a Ag/AgCl wire were inserted into the platelet suspension with the aid of a pair of micromanipulators (Burleigh PCS-5000). Next, the computer-generated waveform (0.2 V to 1 V then to −0.1 V and back to 0.2 V at 1000 V/s, controlled in locally written LabView software) for fast-scan cyclic voltammetry experiments was applied to the electrode via an Axopatch 200B potentiostat (Molecular Devices) at an update frequency of 5 Hz, and the background charging current was allowed to stabilize for ~15 min. Once a stable charging current was achieved, a small volume (typically 5 μL) of a stimulant (0.2~5 U/mL human thrombin, stock 400 U/mL prepared in 150 mM NaCl) was quickly injected into the vial bottom via a syringe, and immediately mixed by a 3-sec stirring at 1150 rpm. Between individual runs, the vials were rinsed thoroughly with copious Tyrode’s buffer and measurements were repeated in the same vial. The pre- and post-calibrations of each electrode were performed in the same manner described above except that serotonin was injected directly into a platelet-free vial. It is noteworthy that serotonin release was often measured from a diluted platelet sample (relative to the physiological platelet count, 1.5~4.5 ×108 platelets/mL) to minimize serotonin fouling of the carbon-fiber microelectrodes.14 A platelet suspension with 1.0 × 107 platelets/mL generates < 1 μM maximum released serotonin, resulting in negligible fouling, and therefore, eliminating the need to pre-treat or replace electrodes after each measurement.
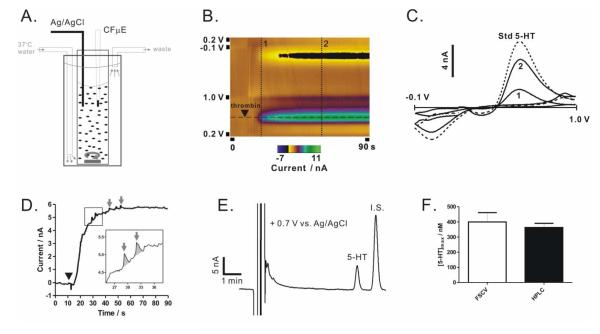
Figure 1. Serotonin release from washed platelet suspension.
(A) Schematic for the experimental setup where CF μE stands for carbon-fiber microelectrode. (B) A false-color plot obtained using FSCV shows serotonin release from a suspension of washed platelets upon thrombin stimulation (symbol ▼). The dotted lines are illustrated below. (C) Cyclic voltammograms obtained from the two time points on the release trace (vertical lines 1 and 2 in B) and a standard serotonin solution (dotted line). (D) The time course of serotonin release replotted at the oxidation peak (current measured at the potential by the horizontal line in B) for each consecutive cyclic voltammogram. Granular secretion of serotonin was occasionally observed (inset and arrows). (E-F) HPLC measurements of serotonin release are compared to FSCV results. [5-HT]max was measured from platelet suspensions of 1.0 × 107 platelets/mL at maximal stimulation (4 U/mL thrombin). Internal standard (I.S.) is N-methylserotonin. Data are represented as mean ± SD, N = 3, and p > 0.3 using the unpaired Student’s t-test.
Pharmacological Manipulations
Washed platelets were resuspended at 2.0 × 108 platelets/mL in Tyrode’s buffer containing 20 μM reserpine (stock 10 mM prepared in DMSO) or 0.5 μM serotonin (stock 10 mM prepared in deoxygenated H2O on the day of experiment), and then incubated for 1 h at room temperature before resuspension in regular Tyrode’s buffer for serotonin release measurements. Incubation with DMSO alone had no effect on serotonin release.
Measurement of Serotonin Release from Single Human Platelets
For single platelet experiments, human platelets were loaded with additional serotonin to facilitate release measurements due to the low physiological serotonin content. The results presented herein were obtained from platelet samples (1.0 × 108 platelets/mL) which had been incubated with 10 μM serotonin for 2 h at room temperature. The procedure for the measurement of serotonin release from single platelets was performed as detailed elsewhere.18 All amperometry measurements were done at room temperature, and platelets were stimulated by a localized 3-sec bolus of 4 U/mL thrombin solution delivered immediately following electrode placement on an individual platelet.
