Abstract
Our previous studies demonstrated that protein kinase D (PKD), a serine/threonine kinase implicated in various cell processes, is up-regulated in basal cell carcinoma (BCC), supporting a possible tumorigenic role for PKD in skin. Since the greatest risk factor for BCC is sun exposure, the ability of ultraviolet B (UVB) irradiation to activate PKD in primary mouse keratinocytes was investigated. Using western analysis with two autophosphorylation-specific antibodies, we show for the first time that UVB activated PKD in a time- and dose-dependent manner. UVB-induced PKD activation was verified using an in vitro kinase assay. Furthermore, activation was reduced by antioxidant pretreatment, suggesting a link with oxidative stress. UVB-induced PKD activation was mediated primarily by Src family tyrosine kinases rather than protein kinase C (PKC), and in fact, UVB did not alter PKC-mediated transphosphorylation. UVB induced apoptosis dose-dependently, and this death could be prevented by overexpression of wild-type PKD, but not mutant PKD or the empty adenovirus. Indeed, a mutant that cannot be phosphorylated by Src kinases exacerbated UVB-elicited apoptosis. Thus, our data indicate that UVB irradiation of keratinocytes induces Src-mediated activation of PKD, which protects cells from UVB-stimulated apoptosis, providing a possible explanation for the observed up-regulation of PKD in BCC.
Keywords: Apoptosis, Epidermis, Protein kinase C (PKC), Protein kinase D (PKD), Skin, Src
INTRODUCTION
Protein kinase D (PKD) is a serine/threonine kinase originally categorized as a member of the protein kinase C (PKC) family (and designated PKCμ), because of its two diacylglycerol-and phorbol ester-binding cysteine-rich domains [reviewed in (Bollag et al., 2004)]. However, further analysis has shown extensive homology to calcium/calmodulin-dependent protein kinases (Bollag et al., 2004), and PKD is, therefore, classified as a member of a new PKD kinase family. Studies have shown a role for PKD in multiple cellular responses. For instance, PKD can promote cellular survival following oxidative stress through its ability to modulate the nuclear factor-κB (NF-κB) pathway (Storz et al., 2003; Storz et al., 2004b; Storz et al., 2004a). Other reports suggest a role of PKD in Golgi trafficking and cell motility [(Liljedahl et al., 2001; Prigozhina and Waterman-Storer, 2004; Yeaman et al., 2004) and reviewed in (Van Lint et al., 2002)]. Still other data indicate that PKD is involved in proliferative responses: PKD is activated by a variety of mitogenic agents (Bollag et al., 2004), and its overexpression in fibroblasts enhances mitogenesis in response to these agents (Zhukova et al., 2001; Sinnett-Smith et al., 2004). Also in epidermal keratinocytes a proproliferative and/or antidifferentiative role for PKD has been proposed (Bollag et al., 2004).
Non-melanoma skin cancers (NMSCs) include squamous cell carcinoma and basal cell carcinoma (BCC). These malignancies are the most common in the United States, with a million new cases diagnosed each year and a rising incidence (Miller and Weinstock, 1994). Exposure to solar ultraviolet radiation (UVR) is the single most important risk factor for developing NMSCs (Kraemer, 1997), which arise from the predominant cells of the epidermis, the keratinocytes. UVR is a potent inducer of oxidative stress in exposed cells and possesses the ability to alter intracellular signaling pathways leading to a pathological state (Marathe et al., 2005). Mitochondria are a major UVR-responsive organelle, and studies have shown that PKD can localize to this organelle [e.g., (Storz et al., 2005)], implying a possible role of PKD in the cellular response of keratinocytes to UVR. Further suggesting a potential link between PKD and epidermal tumorigenesis, PKD levels are increased in mouse epidermal carcinomas (Rennecke et al., 1999) and in human BCC (Ristich et al., 2006). These results raise the possibility of an involvement of PKD in the keratinocyte response to UVR and photocarcinogenesis.
Results from the present study indicate that ultraviolet B (UVB) irradiation of primary mouse keratinocytes resulted in activation of PKD through oxidative stress and the action of Src family tyrosine kinases. Moreover, activated PKD protected keratinocytes from UVB-induced apoptosis, suggesting that alterations in PKD signaling upon UVB irradiation of keratinocytes could promote photocarcinogenesis. These results thus provide a link between our previous observation of elevated levels of PKD in BCC and epidermal tumorigenesis.
RESULTS
UVB induced PKD activation in a time- and dose-dependent manner in primary mouse keratinocytes
Solar UVR is the single most important risk factor for the development of NMSCs, such as BCC, in which we previously observed elevated PKD levels (Ristich et al., 2006). To investigate whether UVB irradiation of primary mouse keratinocytes results in activation of PKD, cells were exposed to 30 mJ/cm2 (in humans a minimal erythemic dose to produce skin burns). Cell lysates were then collected at 15 minutes and 1, 2 and 4 hours post-exposure and processed for western blotting employing an antibody recognizing PKD’s phosphoserine916 (phosphoserine910 in humans) [an autophosphorylation site corresponding to active PKD (Matthews et al., 1999)] and total PKD. UVB irradiation of keratinocytes induced an increase in PKD serine916 phosphorylation observed as early as 2 hrs and remaining elevated at 4 hrs after exposure (Figure 1). Two proteins of nearly equal molecular weights were detected in keratinocytes. Using RT-PCR (not shown) and a PKD2-specific antibody, we have determined that keratinocytes express both PKD1 (upper band) and PKD2 (lower band) isoforms (Supplemental Figure 1), responsible for the double band observed in the immunoblots.
Figure 1. UVB induced activation of PKD in primary mouse keratinocytes.
Near-confluent primary mouse keratinocytes were irradiated with 30 mJ/cm2 UVB and the control cells were sham-irradiated (rinsed and placed into the irradiator box for the appropriate time but without turning on the UV lamp). The cells were lysed at various time points after UVB irradiation as indicated and processed for western blotting employing antibodies against phosphoserine916 PKD (indicative of activated PKD) and total PKD. Actin served as the loading control. Illustrated in the upper panel is a blot representative of 3 separate experiments. Shown in the lower panel are the phosphoserine916 PKD levels from 3 experiments quantified, normalized by total PKD levels and expressed as the means ± SEM; *p<0.01 versus time zero by ANOVA followed by a Dunnett’s post-hoc test. Note that normalizing phosphoserine916 PKD by actin levels gave essentially identical results in this and subsequent experiments.
