Abstract
Background
This study aims to estimate general and racial-ethnic specific cumulative probability of developing dependence among nicotine, alcohol, cannabis or cocaine users, and to identify predictors of transition to substance dependence.
Methods
Analyses were done for the subsample of lifetime nicotine (n=15,918), alcohol (n=28,907), cannabis (n=7,389) or cocaine (n=2,259) users who participated in the first and second wave of the National Epidemiological Survey on Alcohol and Related Conditions (NESARC). Discrete-time survival analyses were implemented to estimate the cumulative probability of transitioning from use to dependence and to identify predictors of transition to dependence.
Results
The cumulative probability estimate of transition to dependence was 67.5% for nicotine users, 22.7% for alcohol users, 20.9% for cocaine users, and 8.9% for cannabis users. Half of the cases of dependence on nicotine, alcohol, cannabis and cocaine were observed approximately 27, 13, 5 and 4 years after use onset, respectively. Significant racial-ethnic differences were observed in the probability of transition to dependence across the four substances. Several predictors of dependence were common across the four substances assessed.
Conclusions
Transition from use to dependence was highest for nicotine users, followed by cocaine, alcohol and cannabis users. Transition to cannabis or cocaine dependence occurred faster than transition to nicotine or alcohol dependence. The existence of common predictors of transition dependence across substances suggests that shared mechanisms are involved. The increased risk of transition to dependence among individuals from minorities or those with psychiatric or dependence comorbidity highlights the importance of promoting outreach and treatment of these populations.
Keywords: nicotine, alcohol, cannabis, cocaine, dependence, racial-ethnic groups, discrete-time time survival analyses
1. Introduction
Although only a small proportion of individuals who use addictive substances develop dependence (United Nations Office on Drugs and Crime., 2007), substance dependence represents a tremendous burden to the individual and to society (World Health Organization., 2002). Estimating the risk and predictors of transition from substance use to dependence can provide information about the etiology and course of addiction, guide clinicians in identifying patients at higher risk of becoming dependent, and assist in the organization of primary and secondary prevention services.
Previous epidemiological studies have found that between one-third to one-half of daily nicotine smokers develop nicotine dependence at some point in their lives (Anthony et al., 1994; Breslau et al., 2001; Dierker et al., 2008; Kandel et al., 1997) and that, within a decade of alcohol, cannabis and cocaine use, 12%-13% develop alcohol dependence, 8% cannabis dependence and 15%-16% cocaine dependence (Wagner and Anthony, 2002a). Several risk factors for the transition from use to dependence have been identified, including being young, male, Black or Native-American, poor, with low levels of educational attained, urban residence, early substance use onset, use of another psychoactive substance, and co-occurrence of a psychiatric disorder (Behrendt et al., 2009; Breslau et al., 2001; Chen et al., 2005; Dawson et al., 2008; Grant and Dawson, 1997, 1998; Kandel et al., 1997; O'Brien and Anthony, 2005; Reardon and Buka, 2002; Wagner and Anthony, 2002a, 2007; Warner et al., 1995).
Despite the significant contributions from previous studies, important questions remain regarding the factors influencing transition from substance use to dependence. For example, most studies have examined all substances together (Kessler et al., 2001; Merikangas et al., 1998) or focused on a single substance (Breslau et al., 2001; Chen et al., 2005; O'Brien and Anthony, 2005), precluding formal examination of similarities and differences of predictors across substances. Psychiatric comorbidity, a consistent predictor of transition in many studies (Kessler et al., 1997; Merikangas et al., 1998) has been often examined as a single category (Merikangas et al., 1998) or analyzed as invariant over time (Breslau, 1995; Sintov et al., 2009). Few studies have examined racial-ethnic differences in the rates and determinants of transitioning from use to dependence (Grant, 1996; Grant et al., 2004b; Kandel et al., 1997; Ridenour et al., 2005).
To fill these gaps in knowledge, we sought to estimate the risk and identify the predictors of transition from substance use to dependence in a large, nationally representative sample of U.S. adults. The specific goals of this study were: 1) to estimate the general and racial-ethnic specific cumulative probability of developing dependence among nicotine, alcohol, cannabis and cocaine users, and, 2) to assess the association between several socio-demographic characteristics, psychiatric comorbidity and drug-use related variables and the risk of transition to dependence among users of these substances.
2. Methods
2.1 Sample and procedures
The 2004-2005 Wave 2 NESARC (Grant et al., 2007b) is the second wave of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC)(Grant et al., 2003a). The target population was the civilian non-institutionalized population 18 years and older residing in households and group quarters (GQ) (e.g., college quarters, group homes, boarding houses, and non-transient hotels). Blacks, Hispanics, and adults ages 18-24 were oversampled.
Of the 43,093 respondents interviewed at Wave 1, 34,653 respondents were re-interviewed at Wave 2. Census-defined eligible for Wave 2 re-interviews included those not deceased (n=1403), deported, mentally or physically impaired (n=781) or on active military duty (n=950). Sample weights were developed to additionally adjust for Wave 2 non-response. Specifically, weights adjusted for the probabilities of selection of a sample housing unit or housing unit equivalent from the GQ, nonresponse at the household and person levels, the selection of one person per household, and oversampling of young adults. Once weighted, data were adjusted to be representative of the US population for region, age, sex, race, and ethnicity. After adjustment, comparisons between Wave 2 respondents and the target population (comprising Wave 2 respondents and eligible non-respondents) indicated that there were no significant differences in terms of a number of baseline (Wave 1) socio-demographic measures or the presence of any lifetime substance, mood, anxiety or personality disorder (PD) (Grant et al., 2007a). This study examines data of the sub-sample of lifetime nicotine (n=15,918), alcohol (n=28,907), cannabis (n=7,389) or cocaine (n=2,259) users.
2.2 Data collection
Data was collected using the National Institute on Alcohol Abuse and Alcoholism Alcohol - Alcohol Use Disorder and Associated Disabilities Interview Schedule-DSM-IV (AUDADIS-IV), (Grant et al., 2001a), version Wave 2 (Grant et al., 2004a). The AUDADIS-IV is a structured diagnostic interview, developed to advance measurement of substance use and mental disorders in large-scale surveys (Grant et al., 2008; Grant et al., 2004e). Computer algorithms produced DSM-IV diagnoses based on AUDADIS-IV data collected at Wave 1 (prevalence cases) and Wave 2 (incident cases between Wave 1 and Wave 2).
2.3 Measures
2.3.1 Substance use and dependence
Extensive AUDADIS-IV questions covered DSM-IV criteria for alcohol and drug-specific abuse and dependence for 10 classes of substances (sedatives, tranquilizers, painkillers, stimulants, cannabis, cocaine or crack, hallucinogens, Inhalants/solvents, Heroin, alcohol and nicotine). Substance use and age of substance use onset was determined by asking respondents about the age at which they first “smoked a first full cigarette” (nicotine use), “had at least 1 drink of any kind of alcohol (not counting small tastes or sips)” (alcohol use), used cannabis (cannabis use), or used cocaine or crack (cocaine use). Dependence on these substances was assessed separately via extensive items covering the DSM-IV criteria (American Psychiatric Association, 1994), which require 3 or more of 7 criteria within a 12-month period. Drug-specific dependence criteria were aggregated to yield diagnoses of drug dependence for each substance. The presence of symptoms for time periods whose duration was greater than 1 year, was assessed by asking the study participants the occurrence of those symptoms “on and off for a few months or longer”, “most days for at least a month,” or “within the same 1-year period”. A previous diagnosis of a substance use disorder (SUDs, abuse or dependence) to any of the 10 substances assessed in the AUDADIS IV was also included as a covariate.
The good to excellent (κ=0.54-0.91) test-retest reliability and validity of AUDADIS-IV SUD diagnoses is well documented in clinical and general population samples (Grant et al., 2003b; Hasin et al., 1997; Hasin et al., 2003; Ruan et al., 2008).
2.3.2 Psychiatric disorders
Mood disorders included DSM-IV primary major depressive disorder, dysthymia, bipolar I, and bipolar II. Anxiety disorders included DSM-IV primary panic disorder (with and without agoraphobia), social anxiety disorder, specific phobias, generalized anxiety disorder and post-traumatic stress disorder (PTSD). AUDADIS-IV methods to diagnose these disorders are described in detail elsewhere (Grant et al., 2005a; Grant et al., 2005b; Grant et al., 2005c; Hasin et al., 2005a; Stinson et al., 2007). Psychotic and conduct disorders were assessed in the Wave 1 of the NESARC and attention-deficit/hyperactivity disorder (ADHD) and PTSD were only assessed in the Wave 2 of the NESARC. Consistent with DSM-IV, “primary” AUDADIS-IV diagnoses excluded disorders that are substance-induced or due to general medical conditions.
Avoidant, dependent, obsessive-compulsive, paranoid, schizoid, histrionic, and antisocial and PDs were assessed on a lifetime basis at Wave 1 and described in detail elsewhere (Grant et al., 2004c). Borderline, schizotypal, and narcissistic PDs were measured at Wave 2. Personality disorder diagnosis required long-term patterns of social and occupational impairment (Grant et al., 2004d).
