Abstract
This study describes the use of methylene blue (MB) plus light (photodynamic inactivation, PDI) in the presence of hydrogen peroxide (H2O2) to kill Staphylococcus aureus, Escherichia coli, and Candida albicans. When H2O2 was added to MB plus light there was an increased antimicrobial effect, which could be due to a change in the type of ROS generated or increased microbial uptake of MB. To clarify the mechanism, the production of ROS was investigated in the presence and absence of H2O2. It was observed that ROS production was almost inhibited by the presence of H2O2 when cells were not present. In addition, experiments using different sequence combinations of MB and H2O2 were performed and MB optical properties inside the cell were analyzed. Spectroscopy experiments suggested that the amount of MB was higher inside the cells when H2O2 was used before or simultaneously with PDI, and ROS formation inside C. albicans cells confirmed that ROS production is higher in the presence of H2O2. Moreover enzymatic reduction of MB by E. coli during photosensitizer uptake to the photochemically inactive leucoMB could be reversed by the oxidative effects of hydrogen peroxide, increasing ROS formation inside the microorganism. Therefore, the combination of a photosensitizer such as MB and H2O2 is an interesting approach to improve PDI efficiency.
1 Introduction
The rapidly increasing emergence of antibiotic resistance amongst pathogenic bacteria may be bringing to an end of a period extending over the past 60 years, termed “the antibiotic era”.1 In the 1940s, with the introduction of penicillin, antibiotic therapy began, but within just two years, pathogenic bacterial strains resistant to the drug were discovered.2 Currently, the epidemiology of infectious diseases is in crisis due to the emergence of bacterial strains with multiple resistances to many conventional antibiotics3,4 and there is an urgent need for new treatments with novel mechanisms of action.5,6 Therefore, an alternative antimicrobial approach that could avoid the appearance of resistance and its side effects is clearly desirable.
Photodynamic therapy (PDT) involves the use of light sources to kill undesired cells or microorganisms and a photosensitizer agent. The excited photosensitizer reacts with molecular oxygen, generating highly reactive oxygen species (ROS) that promote injury and death of microorganisms as well as tumor cells.7,8
PDT has an efficient killing effect upon illumination of different microorganisms, such as Gram-positive bacteria, but less efficient killing of fungi and Gram-negative bacteria.9,10 The structure of the cellular wall seems to be fundamental to determine the difference in susceptibility to PDT between Gram+ and Gram− bacteria. The high susceptibility of Gram+ species is explained by their morphology, with a relatively porous layer of peptidoglycan and lipoproteic acid that allows the photosensitizer to cross their cytoplasmatic membrane. On the other hand, the outer membrane of Gram− microorganisms forms a physical and functional barrier between the cell and its environment.
Hydrogen peroxide (H2O2) is used worldwide for cleaning wounds, removing dead tissue, or as an oral debriding agent, due to its strong oxidizing properties.11 Previous studies from our group12,13 and from McCullagh and Robertson14,15 have indicated that the use of hydrogen peroxide associated with PDT gives increased killing of microorganisms. Therefore, the combination of a well-known antiseptic agent and a potential antimicrobial therapy could improve the efficiency of both treatments, achieving a better result especially for clinical applications. The enhancement of the killing effect may occur due to enhanced photochemistry of the dye, by a chemical reaction between H2O2 and the ROS produced during PDT, or by one treatment (H2O2 or dye + light) damaging the microbial cell and allowing the other treatment to penetrate better.
In this study, our aim was to investigate the enhancement of antimicrobial effect between photodynamic reactions in the presence of hydrogen peroxide. For this purpose, we used spectroscopic methods and fluorescence microscopy to examine ROS formation and photosensitizer uptake by cells. In addition, we also evaluated the optical characteristics of the dye in a biological environment to elucidate the possible mechanisms involved in this association.
2 Results
2.1 Investigation of the microbial reduction in vitro
Three different species of microorganisms with diverse physiology, morphology and different resistance were used in the photoinactivation studies, and all species are important human and animal pathogens. Methylene blue (MB) alone (60 μM) and H2O2 solution (100 mM) were incubated with each of the microorganisms for 30 min to detect any inherent dark toxicity of the compounds. Both of them presented only slight toxicity for the three tested microorganisms.
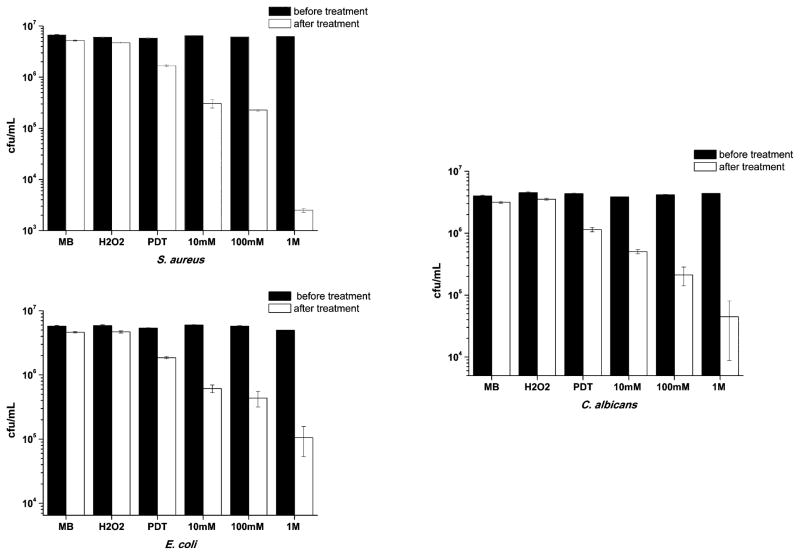
Fig. 1 shows how the viability of the microorganisms changes with PDT in the presence of increasing H2O2 concentration. It can be observed that in the dark or using only H2O2, the bacterial inactivation was insignificant for any microorganism. When MB was diluted in PBS and the dye is photoactivated by red laser (PDT group), microbial reduction was less than 1 log. In fact, the decrease was approximately 70% for Staphylococcus aureus and Candida albicans. For Escherichia coli, the Gram-negative species, the reduction was about 60%. However, for all examined microorganisms, PDT in the presence of increasing hydrogen peroxide concentrations gave increased microbial killing in an H2O2 dose-dependent manner.