HPLC Analysis
The HPLC system consisted of an Agilent 1200-series HPLC equipped with an auto-sampler, a 5 μm, 4.6 × 150 mm C-18 column (Eclipse XDB-C18, Agilent Inc.), and a glassy carbon-based electrochemical detector (2465 electrochemical detector, Waters Inc.). The working potential was set at 0.7 V vs. an in situ Ag/AgCl reference electrode and the current range was set at 50 nA. The mobile phase was an aqueous mixture of 2 mM NaCl, 30 mg/L sodium octylsulphate, 0.17 mL/L dibutylamine, 55.8 mg/L Na2EDTA, 0.1 M citric acid, 0.1 M sodium acetate and 10% methanol, and the flow rate was set at 1.0 mL/min. Stock solutions of 10 mM serotonin and 10 mM N-methylserotonin (internal standard, I.S.) were prepared in 0.1 M perchloric acid (HClO4). The standard solutions were prepared by diluting stock solutions in 0.4 M HClO4 and then supplemented with 1 μM N-methylserotonin. A five-point calibration curve was constructed using serotonin concentrations ranging from 100 nM to 1000 nM and the area ratio of serotonin to the internal standard was plotted against known serotonin concentrations. For platelet release experiments, platelets (1.0 × 107 platelets/mL) were first activated by exposure to 4 U/mL thrombin for 90 s at 37 °C, and then immediately transferred to a pre-cooled Eppendof vial and kept on ice. To quantify the amount of released serotonin, 250 μL of cooled platelet suspension was centrifuged at 4 °C at 2000 g for 10 min, and then 184.52 μL supernatant was transferred without disturbing the platelet pellet, mixed with 4 μL 50 μM internal standard, and 11.48 μL of 70% HClO4. The mixture was sonicated for 5 min and then incubated for 25 min before filtration (0.2 μm PTFE filters) and injection onto the HPLC column. To determine the total amount of serotonin (rather than only secreted serotonin), 184.52 μL cooled platelet suspension was processed similarly without thrombin exposure or centrifugation. 25 μL of this sample was injected for HPLC analysis, and the amount of serotonin was calculated based on the calibration curve.
Transmission Electron Microscopy Analysis
For thin-section images, platelet fixation was accomplished by incubating the cells with an equal volume of 0.1% glutaraldehyde in White’s saline (a 10% solution of a 1:1 mixture of (1) 2.4 mM NaCl, 0.1 mM KCl, 46 mM MgSO4, and 64 mM Ca(NO3)2·4H2O and (2) 0.13 M NaHCO3, 8.4 mM NaH2PO4, and 0.1 g/L of phenol red, pH 7.4). After 15 min, the sample was centrifuged to pellets, and the supernatant fixative was removed and replaced with 3% glutaraldehyde in the same buffer. The samples resuspended in the second aldehyde fixative were maintained at 4°C for 30 min and then sedimented to pellets. The supernatant was removed and replaced with either 1% osmic acid in Zetterquist’s buffer or 1% osmic acid in distilled water containing 1.5% potassium ferrocyanide for 1 h at 4 °C. All samples were dehydrated in a graded series of alcohols and embedded in Epon 812. Thin sections were cut from the plastic blocks on an ultramicrotome and were examined unstained or after staining with uranyl acetate and lead citrate to enhance contrast. For whole-mount images, small drops of PRP were placed on formvar-coated, carbon-stabilized grids, rinsed within 10-15 s with drops of distilled water, dried from the edge with pieces of filter paper and manually agitated to remove residual moisture. The grids were inserted into the electron microscope without fixation or staining. All micrographs were measured on a Phillips 301 electron microscope at an accelerating voltage of 80 keV.