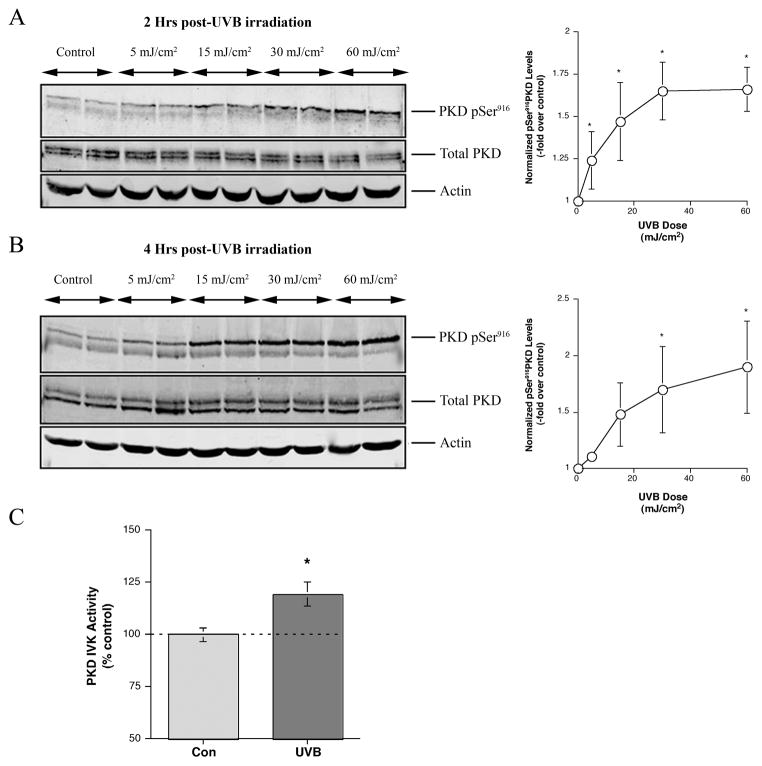
To examine the dose dependence of UVB-induced PKD activation, we irradiated keratinocytes with different doses of UVB (5, 15, 30 and 60 mJ/cm2) and subjected cell lysates to western blot analysis. As shown in Figure 2A and B, UVB irradiation led to an increase in PKD activation (phosphoserine916 immunoreactivity) in a time- and dose-dependent manner with an observable increase at 5 mJ/cm2 noted at 2 hrs post-irradiation. An in vitro kinase activity assay also demonstrated that UVB significantly enhanced PKD activation (Figure 2C). UVB increased PKD activity to a level approximately a third of that enhanced by the phorbol ester 12-O-tetradecanoylphorbol 13-acetate (TPA), an agent often used as a positive control because of its robust stimulation of PKD activity.
Figure 2. Activation of PKD was dependent on time and dosage of UVB.
Near-confluent primary mouse keratinocytes were irradiated with different doses of UVB, and the control cells were sham-irradiated. The cells were lysed at 2 or 4 hours after exposure as indicated and processed for western blotting employing antibodies against phosphoserine916 PKD and total PKD. Actin served as the loading control. Shown is a blot, representative of 3 separate experiments, of (A) 2 hrs or (B) 4 hrs. The right panels show the quantitation of phosphoserine916 PKD normalized to total PKD levels from 3 experiments expressed as the means ± SEM; *p<0.01 versus the zero dose by a repeated measures ANOVA and a Dunnett’s post-hoc test. (C) For the in vitro kinase (IVK) assay keratinocytes were sham-irradiated (Con) or exposed to 30 mJ/cm2. Following PKD immunoprecipitation from control and UVB-treated keratinocyte cell lysates, PKD activity was measured as the transfer of radiolabel from [γ-32P]ATP to the substrate, syntide-2. Radioactivity spotted onto P-81 paper was quantified using a Beckman LS 6500 scintillation counter. Values represent the means ± SEM of 9 samples from 3 separate experiments; *p<0.05 versus the control. Note that a positive control, 100 nM TPA for 2 hours, gave a significant 159 ± 13% increase in PKD IVK activity (means ± SEM of 9 samples from 3 separate experiments; p<0.01).
UVB did not increase serine744 PKD (trans)phosphorylation in mouse keratinocytes, and PKC inhibitors had no effect on UVB-induced PKD activation
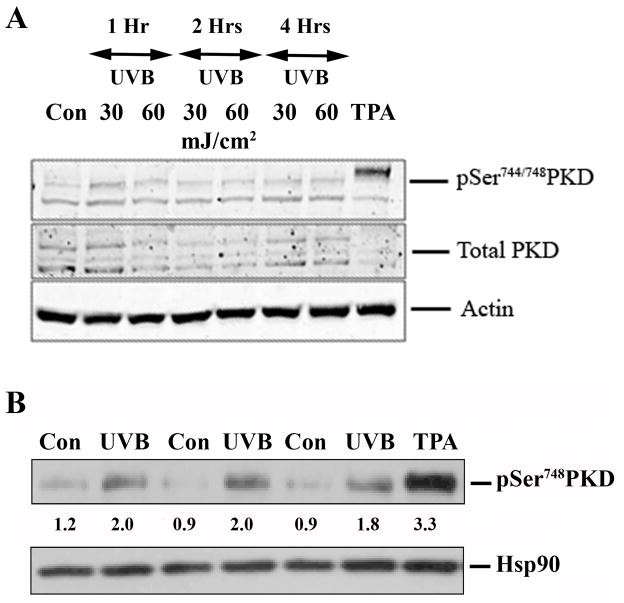
In other studies, PKD activation was examined using an antibody against phosphoserine744/748 within the activation loop of PKD (Iglesias et al., 1998; Song et al., 2006). We examined the effect of UVB irradiation of mouse keratinocytes on the phosphorylation status of serine744/748 (serine738/742 in human) as an additional measure of PKD activation. To our surprise, we were unable to detect any increase in the phosphorylation of serine744/748 residues at any of the time points tested at UV doses yielding significant PKD activation as monitored by serine916 autophosphorylation (Figure 3). TPA (100 nM for 30 minutes) served as the positive control and confirmed our ability to detect an increase in phosphorylation at this site. The Cell Signaling anti-phosphoserine744/748 antibody used here has been reported to primarily detect phosphorylation of serine744 (serine738 in human PKD), the residue transphosphorylated by PKC (Jacamo et al., 2008). We next examined activation loop phosphorylation with the Abcam phosphoserine742 antibody, which has been shown to recognize phosphoserine742 (phosphoserine748 in mouse), a residue that is autophosphorylated upon PKD activation (Jacamo et al., 2008). As anticipated, UVB increased autophosphorylated phosphoserine748 immunoreactivity, consistent with its ability to activate PKD, although the increase was only approximately 40% of that observed with TPA. This effect of UVB on serine748 autophosphorylation was time- and dose-dependent (Supplemental Figure 2).
Figure 3. UVB did not increase phosphoserine744/748 PKD phosphorylation (in particular phosphoserine744 PKD transphosphorylation) in primary mouse keratinocytes, but enhanced serine748 (serine742 in human) autophosphorylation.
(A) Near-confluent primary mouse keratinocytes were irradiated with 30 mJ/cm2 and 60 mJ/cm2 UVB, and the control cells were sham-irradiated. The cells were lysed at various time points after exposure and processed for western blotting employing a Cell Signaling antibody against phosphoserine744/748 PKD, which primarily recognizes phosphoserine744 as well as an antibody recognizing total PKD. Actin served as the loading control, and TPA (100 nM) stimulation for 30 minutes served as a positive control. Illustrated is a blot representative of 3 separate experiments. (B) Near-confluent primary mouse keratinocytes irradiated with 30 mJ/cm2 UVB were lysed 2 h post-UVB and processed for western blotting. Control cells (Con) were sham-irradiated, and a 15-minute treatment with TPA (100 nM) was used as a positive control. Analysis was performed with an Abcam antibody recognizing autophosphorylated phosphoserine742 (phosphoserine748 in mouse). Heat shock protein 90 (Hsp90) served as the loading control. Shown under the blot are the densitometric values (normalized to the loading control) relative to the average (normalized) control value obtained with the Alpha Innotech gel imaging system. The experiment was repeated with similar results.
