Abstract
A [4+2] cycloaddition of α, β-unsaturated imines and isocyanates catalyzed by a phosphoramiditerhodium complex provides pyrimidinones in good yields and high enantioselectivities.
Pyrimidinones are attractive scaffolds due to their biological activity1 and their accessibility via multicomponent reactions. Traditionally, this motif is synthesized via the three-component Biginelli reaction.2 Improvements on Biginelli's initial report3 include the use of chiral acids to achieve high enantioselectivities4 and departure from classic components.5
Recently, our group has reported asymmetric syntheses of nitrogen-containing heterocycles via rhodium catalysis.6,7 Inspired by Hoberg's report8 of a nickelacycle generated from an imine and isocyanate, we envisioned using α, β-unsaturated imines9 and isocyanates10 to generate pyrimidinones.11 By employing α, β-unsaturated imines, we sought to change the typical substitution pattern that accompanies the classic Biginelli reaction. Herein, we report that a rhodium-phosphoramidite complex catalyzes the [4+2] cycloaddition between α, β-unsaturated imines and isocyanates12 to generate pyrimidinones in good yields and high enantioselectivities.13
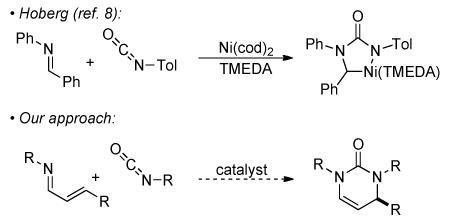
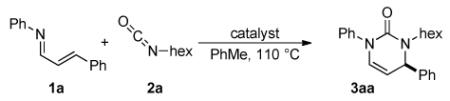
We began our investigations by examining nickel catalysts as per the stoichiometric precedent of Hoberg, but found them ineffective at catalyzing the desired reaction (Table 1, entry 2). Wilkinson's catalyst is only marginally effective, but switching to a Taddol phosphoramidite-rhodium complex provides the target material in moderate yield and enantioselectivity (entries 3 and 4). After exploring various phosphoramidites, we found L2 generates pyrimidinone 3aa in the highest yield and enantioselectivity (entry 5).14
Table 1.
Catalyst Screen.a
 | |||
|---|---|---|---|
| entry | catalyst | yield (%)b | ee (%)c,d |
| 1 | none | 0 | - |
| 2 | Ni(cod)2 (10 mol %), TMEDA (10 mol %) | 0 | - |
| 3 | Rh(PPh3)3Cl (10 mol %) | <5 | - |
| 4 | [Rh(C2H4)2Cl]2 (5 mol %), L1 (10 mol %) | 29 | 81 |
| 5 | [Rh(C2H4)2Cl]2 (5 mol %), L2 (10 mol %) | 56 | 90 |
| 6 | [Rh(C2H4)2Cl]2 (5 mol %), L3 (10 mol %) | 22 | 79 |
| 7 | L2 (10 mol %) | 0 | - |
|
| |||
| |||
Conditions: 1 (0.3 mmol), 2 (1.25 equiv), and catalyst in PhMe at 110 °C for 12 h.
Isolated yield.
Enantiomeric excess determined by HPLC using a chiral stationary phase.
Absolute configuration assigned by analogy to (R)-3af (established by X-ray analysis – see SI).
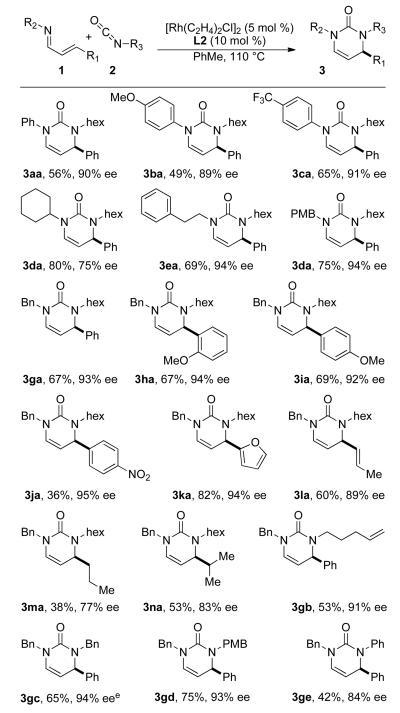
With our optimized catalyst system in hand, we explored the scope of this reaction (Chart 1). Electron-deficient aryl imines (3ca) furnish slightly higher yields and selectivities over electron-rich aryl imines (3ba). Primary alkyl imines generate the highest enantioselectivities with good yield; a secondary alkyl imine is also well-tolerated but provides moderately reduced selectivities (3da). Electron-rich aryl substituents at the 4-position provide products in good yields and high enantioselectivities, while electron-deficient aryl substitution leads to higher enantioselectivity with a decrease in yield. Furyl, vinyl, and alkyl substitution also yields product, with the latter furnishing lower selectivities (3ma). An increase in size of the alkyl substituent only leads to a slight improvement in yield and enantioselectivity (3na). Primary alkyl isocyanates deliver high yields and enantioselectivities.15 Phenyl isocyanate also generates product, but the yield and selectivity is lower. This reaction has been performed on a 4.5 mmol scale using imine 1g and benzyl isocyanate 2c with 2 mol % catalyst loading and the yield and enantioselectivity does not suffer.