Fig. 1.
Effect of MB (60 μM during 10 min) in PBS or H2O2 (100 mM during 10 min) without irradiation and PDT using MB in PBS solution or with crescent concentrations of H2O2 solution under irradiation on the reduction of S. aureus, E. coli and C. albicans. Illumination parameters: λ = 660 nm, P = 40 mW, Δt = 60 s.
2.2 Investigation of the mechanisms involved in the reaction
2.2.1 Singlet oxygen and total ROS detection in cell free system
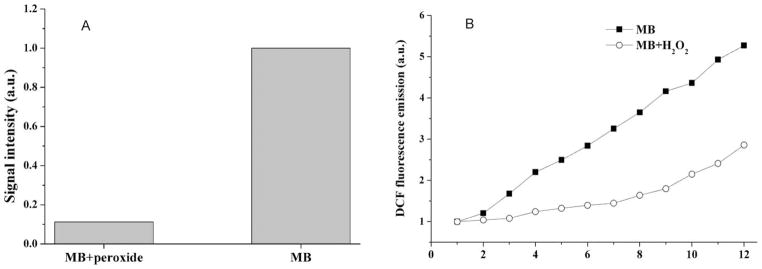
As presented in Fig. 2A and 2B, there are important differences between the rates of ROS generation from illuminated MB in the presence and absence of H2O2, since the formation of singlet oxygen as measured by 1270 nm luminescence was almost inhibited (Fig. 2A) and total ROS production as measured by DCDHF oxidation was diminished (Fig. 2B). These results indicate that altered production of ROS by changes in photochemistry produced by H2O2 does not explain the increased microbial killing. However the photochemical reactions taking place inside the microorganisms may not be the same as those taking place in solution.
Fig. 2.
ROS production by MB in the presence and absence of H2O2. (A) Direct measure of the phosphorescence of 1O2 at λ = 1270 nm (λexc = 532 nm); (B) Indirect measurement of total ROS formation by DCF fluorescence at λemiss 522 nm.
2.2.2 ROS production inside the microorganism
Surprisingly, as shown in the previous sections, the measurement of singlet oxygen and total ROS detection did not confirm our first assumption to explain the increased PDI effect in the presence of H2O2, i.e., H2O2 would provide more molecules of oxygen for the photoreaction than in water solution and this should increase the amount of ROS produced. Thus, we decided to investigate what would happen with the photoreaction in the presence of a biological substance, since our microbiological experiments and also the literature12–15 agreed that H2O2 gave more microbial PDT killing.
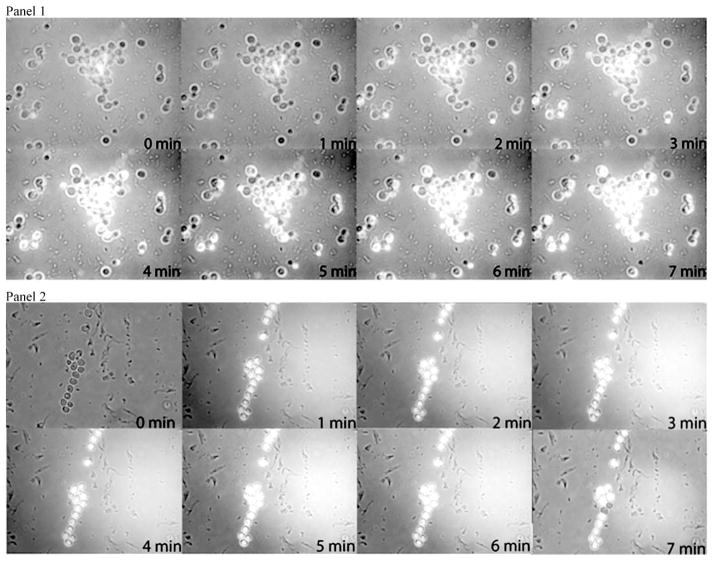
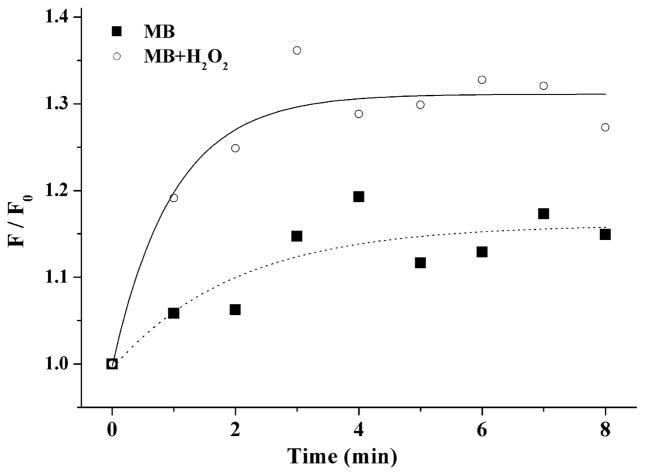
Fig. 3 displays the fluorescence images of DCF (λ > 500 nm) as a function of the irradiation time inside the microorganism due to photo-oxidized MB taken up by C. albicans. Fig. 4 shows the formation of DCFH to DCF due to production of ROS under MB irradiation in presence or not of H2O2. To compare the rates of DCF formation, we assumed that the fluorescence can be written with the expression F(t) = Fmax[1 − βe−t/k],16 where F(t) is the intensity of fluorescence at time, β is a fit parameter, and k is the time constant, i.e., the inverse of the rate of DCF formation. Then, the rate of DCF formation can be calculated from expression: VF = dF(t)/dt. Using this analysis, when t→0, the relative rate (Vperox/VPBS) of DCF formation in the presence of hydrogen peroxide is approximately 4 times faster than in PBS solution.