It has been established that activation of PKD by phorbol esters and growth factors relies mainly on PKC-mediated activation of PKD through serine744/748 (primarily serine744) transphosphorylation [reviewed in (Waldron et al., 1999; Bollag et al., 2004)]. Consistent with the lack of increased serine744 transphosphorylation, we observed no effect of various PKC inhibitors, including the conventional PKC isoform inhibitors Gö6976 and Gö6983 (Supplemental Figure 3), PKCδ inhibitors (Supplemental Figure 4) and a pan-PKC inhibitor Ro31-8220 (Figure 4), on UVB-stimulated PKD serine916 autophosphorylation.
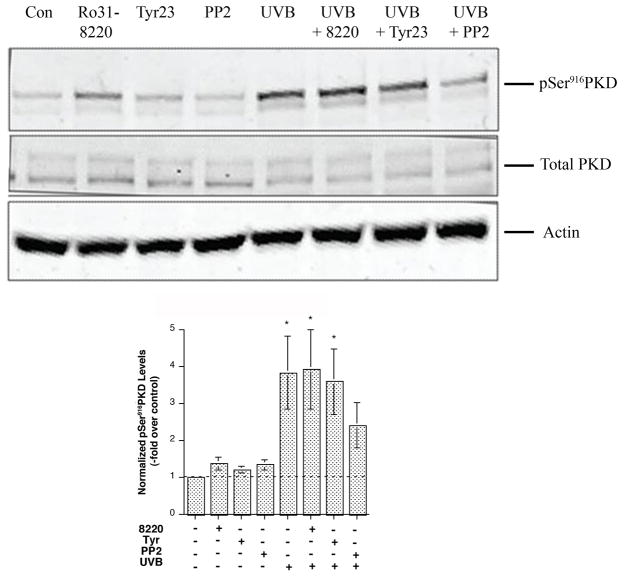
Figure 4. Inhibitors with specificity against Src family tyrosine kinases abrogated UVB-induced PKD activation.
Near-confluent primary mouse keratinocytes were pretreated for 2 hrs with or without the inhibitors Ro 31–8220 (3 μM), tyrphostin 23 (10 μM) or PP2 (10 μM) as indicated and then subjected to sham or UVB irradiation with 30 mJ/cm2. The cells were further incubated for 2 hrs with or without the inhibitors. The cells were lysed and processed for western blotting employing antibodies against phosphoserine916 PKD and total PKD. Actin served as the loading control. Illustrated in the upper panel is a blot representative of 3 separate experiments. Shown in the lower panel are the phosphoserine916 PKD levels from 3 experiments, normalized by total PKD levels and expressed as the means ± SEM; *p<0.05 versus no UVB treatment by a repeated measures ANOVA followed by a Student-Newmann-Keul’s post-hoc test.
Inhibitors with specificity against Src family tyrosine kinases reduced UVB-induced PKD activation
Recent evidence suggests an important role for tyrosine463 (tyrosine469 in mouse) phosphorylation in oxidative stress-induced PKD activation (Storz et al., 2003), with Src and/or Abl as the upstream tyrosine kinases mediating phosphorylation of this residue (Storz and Toker, 2003). To test the possible involvement of tyrosine kinases, as well as PKC, in mediating UVB-induced PKD activation, keratinocytes were treated with tyrphostin 23, a general tyrosine kinase inhibitor, or PP2, a Src family kinase-selective inhibitor (or Ro 31–8220, a general PKC inhibitor) for 2 hrs before subjecting cells to UVB irradiation. Only pre-treatment with PP2 attenuated UVB-induced PKD activation, returning the PKD activation level to a value not significantly different from the control value (Figure 4), suggesting that in keratinocytes PKD activation following UVB is mediated primarily through a Src family tyrosine kinase.
UVB induced tyrosine phosphorylation of PKD
We next determined whether UVB irradiation of primary mouse keratinocytes induced tyrosine phosphorylation of PKD using a co-immunoprecipitation strategy, in which we immunoprecipitated PKD and immunoblotted for phosphotyrosine residues. As seen in Figure 5, UVB irradiation of keratinocytes induced an increase in tyrosine phosphorylation when compared to control cells. Performing the experiment in the reverse direction, that is, immunoprecipitating with the anti-phosphotyrosine antibody and western analysis with anti-PKD (total) antibody, yielded similar results, and for quantitation purposes, the experiments were combined in Figure 5B. In multiple experiments an approximate 1.5-fold increase in tyrosine phosphorylation of PKD was observed in response to UVB irradiation. Studies with a recently available commercial antibody recognizing phosphotyrosine463 confirmed that the observed tyrosine phosphorylation occurred, at least in part, on tyrosine463 (Figure 5C). Furthermore, experiments in which PKD was immunoprecipitated with anti-phosphotyrosine463 and probed for autophosphorylation at serine748 provided evidence that this tyrosine463-phosphorylated PKD was active (Supplemental Figure 5).
Figure 5. UVB induced tyrosine phosphorylation of PKD.
Near-confluent primary mouse keratinocytes were irradiated with 30 mJ/cm2 UVB, and the control cells were sham-irradiated. The cells were lysed 2 hrs post-UVB exposure and immunoprecipitated using total PKD antibody followed by western analysis with a phosphotyrosine antibody. Aliquots representing one-fifth of the protein quantity immunoprecipitated were blotted with total PKD antibody to serve as the loading control (input). Panel (A) shows a representative blot of 4 separate experiments. (B) Values represent the means ± SEM of 4 separate experiments and are expressed as the –fold over the control vlaue;*p<0.05 versus the control value of 1.0 by a two-tailed Student’s t-test. Note that similar results were observed in experiments conducted in the reverse direction, i.e. immunoblotting for total PKD following immunoprecipitation with anti-phosphotyrosine antibody. Therefore, the results were combined for quantitation in Panel B. (C) Near-confluent primary mouse keratinocytes were irradiated with 30 mJ/cm2 UVB (or treated with TPA for 15 minutes as a positive control) and the control cells were sham-irradiated (Con). The cells were lysed 2 hrs post-UVB exposure and western analysis was performed using anti-phosphotyrosine463 and total PKD antibodies. The experiment was repeated with similar results. Shown under the blot are the densitometric values (normalized to the loading control) relative to the average (normalized) control value.
UVB-induced PKD activation was dependent on the redox state of the cells
It has been reported previously (Waldron and Rozengurt, 2000) that the reactive oxygen species (ROS)-scavenging agent, N-acetylcysteine prevents H2O2-induced PKD activation, including the activation loop serine744/748 phosphorylation induced by H2O2 but not that induced by PKC-activating phorbol ester (Waldron et al., 2004). We examined the ability of N-acetylcysteine to alter UVB-induced PKD activation. A 24-hr N-acetylcysteine pre-treatment reduced PKD activation (normalized to total PKD levels) in response to UVB, returning the levels to a value not significantly different from the control and with complete inhibition at 10 mM N-acetylcysteine, as shown in Figure 6.