Test-retest reliabilities for AUDADIS-IV mood, anxiety and PD diagnoses in the general population and clinical settings were fair to good (κ=0.40-0.77) (Canino et al., 1999; Grant et al., 2003b; Ruan et al., 2008). Convergent validity was good to excellent for all affective, anxiety, and PD diagnoses (Grant et al., 2004c; Hasin et al., 2005b), and selected diagnoses showed good agreement (κ=0.64-0.68) with psychiatrist reappraisals (Canino et al., 1999).
2.3.3 Demographic and other substance use-related variables Socio-demographics
Self-reported race/ethnicity was recoded into 5 groups: Whites, Blacks, Hispanics, Native Hawaiians or other Pacific Islanders (NH/PI) and American Indians or Alaskan Natives (AI/AN). Other socio-demographic factors included gender, age, urbanicity (urban vs. rural), nativity (US-born vs. foreign-born), level of education, individual and family income, marital status, and employment status. Early substance use onset (before age 14) and family history of SUDs (any alcohol or drug use disorder among first degree relatives) were also included as substance use-related covariates.
2.4 Analyses
Weighted frequencies and their respective 95% confidence intervals (95% CI) were computed to characterize the sample. Estimated projections of the cumulative probability of transitioning from use to dependence within the first year of substance use onset, the first decade after substance use onset, and lifetime in the general population and by racial-ethnic group were obtained by the standard actuarial method (Machin et al., 2006) as implemented in PROC LIFETEST in SAS (version 9.1.3), (SAS Institute, Cary, N.C.). The log-rank test was used to determine whether survival curves differed statistically across substances and across racial-ethnic groups for each substance.
Univariate and multivariable discrete-time survival analyses (with person-year as the unit of analysis) (Jenkins, 1995) were implemented using SUDAAN version 9.1, (Research Triangle Institute, Research Triangle Park). The models aimed at assessing the association between socio-demographic, psychiatric comorbidity and substance use-related covariates and the hazards of transition to substance dependence. The person-year variable was defined as the number of years from substance use onset to age of dependence onset or age at Wave 2 interview (for censored cases). Education, marital status, and presence of DSM-IV mood, anxiety, and SUDs other than the one under examination were included as time-dependent covariates. Stepwise model selection procedures were used to identify independent correlates based on likelihood-ratio test. Taylor series linearization methods implemented in SUDAAN were used to estimate standard errors and significance, and to accommodate for the complex survey design. All estimates were obtained using Wave 2 weights.
3. Results
3.1 Sociodemographic characteristics
Socio-demographic characteristics of the study populations are presented in table 1. Rates of nicotine, alcohol, cannabis and alcohol use were higher among males, Whites and AI/AN and US-born individuals. While rates of nicotine and cannabis use were higher among individuals in the youngest age group (18 to 29 years old), rates of alcohol and cocaine use were higher among individuals 30 to 44 years old. Respondents less educated and with an individual income lower than $35,000 reported higher rates of nicotine use. Respondents who completed at least some college education or had an individual income equal or higher than $70,000 reported higher rates of alcohol, cannabis or cocaine use. While divorced/separated individuals reported higher rates of nicotine, alcohol and cocaine use, never married individuals reported higher rates of cannabis use.
Table 1.
Socio-demographic characteristics of individuals with lifetime history of nicotine, alcohol, cannabis and cocaine use (weighted percentages).
| Characteristic | Nicotine Use N=15,918 | Alcohol Use N=28,907 | Cannabis Use N=7,389 | Cocaine Use N=2,259 | ||||
|---|---|---|---|---|---|---|---|---|
| |
||||||||
| N | % | N | % | N | % | N | % | |
| Gender | ||||||||
| Male | 8301 | 57.2 | 13197 | 92.5 | 3919 | 26.9 | 1306 | 9.0 |
| Female | 7752 | 38.8 | 15963 | 81.6 | 3490 | 17.4 | 957 | 4.8 |
| Age group | ||||||||
| 18-29 | 1992 | 40.7 | 4281 | 88.1 | 1539 | 31.3 | 328 | 6.7 |
| 30-44 | 4239 | 40.1 | 9358 | 89.7 | 2998 | 28.3 | 1005 | 9.5 |
| ≥45 | 9822 | 51.8 | 15521 | 83.8 | 2872 | 15.0 | 930 | 4.87 |
| Race/ethnicity | ||||||||
| Whites | 10581 | 52.8 | 17805 | 90.3 | 4897 | 24.3 | 1512 | 7.5 |
| Blacks | 2703 | 41.5 | 5169 | 80.8 | 1179 | 17.9 | 275 | 4.2 |
| Hispanics | 2155 | 34.1 | 5033 | 81.3 | 1027 | 16.2 | 383 | 6.0 |
| NH/PI | 268 | 27.9 | 660 | 70.4 | 118 | 12.2 | 33 | 3.4 |
| AI/AN | 346 | 60.3 | 493 | 86.5 | 188 | 32.6 | 60 | 10.4 |
| Urbanicity | ||||||||
| Rural | 2587 | 46.5 | 4679 | 85.6 | 1187 | 21.2 | 391 | 7.0 |
| Urban | 13466 | 46.7 | 24481 | 86.3 | 6222 | 21.4 | 1872 | 6.5 |
| Nativity | ||||||||
| US-Born | 14465 | 49.7 | 25317 | 88.5 | 6990 | 23.9 | 2134 | 7.3 |
| Foreign-born | 1588 | 29.8 | 3843 | 74.0 | 419 | 7.8 | 129 | 2.4 |
| Education | ||||||||
| < High school | 2757 | 50.7 | 3900 | 73.7 | 719 | 13.1 | 246 | 4.5 |
| High school | 3990 | 49.1 | 6672 | 83.6 | 1410 | 17.2 | 451 | 5.5 |
| ≥ College | 9306 | 44.6 | 18588 | 90.5 | 5280 | 25.2 | 1566 | 7.5 |
| Individual income | ||||||||
| $0-$19,999 | 6867 | 45.6 | 11641 | 78.8 | 2631 | 17.3 | 799 | 5.3 |
| $20,000-$34,999 | 3936 | 49.0 | 7045 | 89.4 | 1713 | 21.2 | 512 | 6.3 |
| $35,000-$69,999 | 3849 | 47.3 | 7497 | 93.4 | 2140 | 26.2 | 639 | 7.8 |
| ≥ $70,000 | 1401 | 44.o | 2977 | 94.8 | 925 | 28.9 | 313 | 9.8 |
| Family income | ||||||||
| $0-$19,999 | 3996 | 48.4 | 6144 | 76.0 | 1375 | 16.5 | 479 | 5.7 |
| $20,000-$34,999 | 3421 | 49.3 | 5748 | 84.7 | 1308 | 18.7 | 376 | 5.4 |
| $35,000-$69,999 | 5002 | 47.2 | 9309 | 89.2 | 2385 | 22.4 | 717 | 6.7 |
| ≥ $70,000 | 3634 | 42.1 | 7959 | 93.5 | 2341 | 27.1 | 691 | 8.0 |
| Marital Status | ||||||||
| Married | 8536 | 45.5 | 16137 | 87.3 | 3865 | 20.5 | 1142 | 6.1 |
| Divorced/separated | 3217 | 56.4 | 5027 | 89.6 | 1503 | 26.8 | 525 | 9.1 |
| Widowed | 1533 | 45.7 | 2290 | 71.0 | 125 | 3.7 | 30 | 0.9 |
| Never married | 2767 | 41.9 | 5706 | 87.9 | 1916 | 28.9 | 566 | 8.5 |
| Employment status | ||||||||
| Never employed | 2542 | 47.0 | 3803 | 72.3 | 381 | 7.0 | 118 | 2.2 |
| Ever employed | 13511 | 46.6 | 25357 | 88.8 | 7028 | 24.1 | 2145 | 7.4 |
Note: NH/PI=Native Hawaiians or other Pacific Islanders and AI/AN=American Indians or Alaskan Natives
3.2 Psychiatric and Substance Use Comorbid Disorders
Psychiatric and substance use comorbid disorders as well as other substance use-related characteristics of the study populations are presented in table 2. Rates of nicotine, alcohol, cannabis and cocaine use were higher among individuals reporting any lifetime psychiatric disorder, mood disorder, anxiety disorder, conduct disorder, personality disorder or ADHD. Individuals with a family history of a SUD or having a diagnosis of a SUD reported higher rates of use of all the substances assessed. Characteristics of individuals who developed dependence on the four substances are presented in supplementary tables available with the online version of this article (Appendix A).
Table 2.