Fig. 3.
Panel 1: Photomicrographs of suspension of C. albicans loaded with DCFH in the presence of MB in PBS solution. Compositions of fluorescence and optical microscopy of the cells following irradiation. Under irradiation, the DFC fluorescence was increased in function of irradiation time (increased brightness due to DCFH oxidation by ROS formation). Panel 2: Photomicrographs of suspension of C. albicans loaded with DCFH in the presence of MB in H2O2 solution. Compositions of fluorescence and optical microscopy of the cells following irradiation. Under irradiation, the DFC fluorescence was increased in function of irradiation time (increased brightness due to DCFH oxidation by ROS formation).
Fig. 4.
Dynamic of relative ROS formation inside C. albicans cells by MB in PBS solution and MB in H2O2 solution evaluated by fluorescence intensity (F) of DCF formation under irradiation. F0 is the fluorescence at initial irradiation time. Illumination parameters: λ = 660 nm, P = 40 mW, Δt = 60 s. Solid and dashed lines are the exponential data fits (see text).
2.3 Investigation of the mechanisms involved
2.3.1 MB uptake by bacteria
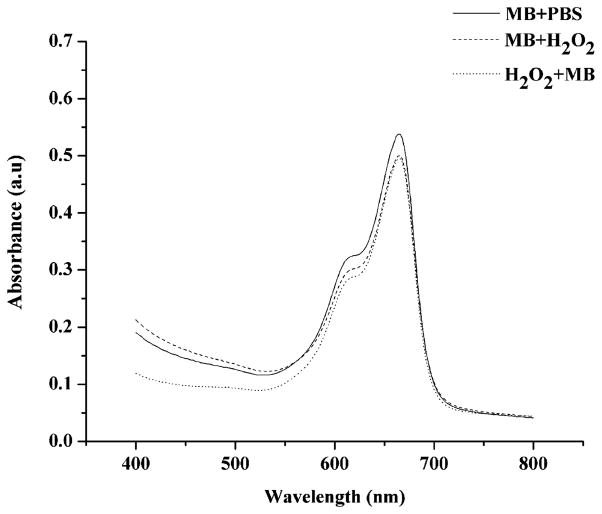
MB concentration from the supernatant removed from the E. coli suspension treated (experiment) or not (control) with H2O2 was calculated from absorbance spectra. The optical density at λ = 660 nm is higher when MB is diluted in PBS solution with a calculated concentration of approximately 22 μM and lower when the dye is in a peroxide solution (around 17 μM), which means that the amount of MB uptake by bacteria increases when H2O2 is combined with MB, i.e., 72% and 63% of MB is taken up by cells in the presence of H2O2 and in PBS solution, respectively. These data (Fig. 5) indicate that there is more MB bound to the microorganism when they are treated with a peroxide solution, therefore maybe there is an increased probability of generating ROS inside the cells.
Fig. 5.
Absorbance spectrum of E. coli supernatant after incubation with MB in PBS solution, incubation with MB in H2O2 solution, and incubation with H2O2 and then with MB.
2.3.2 Reduction state of the dye
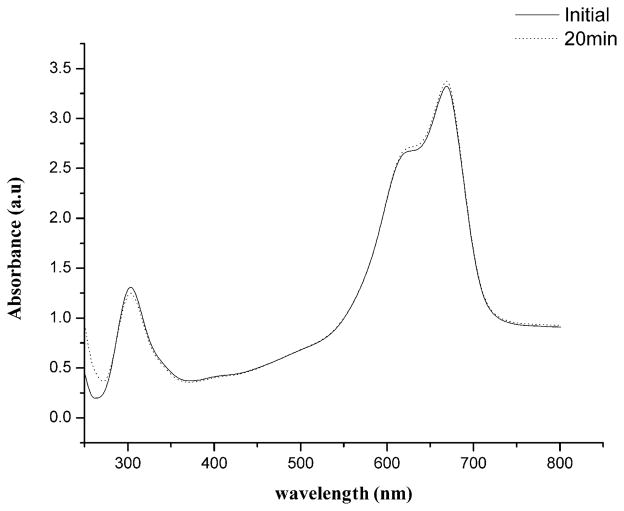
The contact of MB with biological environments containing reductase enzymes usually causes a small but reversible chemical reduction of the dye leading to an increase in the concentration of leucoMB.17,18 In our experimental conditions, this effect was visualized by putting MB in contact with E. coli and observing an increased absorption at 290 nm and a decrease in the absorption peak at 660 nm. Interestingly, when H2O2 was added to the cuvette there was a progressive inversion in the optical characteristics of the dye better visualized after 20 min of peroxide incubation (Fig. 6). The absorption peak at 290 nm diminished and the absorption peak at 660 nm increased showing oxidation of leucoMB and reappearance of “colored” form. In fact, the initial ratio 660/290 was 2.5 while following 20 min, the ratio increased to 2.7 (p = 0.001686). This finding could also be important to explain the enhancement in cell killing when MB and H2O2 are used during PDT.
Fig. 6.