Data Analysis
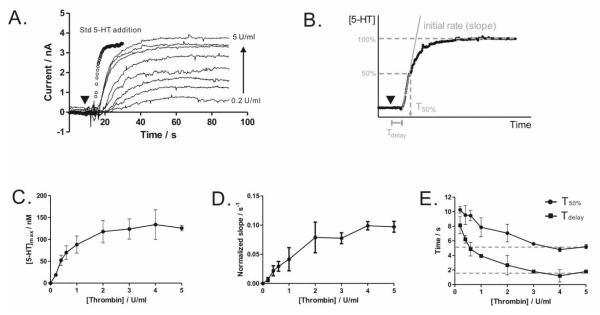
Parameters used to characterize serotonin release from platelet suspensions include [5-HT]max, slope, Tdelay, and T50% shown in Figure 3B. The [5-HT]max was determined by averaging the signal during the last 10 s in the plateau region, and slope was calculated from the first 5 s (at 37 °C) or 10 s (at room temperature) time period following release onset. Tdelay and T50% were determined based on the following events: (1) the time of stimulation was approximated to occur at 9 s based on the fact that the stimulant was manually injected at 5-6 s and mixed by 3 s stirring; (2) the time of release onset and half-height of maximal release were manually determined by identifying the inflection point marking the beginning of the release and the time point corresponding to the half [5-HT]max value, respectively; (3) Tdelay , the time between stimulation and release onset, and T50%, the time to reach half-height of maximal release, were then calculated accordingly. For the data analysis of single platelet amperometric traces, previously described procedures were followed.18, 19 Briefly, the spike area was determined by integrating the spike current from 0.5% of maximal amplitude on the rising phase to 1.0% of maximal amplitude on the decaying phase using commercially available software (Minianalysis, Synaptosoft Inc). All data are reported as mean ± standard deviation (mean ± SD) and subject to unpaired and two-tailed Student’s t-test to determine significant differences (p<0.05).
Figure 3. Thrombin-stimulated serotonin release.
(A) Washed platelets release more serotonin at a faster rate when stimulated with increasing concentrations of thrombin (symbol ▼). (B) Release trace analysis includes the determination of [5-HT]max, slope (initial rate normalized to the saturating [5-HT]max ), Tdelay, and T50%. (C-E) Quantitative analysis of stimulation and secretion coupling. Measurements were performed at 37 ± 1 °C in platelet suspensions of 2.0 × 108 platelets/mL. Data are represented as mean ± SD, N = 3 for all measurements.
RESULTS AND DISCUSSION
Serotonin Release from Platelet Suspension
Platelets sequester highly concentrated serotonin along with other small molecules, including Ca2+ and ATP, within dense-body granules and rapidly release them following activation by physiological stimuli including thrombin.1 To measure real-time serotonin release, a cylindrical carbon-fiber microelectrode was directly immersed in a suspension of washed platelets (Figure 1A). Here, washed platelets, a common preparation of blood platelets for functional assessments, are preferred to whole blood for electrochemical measurements based on their clearly defined solution composition and the absence of electroactive interferants found in the whole blood such as uric acid. Following thrombin addition to the suspension, serotonin release begins after a brief delay (several seconds), and reaches a plateau within ~20 s (Figure 1B and 1D). Occasionally, interesting release features (arrows, Figure 1D) are observed on top of the gradually rising current for newly immersed electrodes. These individual features often exhibit a sharp rise followed by a slow delay, characteristic of granular secretion of serotonin; they are likely resultant from those platelets adjacent to or adhering onto the microelectrodes surface. These individual granule secretion events will be discussed in detail below. To verify the selectivity and quantitative ability of FSCV in this experimental setup, the supernatant from the platelet suspension following thrombin activation was isolated by centrifugation and subject to HPLC analysis (Figure 1E). The chromatogram clearly shows only one serotonin peak in addition to the spiked internal standard and solvent front (containing HClO4 used for deproteinization during sample preparation). Independent quantification from both the electrochemical and HPLC measurements yielded consistent results for the released serotonin amount (Figure 1F) and, based on the HPLC measurements, this amount (0.36 ± 0.03 nmole 5-HT/107 platelets) accounts for ~95% of the total serotonin (0.38 ± 0.01 nmole 5-HT/107 platelets) stored in platelets.