Psychopathologic and substance use-related characteristics of individuals with lifetime history of nicotine, alcohol, cannabis and cocaine use. Weighted percentages.
| Characteristic | Nicotine Use N=15,918 | Alcohol Use N=28,907 | Cannabis Use N=7,389 | Cocaine Use N=2,259 | ||||
|---|---|---|---|---|---|---|---|---|
| |
||||||||
| N | % | N | % | N | % | N | % | |
| Any lifetime psychiatric disorder | ||||||||
| No | 11458 | 27.4 | 21406 | 76.3 | 4573 | 5.1 | 1265 | 0.6 |
| Yes | 4595 | 58.0 | 7754 | 91.9 | 2836 | 31.0 | 998 | 10.0 |
| Any lifetime mood disorder | ||||||||
| No | 11458 | 44.5 | 21406 | 88.9 | 4573 | 17.7 | 1265 | 4.9 |
| Yes | 4595 | 53.0 | 7754 | 90.1 | 2836 | 32.5 | 998 | 11.4 |
| Any lifetime anxiety disorder | ||||||||
| No | 10544 | 44.2 | 19852 | 84.9 | 4328 | 18.0 | 1251 | 5.2 |
| Yes | 5509 | 52.2 | 9308 | 89.1 | 3081 | 29.0 | 1012 | 9.54 |
| Any lifetime personality disorder | ||||||||
| No | 11772 | 44.1 | 22186 | 84.8 | 4651 | 17.3 | 1229 | 4.6 |
| Yes | 4281 | 55.4 | 6974 | 91.3 | 2758 | 35.5 | 1034 | 13.3 |
| Any lifetime conduct disorder | ||||||||
| No | 15878 | 46.6 | 28864 | 86.2 | 7293 | 21.3 | 2232 | 6.5 |
| Yes | 175 | 54.2 | 296 | 92.5 | 116 | 35.8 | 31 | 9.6 |
| Any lifetime psychotic disorder | ||||||||
| No | 14945 | 46.4 | 27387 | 86.4 | 6866 | 21.2 | 2051 | 6.3 |
| Yes | 673 | 56.6 | 992 | 84.7 | 332 | 27.6 | 135 | 11.2 |
| Any lifetime diagnosis of ADHD | ||||||||
| No | 15533 | 46.2 | 28424 | 86.1 | 7031 | 20.8 | 2113 | 6.3 |
| Yes | 520 | 65.0 | 736 | 92.8 | 378 | 46.9 | 150 | 18.7 |
| Any lifetime diagnosis of nicotine dependence | ||||||||
| No | 8580 | 31.9 | 21996 | 83.4 | 4221 | 15.6 | 1096 | 4.1 |
| Yes | 7473 | 100.0 | 7164 | 96.3 | 3188 | 42.3 | 1167 | 15.5 |
| Any lifetime diagnosis of alcohol dependence | ||||||||
| No | 12547 | 42.5 | 24291 | 83.9 | 4672 | 15.7 | 1104 | 3.7 |
| Yes | 3506 | 71.8 | 4869 | 100.0 | 2737 | 55.8 | 1159 | 23.6 |
| Any lifetime diagnosis of cannabis dependence | ||||||||
| No | 15595 | 46.1 | 28608 | 86.0 | 6845 | 20.1 | 1960 | 5.8 |
| Yes | 458 | 81.8 | 552 | 98.9 | 564 | 100.0 | 303 | 53.9 |
| Any lifetime diagnosis of cocaine dependence | ||||||||
| No | 15707 | 46.2 | 28767 | 86.1 | 7032 | 20.6 | 1865 | 5.5 |
| Yes | 346 | 87.2 | 393 | 99.0 | 377 | 94.5 | 398 | 100.0 |
| Any lifetime Substance Use Disorder (SUD)a | ||||||||
| No | 7922 | 35.4 | 20198 | 82.3 | 1442 | 7.2 | 165 | 0.8 |
| Yes | 8131 | 67.7 | 8962 | 96.6 | 5967 | 40.7 | 2098 | 14.1 |
| Family history of SUDa | ||||||||
| No | 8466 | 41.5 | 16785 | 84.1 | 3313 | 16.1 | 840 | 4.1 |
| Yes | 7587 | 54.1 | 12375 | 89.2 | 4096 | 29.1 | 1423 | 10.1 |
| Specific SU onset | ||||||||
| Before age 14 | 4974 | 2092 | 1051 | 51 | ||||
| At/after age 14 | 11079 | 27068 | 6339 | 2208 | ||||
SUD for 10 substances included in AUDADIS IV (Sedatives, tranquilizers, painkillers, stimulants, cannabis, cocaine or crack, hallucinogens, inhalants/solvents, heroin, other) and alcohol and nicotine. The substance of interest described in the column (i.e. nicotine, alcohol, cannabis or cocaine) was excluded from the list
# Use onset for the substance of interest described in the column (i.e. nicotine, alcohol, cannabis or cocaine)
3.3 Probability of transitioning to substance dependence among substance users
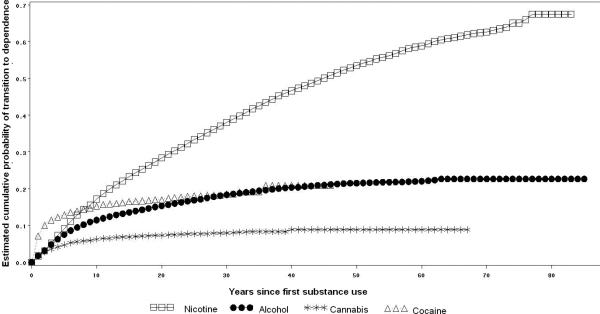
After the first year of substance use onset the probability of transition to dependence was almost 2.0% for nicotine, alcohol and cannabis users and 7.1% for cocaine users. The probability estimates of transition to dependence a decade after use onset was 15.6% among nicotine users, 14.8% among cocaine users, 11.0% among alcohol users, and 5.9% among cannabis users. Lifetime cumulative probability estimates indicated that 67.5% of nicotine users, 22.7% of alcohol users, 20.9% of cocaine users, and 8.9% of cannabis users would become dependent on those substances at some time in their life. Half of the cases of nicotine, alcohol, cannabis and cocaine dependence were observed approximately 27, 13, 5 and 4 years after use onset, respectively (Figure 1).
Figure 1.
Cumulative probability of transitioning to dependence on nicotine, alcohol, cannabis and cocaine among users of these substances
3.3.1 Racial differences in transition to nicotine dependence
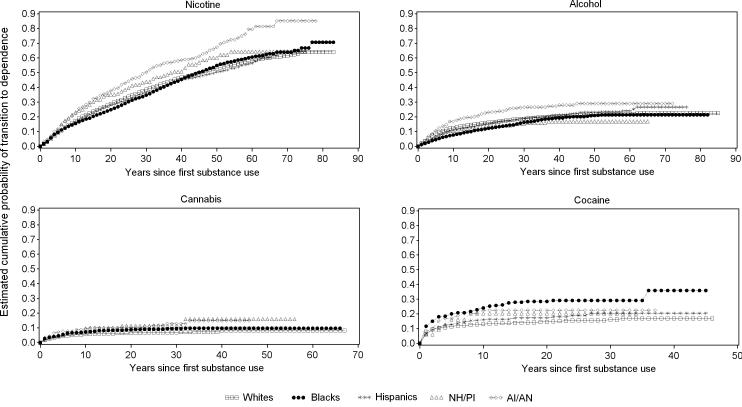
Among nicotine users, 64.1% of Whites, 70.7% of Blacks, 64.7% of Hispanics, 64.5% of NH/PI, and 85.1% of AI/AN transitioned to dependence at some time in their life (log rank test= 41.53, p<0.01). Half of the cases of nicotine dependence among Whites, Blacks, Hispanics, NH/PI and AI/AN developed approximately 23, 30, 26, 18 and 24 years after use onset, respectively (Figure 2).
Figure 2.
Cumulative probability of transitioning to dependence among nicotine, alcohol, cannabis and cocaine users by racial-ethnic group
3.3.2 Racial differences in transition to alcohol dependence
Among alcohol users, 22.6% of Whites, 21.4% of Blacks, 26.7% of Hispanics, 16.9% of NH/PI and 29.0% of AI/AN transitioned to dependence at some time in their life (log rank test= 47.16, p<0.01). Half of the cases of alcohol dependence among Whites, NH/PI and AI/AN developed approximately 8 years after the first use, whereas among Blacks and Hispanics alcohol dependence developed approximately 16 years after the first use (Figure 2).
3.3.3 Racial differences in transition to cannabis dependence
Among cannabis users, 8.2% of Whites, 9.7% of Blacks, 10.0% of Hispanics, 15.9% of NH/PI, and 14.8% of AI/AN (log rank test= 17.57, p<0.01) transitioned to dependence at some time in their life. Half of the cases of cannabis dependence among Whites, Blacks, Hispanics, and AI/AN developed approximately 5 years after the first use. NH/PI developed cannabis dependence approximately 10 years after the first use (Figure 2).