Absorbance spectra of methylene blue inside E. coli (initial) and after 20 min of incubation in peroxide solution. Note the variation of the leucoMB and MB absorbance peaks (p < 0.01).
2.3.3 Changing the sequence of addition of the components
The presence of biological substrates affects the photoreaction of MB in a peroxide environment, as we can see at panel 2. We then asked if, as suggested by Funk and Krise,19 the H2O2 could alter the membrane permeability and improve the cellular accumulation of MB or, as suggested by Caetano and co-workers,26 that the photoreaction would promote membrane disruption and facilitate the penetration of H2O2 into the cell. A possible explanation of the higher effect of PDT in the presence of H2O2 could be that MB has an easier transit across the cell membrane.20 To test this hypothesis, we used a Gram-negative bacterium since these species have an outer membrane that provides a physical and functional barrier between the cell and its internal environment.
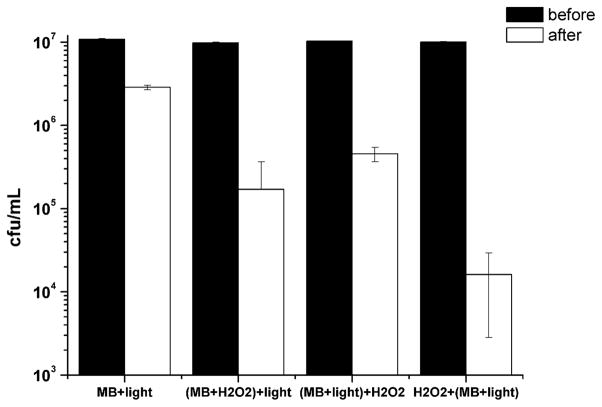
As shown in Fig. 7, the bacterial inactivation using MB in a PBS solution was only about 0.5 log and this value was significantly different from all the other groups. Concerning the other tested sequences of addition of H2O2 and PDT, a remarkable difference was observed among the groups. The group (MB+H2O2)+light showed a reduction of 1.8 log while the decrease for the group (MB+light)+H2O2 was about 1.4 log. Interestingly, the group H2O2+(MB+light) presented a diminution of 2.8 log suggesting that the H2O2 damages the bacterial outer membrane and improves the dye uptake.
Fig. 7.
Effects of different sequences of H2O2, MB diluted in PBS or H2O2 and light on in vitro killing of E. coli. Illumination parameters: λ = 660 nm, P = 40 mW, Δt = 60 s.
3 Discussion
McCullagh and Robertson first reported a possible improvement of microbial killing using H2O2 and PDT against cyanobacteria.14 Also in 2006, the same authors showed the photodestruction of Chlorella vulgaris by MB and nuclear fast red (NFR) combined with H2O2 under irradiation,15 presenting this interaction as an improvement in antimicrobial PDT. Hayek et al.12 and Garcez et al.13 found similar results working with a urea peroxide paste associated with a photosensitizer against oral bacteria in vitro and in vivo. However, those studies did not compare PDT killing with PDT killing in the presence of H2O2.
To test the combination of MB-PDT and H2O2, we used more challenging conditions such as an increased number of bacteria, which diminishes the efficiency of the PDT, the microorganisms were used in stationary growth phase, when they have increased defenses against ROS, and sub-lethal PDT parameters were chosen in order to enhance the differences among the tested conditions. Our results showed that the combination of MB with H2O2 solution can enhance the antimicrobial effectiveness of PDT for all species of microorganism tested, such as S. aureus (Gram+), E. coli (Gram−) and for the fungus C. albicans. The higher susceptibility of S. aureus was expected, since many reports have shown that Gram+ bacteria are more susceptible to PDT than other microbial cells.7–10 In addition, our findings indicate that the progressive increase in H2O2 concentration progressively increases microbial killing by PDT.
The first hypothesis to explain our results was that the increased amount of molecular oxygen, produced by H2O2 dissociation, could improve the likelihood of ROS formation. Because it is generally accepted that the type II reaction predominates in oxygenated system,21 singlet oxygen could be generated via an energy transfer process during a collision of the excited sensitizer with triplet oxygen and this process typically produces 103 to 105 molecules of singlet oxygen before being degraded.22 The amount of molecular oxygen in a H2O2 solution (at 1 M, normally used as an antimicrobial agent) is approximately 30% higher than in a PBS solution (data not shown). However, our findings undoubtedly demonstrate that ROS production in solution decreases rather than increases in the presence of H2O2 and the production of singlet oxygen is almost inhibited compared to the production of singlet oxygen in PBS solution. Therefore, the possibility of a type I mechanism was tested.
We tried to explain the enhanced microbial reduction by photochemical reaction. It could occur that the photo-excited MB reacts directly with H2O2 in a type I process via electron transfer generating either hydroxyl or more likely hydroperoxide radicals and that these ROS could attack the microorganisms. This hypothesis was supported by the report from Gak et al.23 that investigated the quenching of triplet excited MB by H2O2. The authors suggested two mechanisms for the quenching: electron transfer from the excited MB to H2O2 with the formation of a MB+ radical and OH− or electron transfer from H2O2 to the MB molecule with the formation of the MB− radical and HO2* radical. McCullagh and Robertson14 found an increase in the antimicrobial activity of MB/H2O2 system when investigating the antimicrobial photodynamic effect of MB and H2O2 against cyanobacteria in an oxygen-free atmosphere. It was proposed that the process involved a type I mechanism with electron transfer from MB to H2O2 generating active radical species. However, the total ROS production measured in the present study indicated a decrease in ROS formation in a H2O2 environment.