Comparison to Quantal Secretion from Single Human Plateles
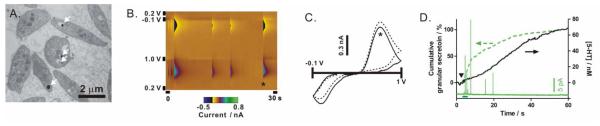
Platelets store serotonin within individual dense-body granules (Figure 2A), and therefore, the measured serotonin release in a platelet suspension is the result of cumulative granular secretion from activated single platelets. Herein, we adopted the experimental protocol of single platelet measurement developed previously17, 18 to verify this origin and gain insight into human platelet serotonin release at the single cell level. As described above, the granular secretion from human platelets was occasionally observed in platelet suspension experiments (Figure 1D); here, on the basis of single platelet measurements, it is clearly demonstrated (Figure 2B). In fact, this is the first dynamic evidence directly supporting human platelet quantal secretion behavior, a phenomenon previously demonstrated for rabbit platelets.18 In the single platelet experiment, a disk-shaped carbon-fiber microelectrode is placed on a single human platelet to measure the released serotonin molecules from individual granules when the platelet is activated by thrombin. In Figure 2B, the FSCV data clearly shows that an activated platelet releases discrete granules, and the extracted cyclic voltammogram confirms the molecular identity of the released serotonin (Figure 2C). To quantify the number of molecules released from each granule, amperometry measurements were subsequently employed due to its superior time resolution (sub-millisecond). Platelets were pre-loaded with additional serotonin to facilitate single-platelet amperometry measurements as the physiological serotonin content in human platelets is generally low, making amperometric measurements challenging. Individual secretion events from these serotonin-loaded platelets were observed as sharp current spikes resulting from the oxidation of released serotonin molecules. The average spike area and total spike area per platelet are 0.22 ± 0.02 pC and 1.1 ± 0.1 pC (mean ± SEM), respectively, which, based on Faraday’s law, translates to 6.9(±0.6) × 105 serotonin molecules released per granule and 3.4(±0.3) × 106 serotonin molecules released per platelet. Though this number of molecules per platelet only accounts for ~69% of the molecules estimated from bulk serotonin release measurement, it is a reasonable percentage based on the fact that single platelet measurements only sample the top half of the surface-adherent platelets and release events may also occur on the bottom half. Furthermore, the average delivery rate of these serotonin-enriched granules to the cell exterior should, in principle, agree with the rate of serotonin release measured from the platelet suspension. Indeed, the time course of the cumulative granular secretion obtained from single platelet measurements matches fairly well with that determined from a platelet suspension (Figure 2D). The apparent deviation is, at least in part, due to the fact that individual platelets were stimulated both chemically and mechanically because the microelectrode was tightly placed on platelets during single platelet amperometry measurements. In fact, mechanically stimulated release was occasionally observed prior to chemical stimulant delivery. Based on the measurements of serotonin release from single platelets and platelet suspensions, it is clear that fast-scan cyclic voltammetry at the carbon-fiber microelectrodes can quantitatively monitor the real-time serotonin release from human platelet suspensions.
Figure 2. Quantal secretion of serotonin from human platelets.
(A) An electron micrograph shows the “bull’s eye”-shaped dense-body granules (arrows). (B) A false-color plot obtained using FSCV shows quantal serotonin release from an individual thrombin-stimulated human platelet. Waveform update frequency is 10 Hz. (C) Cyclic voltammograms obtained from a secretion event (indicated by the * on the color plot) and a standard serotonin solution (dotted line) are overlaid. (D) Cumulative granular serotonin secretion (dotted green line) based on single platelet amperometry measurements (solid green representative amperometric trace at the bottom) was plotted on the left y-axis, while the serotonin release measured from the same serotonin-loaded platelet suspension (1.0 × 107 platelets/mL) was plotted on the right y-axis. Thrombin stimulation times were represented by the symbol ▼and a 3-sec solid bar for platelet suspension and single platelet measurements, respectively. The cumulative release line was constructed using the spike time of 221 spikes collected from 42 single platelets. All measurements were performed at room temperature.