Figure 6. UVB-induced PKD activation was dependent on the redox state of the cells.
Near-confluent primary mouse keratinocytes were pre-treated for 24 hrs with different doses of N-acetylcysteine (NAC). Cells were then subjected to UVB irradiation with 30 mJ/cm2 followed by a 2-hr incubation. Control cells were sham-irradiated. The cells were lysed and processed for western blotting using antibodies against phosphoserine916 PKD and total PKD. Actin served as the loading control. Illustrated is a blot representative of 3 separate experiments. Shown in the lower panel are the phosphoserine916 PKD levels from 3 experiments, normalized by total PKD levels and expressed as the means ± SEM; *p<0.05 versus no UVB treatment by a repeated measures ANOVA followed by a Student-Newmann-Keul’s post-hoc test.
UVB induced apoptosis in primary mouse keratinocytes
UVB irradiation has been reported to induce apoptosis in several cell types including keratinocytes (Assefa et al., 2005). To examine this possibility in our cells, primary mouse keratinocytes were irradiated with different doses of UVB, and apoptosis was determined by measuring caspase-3 activity. UVB irradiation induced a dose-dependent increase in apoptosis (Figure 7A). UVB-dependent apoptosis was confirmed by western analysis monitoring poly-ADP ribose polymerase (PARP) cleavage as a downstream marker of caspase-3 activity (Figure 7B). Interestingly, there was also a decrease in phosphoserine916 and total PKD immunoreactivity observed at higher UVB doses at 24 hrs post-irradiation (data not shown), likely the result of apoptosis-related cleavage of the enzyme (Endo et al., 2000).
Figure 7. UVB induced apoptosis in primary mouse keratinocytes.
Near-confluent primary mouse keratinocytes were irradiated with different doses of UVB, and control cells were sham-irradiated. The cells were lysed 24 hrs post-UVB exposure and processed for the caspase-3 activity assay as described in Methods. Values are expressed relative to the control and represent the means ± SEM of 3 independent experiments performed in triplicate; **p<0.05 versus the control by ANOVA followed by a Dunnett’s post-hoc test. (B) Samples were processed for western blotting employing an antibody recognizing PARP to monitor its cleavage (a marker of apoptosis and caspase-3 activation). Actin served as the loading control. Illustrated is a blot representative of 3 separate experiments.
Adenovirus-mediated overexpression of wild-type PKD but not mutant PKD, protected keratinocytes from UVB-induced apoptosis
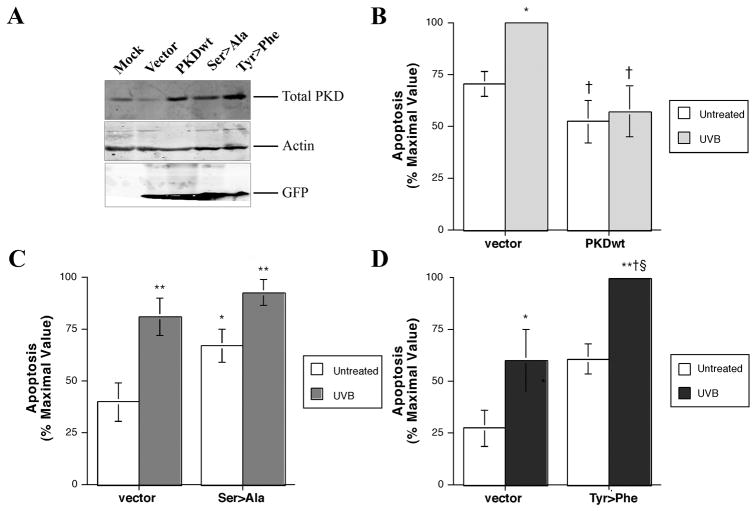
To determine the role of PKD in the apoptotic response to UVB irradiation, we overexpressed PKD and its mutants in the primary keratinocytes prior to UVB exposure. To do so, we obtained constructs of vector alone, wild-type PKD and PKD mutants with serines738/742 mutated to alanines (ser738/742ala PKD) and with tyrosine463 mutated to phenylalanine (tyr463phe PKD) from Dr. Alex Toker (Harvard University, Boston, MA). These constructs were cut from pcDNA3 and cloned into the pAdTrack shuttle vector, which was allowed to recombine with the viral backbone in an appropriate bacterial strain. The resultant viruses were amplified and purified. Keratinocytes were infected with viruses, and overexpression of PKD and its mutants was verified by western blotting employing total PKD antibody, with infection confirmed using an anti-GFP antibody. Figure 8A illustrates the successful infection of the keratinocytes and overexpression of PKD and its mutants.
Figure 8. Overexpression of wild-type PKD protected keratinocytes from UVB-induced apoptosis whereas the Ser738/742Ala and Tyr463Phe mutants did not.
Primary mouse keratinocytes were infected with adenovirus expressing vector alone, wild-type PKD or the ser738/742ala (Ser>Ala) or the tyr463phe (Tyr>Phe) PKD mutants in SFKM for 24 hrs. After 24 hrs the medium was changed to fresh SFKM, and cells were cultured for an additional 48 hrs to attain confluency. (A) The cells were lysed and processed for western blotting employing antibodies against total PKD to confirm overexpression and GFP to verify successful viral infection. Actin served as the loading control. Illustrated is a blot representative of at least 3 separate experiments. Alternatively, primary mouse keratinocytes infected with adenovirus expressing vector alone, (B) wild-type PKD, or the (C) ser738/742ala (Ser>Ala) or (D) tyr463phe (Tyr>Phe) PKD mutants were untreated (sham-irradiated) or subjected to 10 mJ/cm2 UVB irradiation. The cells were lysed 24 hrs post-UVB exposure and processed for the caspase-3 activity assay as described in Methods. Values are expressed relative to the maximal activity observed and represent the means ± SEM of (A) 3, (B) 5 and (C) 5 independent experiments performed in triplicate; *p<0.01, **p<0.01 versus the vector-infected, untreated (sham-irradiated) control cells; †p<0.05 versus the vector-infected, UVB-irradiated cells; and §p<0.05 versus the PKD mutant-infected, untreated cells as determined by ANOVA followed by a Student-Newmann-Keul’s post-hoc test.
To determine whether overexpression of PKD and its various mutants affects UVB-induced apoptosis, keratinocytes were infected with vector or wild-type PKD, or the ser738/742ala or tyr463phe PKD mutants and subjected to 10 mJ/cm2 UVB irradiation. This submaximal dose of UVB was used to facilitate our ability to detect protective or exacerbating effects of PKD and its mutants on UVB-elicited apoptosis. Our results, illustrated in Figure 8B–D, indicate that compared to adenoviral vector infection, wild-type PKD overexpression protected against UVB-induced apoptosis, whereas the tyr463phe and ser738/742ala PKD mutants did not. Indeed, the tyr463phe mutant exacerbated the apoptotic response to UVB, as UVB-induced apoptosis in the tyr463phe PKD-infected cells was significantly enhanced relative to the UVB-exposed vector-infected control, although there was no statistically significant difference with this mutant in the absence of UVB exposure. This result is consistent with a dominant-negative action of the tyr463phe mutant in UVB-elicited apoptosis and provides further support for the importance of tyrosine phosphorylation in PKD activation in response to UVB. On the other hand, the ser738/742ala PKD mutant was able to increase apoptosis basally and blunt the response to UVB, suggesting a role for activation loop phosphorylation in basal keratinocyte survival.