Investigating the behavior and resistance of bacteria under extreme conditions and the physiological changes associated with oxidative stress, Baatout and collaborators24 showed that bacterial strains exhibited varying sensitivities towards exogenous H2O2. For all bacterial strains some physiological damage was noted, in particular with regard to their membrane permeability. Depending on the bacterial strains, moderate to high physiological damage was observed. Membrane potential, esterase activity, intracellular pH were considerably modified at high H2O2 concentrations. In addition, a low micromolar, single dose of hydrogen peroxide was shown to cause dramatic increases in the apparent intracellular accumulation of fluorophores with different physicochemical properties in different cell types.19 The results were consistent with changes in lateral membrane diffusion caused by hydrogen peroxide.
In fact, Funk and Krise19 reported that exposure to H2O2 facilitated the accumulation of the test substrates (lucifer yellow, oregon green and daunorubincin) in different cell types (HL-60 and human foreskin fibroblasts). Our results agree with those of Funk and Krise, since the amount of MB uptake after incubation with H2O2 is higher (5 μM more compared to PBS solution). The enhancement on the amount of MB uptake by the microorganism results in a higher concentration of intracellular MB, therefore MB might be closer to the intracellular sites sensitive to PDT damage. It has been recognized for many years that MB binds to DNA, preferentially to G-C base pairs and it has been assumed that MB, being a tricyclic heterocyclic compound, would intercalate between the base pairs of DNA.24 MB can also photo-oxidize several amino acids, specifically tryptophan, tyrosine, histidine, methionine and cysteine and this reaction can occur by type I or type II mechanisms. Some organelles as E. coli ribosomes are inactivated by MB photodynamic treatment and biological membrane contains proteins, phospholipids and cholesterol, which are susceptible to photodamage.25
Increased MB uptake after H2O2 can explain the results found when changing the sequence of treatments. It is clear that using H2O2 before PDT or using MB in H2O2 solution improves the uptake of MB by the microorganism and leads to increased microbial killing. However, it is not totally clear how to explain improved killing when PDT was used first and subsequently H2O2 solution.
Caetano and coworkers26 investigated the photodecomposition of phospholipid bilayers in aqueous solutions of methylene blue and observed giant unilamellar vesicles under an optical microscope, which revealed a consistent pattern of membrane disruption as a function of MB concentration and photon density for different substrates supporting the vesicles. Also, Giroldo et al.27 studying the effect of antimicrobial PDT in C. albicans propose that the use of PDT in microorganisms results in an increase of membrane permeabilization, suggesting that antimicrobial PDT mechanism using MB may be related to damage in the plasma membrane of the cells.
Using this hypothesis, PDT alone could promote minor membrane disruption and this could increase the exposure of internal components of the bacteria to H2O2. Since H2O2 is currently used as antimicrobial agent,15 it was expected that facilitating the access of this agent to the interior of bacteria could increase the bacterial killing.
Investigating the production of ROS inside the microorganism and comparing the pictures in panel 1 and 2, the DCF fluorescence is higher inside the cells when the MB is in a H2O2 solution than in PBS solution. The dynamic of ROS production is different between the solutions. In a H2O2 environment, the intracellular concentration of MB may result in a faster and a higher amount of ROS production inside the microorganism and therefore a more efficient antimicrobial effect.
Apparently, these data are not consistent with the results of in vitro analysis of ROS and singlet oxygen photo-production in the presence of H2O2. However, the results of the optical characteristics of the dye showed that the amount of leucoMB decreases when in contact with H2O2 and the oxidized “colored” form increases. May et al.28 suggested that a reduction occurs on MB at the external face of the membrane through an interaction with reductase enzymes. The dye enters the cell by diffusion, and entry is facilitated because leucoMB is more lipophilic and it lacks the charge possessed by MB. It is proposed that once inside the cell, the leuco form is reoxidized.29 Moreover, when in a H2O2 solution, the oxidation process is more intense and the amount of oxidized “colored” molecules inside the bacteria is enhanced. In aqueous solution, MB should be found in its leuco form, which is incapable of absorbing visible light (λ = 660 nm) and consequently initiate a photodynamic reaction.27
Also, C. albicans is an aerobic microorganism and can deal with H2O2 by its expression of peroxidase and catalase enzymes, probably during the pre-irradiation time when the microorganism is in contact with MB and H2O2. The hydrogen peroxide improves the uptake of the photosensitizer and part of the H2O2 may be inactivated by the yeast enzymes. Moreover, there are a lot of different sites to be oxidized by H2O2 or PDT inside the cells and the quenching proposed by Gak et al.23 (electron transfer from the excited MB to H2O2 or electron transfer from H2O2 to the MB molecule) is probably lower than in vitro or even there was no quenching. Therefore, the photoreaction inside the microorganism was not decreased by the presence of H2O2. Indeed, the superior amount of MB inside the cells produces an enhanced photoreaction.
These data indicate that the photochemistry of the MB is most unfavorable in the presence of H2O2 in cell free systems. However, the exposure of microorganisms to H2O2 leads to a higher uptake of MB inside the cells resulting in an improved ROS production close to vital structures and, consequently, to a higher microbial death.
4 Experimental procedure
4.1 In vitro antimicrobial experiments
Suspensions of three microorganisms, Gram-positive (Staphylococcus aureus, ATCC 27659), Gram-negative (Escherichia coli, HB 101) and yeast (Candida albicans, 51393) were grown in brain heart infusion (BHI) broth at 37 °C with shaking (150 rpm) for 48 h, until they formed a stationary growth phase suspension.
The suspensions were diluted in PBS to a cell density of 107 per mL, and 1 mL aliquots were added to wells of a 24-well plate. The three microorganisms were incubated with 60 μM of methylene blue (MB) (Sigma Aldrich, Milwaukee, USA) in a PBS solution and in 100 mM solution of H2O2 for 30 min in the absence of light to verify intrinsic (dark) toxicity.