3.3.4 Racial differences in transition to cocaine dependence
Among cocaine users, 16.8% of Whites, 35.9% of Blacks, 20.5% of Hispanics, 20.3% of NH/PI, and 22.5% of AI/AN transitioned to dependence at some time in their life (log rank test= 30.40, p<0.01). Half of the cases of cocaine dependence among Whites, Blacks, Hispanics, NH/PI and AI/AN developed approximately 2, 3, 4, 5 and 4 years, after the first use, respectively (Figure 2).
3.4 Predictors of transition from substance use to dependence
In univariate and multivariable discrete-time survival models several socio-demographic, psychopathological and substance use-related variables predicted transition from substance use to dependence for most of the substances assessed (Tables 3 and 4).
Table 3.
Predictors of transition from first use to dependence on nicotine, alcohol, cannabis and cocaine among specific substance users. Univariate discrete-time survival analyses.
| Characteristics | Nicotine Dependence N=7,320 HR (95%CI) | Alcohol Dependence N=4,822 HR (95%CI) | Cannabis Dependence N=529 HR (95%CI) | Cocaine Dependence N=376 HR (95%CI) |
|---|---|---|---|---|
| Gender (male) | 0.88 (0.8-0.9) | 1.89 (1.8-2.0) | 1.39 (1.1-1.7) | 0.79 (0.6-1.0) |
| Age group | ||||
| 18-29 | 15.46 (13.9-17.2) | 6.68 (5.9-7.6) | 5.37 (3.9-7.4) | 1.97 (1.4-2.9) |
| 30-44 | 4.59 (4.2-5.0) | 2.93 (2.7-3.2) | 2.67 (2.0-3.6) | 1.35 (1.0-1.8) |
| >45* | 1.0 | 1.0 | 1.0 | 1.0 |
| Race/ethnicity | ||||
| Whites* | 1.0 | 1.0 | 1.0 | 1.0 |
| Blacks | 1.07 (0.9-1.2) | 0.91 (0.8-1.0) | 1.27 (0.9-1.7) | 2.05 (1.5-2.8) |
| Hispanics | 0.99 (0.9-1.1) | 1.09 (0.9-1.3) | 1.23 (0.9-1.7) | 1.34 (0.9-1.9) |
| NH/PI | 1.19 (0.9-1.5) | 0.74 (0.6-1.0) | 2.4 (1.2-4.8) | 1.57 (0.6-4.2) |
| AI/AN | 1.55 (1.3-1.8) | 1.45 (1.2-1.8) | 2.02 (1.2-3.3) | 1.82 (0.9-3.7) |
| Urbanicity (Urban) | 1.0 (0.9-1.09) | 1.02 (0.9-1.1) | 1.2 (0.8-1.6) | 0.99 (0.7-1.4) |
| US-Born | 1.41 (1.2-1.6) | 1.7 (1.5-2.0) | 0.75 (0.4-1.4) | 1.09 (0.6-2.1) |
| Education (years) | 1.0 (0.99-1.0) | 0.99 (0.9-1.0) | 0.89 (0.8-0.9) | 0.85 (0.8-0.9) |
| Individual income | ||||
| $0-$19,999 | 1.39 (1.3-1.6) | 1.23 (1.1-1.4) | 2.77 (1.8-4.2) | 2.76 (1.7-4.5) |
| $20,000-$34,999 | 1.39 (1.2-1.6) | 1.35 (1.2-1.6) | 2.16 (1.4-3.4) | 1.97 (1.2-3.4) |
| $35,000-$69,999 | 1.25 (1.1-1.4) | 1.27 (1.1-1.5) | 1.33 (0.8-2.1) | 1.61 (0.9-2.7) |
| ≥ $70,000* | 1.0 | 1.0 | 1.0 | 1.0 |
| Family income | ||||
| $0-$19,999 | 1.09 (0.9-1.2) | 1.38 (1.2-1.5) | 2.4 (1.7-3.5) | 2.25 (1.6-3.3) |
| $20,000-$34,999 | 1.03 (0.9-1.1) | 1.16 (1.0-1.3) | 2.36 (1.6-3.4) | 2.14 (1.4-3.2) |
| $35,000-$69,999 | 1.05 (0.9-1.1) | 1.19 (1.1-1.3) | 1.41 (1.0-1.9) | 1.45 (1.0-2.1) |
| ≥ $70,000* | 1.0 | 1.0 | 1.0 | 1.0 |
| Marital Status | ||||
| Married/living with someone* | 1.0 | 1.0 | 1.0 | 1.0 |
| Never married | 2.69 (2.5-2.9) | 2.64 (2.4-2.9) | 2.44 (1.9-3.1) | 1.15 (0.8-1.6) |
| Divorced/Separated/Widowed | 0.97 (0.9-1.1) | 1.05 (0.9-1.2) | 1.59 (1.2-2.1) | 1.34 (1.1-1.8) |
| Ever employed* | 1.0 | 1.0 | 1.0 | 1.0 |
| Employment status (Never employed) | 0.36 (0.3-0.4) | 0.31 (0.3-0.4) | 0.8 (0.5-1.3) | 1.55 (1.1-2.3) |
| Diagnosed with a mood disorder | 2.06 (1.9-2.2) | 2.47 (2.3-2.7) | 3.29 (2.6-4.2) | 2.92 (2.3-3.8) |
| Diagnosed with an anxiety disorder | 1.90 (1.8-2.0) | 2.06 (1.9-2.2) | 2.73 (2.2-3.5) | 2.53 (2.0-3.3) |
| Any lifetime personality disorder | 2.17 (2.0-2.3) | 3.03 (2.8-3.3) | 4.21 (3.3-5.4) | 3.58 (2.8-4.6) |
| Any lifetime conduct disorder | 1.34 (1.1-1.7) | 1.58 (1.2-2.1) | 1.81 (0.9-3.4) | 1.11 (0.4-3.0) |
| Any lifetime psychotic disorder | 1.38 (1.2-1.6) | 1.53 (1.3-1.8) | 1.91 (1.2-3.0) | 1.78 (1.2-2.6) |
| Any lifetime diagnosis of ADHD | 2.14 (1.9-2.4) | 3.01 (2.6-3.5) | 2.69 (2.0-3.7) | 2.63 (1.8-3.9) |
| Diagnosed with nicotine dependence | 3.52 (3.3-3.8) | 2.99 (2.3-3.9) | 2.74 (2.0-3.7) | |
| Diagnosed with alcohol dependence | 3.05 (2.9-3.2) | 4.24 (3.3-5.4) | 3.32 (2.5-4.5) | |
| Diagnosed with cannabis dependence | 4.31 (3.8-4.8) | 7.39 (6.5-8.4) | 3.83(3.0-5.0) | |
| Diagnosed with cocaine dependence | 3.08 (2.7-3.5) | 6.64 (5.9-7.5) | 6.33 (4.9-8.1) | |
| Family history of SUD | 1.31 (1.2-1.4) | 1.77 (1.7-1.9) | 1.60 (1.3-2.0) | 1.72 (1.3-2.2) |
| Specific SU onset (before age 14) | 1.29 (1.2-1.4) | 2.51 (2.3-2.7) | 2.47 (2.0-3.1) | 1.65 (0.9-3.0) |
Note: NH/PI=Native Hawaiians or other Pacific Islanders and AI/AN=American Indians or Alaskan Natives
Reference category
Table 4.
Socio-demographic, psychopathologic and substance use-related predictors of transition from first use to Dependence on nicotine, alcohol, cannabis and cocaine among specific substance users. Multivariable discrete-time survival analyses.