DISCUSSION
Much of UVR’s cellular insult to the skin is brought about by UVA and UVB wavelengths. In addition, with longer wavelength UVR, the direct effect upon target molecules decreases, while the indirect effects elicited by generation of ROS increases [reviewed in (Assefa et al., 2005)]. Solar UVR has been shown to activate many vital signaling pathways involved in cell survival and apoptosis (Assefa et al., 2005), including NF-κB (Li and Karin, 1998; Reelfs et al., 2004) and stress-related kinases (Chen et al., 2001). On the other hand, UVR can also trigger cell death through its ability to activate the intrinsic pathway of apoptosis and eliminate cells with DNA damage (Brash, 1996; Sitailo et al., 2002; Assefa et al., 2003). PKC has also been implicated in UVR-related intracellular signaling events. Activation of PKCδ induces apoptosis by activation of caspases (Denning et al., 2002), whereas activation of PKCε protects cells from death, suggesting the complex nature of PKC signaling and emphasizing that various upstream activators can differ in their ability to recruit downstream signaling molecules, resulting in disparate cellular responses (Matsumara et al., 2003).
Previous studies in our laboratory have shown that PKD is localized to the highly proliferative basal layer of the skin (Dodd et al., 2005b; Ristich et al., 2006), and its levels are up-regulated in human BCC and in a neoplastic mouse keratinocyte cell line (Ristich et al., 2006). Because UVR is the single most important risk factor in the development of NMSCs, we undertook this study to determine the role of PKD in the cellular response to UVB in primary mouse keratinocytes, with the idea that misregulated PKD signaling during UVB exposure could potentially modulate epidermal photocarcinogenesis. Indeed, UVB irradiation led to PKD activation, monitored by autophosphorylation of serine916, in a time- and dose-dependent manner (Figures 1 and 2).
Studies performed by other laboratories have used serine744/748 phosphorylation as an additional marker of PKD activation. In our experiments, we detected no increase in phosphoserine744/748 immunoreactivity, as monitored by an antibody recognizing primarily transphosphorylated serine744 (Figure 3A). Nevertheless, activation of PKD was confirmed by both the in vitro kinase assay (Figure 2C) and an increase in serine742 autophosphorylation (Figure 3B and Supplemental Figure 2). It should be noted that UVB-induced PKD activation was not as robust as that elicited by TPA, likely because TPA stimulates PKD activity via both PKC-mediated transphosphorylation and the generation of ROS [e.g., (Przybyszewski et al., 1998)].
To explore further the mechanism of activation of PKD, and more specifically the role of PKC in the activation of PKD, we employed inhibitors with selectivity towards various isoforms of PKC. None of the PKC inhibitors tested had any effect on UVB-elicited PKD activation, suggesting that this process is independent of PKC activation. On the other hand, our data suggest that basal activation loop phosphorylation is required to prevent apoptosis under control conditions. Thus, the ser738/742ala PKD mutant increased apoptosis basally (in the absence of UVB), thereby essentially blunting the response to UVB (Figure 8B). Together with the lack of effect of Ro31-8220 on basal PKD activation (Figure 4), this result suggests the possibility that autophosphorylation of serine742 is a critical factor in basal PKD activation to prevent apoptosis under control conditions.
Reports from various laboratories suggest a role for non-receptor tyrosine kinases, such as the Src-Abl pathway, in inducing tyrosine phosphorylation of PKD and leading to its activation. This idea was tested in our system using the inhibitor PP2 (a Src family selective inhibitor). PP2 decreased PKD activation, favoring the idea that a Src family tyrosine kinase may be involved in UVB-induced PKD activation (Figure 4). This conclusion was further supported by experiments showing that UVB increases tyrosine phosphorylation of PKD as well as western analysis using an antibody recognizing phosphotyrosine463 (Figure 5). This result is also consistent with the ability of the tyr463phe PKD mutant to act in a dominant-negative manner to exacerbate UVB’s apoptotic effect (and prevent PKD’s ability to promote survival) (Figure 8).
The generation of ROS has been implicated in the pathophysiology of many human diseases. A link between ROS generation and PKD activation has already been established in other cell types (Waldron and Rozengurt, 2000). To test the role of oxidative stress in PKD activation in response to UVB, we pre-treated keratinocytes with the ROS-scavenging agent N-acetylcysteine. This anti-oxidant abrogated UVB-induced PKD activation (Figure 7), emphasizing the dependence of UVB-elicited PKD activation on ROS. In addition, this result is consistent with Toker’s demonstration of the ability of oxidative stress to activate PKD via Src-mediated phosphorylation of tyrosine463 in HeLa cells, resulting in their protection from apoptosis (Storz et al., 2003; Storz and Toker, 2003; Storz et al., 2005).
As observed in other systems (Assefa et al., 2005), irradiation of mouse keratinocytes with UVB led to a dose-dependent increase in apoptosis as measured by caspase-3 activity and PARP cleavage (Figure 7). To test the photoprotective effects of PKD in UVB, we overexpressed adenoviruses encoding wild-type PKD and mutant versions of PKD in keratinocytes (Figure 8). Wild-type PKD overexpression abrogated UVB-elicited apoptosis, completely preventing apoptosis at a low UVB dose (10 mJ/cm2). On the other hand, the ser738/742ala PKD mutant induced some apoptosis basally, suggesting that PKD is a survival signal and phosphorylation of its activation loop is required under basal conditions. However, this mutant appeared not to prevent the anti-apoptotic action of PKD, since there was no difference in UVB-induced apoptosis with versus without ser738/742ala PKD overexpression. On the other hand, the tyr463phe mutant appeared to act as a dominant negative, since UVB-stimulated apoptosis was exacerbated with overexpression of this mutant (Figure 8). This result is thus entirely consistent with the idea that PKC-mediated activation of PKD plays little role in PKD’s activation by UVB but that the key activator is tyrosine phosphorylation, likely by a Src family tyrosine kinase member.
Data from the Rozengurt laboratory showed that PKD’s tyrosine463 residue was dispensable for PKD activation, and mutation of this tyrosine had no effect on activation loop phosphorylation (Waldron et al., 2004). In contrast, Toker and colleagues have suggested the possibility that the phosphorylation of tyrosine463 of PKD by the Src-Abl pathway leads to release of inhibition by the pleckstrin homology domain and exposes the activation loop phosphorylation sites (Storz et al., 2003). These differences could be due to differences in cell type, in activating stimuli and/or in the nature of the recruitment of upstream signaling molecules. Storz et al. (Storz et al., 2004a) have demonstrated that full activation of PKD in response to oxidative stress requires two sequential events: tyrosine phosphorylation by Src-Abl leads to activation of PKCδ and the activated PKCδ promotes activation loop phosphorylation of the sites exposed upon Src-Abl-mediated phosphorylation of PKD tyrosine463. In our study, pre-treatment of keratinocytes with PKCδ inhibitors did not attenuate UVB-induced PKD activation, suggesting the possibility that UVB signals can be relayed to activate PKD without involving PKCδ. Recently, PKC-independent activation loop serine744/748 phosphorylation in response to mitogen-induced stimulation was demonstrated (Sinnett-Smith et al., 2009), and differentiating keratinocytes re-entering the cell cycle exhibit PKD activation without an increase in serine744 phosphorylation (Jadali and Ghazizadeh, 2010). Thus, our results are consistent with the idea that enhanced PKC-mediated phosphorylation of PKD is not required for its activation by UVB irradiation. Nevertheless, the ability of the ser738/742ala mutant to promote apoptosis basally, and its inability to prevent UVB-induced apoptosis, suggest that activation loop phosphorylation, presumably at serine748 (serine742 in human PKD), is necessary for PKD’s ability to protect cells from UVB.