When the results showed a similar microbial killing for the dye and for the H2O2, around 20% after 30 min (data not shown), we tested the effect of photoreaction when 60 μM of MB were incubated in a solution of PBS or in a solution of H2O2 (10 mM, 100 mM and 1 M) for 10 min (pre-irradiation time) followed by illumination with a diode laser at λ = 660 nm, output power of 40 mW (MMOptics, São Carlos, Brazil) and exposure time of 60 s corresponding to the delivery of 60 J cm−2.
Since 30 min of contact with a solution of 100 mM H2O2 did not affect the viability of the microorganisms more than the dark toxicity of the MB, we tested the microbial reduction in the presence of 10 times less and 10 times more H2O2 than the dark exposure to verify if more or less oxygen available in the solution could kill more microorganisms.
A control group was incubated by the same time without any irradiation or H2O2 challenge. Survival fractions were determined from the colony forming units (CFU) in the initial inoculums and compared with the experimental CFU.
4.2 In vitro singlet oxygen and total ROS detection
The first hypothesis that we tested concerned about oxygen concentration. In H2O2 solution the molecules of oxygen available for a photoreaction could be greater than in water solution, as a consequence, this would increase the amount of ROS, especially singlet oxygen.
To validate this hypothesis, a direct singlet oxygen detection was performed, based on its phosphorescence at λ = 1270 nm. A cuvette containing 30 μM of MB in a deutered (D2O) solution or D2O plus H2O2 solution was irradiated using a Nd:YAG laser (Surelite III, Continuum, Santa Clara, USA) at λ = 532 nm (pulse-energy of 30 mJ and pulse-length of 10 ns). The transient emission of singlet oxygen phosphorescence was accumulated and integrated over 20 pulses by a fluorimeter linked to an infrared photomultiplier tube.30 A D2O solution was used to enhance the lifetime of singlet oxygen and therefore increase its detection by the equipment.
In addition, an indirect total ROS detection was performed, based on the oxidation of diacetate of dichlorofluorescein (DCFH-DA) to its fluorescent form dichlorofluorescein (DCF form). The fluorescence was measured (λ = 522 nm) with a standard fluorimeter. Initially, 10 μL of sulfuric acid (Aldrich, Milwaukee, USA) was added to a cuvette containing 20 μL of DCFH-DA for 20 min in dark conditions. Then, ten-μL of sodium hydroxide was added to neutralize the acid. MB at 60 μM in PBS or H2O2 solution completed the cuvette and a baseline was registered. Illumination was performed using a λ = 660 nm diode laser with P = 40 mW for periods of 1 min performing 12 min of irradiation and DCF fluorescence was measured (at λ = 522 nm) following excitation with a λ = 490 nm lamp.16
4.3 Total ROS detection inside C. albicans
To evaluate if biological material could affect the photoreaction of MB in a peroxide environment, a suspension of C. albicans was incubated with 60 μM of MB in PBS solution for 10 min. First, the microorganism incubated with MB was washed and centrifuged for 10 min at 4000 rpm; the supernatant was removed and the cells re-suspended in a PBS solution. Then, a solution of 10 μM of DCFH-DA was added for 15 min and again washed to remove all DCFH-DA not bound to the cells The cells were irradiated using the same laser as above mentioned with a fluence of 60 J cm−2 (Δt = 1 min) and the rate of DCF formation was evaluated at λ = 522 nm by fluorescence microscopy images (inverted Zeiss microscope). The images were analyzed using the software Image J (National Institute of Health, USA) and Adobe Photoshop CS3 for Macintosh (Adobe, USA). Each picture has 640 × 480 pixels and 8 bits black and white image. The total area of each image was selected and analyzed by fluorescence intensity through the histogram and the values were analyzed as a function of time. The relative brightness (maximum and extension) of fluorescence images was evaluated as a function of irradiation time, and plotted as intensity versus irradiation time. The same experimental procedure was repeated using MB in a 100 mM H2O2 solution. The use of yeast instead of bacteria is justified because the morphology of C. albicans is more complex and it is bigger than S. aureus or E. coli to be observed by optical microscopy.
4.4 Mechanisms involved in the combination
4.4.1 MB uptake by bacteria
Suspensions of E. coli were diluted in 60 μM MB in PBS solution to a cell density of 107 per mL for 10 min. The suspension was centrifuged for 10 min at a rate of 4000 rpm sedimenting bacteria with bound dye. In the other group, suspension of E. coli was diluted in 100 mM of H2O2 associated to 60 μM MB solution to a cell density of 107 per mL for 10 min, and then the suspension was also centrifuged for 10 min at 4000 rpm and the supernatant was removed. In the third group, bacterial suspension was firstly incubated with 100 mM of H2O2 for 10 min. The peroxide solution was washed and replaced by MB at 60 μM in PBS solution. The suspension was again centrifuged for another 10 min at 4000 rpm. The supernatant of all groups was removed and analyzed by optical spectroscopy to evaluate the MB uptake by bacteria.
4.4.2 Reduction state of the dye
To evaluate if H2O2 would alter the balance between MB and leucoMB,18 the optical characteristics of the MB inside an E. coli suspension in aqueous or H2O2 solution were analyzed via absorbance spectroscopy in a computer-interfaced double-beam spectrophotometer (Cary – 17D Spectrophotometer Conversion, On-Line Instrument System Inc, USA) in 1.0 cm optical path length quartz cuvettes. The dye was dissolved in 3 mL of de-ionized water or H2O2 at the concentration of 60 μM in the presence or absence of 104 cfu mL−1. The optical density at λ = 290 nm to λ = 660 nm was obtained from each wavelength. A second assay was performed replacing 1 mL of aqueous solution by peroxide solution to keep the same MB molarity.