Quantifying Secretion with Varied Stimulation Strength
With the ability to measure real-time serotonin release from platelet suspensions, we next examined the stimulation-secretion coupling by varying the applied thrombin concentration. When the added thrombin concentration was varied from 0.2 to 5 U/ml, the serotonin release was clearly enhanced at higher thrombin concentrations and approached a saturating concentration at ~5 U/ml (Figure 3A). The electrode response to addition of a standard serotonin solution is also superimposed on the release traces, indicating that the maximum slope of the release traces is a measure of platelet release behavior, rather than the electrode response time. Quantitative analysis (Figure 3B-E) shows a clear dose-dependence of serotonin release on thrombin concentration. The maximum serotonin release, [5-HT]max (Figure 3C), rises nonlinearly with increasing concentrations of thrombin and reaches its maximum at ~4 U/mL. A similar trend was observed for the initial slope (Figure 3D). Contrarily, Tdelay and T50% show an inverse dependence on thrombin concentration (Figure 3E), and both time parameters level off at a similar thrombin concentration. These kinetic parameters are comparable to those determined based on fluorescence measurements of released dyes preloaded in platelet dense-body granules.9 In this study, only a brief agitation for stimulus mixing (3 s at 1150 rpm) was used following thrombin addition and platelets were allowed to release serotonin in static conditions. Constant stirring and extracellular Ca2+ were avoided intentionally in this experiment to minimize platelet aggregation on the electrode tip, because measurement artifacts could be introduced by local serotonin concentration changes within the adherent aggregates. While extracellular Ca2+ is essential for a number of platelet behaviors including aggregation, the absence of extracellular Ca2+ does not influence the amount and rate of thrombin-stimulated platelet serotonin release (supporting info, Figure S-1). Overall, the electrochemical method developed herein clearly reveals the quantitative stimulation-secretion coupling by measuring the real-time release of endogenous serotonin from platelets.
Pharmacological Manipulation of Serotonin Storage and Release
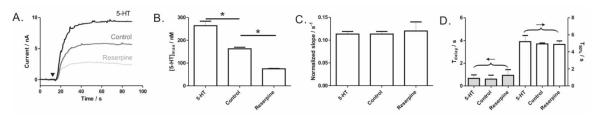
To further demonstrate the ability of this method to reveal fundamental platelet secretory behavior, we next investigated the dynamics of serotonin storage and release by platelets following pharmacological manipulation. In the blood stream, platelets are the primary cell type responsible for regulating blood serotonin levels.22 These cells possess a set of protein machinery, including serotonin transporters on the cell membrane and monoamine transporters on the granule membrane, to efficiently take up serotonin from the bloodstream and ultimately package it into the dense-body granules.23, 24 Herein, washed platelets treated with exogenous serotonin and reserpine, a drug known to act on the monoamine transporter and deplete granule-stored monoamine, clearly showed (Figure 4A-B) increased and decreased maximum releasable serotonin content, respectively; however, the time course of the release, characterized by the normalized slope, Tdelay and T50%, was not altered (Figure 4C-D). These results indicate that the ability of platelets to deliver dense-body granules to the cell exterior was not comprised upon serotonin content manipulation, and evidently suggest that the observed difference in serotonin release was due to changes in granule serotonin storage only. Indeed, the current best method of assessing platelet dense-body granule secretion, the [14C]-serotonin assay, takes advantage of this mechanism by loading platelets with radiolabeled serotonin and then monitoring the radioactivity of the released radiolabeled serotonin from stimulated platelets.5 In comparison, the electrochemical method detailed herein clearly offers a superior alternative to study serotonin storage and release dynamics in platelets because the radioactive isotopes are not necessary and kinetic information is readily available.
Figure 4. Reserpine and serotonin effects.
Reserpine and exogenous serotonin treatment of platelets alters (A-B) the amounts of serotonin released, but not (C-D)the time courses of platelet release. Slope in C was normalized to its own [5-HT]max. Maximum thrombin stimulation (4 U/mL, symbol▼) was used and measurements were performed at 37 ± 1 °C in platelet suspensions of 2.0 × 108 platelets/mL. Data are represented as mean ± SD, N = 3 and *p < 0.002 using unpaired Student’s t-test.