In summary, our results indicate that activation of PKD appears to confer a survival advantage to UVB-damaged keratinocytes, with the result that any genetic mutation induced by irradiation could potentially be amplified as a result of increased PKD activation and action. Thus, our study provides a direct link between UVB exposure and PKD activation and points towards a role for PKD in the development of NMSCs, for which exposure to solar UVR represents the most important risk factor. Thus, identification of PKD as a potential pharmaceutical target suggests that appropriate inhibitory compounds might be useful for intervention in disease progression. Since PKD plays numerous roles in other cellular processes systemically, any generalized inhibition of the enzyme could lead to undesired side effects. The promising aspect of skin biology is that many of the inhibitors identified to date could be applied topically, thereby circumventing the difficulty of possible systemic side effects. Therefore, the development and/or identification of specific or selective inhibitors of PKD could lead to effective weapons in the pharmaceutical arsenal for treatment of epidermal tumorigenesis.
MATERIALS AND METHODS
Materials
All reagents used were of the highest quality available. Calcium-free minimum essential medium (MEM)-alpha was from Biologos, Inc. (Montgomery, IL), ITS+ (6.25 μg/ml insulin, 6.25 μg/ml transferrin, 6.25 ng/ml selenous acid, 1.25 mg/ml BSA and 5.35 μg/ml linoleic acid) from Collaborative Biomedical Products (Bedford, MA), and bovine pituitary extract and epidermal growth factor from Gibco Invitrogen (Carlsbad, CA). DC protein assay reagents were from Bio-Rad (Hercules, CA). Bovine serum albumin (BSA) was from Sigma, and Immobilon–P PVDF membrane was from Millipore (Bellerica, MA). Antibodies recognizing phosphoserine916 PKD (#2051), phosphoserine744/748 PKD (#2054) and total PKD (#2052) were from Cell Signaling Technology (Boston, MA), anti-phosphoserine742 PKD (#ab17945), anti-phosphotyrosine463 PKD (#ab59415) and anti-PKD2 (#ab7281) from Abcam (Cambridge, MA) and anti-actin (#sc1616), anti-Hsp90 (#sc7947) and anti-PKD (#sc638, 639 and 935) antibodies from Santa Cruz Biotechnology (Santa Cruz, CA). AlexaFluor IR680-conjugated secondary antibodies were from LiCor Biosciences (Lincoln, NE) and horseradish peroxidase-coupled secondary antibodies from Cell Signaling Technology or Thermo Scientific (Waltham, MA).
Cell culture
Primary epidermal mouse keratinocytes were prepared from 1–3 day old neonatal ICR mice and cultured in serum-free keratinocyte medium (SFKM) as described in (Jung et al., 1999). Cells were refed every 1–2 days.
Western blot analysis
Near confluent (>80%) cultures of primary mouse keratinocytes plated in the top row of 6-well plates were washed with phosphate-buffered saline lacking calcium and magnesiuim (PBS-), followed by a second rinse with PBS-, which was aspirated, leaving a thin film to prevent drying of the cells. The cells were then exposed to UVB radiation using a Philips UVB broadband lamp (Solarc Systems, Barrie, ON Canada), which was calibrated using an IL1700 research radiometer with a UVB-1/W phototherapy sensor (International Light Technologies, Peabody, MA). Post-irradiation, cells were refed with SFKM, incubated in a 5% CO2 incubator at 37°C for the indicated times and harvested using heated lysis buffer [0.1875 M Tris·HCl (pH 8.5), 3% sodium dodecyl sulfate, and 1.5 mM ethylenediaminetetraacetic acid]. Protein concentration was determined using the DC protein assay with BSA as the standard, and 3× sample buffer (30% glycerol, 15% β-mercaptoethanol, and 1% bromophenol blue) was added to the remaining lysate to constitute loading buffer (Laemmli, 1970). Equal amounts of protein were separated by electrophoresis through 8% polyacrylamide gels and transferred to Immobilon FL. Membranes were blocked with LiCor blocking buffer and incubated overnight at 4°C with the desired antibodies. Immunoreactive bands were visualized using the appropriate AlexaFluor IR680-conjugated secondary antibodies and the Odyssey Infrared imaging system (Licor Biosciences, Lincoln, NE) and quantified using Odyssey application software (version 2.1). Alternatively, western blots were incubated with horseradish peroxidase-coupled secondary antibodies, visualized with enhanced chemiluminescence using SuperSignal West Femto chemiluminescence substrate (Thermo Scientific) on an Alpha Innotech Fluorchem 8900 (San Leandro, CA) and quantified using the instrument’s software, or with ECL Plus Western Blotting Detection Reagent from GE Healthcare (Piscataway, NJ) and Blue Basic Autorad film (ISC Bioexpress, Kaysville, UT).
In vitro kinase assay
The PKD in vitro kinase activity assay was performed as in Dodd et al. (Dodd et al., 2005a), following immunoprecipitation of PKD using the Santa Cruz PKD antibodies.
Caspase-3 activity assay
Caspase-3 activity was measured using a Caspase-3 Apoptosis Detection kit (Santa Cruz Biotechnology, Inc.) according to the manufacturer’s protocol. Briefly, primary mouse keratinocytes were UVB-irradiated and 24 hours later after washing with PBS-, cells were lysed in cell lysis buffer (provided by the manufacturer and used at a ratio of 1 ml per 2 × 106 cells). An aliquot of the cell lysate was transferred to a microcentrifuge tube, and master mix [2× reaction buffer (provided), dithiothreitol (10 mM final concentration) and 5 μL DEVD-AFC substrate per reaction] was added to each sample. After incubation of the reaction mixtures for 1 hr at 37°C, an aliquot of each sample was transferred to a 96-well plate (Microlite 2+ FLT BTM Thermo Labsystems, catalog #7572) and the levels of free AFC measured using a fluorescent microplate reader (SPECTRAFluor Plus, Tecan, Männedorf, Switzerland) with 400 nm excitation and 505 nm emission filters.
Co-immunoprecipitation
Cells were scraped into immunoprecipitation (IP) lysis buffer containing 50 mM Tris (pH 7.5), 150 mM NaCl, 1% NP-40, 5 mM NaF, 1mM Na3VO3, 1% PMSF and 1% aprotinin on ice. Protein concentration was determined and the lysates were pre-cleared with rabbit IgG beads followed by incubation with the appropriate primary antibodies (with rocking). To collect the immunoprecipitates, rabbit IgG beads were incubated with the samples, collected by centrifugation and washed three times with IP lysis buffer. After addition of loading buffer, proteins were separated by SDS-PAGE and visualized by western blot analysis, as described earlier.