Optical densities were measured every 1 min and analyzed regarding MB/leucoMB ratio; the ratio was calculated by absorbance at 660 nm/absorbance at 290 nm, since the absorbance of MB monomers is around λ = 660 nm and for leucoMB is around λ = 290 nm. The different values found at each sample time were statistically compared using t-test with Microsoft Excel. Results were considered significant when p < 0.01.
4.4.3 Changing incubation sequence
Different combination sequences of MB and H2O2 were performed to verify the hypothesis that different mechanism of killing could occurs depending on the sequence used for the antimicrobial effect. Suspensions of E. coli in the stationary phase were diluted in PBS to a cell density of 107 per mL, and 100 μL aliquots were added to wells of a 96-well plate. Regarding to the experimental groups, one group [(MB+H2O2)+light] received the 60 μM MB in a 100 mM solution of H2O2 for 10 min and was irradiated by the same diode laser used above (light fluence: 60 J cm−2). The second group [H2O2+(MB+light)] received 100 mM of H2O2 for 10 min, and then the suspension was centrifuged for 10 min at 4000 rpm. The supernatant was removed and replaced by a 60 μM of MB solution in PBS for further 10 min and then irradiated (light fluence: 60 J cm−2). The third group [(MB+light)+H2O2] was incubated with 60 μM of MB for 10 min in a PBS solution followed by irradiation. Then, this group received a 100 mM solution of H2O2 in the absence of light for further 10 min. Survival fractions were determined from the CFU in the initial inoculums and compared with the experimental CFU.
Conclusion
The combination of methylene blue and H2O2 has been shown to be an interesting approach to improve PDT efficiency against Gram+ bacteria, Gram− bacteria and fungi. The mechanism involved seems to be related with the increase in cell membrane permeability, MB reduction by the cell during photosensitizer uptake followed by oxidation of the leucoMB form as a result of the hydrogen peroxide, therefore increasing ROS formation inside the microorganism.
Acknowledgments
The authors kindly thank Prof. Karin do Amaral Riske for the use of the optical microscope, Helena Junqueira for her collaboration, and FAPESP (grant 05/51598-7) for financial support. R. Itri, M. S. Baptista and M. S. Ribeiro also acknowledge CNPq for research fellowship. N. A. Daghastanli was supported by a post-doctorate fellowship from FAPESP (05/00253-0). Research in Dr Hamblin’s laboratory is supported by US NIH grant R01AI050875, US Air Force MFEL Program (FA9550-04-1-0079).
References
- 1.Yoshikawa TT. Antimicrobial resistance and aging: beginning of the end of the antibiotic era? J Am Geriatr Soc. 2002;50:226–229. doi: 10.1046/j.1532-5415.50.7s.2.x. [DOI] [PubMed] [Google Scholar]
- 2.Wise R, Hart T, Cars O. Antimicrobial resistance. Br Med J. 1998;37:609–610. doi: 10.1136/bmj.317.7159.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hancock RE, Bell A. Antibiotic uptake into gram-negative bacteria. Eur J Clin Microbiol Infect Dis. 1988;7:713–720. doi: 10.1007/BF01975036. [DOI] [PubMed] [Google Scholar]
- 4.Livermore DM. Antibiotic resistance in staphylococci. Int J Antimicrob Agents. 2001;16:3–10. doi: 10.1016/s0924-8579(00)00299-5. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal R, Athar M, Urban SA, Bickers DR, Mukhtar H. Involvement of singlet oxygen in chloroaluminium phthalocyanine tetrasulfonate-mediated photoenhancement of lipid peroxidation in rat epidermal microsomes. Cancer Lett. 1991;56:125–129. doi: 10.1016/0304-3835(91)90086-w. [DOI] [PubMed] [Google Scholar]
- 6.Phoenix DA, Sayed Z, Hussain S, Harris F, Wainwright M. The phototoxicity of phenothiazinium derivates against Escherichia coli and Staphylococcus aureus. FEMS Immunol Med Microbiol. 2003;39:17–22. doi: 10.1016/S0928-8244(03)00173-1. [DOI] [PubMed] [Google Scholar]
- 7.Hamblin MR, O’Donnell DA, Murthy N, Rajagopalan K, Michaud N, Sherwood ME, Hasan T. Polycationic photosensitizer conjugates: effects of chain length and Gram classification on the photodynamic inactivation of bacteria. J Antimicrob Chemother. 2002;49:941–951. doi: 10.1093/jac/dkf053. [DOI] [PubMed] [Google Scholar]
- 8.Wainwright M, Phoenix DA, Marland J, Wareing DRA, Bolton FJ. A study of photobactericidal activity in phenothiazinium series. FEMS Immunol Med Microbiol. 1997;19:75–83. doi: 10.1111/j.1574-695X.1997.tb01074.x. [DOI] [PubMed] [Google Scholar]
- 9.Dahl TA, Midden WR, Hartman PE. Comparison of killing of gram-negative and gram-positive bacteria by pure singlet oxygen. J Bacteriol. 1989;171:2188–2194. doi: 10.1128/jb.171.4.2188-2194.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedberg JS, Skema C, Baum ED, Burdick J, Vinogradov SA, Wilson DF, Horan AD, Nachamkin I. In vitro effects of photodynamic therapy on Aspergillus fumigatus. J Antimicrob Chemother. 2001;48:105–107. doi: 10.1093/jac/48.1.105. [DOI] [PubMed] [Google Scholar]