Serotonin Release from Hermansky-Pudlak Syndrome Platelets
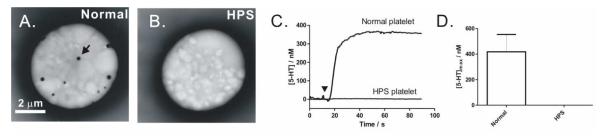
Finally, we exploited this method to study serotonin release from clinically relevant platelets. Chemical messengers stored in and released from dense-body granules play an important role in promoting blood clotting, and therefore, platelets that do not have dense-body granules or do not have the ability to retain critical granule contents may cause bleeding disorders. Hermansky-Pudlak Syndrome, an autosomal recessive disorder, is such a disease.25, 26 One hallmark of clinical diagnosis for HPS is the absence of dense-body granules in patients’ platelets. When platelets are air-dried and viewed intact by electron microscopy, normal platelets from healthy donors typically exhibit several opaque granules (Figure 5A) which are the electron-dense cores of dense-body granules; however, such features are absent in HPS platelets (Figure 5B). Previous studies using techniques such as HPLC have reported that HPS platelets do not store serotonin, and thus, are incapable of releasing it when needed.27 Here, we exploited the electrochemical method to confirm the absence of serotonin release from HPS platelets. Indeed, HPS platelets do not release serotonin (Figure 5C-D). Though, in some cases, minimal serotonin release was measured from highly concentrated platelet suspensions due to the exquisite sensitivity of this electrochemical method (supporting info, Figure S-2). From the normalized time course obtained from these highly concentrated platelet samples, the delivery of granules to the cell exterior appears normal, but its stored serotonin content is not. This study clearly confirms the abnormal storage behavior of HPS dense-body granules and also demonstrates the potential of this electrochemical method in assisting with the diagnosis of diseased platelets.
Figure 5. Serotonin release from normal and Hermansky-Pudlak Syndrome platelets.
When viewed using whole-mount TEM, (A) normal platelets typically exhibit several dense-body granules (one is highlighted by a black arrow) within the platelet cytoplasm, while this feature is absent for (B) HPS platelets. (C-D) No detectable serotonin release was measured from HPS platelet samples. Data are represented as mean ± SD, N = 7 and 5 donors for normal and HPS platelets, respectively. Maximal thrombin stimulation (4 U/mL, symbol▼) was used and measurements were performed at 37 ± 1 °C in platelet suspensions of 1.0 × 107 platelets/mL.
CONCLUSION
With the ability to measure dense-body granule secretion from single human platelets and human platelet suspensions, there has never been a better opportunity to unravel the inner-workings of platelets as they deliver critical chemical messengers. Based on the ubiquitous presence of serotonin in the platelets of various animal species, this electrochemical method can be broadly applied to gain mechanistic understanding of secretion behavior in both normal and diseased platelets. Not only uniquely positioned to give insights into the fundamental platelet secretion process, this electrochemical method also has the potential to be used as a diagnostic tool in place of the gold-standard [14C]-serotonin assay based on its simplicity and environment-friendly nature, and therefore, should prove to be useful in both research and clinical settings.
Supplementary Material
ACKNOWLEDGEMENT
We gratefully thank M. Krumwiede for her help with TEM studies. This work is generously supported by the National Institute of Health New Innovator Award and Camille Dreyfus Teacher-Scholar Award to CLH.