Production of PKD-expressing adenoviruses
Adenoviruses containing various recombinant PKD constructs were made using the AdEasy adenoviral system provided by Dr. Bert Vogelstein (Johns Hopkins University, Baltimore, MD), as described (He et al., 1998). Briefly, PKD constructs obtained from Dr. Alex Toker (Harvard University, Boston, MA) were cut from pcDNA3 and ligated into the pAdTrack-CMV shuttle vector containing green fluorescent protein (GFP). The resultant plasmids were linearized and electroporated into recombination-proficient BJ5183 cells. Cells containing the correct inserts were selected using kanamycin, and the plasmid DNA was isolated using Qiagen mini-prep kits. The recombined plasmids were chemically transformed into XL10 Gold cells, and DNA was obtained from these cells. To produce the viruses expressing PKD, the above DNA was transfected into Ad-293 cells using Lipofectamine. The infected cells exhibiting cytopathic effects greater than 70% were harvested into PBS- and collected by centrifugation. Cell pellets were subjected to repeated freeze-thaw cycles in liquid nitrogen followed by centrifugation. The supernatants were collected and virus particles purified using cesium-chloride gradient ultracentrifugation. The resulting viral particles were dialyzed against several changes of buffer and stored in a virus storage solution (10 mM Tris, pH 8.1 in 0.9% NaCl containing 10% glycerol) at −20°C until use. The number of viral particles was determined by protein quantitation.
Adenovirus infection
Virus-packaged PKD constructs were added to primary mouse keratinocyte cultures on the second day after plating, concominant with the change to SFKM. The virus-containing media was removed 24 hr post-infection and cells refed with SFKM. Cells were incubated for an additional 24 hours before exposure to UVB. PKD overexpression was confirmed by western analysis using total PKD antibody and viral infection verified by monitoring GFP levels.
Statistical Analysis
Experiments were performed a minimum of three times on separate keratinocyte preparations. Data were statistically evaluated with two-way, or repeated measures if matching was shown to be effective, analysis of variance (ANOVA) and a Student-Newmann-Keul’s or Dunnett’s post-hoc test using Instat (GraphPad Software, San Diego, CA), as indicated.
Supplementary Material
Acknowledgments
This project was supported by a Merit Award from the Veterans’ Administration and a grant from the National Institutes of Health/National Institute of Arthritis, Musculoskeletal and Skin Diseases #AR45212 (WBB). We thank Dr. Alex Toker for generously providing the various PKD constructs as well as for his useful discussions, and Dr. Bert Vogelstein for his kind gift of the AdEasy adenovirus system. We greatly appreciate the expert technical assistance of Mr. Peter Parker and Ms. Mariya Wilson. This work was submitted in partial fulfillment of the requirements for a doctoral degree from the Medical College of Georgia (SNA).
References
- Assefa Z, Garmyn M, Vantieghem A, Declercq W, Vandenabeele P, Vandenheede JR, Agostinis P. Ultraviolet B radiation-induced apoptosis in human keratinocytes: cytosolic activation of procaspase-8 and the role of Bcl-2. FEBS Lett. 2003;540:125–132. doi: 10.1016/s0014-5793(03)00238-2. [DOI] [PubMed] [Google Scholar]
- Assefa Z, Van Laethem A, Garmyn M, Agostinis P. Ultraviolet radiation-induced apoptosis in keratinocytes: on the role of cytosolic factors. Biochim Biophys Acta. 2005;1755:90–106. doi: 10.1016/j.bbcan.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Bollag WB, Dodd ME, Shapiro BA. Protein kinase D and keratinocyte proliferation. Drug News Persp. 2004;17:117–126. doi: 10.1358/dnp.2004.17.2.829045. [DOI] [PubMed] [Google Scholar]
- Brash DE. Cellular proofreading. Nat Med. 1996;2:525–526. doi: 10.1038/nm0596-525. [DOI] [PubMed] [Google Scholar]
- Chen W, Tang Q, Gonzales MS, Bowden GT. Role of p38 MAP kinases and ERK in mediating ultraviolet-B induced cyclooxygenase-2 gene expression in human keratinocytes. Oncogene. 2001;20:3921–3926. doi: 10.1038/sj.onc.1204530. [DOI] [PubMed] [Google Scholar]
- Denning MF, Wang Y, Tibudan S, Alkan S, Nickoloff BJ, Qin JZ. Caspase activation and disruption of mitochondrial membrane potential during UV radiation-induced apoptosis of human keratinocytes requires activation of protein kinase C. Cell Death Differ. 2002;9:40–52. doi: 10.1038/sj.cdd.4400929. [DOI] [PubMed] [Google Scholar]
- Dodd EM, Ristich VL, Ray S, Lober RM, Bollag WB. Regulation of protein kinase D during differentiation and proliferation of primary mouse keratinocytes. J Invest Dermatol. 2005a;125:294–306. doi: 10.1111/j.0022-202X.2005.23780.x. [DOI] [PubMed] [Google Scholar]
- Dodd ME, Ristich VL, Ray S, Lober RM, Bollag WB. Regulation of protein kinase D during differentiation and proliferation of primary mouse keratinocytes. J Invest Dermatol. 2005b;125:294–306. doi: 10.1111/j.0022-202X.2005.23780.x. [DOI] [PubMed] [Google Scholar]
- Endo K, Oki E, Biedermann V, Kojima H, Yoshida K, Johannes F-J, Kufe D, Datta R. Proteolytic cleavage and activation of protein kinase Cμ by caspase-3 in the apoptotic response of cells to 1-beta-D-arabinofuranosylcytosine and other genotoxic agents. J Biol Chem. 2000;275:18476–18481. doi: 10.1074/jbc.M002266200. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenovirus. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias T, Waldron RT, Rozengurt E. Identification of in vivo phosphorylation sites required for protein kinase D activation. J Biol Chem. 1998;273:27662–27667. doi: 10.1074/jbc.273.42.27662. [DOI] [PubMed] [Google Scholar]
- Jacamo R, Sinett-Smith J, Rey O, Waldron RT, Rozengurt E. Sequential protein kinase C (PKC)-dependent and PKC-independent protein kinase D catalytic activation via Gq-coupled receptors: differential regulation of activation loop Ser(744) and Ser(&$*) phosphorylation. J Biol Chem. 2008;283:12877–12887. doi: 10.1074/jbc.M800442200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung EM, Betancourt-Calle S, Mann-Blakeney R, Griner RD, Bollag WB. Sustained phospholipase D activation is associated with keratinocyte differentiation. Carcinogenesis. 1999;20:569–576. doi: 10.1093/carcin/20.4.569. [DOI] [PubMed] [Google Scholar]