- 11.Feuerstein O, Moreinos D, Steinberg D. Synergic antibacterial effect between visible light and hydrogen peroxide on Streptococcus mutans. J Antimicrob Chemother. 2006;57:872–876. doi: 10.1093/jac/dkl070. [DOI] [PubMed] [Google Scholar]
- 12.Hayek RR, Araujo NS, Gioso MA, Ferreira J, Baptista-Sobrinho CA, Yamada AM, Ribeiro MS. Comparative study between the effects of photodynamic therapy and conventional therapy on microbial reduction in ligature-induced peri-implantitis in dogs. J Periodontol. 2005;76:1275–1281. doi: 10.1902/jop.2005.76.8.1275. [DOI] [PubMed] [Google Scholar]
- 13.Garcez AS, Núñez SC, Lage-Marques JL, Jorge AOC, Ribeiro MS. Efficiency of NaOCl and laser-assisted photosensitization on the reduction of Enterococcus faecalis in vitro. Oral Surg, Oral Med, Oral Pathol, Oral Radiol Endodontol. 2006;102:e93–98. doi: 10.1016/j.tripleo.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 14.McCullagh C, Robertson PKJ. Photo-dynamic biocidal action of methylene blue and hydrogen peroxide on the cyanobacterium Syne-chococcus leopoliensis under visible light irradiation. J Photochem Photobiol, B. 2006;83:63–68. doi: 10.1016/j.jphotobiol.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 15.McCullagh C, Robertson PKJ. Photodestruction of Chlorella vulgaris by methylene blue or nuclear fast red combined with hydrogen peroxide under visible light irradiation. Environ Sci Technol. 2006;40:2421–2425. doi: 10.1021/es052542s. [DOI] [PubMed] [Google Scholar]
- 16.Daghastanli N, Itri R, Baptista MS. Singlet oxygen reacts with 2′,7′-Dichlorodihydrofluorescein and contributes to the formation of 2′,7′-Dichlorofluorescein. Photochem Photobiol. 2008;84:1238–1243. doi: 10.1111/j.1751-1097.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- 17.Gabrieli D, Belisle E, Severino D, Kowaltowski AJ, Baptista MS. Binding, aggregation and photochemical properties of methylene blue in mitochondrial suspensions. Photochem Photobiol. 2004;79:227–232. doi: 10.1562/be-03-27.1. [DOI] [PubMed] [Google Scholar]
- 18.Blázquez-Castro A, Stockert JC, Sanz-Rodríguez F, Zamarrón A, Juarranz A. Differential photodynamic response of cultured cells to methylene blue and toluidine blue: role of dark redox processes. Photochem Photobiol Sci. 2009;8:371–376. doi: 10.1039/b818585a. [DOI] [PubMed] [Google Scholar]
- 19.Funk RS, Krise JP. Exposure to hydrogen peroxide can increase the intracellular accumulation of drugs. Mol Pharmaceutics. 2007;4:154–159. doi: 10.1021/mp060071q. [DOI] [PubMed] [Google Scholar]
- 20.Seaver LC, Imlay JA. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J Bacteriol. 2001;183:7182–7189. doi: 10.1128/JB.183.24.7182-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foote CS. Definition of type I and type II photosensitized oxidation. Photochem Photobiol. 1991;54:659. doi: 10.1111/j.1751-1097.1991.tb02071.x. [DOI] [PubMed] [Google Scholar]
- 22.De Rosa MC, Crutchley RJ. Photosensitized singlet oxygen and its applications. Coord Chem Rev. 2002;233–234:351–371. [Google Scholar]
- 23.Gak VY, Nadtochenko VA, Kiwi J. Triplet-excited dye molecules (eosine and methylene blue) quenching by H2O2 in aqueous solution. J Photochem Photobiol, A. 1998;116:57–62. [Google Scholar]
- 24.Baatout S, De Boever P, Mergeay M. Physiological changes induced in four bacterial strains following oxidative stress. Prikl Biokhim Mikrobiol. 2006;42:418–427. [PubMed] [Google Scholar]
- 25.Wainwright M. Photodynamic antimicrobial chemotherapy (PACT) J Antimicrob Chemother. 1998;42:13–28. doi: 10.1093/jac/42.1.13. [DOI] [PubMed] [Google Scholar]
- 26.Caetano W, Haddad PS, Itri R, Severino D, Vieira VC, Baptista MS, Schroder AP, Marques CM. Photo-induced destruction of giant vesicles in methylene blue solutions. Langmuir. 2007;23:1307–1314. doi: 10.1021/la061510v. [DOI] [PubMed] [Google Scholar]
- 27.Giroldo LM, Felipe MP, de Oliveira MA, Munin E, Alves LP, Costa MS. Photodynamic antimicrobial chemotherapy (PACT) with methylene blue increases membrane permeability in Candida albicans. Lasers Med Sci. 2009;24:109–112. doi: 10.1007/s10103-007-0530-2. [DOI] [PubMed] [Google Scholar]
- 28.May JM, Qu ZC, Cobb CE. Reduction and uptake of methylene blue by human erythrocytes. Am J Physiol: Cell Physiol. 2004;286:C1390–C1398. doi: 10.1152/ajpcell.00512.2003. [DOI] [PubMed] [Google Scholar]
- 29.Wondrak GT. NQO1-activated phenothiazinium redox cyclers for the targeted bioreductive induction of cancer cell apoptosis. Free Radical Biol Med. 2007;43:178–190. doi: 10.1016/j.freeradbiomed.2007.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveira MS, Lima M, Severino D, Baptista MS, Di Mascio P, Tabak M. Quenching of singlet molecular oxygen, O2 (1Δg), by dipyridamole and derivatives. Photochem Photobiol. 2007;83:1379–1385. doi: 10.1111/j.1751-1097.2007.00174.x. [DOI] [PubMed] [Google Scholar]