REFERENCES
- 1.Holmsen H, Weiss HJ. Annu.Rev.Med. 1979;30:119–134. doi: 10.1146/annurev.me.30.020179.001003. [DOI] [PubMed] [Google Scholar]
- 2.White JG, Krumwiede M. Thromb.Haemost. 2007;98:69–72. [PubMed] [Google Scholar]
- 3.Anderson GM, Hall LM, Yang JX, Cohen DJ. Anal.Biochem. 1992;206:64–67. doi: 10.1016/s0003-2697(05)80011-9. [DOI] [PubMed] [Google Scholar]
- 4.Bossant MJ, Ninio E, Delautier D, Bessou G, Trouvin JH, Benveniste J. Anal.Biochem. 1989;182:419–423. doi: 10.1016/0003-2697(89)90617-9. [DOI] [PubMed] [Google Scholar]
- 5.Gear AR, Burke D. Blood. 1982;60:1231–1234. [PubMed] [Google Scholar]
- 6.Neufeld HA, Towner RD, Pace J. Experientia. 1975;31:391–392. doi: 10.1007/BF01922604. [DOI] [PubMed] [Google Scholar]
- 7.Detwiler TC, Feinman RD. Biochemistry. 1973;12:2462–2468. doi: 10.1021/bi00737a015. [DOI] [PubMed] [Google Scholar]
- 8.Ford SR, Leach FR. Methods Mol.Biol. 1998;102:3–20. doi: 10.1385/0-89603-520-4:3. [DOI] [PubMed] [Google Scholar]
- 9.Wuthrich C, Deranleau DA, Dubler D, Luscher EF. Biochemistry. 1984;23:1224–1229. doi: 10.1021/bi00301a031. [DOI] [PubMed] [Google Scholar]
- 10.McCrea JM, Robinson P, Gerrard JM. Biochim.Biophys.Acta. 1985;842:189–194. doi: 10.1016/0304-4165(85)90202-8. [DOI] [PubMed] [Google Scholar]
- 11.Kissinger PT, Hart JB, Adams RN. Brain Res. 1973;55:209–213. doi: 10.1016/0006-8993(73)90503-9. [DOI] [PubMed] [Google Scholar]
- 12.Robinson DL, Hermans A, Seipel AT, Wightman RM. Chem.Rev. 2008;108:2554–2584. doi: 10.1021/cr068081q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez XA, Andrews AM. Anal.Chem. 2005;77:818–826. doi: 10.1021/ac049103g. [DOI] [PubMed] [Google Scholar]
- 14.Singh YS, Sawarynski LE, Michael HM, Ferrell RE, Murphey-Corb MA, Swain GM, Patel BA, Andrews AM. ACS Chem.Neurosci. 2010;1:49–64. doi: 10.1021/cn900012y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leszczyszyn DJ, Jankowski JA, Viveros OH, Diliberto EJ, Jr, Near JA, Wightman RM. J.Biol.Chem. 1990;265:14736–14737. [PubMed] [Google Scholar]
- 16.Chen TK, Luo G, Ewing AG. Anal.Chem. 1994;66:3031–3035. doi: 10.1021/ac00091a007. [DOI] [PubMed] [Google Scholar]
- 17.Ge S, Wittenberg NJ, Haynes CL. Biochemistry. 2008;47:7020–7024. doi: 10.1021/bi800792m. [DOI] [PubMed] [Google Scholar]
- 18.Ge S, White JG, Haynes CL. Anal.Chem. 2009;81:2935–2943. doi: 10.1021/ac8024202. [DOI] [PubMed] [Google Scholar]
- 19.Ge S, White JG, Haynes CL. ACS Chem.Biol. 2010;5:819–828. doi: 10.1021/cb100130b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcenac F, Blache D. Biochim.Biophys.Acta. 1985;840:377–382. doi: 10.1016/0304-4165(85)90218-1. [DOI] [PubMed] [Google Scholar]
- 21.Hochstetler SE, Puopolo M, Gustincich S, Raviola E, Wightman RM. Anal.Chem. 2000;72:489–496. doi: 10.1021/ac991119x. [DOI] [PubMed] [Google Scholar]
- 22.Rand M, Reid G. Nature. 1951;168:385. doi: 10.1038/168385b0. [DOI] [PubMed] [Google Scholar]
- 23.Lesch KP, Wolozin BL, Murphy DL, Reiderer P. J.Neurochem. 1993;60:2319–2322. doi: 10.1111/j.1471-4159.1993.tb03522.x. [DOI] [PubMed] [Google Scholar]
- 24.Holtje M, Winter S, Walther D, Pahner I, Hortnagl H, Ottersen OP, Bader M, Ahnert-Hilger G. J.Biol.Chem. 2003;278:15850–15858. doi: 10.1074/jbc.M212816200. [DOI] [PubMed] [Google Scholar]
- 25.Hermansky F, Pudlak P. Blood. 1959;14:162–169. [PubMed] [Google Scholar]
- 26.Huizing M, Anikster Y, Gahl WA. Traffic. 2000;1:823–835. doi: 10.1034/j.1600-0854.2000.011103.x. [DOI] [PubMed] [Google Scholar]
- 27.Maurer-Spurej E, Dyker K, Gahl WA, Devine DV. Br.J.Haematol. 2002;116:604–611. doi: 10.1046/j.0007-1048.2001.03302.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.