Chart 1.
Reaction scope.a
a Conditions: 1 (0.3 mmol), 2 (1.25 equiv), [Rh(C2H4)2Cl]2 (5 mol %), and L2 (10 mol %) in PhMe at 110 °C for 12 h. b Isolated yield. c Enantiomeric excess determined by HPLC using a chiral stationary phase. d Absolute configuration assigned by analogy to (R)-3af (established by X-ray analysis – see SI). e On 4.5 mmol scale with [Rh(C2H4)2Cl]2 (1 mol %) and L2 (2 mol %): 71% yield and 94% ee.
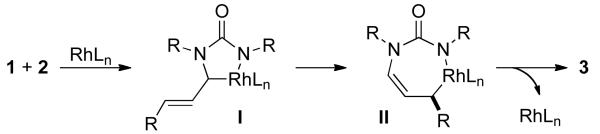
We propose the following mechanism (Scheme 1). Initial coordination of the α, β-unsaturated imine and isocyanate is followed by oxidative cyclization to generate rhodacycle I, which resembles the nickelacycle isolated by Hoberg.8 An η1-η3-η1 shift forms rhodacycle II that reductively eliminates to furnish the pyrimidinone and regenerate catalyst. An alternative mechanism involving a [4+1] cycloaddition between the α, β-unsaturated imine and rhodium can also be envisioned. If a [4+1] cycloaddition occurs first, the stereocenter would be set before the isocyanate is incorporated. Variance in enantioselectivities using different isocyanates suggests that oxidative cyclization of the imine and isocyanate takes place before the enantiodetermining step.
Scheme 1.
Mechanistic Proposal.
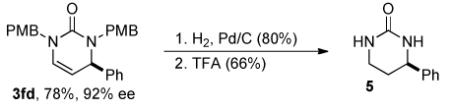
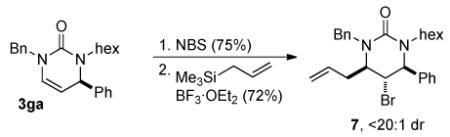
The pyrimidinones generated from this reaction may serve as useful chiral building blocks. After reduction of 3fd, the resulting bis(4-methoxybenzyl)-tetrahydropyrimidinone can be deprotected using neat trifluoroacetic acid (eq 1). In the presence of N-bromosuccinimide and wet dimethylformamide, 3ga generates the bromohydrin that can be converted to 7 using boron trifluoride etherate and allyltrimethylsilane (eq 2).5b
 |
(1) |
 |
(2) |
In conclusion, we report the synthesis of pyrimidinones from α, β-unsaturated imines and isocyanates using a rhodiumphosphoramidite catalyst, affording a substitution pattern complementary to that of Biginelli adducts. This reaction proceeds in moderate to good yields and high enantioselectivities, and the products are useful chiral building blocks.
Supplementary Material
ACKNOWLEDGMENT
We thank NIGMS (GM80442) for support. We thank Johnson Matthey for a loan of rhodium salts. T. R. thanks Roche for an Excellence in Chemistry Award and Amgen for unrestricted support.
Footnotes
SUPPORTING INFORMATION Experimental procedures, characterization, 1H and 13C NMR spectra, and CIF file for 3ge. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.(a) Kappe CO. Eur. J. Med. Chem. 2000;35:1043–1052. doi: 10.1016/s0223-5234(00)01189-2. [DOI] [PubMed] [Google Scholar]; (b) Singh K, Arora D, Singh K, Singh S. Mini-Rev. Med. Chem. 2009;9:95–106. doi: 10.2174/138955709787001686. [DOI] [PubMed] [Google Scholar]
- 2.(a) Kappe CO. J. Org. Chem. 1997;62:7201–7204. doi: 10.1021/jo971010u. [DOI] [PubMed] [Google Scholar]; (b) Kappe CO. Acc. Chem. Res. 2000;33:879–888. doi: 10.1021/ar000048h. [DOI] [PubMed] [Google Scholar]
- 3.Biginelli P. Gazz. Chim. Ital. 1893;23:360–413. [Google Scholar]
- 4.(a) Huang Y, Yang F, Zhu C. J. Am. Chem. Soc. 2005;127:16386–16387. doi: 10.1021/ja056092f. [DOI] [PubMed] [Google Scholar]; (b) Li N, Chen X-H, Song J, Luo S-W, Fan W, Gong L-Z. J. Am. Chem. Soc. 2009;131:15301–15310. doi: 10.1021/ja905320q. [DOI] [PubMed] [Google Scholar]; (c) Cai Y-F, Yang H-M, Li L, Jiang K-Z, Lai G-Q, Jiang J-X, Xu L-W. Eur. J. Org. Chem. 2010:4986–4990. [Google Scholar]; (d) Saha S, Moorthy JN. J. Org. Chem. 2011;76:396–402. doi: 10.1021/jo101717m. [DOI] [PubMed] [Google Scholar]
- 5.(a) Wan J-P, Liu Y. Synthesis. 2010;23:3943–3953. [Google Scholar]; (b) He Z-Q, Zhou Q, Wu L, Chen Y-C. Adv. Synth. Catal. 2010;352:1904–1908. [Google Scholar]