- Kraemer KH. Sunlight and skin cancer: another link revealed. Proc Natl Acad Sci U S A. 1997;94:11–14. doi: 10.1073/pnas.94.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li N, Karin M. Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Proc Natl Acad Sci USA. 1998;95:13012–13017. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljedahl M, Maeda Y, Colanzi A, Ayala I, Van Lint J, Malhotra V. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 2001;104:409–420. doi: 10.1016/s0092-8674(01)00228-8. [DOI] [PubMed] [Google Scholar]
- Marathe GK, Johnson C, Billings SD, Sothall MD, Pei Y, Spandau D, Murphy RC, Zimmerman GA, McIntyre TM, Travers JB. Ultraviolet B radiation generates platelet-activating factor-like phospholipids underlying cutaneous damage. J Biol Chem. 2005;280:35448–35457. doi: 10.1074/jbc.M503811200. [DOI] [PubMed] [Google Scholar]
- Matsumara M, Tanaka N, Kuroki T, Ichihashi M, Ohba M. Biochem Biophys Res Commun. 2003;303:350–356. doi: 10.1016/s0006-291x(03)00345-0. [DOI] [PubMed] [Google Scholar]
- Matthews SA, Rozengurt E, Cantrell D. Characterization of serine 916 as an in vivo autophosphorylation site for protein kinase D/Protein kinase Cmu. J Biol Chem. 1999;274:26543–26549. doi: 10.1074/jbc.274.37.26543. [DOI] [PubMed] [Google Scholar]
- Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. Journal of the American Academy of Dermatology. 1994;30:774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- Prigozhina NL, Waterman-Storer CM. Protein kinase D-mediated anterograde membrane trafficking is required for fibroblast motility. Curr Biol. 2004;14:88–98. doi: 10.1016/j.cub.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Przybyszewski J, Box HC, Kulesz-Martin M. Induction of reactive oxygen species without 8-hydroxydeoxyguanosine formation in DNA of initiated mouse keratinocytes treated with 12-O-tetradecanoylphorbol-13-acetate. Carcinogenesis. 1998;19:1467–1474. doi: 10.1093/carcin/19.8.1467. [DOI] [PubMed] [Google Scholar]
- Reelfs O, Tyrrell RM, Pourzand C. Ultraviolet a radiation-induced immediate iron release is a key modulator of the activation of NF-kappaB in human skin fibroblasts. J Invest Dermatol. 2004;122:1440–1447. doi: 10.1111/j.0022-202X.2004.22620.x. [DOI] [PubMed] [Google Scholar]
- Rennecke J, Rehberger PA, Fürstenberger G, Johannes F-J, Stöhr M, Marks F, Richter KH. Protein kinase-Cμ expression correlates with enhanced keratinocyte proliferation in normal and neoplastic mouse epidermis and in cell culture. Int J Cancer. 1999;80:98–103. doi: 10.1002/(sici)1097-0215(19990105)80:1<98::aid-ijc19>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Ristich VL, Bowman PH, Dodd ME, Bollag WB. Protein kinase D distribution in normal human epidermis, basal cell carcinoma and psoriasis. Br J Dermatol. 2006;154:586–593. doi: 10.1111/j.1365-2133.2005.07073.x. [DOI] [PubMed] [Google Scholar]
- Sinnett-Smith J, Jacamo R, Kui R, Wang YM, Young SH, Rey O, Waldron RT, Rozengurt E. Protein Kinase D Mediates Mitogenic Signaling by Gq-coupled Receptors through Protein Kinase C-independent Regulation of Activation Loop Ser744 and Ser748 Phosphorylation. J Biol Chem. 2009;284:13434–13445. doi: 10.1074/jbc.M806554200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnett-Smith J, Zhukova E, Hsieh N, Jiang X, Rozengurt E. Protein kinase D potentiates DNA synthesis induced by Gq-coupled receptors by increasing the duration of ERK signaling in Swiss 3T3 cells. J Biol Chem. 2004;279:16883–16893. doi: 10.1074/jbc.M313225200. [DOI] [PubMed] [Google Scholar]
- Sitailo LA, Tibudan SS, Denning MF. Activation of caspase-9 is required for UV-induced apoptosis of human keratinocytes. J Biol Chem. 2002;277:19346–19352. doi: 10.1074/jbc.M200401200. [DOI] [PubMed] [Google Scholar]
- Song J, Li J, Lulla A, Evers BM, Chung DH. Protein kinase D protects against oxidative stress-induced intestinal epithelial cell injury via Rho/ROK/PKC-delta pathway activation. Am J Cell Physiol. 2006;290:C1469–C1476. doi: 10.1152/ajpcell.00486.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz P, Doppler H, Johannes F-J, Toker A. Tyrosine phosphorylation of protein kinase D in the pleckstrin homology domain leads to activation. J Biol Chem. 2003;278:17969–17976. doi: 10.1074/jbc.M213224200. [DOI] [PubMed] [Google Scholar]
- Storz P, Doppler H, Toker A. Protein kinase D mediates mitochondrion-to-nucleus signaling and detoxification from mitochondrial reactive oxygen species. Mol Cell Biol. 2005;25:8520–8530. doi: 10.1128/MCB.25.19.8520-8530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz P, Döppler H, Toker A. Activation loop phosphorylation controls protein kinase D-dependent activation of nuclear factor kB. Mol Pharmacol. 2004a;66:870–879. doi: 10.1124/mol.104.000687. [DOI] [PubMed] [Google Scholar]
- Storz P, Döppler H, Toker A. Protein kinase Cdelta selectively regulates protein kinase D-dependent activation of NF-kappaB in oxidative stress signaling. Mol Cell Biol. 2004b;24:2614–2626. doi: 10.1128/MCB.24.7.2614-2626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz P, Toker A. Protein kinase D mediates a stress-induced NF-kB activation and survival pathway. EMBO J. 2003;22:109–120. doi: 10.1093/emboj/cdg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lint J, Rykx A, Maeda Y, Vantus T, Sturany S, Malhotra V, Vandenheede JR, Seufferlein T. Protein kinase D: an intracellular traffic regulator on the move. Trends Cell Biol. 2002;12:193–200. doi: 10.1016/s0962-8924(02)02262-6. [DOI] [PubMed] [Google Scholar]
- Waldron RT, Iglesias T, Rozengurt E. Phosphorylation-dependent protein kinase D activation. Electrophoresis. 1999;20:382–390. doi: 10.1002/(SICI)1522-2683(19990201)20:2<382::AID-ELPS382>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Waldron RT, Rey O, Zhukova E, Rozengurt E. Oxidative stress induces protein kinase C-mediated activation loop phosphorylation and nuclear redistribution of protein kinase D. J Biol Chem. 2004;279:27482–27493. doi: 10.1074/jbc.M402875200. [DOI] [PubMed] [Google Scholar]
- Waldron RT, Rozengurt E. Oxidative stress induces protein kinase D activation in intact cells. J Biol Chem. 2000;275:17114–17121. doi: 10.1074/jbc.M908959199. [DOI] [PubMed] [Google Scholar]
- Yeaman C, Ayala MI, Wright JR, Bard F, Bossard C, Ang A, Maeda Y, Seufferlein T, Mellman I, Nelson WJ, Malhotra V. Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network. Nat Cell Biol. 2004;6:106–112. doi: 10.1038/ncb1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhukova E, Sinnett-Smith J, Rozengurt E. Protein kinase D potentiates DNA synthesis and cell proliferation induced by bombesin, vasopressin, or phorbol esters in Swiss 3T3 cells. J Biol Chem. 2001;276:40298–40305. doi: 10.1074/jbc.M106512200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.