| Characteristics | Nicotine Dependence N=7,320 HR (95%C.I.) | Alcohol Dependence N=4,822 HR (95%C.I.) | Cannabis Dependence N=529 HR (95%C.I.) | Cocaine Dependence N=376 HR (95%C.I.) |
|---|---|---|---|---|
| Gender (Male) | 0.9 (0.8-0.9) | 1.93 (1.8-2.1) | 1.44 (1.1-1.9) | |
| Age group | ||||
| 18-29 | 15.78 (13.9-17.9) | 3.85 (3.4-4.4) | 3.09 (1.9-4.9) | |
| 30-44 | 4.23 (3.9-4.6) | 2.12 (1.9-2.4) | 2.46 (1.7-3.5) | |
| ≥45* | 1.0 | 1.0 | 1.0 | |
| Race/ethnicity | ||||
| Whites* | 1.0 | 1.0 | 1.0 | 1.0 |
| Blacks | 0.96 (0.9-1.1) | 0.89 (0.8-0.9) | 1.18 (0.9-1.6) | 3.45 (2.2-5.3) |
| Hispanics | 0.80 (0.7-0.9) | 1.08 (0.9-1.2) | 0.82 (0.5-1.2) | 1.19 (0.7-1.9) |
| NH/PI | 1.29 (1.0-1.7) | 1.02 (0.7-1.5) | 2.31 (0.8-6.7) | 2.33 (0.6-8.7) |
| AI/AN | 1.3 (1.1-1.5) | 1.05 (0.8-1.3) | 1.91 (1.2-3.0) | 1.79 (0.6-5.3) |
| US-Born | 1.35 (1.2-1.6) | 1.55 (1.3-1.9) | ||
| Education years (continuous) | 0.98 (0.9-1.0) | |||
| Marital Status | ||||
| Married/living with someone* | 1.0 | 1.0 | 1.0 | |
| Never married | 1.33 (1.2-1.5) | 1.84 (1.6-2.1) | 1.07 (0.6-1.8) | |
| Divorced/Separated/Widowed/ | 1.32 (1.2-1.4) | 2.29 (2.0-2.6) | 1.86 (1.1-3.3) | |
| Employment status (Never employed) | 0.49 (0.4-0.6) | 0.54 (0.5-0.6) | ||
| Diagnosed with a mood disorder | 1.62 (1.4-1.8) | 2.29 (2.0-2.6) | 2.69 (1.9-3.9) | 2.58 (1.5-4.4) |
| Diagnosed with an anxiety disorder | 1.96 (1.8-2.2) | 1.98 (1.7-2.3) | 2.31 (1.6-3.4) | |
| Any lifetime personality disorder | 1.42 (1.3-1.5) | 1.64 (1.5-1.8) | 2.3 (1.7-3.1) | 1.99 (1.4-2.9) |
| Any lifetime psychotic disorder | 1.17 (1.0-1.3) | 1.46 (0.9-2.5) | ||
| Diagnosed with nicotine dependence | 3.29 (2.9-3.7) | 2.06 (1.4-3.1) | ||
| Diagnosed with alcohol dependence | 2.35 (2.1-2.6) | 2.55 (1.8-3.5) | 2.56 (1.7-3.8) | |
| Diagnosed with cannabis dependence | 1.66 (1.2-2.3) | 2.99 (2.3-3.9) | 3.84 (1.8-8.2) | |
| Diagnosed with cocaine dependence | 1.44 (1.0-2.1) | 3.27 (2.1-5.2) | 4.14 (2.3-7.6) | |
| Family history of SUD | 1.14 (1.1-1.2) | 1.49 (1.4-1.6) | ||
| Specific SU onset (before age 14) | 1.13 (1.1-1.2) | 0.63 (0.5-0.9) | 0.34 (0.1-0.9) |
Reference categories
3.4.1 Socio-demographic predictors
According to the adjusted models (Table 4), males were less likely than females to transition from nicotine use to dependence, and more likely to transition from alcohol and cannabis use to dependence. Individuals younger than 45 years old were significantly more likely to transition to dependence for any of the substances assessed than those older than 45 years old. Compared to White nicotine users, Hispanic nicotine were less likely to transition to dependence and NH/PI and AI/AN nicotine users were more likely to transition to dependence. Compared to White alcohol users, Black alcohol users were less likely to transition to dependence. Compared to White cannabis users, AI/AN cannabis users were more likely to transition to dependence. Compared to White cocaine users, Black cocaine users were notably more likely to transit to dependence.
US-born Individuals were more likely than foreign-born individuals to report transition from nicotine and alcohol use to dependence. Compared to nicotine or alcohol users married or living with someone, those never married were more likely to report transition to dependence. Compared to cocaine users married or living with someone, those widowed, divorced or separated were more likely to report transition to dependence. Nicotine and alcohol users who reported ever having been employed showed a lower likelihood of transitioning to dependence. Neither education, nor income predicted transition to dependence.
3.4.2 Psychopathological and substance use-related predictors
A history of any mental disorder strongly predicted the development of substance dependence. For instance, nicotine, alcohol, cannabis or cocaine users diagnosed with a mood disorder or a PD were more likely to become dependent on those substances than individuals who did not report having had any of these disorders. As presented in table 4, controlling for the effect of other covariates did not appreciably modified the results. Nicotine, alcohol or cannabis users diagnosed with an anxiety disorder showed an increased risk of becoming dependent on these substances in the adjusted models. A lifetime diagnosis of a psychotic disorder increased the risk of developing nicotine dependence among nicotine users in the adjusted model.
Having a history of SUD predicted a further development of an additional SUD (Table 4). Individuals diagnosed with nicotine dependence were more likely to develop alcohol dependence among alcohol users, and cannabis dependence among cannabis users. Nicotine, cannabis and cocaine users diagnosed with alcohol dependence showed a higher risk of developing dependence on these substances. Nicotine, alcohol and cocaine users diagnosed with cannabis dependence showed increased hazards of developing dependence on these substances. Nicotine, alcohol and cannabis users diagnosed with cocaine dependence were more likely to develop dependence on these substances.
Family history of SUD increased the risk of transition from nicotine or alcohol use to dependence (table 4). Individuals who used nicotine, before age 14 exhibited higher hazards of becoming nicotine dependent than individuals who started to use it after that age. Individuals who used cannabis or cocaine before age 14 were less likely to transit to dependence on these substances (table 4).
4. Discussion
In a large, nationally representative sample of US adults, the cumulative probability of transition to dependence was highest for nicotine users, followed by cocaine users, alcohol users and, lastly, cannabis users. The transition to cannabis or cocaine dependence occurred faster than the transition to nicotine or alcohol dependence. Furthermore, there were important variations in the probability of becoming dependent across the different racial-ethnic groups. Most predictors of transition were common across substances.
Consistent with previous estimates from the National Comorbidity Survey (Wagner and Anthony, 2002a), the cumulative probability of transition from use to dependence a decade after use onset was 14.8% among cocaine users, 11.0% among alcohol users, and 5.9% among cannabis users. This probability was 15.6% among nicotine users. Furthermore, lifetime cumulative probability estimates indicated that 67.5% of nicotine users, 22.7% of alcohol users, 20.9% of cocaine users, and 8.9% of cannabis users would become dependent at some time in their life. Pharmacokinetic, environmental and physiological factors may contribute to explain the higher rates of transition from nicotine use to dependence compared to transition from use to dependence of other substances. For instance, the rate of absorption in the extensive surface area of alveoli attained from smoking nicotine (Henningfield and Keenan, 1993; Samaha and Robinson, 2005) is far greater than the rate of absorption of alcohol or cocaine in the nasal and gastro-intestinal mucosae (Fattinger et al., 2000; Norberg et al., 2003). The higher social acceptability of nicotine use compared to other substances also increases the exposure to environmental, situational and sensorial cues that evoke craving for its consumption (Benowitz, 2008; Hatsukami et al., 2008). Furthermore, nicotine use does not produce the notorious disruptive behavioral changes that alcohol, cannabis and cocaine use often engender (Benowitz, 2008; Hatsukami et al., 2008).
Variations in the effect of molecular mechanisms underlying addiction-associated neuroadaptations in several regions of the brain (Adinoff, 2004; Benowitz, 2008; Lupica et al., 2004; Moussas et al., 2009; Nestler, 2005), may also contribute to explain differences in the probability of transition across substances (Renthal and Nestler, 2008). Chronic drug exposure results in the accumulation of ΔFosB, which increases the sensitivity to the reinforcing effects of drugs (Perrotti et al., 2008; Renthal and Nestler, 2008; Wallace et al., 2008). Chronic exposure to Δ9-tetrahydrocannabinol, the active ingredient in cannabis, produces a less dramatic effects on ΔFosB induction in the nucleus accumbens shell and dorsal striatum than the effect observed after chronic exposure to cocaine and alcohol (Perrotti et al., 2008).
Consistent with previous studies (Anthony et al., 1994; Behrendt et al., 2009; Breslau et al., 2001; Chen et al., 2005; O'Brien and Anthony, 2005; Ridenour et al., 2003; Ridenour et al., 2005; Wagner and Anthony, 2002a, 2007), the lag period from substance use onset to dependence was greater for nicotine and alcohol than for cannabis or cocaine. Differences in the speed of transition from use to dependence may be related to addictive liability and pharmacokinetic properties of the substances (Koob and Volkow, 2009; Lupica et al., 2004; Nestler, 2005; Ridenour et al., 2005), as well as their availability, legality and social acceptability (Ridenour et al., 2005). Confirming prior findings (Agrawal et al., 2008; Drgon et al., 2006; Kandel, 2002; Stinson et al., 2005), use of and dependence on other substance increased the risk of transition to dependence. In our sample, one third of alcohol users and half of nicotine users had an additional SUD, compared to more than 80% of cannabis users and 90% of cocaine users. The accelerated speed of transition from cannabis or cocaine use to dependence given a previous history of a SUD may be mediated by conditioned learning processes, faster neuroadaptations (Leri et al., 2003; Schlaepfer et al., 2008) and drug interactions leading to slower biotransformation, decreases in adverse drug effects and overall enhanced drug effects (Bradberry et al., 1999; Kapusta et al., 2007; Leri et al., 2003; Mayer, 1984). Individuals dependent on more than one substance appear to have higher genetic liability and alleles of several genes have been associated with polysubstance abuse (Agrawal et al., 2008; Drgon et al., 2006; Schlaepfer et al., 2008). Yet, the simultaneous use of multiple drugs is not entirely explained by genetic factors, with environmental influences playing an important role (Agrawal et al., 2004). Exposure to areas with high levels of social disadvantage facilitates access not only to one but to several drugs, (Storr et al., 2004; Wagner and Anthony, 2002b), which may normalize polysubstance use behaviors. The rapid transition from cannabis and cocaine use to dependence emphasizes the need for aggressive preventive interventions among users of these substances.