- 6.(a) Perreault S, Rovis T. Chem. Soc. Rev. 2009;38:3149–3159. doi: 10.1039/b816702h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Friedman RK, Oberg KM, Dalton DM, Rovis T. Pure Appl. Chem. 2010;82:1353–1364. doi: 10.1351/PAC-CON-09-12-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Yu RT, Rovis T. J. Am. Chem. Soc. 2006;128:2782–2783. doi: 10.1021/ja057803c. [DOI] [PubMed] [Google Scholar]; (b) Yu RT, Rovis T. J. Am. Chem. Soc. 2006;128:12370–12371. doi: 10.1021/ja064868m. [DOI] [PubMed] [Google Scholar]; (c) Lee EE, Rovis T. Org. Lett. 2008;10(6):1231–1234. doi: 10.1021/ol800086s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yu RT, Rovis T. J. Am. Chem. Soc. 2008;130:3262–3263. doi: 10.1021/ja710065h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Oberg KM, Lee EE, Rovis T. Tetrahedron. 2009;65:5056–5061. doi: 10.1016/j.tet.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Yu RT, Lee EE, Malik G, Rovis T. Angew. Chem. Int. Ed. 2009;48:2379–2382. doi: 10.1002/anie.200805455. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Keller Friedman R, Rovis T. J. Am. Chem. Soc. 2009;131:10775–10782. doi: 10.1021/ja903899c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Yu RT, Friedman RK, Rovis T. J. Am. Chem. Soc. 2009;131:13250–13251. doi: 10.1021/ja906641d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Dalton DM, Oberg KM, Yu RT, Lee EE, Perreault S, Oinen ME, Pease ML, Malik G, Rovis T. J. Am. Chem. Soc. 2009;131:15717–15728. doi: 10.1021/ja905065j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Oinen ME, Yu RT, Rovis T. Org. Lett. 2009;11:4934–4937. doi: 10.1021/ol9020805. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Hyster TK, Rovis T. J. Am. Chem. Soc. 2010;132:10565–10569. doi: 10.1021/ja103776u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoberg H, Sümmermann K. J. Organomet. Chem. 1983;253:383–389. [Google Scholar]
- 9.(a) Behforouz M, Ahmadian M. Tetrahedron. 2000;56:5259–5288. [Google Scholar]; (b) Jayakumar S, Ishar MPS, Mahajan MP. Tetrahedron. 2002;58:379–471. [Google Scholar]; (c) Groenendaal B, Ruijter E, Orru RVA. Chem. Commun. 2008:5474–5489. doi: 10.1039/b809206k. [DOI] [PubMed] [Google Scholar]
- 10.For a similar reaction of α, β-unsaturated imines with ketenes, see; Jian T-Y, Shao P-L, Ye S. Chem. Commun. 2011;47:2381–2383. doi: 10.1039/c0cc04839a.
- 11.Elliot demonstrated that alkenyloxazolines and isocyanates react thermally to generate oxazolopyrimidines stereoselectively; Elliott MC, Kruiswijk E. J. Chem. Soc., Perkin Trans. 1. 1999:3157–3166. Elliott MC, Kruiswijk E, Willock D. J. Tetrahedron. 2001;57:10139–10146. Orru showed that in situ generated 1-H-α, β-unsaturated imines react with isocyanates to generate pyrimidinones; Vugts DJ, Koningstein MM, Schmitz RF, de Kanter FJJ, Groen MB, Orru RVA. Chem. Eur. J. 2006;12:7178–7189. doi: 10.1002/chem.200600168.
- 12.For reviews on cycloadditions involving isocyanates, see: Braunstein P, Nobel D. Chem. Rev. 1989;89:1927–1945. Louie J. Curr. Org. Chem. 2005;9:605–623.
- 13.For related metal-catalyzed reactions generating nitrogen-heterocycles, see: Wender PA, Pedersen TM, Scanio MJC. J. Am. Chem. Soc. 2002;124:15154–15155. doi: 10.1021/ja0285013. Ogoshi S, Ikeda H, Kurosawa H. Angew. Chem. Int. Ed. 2007;46:4930–4932. doi: 10.1002/anie.200700688. Mizuno A, Kusama H, Iwasawa N. Angew. Chem. Int. Ed. 2009;48:8318–8320. doi: 10.1002/anie.200904402. Miura T, Morimoto M, Murakami M. J. Am. Chem. Soc. 2010;132:15836–15838. doi: 10.1021/ja105541r. Adak L, Chan WC, Yoshikai N. Chem. Asian J. 2011;6:359–362. doi: 10.1002/asia.201000564.
- 14.The 1H NMR of the unpurified reaction mixture typically shows unreacted starting material and product only, with occasional urea derived from isocyanate hydrolysis.
- 15.Cyclohexyl isocyanate provides trace product and tert-butyl isocyanate does not generate any product.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.