Blacks, NH/PI and AI/AN had the highest rates of transition from use to dependence. Acculturative stress and racial/ethnic discrimination may help explain the increased risk of transition from use to dependence among these minority groups (Caraballo et al., 2008; Chae et al., 2008; Kim et al., 2007). Experiencing a stressful event, like discrimination, may promote the utilization of maladaptive coping mechanisms, such as substance use (Gibbons et al., 2004; Whitbeck et al., 2001), or produce enduring neurochemical changes leading to develop a SUD (Koob, 2009). Low social capital, common among AI/AN and Blacks may further increase the risk of transition (Beauvais et al., 2004; Fothergill et al., 2009; U.S. Department of Health and Human Services, 1998). Furthermore, several biological mechanisms and genetic factors may also contribute to explain racial-ethnic variations in the risk of transition to SUD. Genetic influences have accounted for a significant amount of the variance in the development of SUD among AI/AN (Ehlers et al., 2009; Wilhelmsen and Ehlers, 2005). Genetic factors involved in nicotine and cotinine metabolism may contribute to variations in the amount of daily tobacco consumption in several racial-ethnic groups (Benowitz et al., 2009). For instance, Hawaiian (Derby et al., 2008) and Canadian Native-Americans (Hukkanen et al., 2005; Schoedel et al., 2004) smokers exhibit increased activity of the cytochrome P450 2A6 (CYP2A6), the primary enzyme that inactivates nicotine to cotinine. This increased activity may lead them to increased daily tobacco consumption and vulnerability to develop nicotine dependence.
Psychiatric comorbidity predicted transition to dependence, as previously reported (Breslau et al., 2004; Glantz et al., 2008; Kessler, 2004; Kessler et al., 1996; Zimmermann et al., 2003). The co-occurrence of mental and SUD may be due to self-medication practices or the existence of common liability factors (Leonard et al., 2007; Neale and Kendler, 1995; van Os et al., 2002). Family, twin and genome-wide studies suggest that genetic factors are largely responsible for the pattern of comorbidity of common psychiatric and substance use disorders (Burmeister et al., 2008; Kendler et al., 2003; Li and Burmeister, 2009). For example, individuals with a lifetime history of a psychotic disorder had an increased risk of transition to nicotine dependence. Recent findings have linked mutations in the neurexin 1 gene, a cell adhesion-related gene, to an increased risk for nicotine dependence or schizophrenia (Leonard et al., 2007; Nussbaum et al., 2008; Rujescu et al., 2009). Heightened impulsivity and disturbances in reward motivation are also common risk factors across several psychiatric disorders that can explain their co-occurrence (Agrawal et al., 2004; Chambers and Potenza, 2003). Prevention and treatment of mental disorders may decrease the risk of transition from substance use to dependence.
Results of the unadjusted models indicate that individuals with an early use onset had an increased risk of transition to dependence, which is consistent with the result of previous epidemiological studies (Behrendt et al., 2009; Dawson et al., 2008; Grant et al., 2001b). However, after controlling for the effect of socio-economic, psychiatric comorbidity and drug-use covariates, the direction of this association changed for cannabis and cocaine, indicating that the higher risk of developing dependence on these substances among those who reported an early use onset was mostly explained by those factors. Therefore, the risk of transitioning to dependence may not be a consequence of early substance use onset per se, but rather of the existence of a concomitant psychiatric and SUD that increase the risk of developing a SUD (Behrendt et al., 2009; Degenhardt et al., 2009; Wittchen et al., 2007). The higher risk of transitioning to cannabis and cocaine dependence among those individuals with later use onset, may also be due to the emergence of more intense patterns of use, greater availability and affordability of these drugs in late adolescence and higher social acceptability (Bachman et al., 1998; Behrendt et al., 2009; Kandel, 2002). Consistent with previous findings (Behrendt et al., 2009), the positive association between early onset of nicotine use and transition to dependence remained significant after controlling for other covariates.
The present findings should be interpreted in light of common limitations in most large-scale surveys. First, information on substance use and SUD was based on self-report and not confirmed by objective methods. Second, diagnoses may be subject to recall bias (the longer the time interval between the event and assessment, the higher the probability of incorrect recalls) and to cognitive impairment associated with the use of drugs (Grant et al., 2003c; Hasin and Liu, 2003). Third, in some cases, substance use may have been intermittent, rather than continuous, which would produce inexact estimates of the time elapsed from use onset to dependence. Fourth, the NESARC excludes institutionalized populations, who have an increased probability of having a diagnosis of substance dependence (McCutcheon et al., 2009). Fifth, factors that may help explain the racial-ethnic differences, such as income could not be included in the models as time-dependent covariates since the NESARC, as most large scale surveys, does not include information on changes in income overtime.
In summary, the rate of transition from use to dependence is higher for nicotine user than for alcohol, cannabis or cocaine users. Individuals from some ethnic minority groups and those with a history of psychiatric and substance dependence comorbidity showed an increased risk of transitioning from use to dependence. Several predictors of dependence were common across the substances assessed, suggesting the existence of shared mechanisms. Promoting outreach and treatment of individuals from minority population groups or those with a primary mental disorder may constitute an important strategy to prevent transition to substance dependence.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data tables are available with the online version of this article. See Appendix A.
Appendix A. Supplementary data tables 5 and 6 associated with this article can be found in the online version of this article at doi:10.1016/j.drugalcdep.xxxx.xx.xxx.
References
- Adinoff B. Neurobiologic processes in drug reward and addiction. Harvard review of psychiatry. 2004;12:305–320. doi: 10.1080/10673220490910844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Neale MC, Prescott CA, Kendler KS. Cannabis and other illicit drugs: comorbid use and abuse/dependence in males and females. Behavior genetics. 2004;34:217–228. doi: 10.1023/B:BEGE.0000017868.07829.45. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Pergadia ML, Saccone SF, Hinrichs AL, Lessov-Schlaggar CN, Saccone NL, Neuman RJ, Breslau N, Johnson E, Hatsukami D, Montgomery GW, Heath AC, Martin NG, Goate AM, Rice JP, Bierut LJ, Madden PA. Gamma-aminobutyric acid receptor genes and nicotine dependence: evidence for association from a case-control study. Addiction (Abingdon, England) 2008;103:1027–1038. doi: 10.1111/j.1360-0443.2008.02236.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders : DSM-IV. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Anthony J, Warner L, Kessler R. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Experimental and Clinical Psychopharmacology. 1994;2:244–268. [Google Scholar]
- Bachman JG, Johnson LD, O'Malley PM. Explaining recent increases in students’ marijuana use: impacts of perceived risks and disapproval, 1976 through 1996. American journal of public health. 1998;88:887–892. doi: 10.2105/ajph.88.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais F, Jumper-Thurman P, Helm H, Plested B, Burnside M. Surveillance of drug use among American Indian adolescents: patterns over 25 years. J Adolesc Health. 2004;34:493–500. doi: 10.1016/j.jadohealth.2003.07.019. [DOI] [PubMed] [Google Scholar]
- Behrendt S, Wittchen HU, Hofler M, Lieb R, Beesdo K. Transitions from first substance use to substance use disorders in adolescence: is early onset associated with a rapid escalation? Drug and alcohol dependence. 2009;99:68–78. doi: 10.1016/j.drugalcdep.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Neurobiology of nicotine addiction: implications for smoking cessation treatment. The American journal of medicine. 2008;121:S3–10. doi: 10.1016/j.amjmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handbook of experimental pharmacology. 2009:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW, Lee T, Jatlow P. Rapid induction of behavioral and neurochemical tolerance to cocaethylene, a model compound for agonist therapy of cocaine dependence. Psychopharmacology. 1999;146:87–92. doi: 10.1007/s002130051092. [DOI] [PubMed] [Google Scholar]
- Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behavior genetics. 1995;25:95–101. doi: 10.1007/BF02196920. [DOI] [PubMed] [Google Scholar]
- Breslau N, Johnson EO, Hiripi E, Kessler R. Nicotine dependence in the United States: prevalence, trends, and smoking persistence. Archives of general psychiatry. 2001;58:810–816. doi: 10.1001/archpsyc.58.9.810. [DOI] [PubMed] [Google Scholar]
- Breslau N, Novak SP, Kessler RC. Psychiatric disorders and stages of smoking. Biological psychiatry. 2004;55:69–76. doi: 10.1016/s0006-3223(03)00317-2. [DOI] [PubMed] [Google Scholar]
- Burmeister M, McInnis MG, Zollner S. Psychiatric genetics: progress amid controversy. Nature reviews. 2008;9:527–540. doi: 10.1038/nrg2381. [DOI] [PubMed] [Google Scholar]
- Canino G, Bravo M, Ramirez R, Febo VE, Rubio-Stipec M, Fernandez RL, Hasin D. The Spanish Alcohol Use Disorder and Associated Disabilities Interview Schedule (AUDADIS): reliability and concordance with clinical diagnoses in a Hispanic population. Journal of studies on alcohol. 1999;60:790–799. doi: 10.15288/jsa.1999.60.790. [DOI] [PubMed] [Google Scholar]
- Caraballo RS, Yee SL, Gfroerer J, Mirza SA. Adult tobacco use among racial and ethnic groups living in the United States, 2002-2005. Preventing chronic disease. 2008;5:A78. [PMC free article] [PubMed] [Google Scholar]
- Chae DH, Takeuchi DT, Barbeau EM, Bennett GG, Lindsey J, Krieger N. Unfair treatment, racial/ethnic discrimination, ethnic identification, and smoking among Asian Americans in the National Latino and Asian American Study. American journal of public health. 2008;98:485–492. doi: 10.2105/AJPH.2006.102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Potenza MN. Neurodevelopment, impulsivity, and adolescent gambling. Journal of gambling studies / co-sponsored by the National Council on Problem Gambling and Institute for the Study of Gambling and Commercial Gaming. 2003;19:53–84. doi: 10.1023/a:1021275130071. [DOI] [PubMed] [Google Scholar]
- Chen CY, O'Brien MS, Anthony JC. Who becomes cannabis dependent soon after onset of use? Epidemiological evidence from the United States: 2000-2001. Drug and alcohol dependence. 2005;79:11–22. doi: 10.1016/j.drugalcdep.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcoholism, clinical and experimental research. 2008;32:2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Chiu WT, Conway K, Dierker L, Glantz M, Kalaydjian A, Merikangas K, Sampson N, Swendsen J, Kessler RC. Does the ‘gateway’ matter? Associations between the order of drug use initiation and the development of drug dependence in the National Comorbidity Study Replication. Psychological medicine. 2009;39:157–167. doi: 10.1017/S0033291708003425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derby KS, Cuthrell K, Caberto C, Carmella SG, Franke AA, Hecht SS, Murphy SE, Le Marchand L. Nicotine metabolism in three ethnic/racial groups with different risks of lung cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:3526–3535. doi: 10.1158/1055-9965.EPI-08-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierker L, He J, Kalaydjian A, Swendsen J, Degenhardt L, Glantz M, Conway K, Anthony J, Chiu WT, Sampson NA, Kessler R, Merikangas K. The importance of timing of transitions for risk of regular smoking and nicotine dependence. Ann Behav Med. 2008;36:87–92. doi: 10.1007/s12160-008-9051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drgon T, D'Addario C, Uhl GR. Linkage disequilibrium, haplotype and association studies of a chromosome 4 GABA receptor gene cluster: candidate gene variants for addictions. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:854–860. doi: 10.1002/ajmg.b.30349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Gilder DA, Gizer IR, Wilhelmsen KC. Heritability and a genome-wide linkage analysis of a Type II/B cluster construct for cannabis dependence in an American Indian community. Addiction biology. 2009;14:338–348. doi: 10.1111/j.1369-1600.2009.00160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattinger K, Benowitz NL, Jones RT, Verotta D. Nasal mucosal versus gastrointestinal absorption of nasally administered cocaine. European journal of clinical pharmacology. 2000;56:305–310. doi: 10.1007/s002280000147. [DOI] [PubMed] [Google Scholar]
- Fothergill KE, Ensminger ME, Green KM, Robertson JA, Juon HS. Pathways to adult marijuana and cocaine use: a prospective study of African Americans from age 6 to 42. Journal of health and social behavior. 2009;50:65–81. doi: 10.1177/002214650905000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons FX, Gerrard M, Cleveland MJ, Wills TA, Brody G. Perceived discrimination and substance use in African American parents and their children: a panel study. Journal of personality and social psychology. 2004;86:517–529. doi: 10.1037/0022-3514.86.4.517. [DOI] [PubMed] [Google Scholar]
- Glantz MD, Anthony JC, Berglund PA, Degenhardt L, Dierker L, Kalaydjian A, Merikangas KR, Ruscio AM, Swendsen J, Kessler RC. Mental disorders as risk factors for later substance dependence: estimates of optimal prevention and treatment benefits. Psychological medicine. 2008:1–13. doi: 10.1017/S0033291708004510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B, Dawson D, Hasin D. The Alcohol Use Disorders and Associated Disabilities Interview Schedule—Version for DSM-IV (AUDADIS-IV) National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2001a. [Google Scholar]
- Grant B, Kaplan K, Moore T, Kimball J. 2004–2005 Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions: Source and Accuracy Statement. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2007a. [Google Scholar]
- Grant B, Moore T, Shepard J, Kaplan K. Source and Accuracy Statement: Wave 1 of the 2001–2002 National Epidemiologic Survey of Alcohol and Related Conditions (NESARC) National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2003a. [Google Scholar]
- Grant BF. Prevalence and correlates of drug use and DSM-IV drug dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. Journal of substance abuse. 1996;8:195–210. doi: 10.1016/s0899-3289(96)90249-7. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of substance abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of substance abuse. 1998;10:163–173. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Hasin DS. The Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions Alcohol Use Disorder and Associated Disabilities Interview Schedule - DSMIV Version.accessed on. 2004a.
- Grant BF, Dawson DA, Stinson FS, Chou PS, Kay W, Pickering R. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV (AUDADIS-IV): reliability of alcohol consumption, tobacco use, family history of depression and psychiatric diagnostic modules in a general population sample. Drug and alcohol dependence. 2003b;71:7–16. doi: 10.1016/s0376-8716(03)00070-x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug and alcohol dependence. 2004b;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Chou SP, Huang B, Stinson FS, Dawson DA, Saha TD, Smith SM, Pulay AJ, Pickering RP, Ruan WJ, Compton WM. Sociodemographic and psychopathologic predictors of first incidence of DSM-IV substance use, mood and anxiety disorders: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. Molecular Psychiatry. 2008 doi: 10.1038/mp.2008.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Blanco C, Stinson FS, Chou SP, Goldstein RB, Dawson DA, Smith S, Saha TD, Huang B. The epidemiology of social anxiety disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. The Journal of clinical psychiatry. 2005a;66:1351–1361. doi: 10.4088/jcp.v66n1102. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Stinson FS, Dawson DA, Chou SP, Ruan WJ, Pickering RP. Prevalence, correlates, and disability of personality disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2004c;65:948–958. doi: 10.4088/jcp.v65n0711. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Stinson FS, Dawson DA, Ruan WJ, Goldstein RB, Smith SM, Saha TD, Huang B. Prevalence, correlates, co-morbidity, and comparative disability of DSM-IV generalized anxiety disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychol Med. 2005b;35:1747–1759. doi: 10.1017/S0033291705006069. [DOI] [PubMed] [Google Scholar]
- Grant BF, Kaplan KK, Stinson FS. Source and Accuracy Statement: The Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions.accessed on. 2007b.
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of general psychiatry. 2004d;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Ruan WJ, Pickering RP. Co-occurrence of 12-month alcohol and drug use disorders and personality disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2004e;61:361–368. doi: 10.1001/archpsyc.61.4.361. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Harford TC. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. Journal of substance abuse. 2001b;13:493–504. doi: 10.1016/s0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Hasin DS, Dawson DA, Chou SP, Ruan WJ, Huang B. Prevalence, correlates, and comorbidity of bipolar I disorder and axis I and II disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. The Journal of clinical psychiatry. 2005c;66:1205–1215. doi: 10.4088/jcp.v66n1001. [DOI] [PubMed] [Google Scholar]
- Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. J Int Neuropsychol Soc. 2003c;9:679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- Hasin D, Carpenter KM, McCloud S, Smith M, Grant BF. The alcohol use disorder and associated disabilities interview schedule (AUDADIS): reliability of alcohol and drug modules in a clinical sample. Drug and alcohol dependence. 1997;44:133–141. doi: 10.1016/s0376-8716(97)01332-x. [DOI] [PubMed] [Google Scholar]
- Hasin D, Liu X. High-risk community drinkers: a 10-year follow-up study [Abstract 103]. Alcohol: Clinical and Experimental Research. 2003;27:180A. [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. The epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005a;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Archives of general psychiatry. 2005b;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Schuckit MA, Martin CS, Grant BF, Bucholz KK, Helzer JE. The validity of DSM-IV alcohol dependence: what do we know and what do we need to know? Alcoholism, clinical and experimental research. 2003;27:244–252. doi: 10.1097/01.ALC.0000060878.61384.ED. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Stead LF, Gupta PC. Tobacco addiction. Lancet. 2008;371:2027–2038. doi: 10.1016/S0140-6736(08)60871-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. Journal of consulting and clinical psychology. 1993;61:743–750. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacological reviews. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Jenkins SP. Easy estimation methods for discrete-time duration models. Oxford Bull Econ Stat. 1995;57:129–138. [Google Scholar]
- Kandel D, Chen K, Warner LA, Kessler RC, Grant B. Prevalence and demographic correlates of symptoms of last year dependence on alcohol, nicotine, marijuana and cocaine in the U.S. population. Drug and alcohol dependence. 1997;44:11–29. doi: 10.1016/s0376-8716(96)01315-4. [DOI] [PubMed] [Google Scholar]
- Kandel DB. Stages and pathways of drug involvement : examining the gateway hypothesis. Cambridge University Press; Cambridge, UK ; New York: 2002. [Google Scholar]
- Kapusta ND, Plener PL, Schmid R, Thau K, Walter H, Lesch OM. Multiple substance use among young males. Pharmacology, biochemistry, and behavior. 2007;86:306–311. doi: 10.1016/j.pbb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of general psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kessler R, Aguilar-Gaxiola S, Andrade L, Bijl R, Borges G, Caraveo-Anduaga J, DeWit D, Kolody B, Merikangas K, Molnar B, Vega W, Walters E, Wittchen H, Ustun T. Mental-substance comorbidities in the ICPE surveys (English). Psychiatria Fennica. 2001;32:62–79. [Google Scholar]
- Kessler RC. The epidemiology of dual diagnosis. Biological psychiatry. 2004;56:730–737. doi: 10.1016/j.biopsych.2004.06.034. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Archives of general psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Nelson CB, McGonagle KA, Edlund MJ, Frank RG, Leaf PJ. The epidemiology of co-occurring addictive and mental disorders: implications for prevention and service utilization. The American journal of orthopsychiatry. 1996;66:17–31. doi: 10.1037/h0080151. [DOI] [PubMed] [Google Scholar]
- Kim SS, Ziedonis D, Chen KW. Tobacco use and dependence in Asian Americans: a review of the literature. Nicotine Tob Res. 2007;9:169–184. doi: 10.1080/14622200601080323. [DOI] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2009 doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of Addiction. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard S, Mexal S, Freedman R. Smoking, Genetics and Schizophrenia: Evidence for Self Medication. Journal of dual diagnosis. 2007;3:43–59. doi: 10.1300/J374v03n03_05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Bruneau J, Stewart J. Understanding polydrug use: review of heroin and cocaine co-use. Addiction (Abingdon, England) 2003;98:7–22. doi: 10.1046/j.1360-0443.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- Li MD, Burmeister M. New insights into the genetics of addiction. Nature reviews. 2009;10:225–231. doi: 10.1038/nrg2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC, Hoffman AF. Marijuana and cannabinoid regulation of brain reward circuits. British journal of pharmacology. 2004;143:227–234. doi: 10.1038/sj.bjp.0705931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machin D, Cheung YB, Parmar MKB, Parmar MKB. Survival analysis : a practical approach. Wiley; Chichester, England ; Hoboken, NJ: 2006. [Google Scholar]
- Mayer J. Mechanisms of drug interactions with alcohol. In: Kreek MJ, Stimmel B, editors. Dual Addiction. Hawarth Press; New York: 1984. [Google Scholar]
- McCutcheon VV, Heath AC, Edenberg HJ, Grucza RA, Hesselbrock VM, Kramer JR, Bierut LJ, Bucholz KK. Alcohol criteria endorsement and psychiatric and drug use disorders among DUI offenders: greater severity among women and multiple offenders. Addict Behav. 2009;34:432–439. doi: 10.1016/j.addbeh.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Mehta RL, Molnar BE, Walters EE, Swendsen JD, Aguilar-Gaziola S, Bijl R, Borges G, Caraveo-Anduaga JJ, DeWit DJ, Kolody B, Vega WA, Wittchen HU, Kessler RC. Comorbidity of substance use disorders with mood and anxiety disorders: results of the International Consortium in Psychiatric Epidemiology. Addictive behaviors. 1998;23:893–907. doi: 10.1016/s0306-4603(98)00076-8. [DOI] [PubMed] [Google Scholar]
- Moussas G, Christodoulou C, Douzenis A. A short review on the aetiology and pathophysiology of alcoholism. Annals of general psychiatry. 2009;8:10. doi: 10.1186/1744-859X-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Kendler KS. Models of comorbidity for multifactorial disorders. American journal of human genetics. 1995;57:935–953. [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. The neurobiology of cocaine addiction. Science & practice perspectives / a publication of the National Institute on Drug Abuse. National Institutes of Health. 2005;3:4–10. doi: 10.1151/spp05314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg A, Jones AW, Hahn RG, Gabrielsson JL. Role of variability in explaining ethanol pharmacokinetics: research and forensic applications. Clinical pharmacokinetics. 2003;42:1–31. doi: 10.2165/00003088-200342010-00001. [DOI] [PubMed] [Google Scholar]
- Nussbaum J, Xu Q, Payne TJ, Ma JZ, Huang W, Gelernter J, Li MD. Significant association of the neurexin-1 gene (NRXN1) with nicotine dependence in European- and African-American smokers. Human molecular genetics. 2008;17:1569–1577. doi: 10.1093/hmg/ddn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000-2001. Neuropsychopharmacology. 2005;30:1006–1018. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- Perrotti LI, Weaver RR, Robison B, Renthal W, Maze I, Yazdani S, Elmore RG, Knapp DJ, Selley DE, Martin BR, Sim-Selley L, Bachtell RK, Self DW, Nestler EJ. Distinct patterns of DeltaFosB induction in brain by drugs of abuse. Synapse (New York, NY. 2008;62:358–369. doi: 10.1002/syn.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon SF, Buka SL. Differences in onset and persistence of substance abuse and dependence among whites, blacks, and Hispanics. Public Health Rep. 2002;117(Suppl 1):S51–59. [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends in molecular medicine. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridenour TA, Cottler LB, Compton WM, Spitznagel EL, Cunningham-Williams RM. Is there a progression from abuse disorders to dependence disorders? Addiction (Abingdon, England) 2003;98:635–644. doi: 10.1046/j.1360-0443.2003.00350.x. [DOI] [PubMed] [Google Scholar]
- Ridenour TA, Maldonado-Molina M, Compton WM, Spitznagel EL, Cottler LB. Factors associated with the transition from abuse to dependence among substance abusers: implications for a measure of addictive liability. Drug and alcohol dependence. 2005;80:1–14. doi: 10.1016/j.drugalcdep.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan WJ, Goldstein RB, Chou SP, Smith SM, Saha TD, Pickering RP, Dawson DA, Huang B, Stinson FS, Grant BF. The alcohol use disorder and associated disabilities interview schedule-IV (AUDADIS-IV): reliability of new psychiatric diagnostic modules and risk factors in a general population sample. Drug and alcohol dependence. 2008;92:27–36. doi: 10.1016/j.drugalcdep.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rujescu D, Ingason A, Cichon S, Pietilainen OP, Barnes MR, Toulopoulou T, Picchioni M, Vassos E, Ettinger U, Bramon E, Murray R, Ruggeri M, Tosato S, Bonetto C, Steinberg S, Sigurdsson E, Sigmundsson T, Petursson H, Gylfason A, Olason PI, Hardarsson G, Jonsdottir GA, Gustafsson O, Fossdal R, Giegling I, Moller HJ, Hartmann AM, Hoffmann P, Crombie C, Fraser G, Walker N, Lonnqvist J, Suvisaari J, Tuulio-Henriksson A, Djurovic S, Melle I, Andreassen OA, Hansen T, Werge T, Kiemeney LA, Franke B, Veltman J, Buizer-Voskamp JE, Sabatti C, Ophoff RA, Rietschel M, Nothen MM, Stefansson K, Peltonen L, St Clair D, Stefansson H, Collier DA. Disruption of the neurexin 1 gene is associated with schizophrenia. Human molecular genetics. 2009;18:988–996. doi: 10.1093/hmg/ddn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Robinson TE. Why does the rapid delivery of drugs to the brain promote addiction? Trends in pharmacological sciences. 2005;26:82–87. doi: 10.1016/j.tips.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Schlaepfer IR, Hoft NR, Ehringer MA. The genetic components of alcohol and nicotine co-addiction: From genes to behavior. Current drug abuse reviews. 2008;1:124–134. doi: 10.2174/1874473710801020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14:615–626. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- Sintov ND, Kendler KS, Walsh D, Patterson DG, Prescott CA. Predictors of illicit substance dependence among individuals with alcohol dependence. Journal of studies on alcohol and drugs. 2009;70:269–278. doi: 10.15288/jsad.2009.70.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson FS, Dawson DA, Chou SP, Smith S, Goldstein RB, Ruan WJ, Grant BF. The epidemiology of specific phobia in the USA: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychol Med. 2007;37:1–13. doi: 10.1017/S0033291707000086. [DOI] [PubMed] [Google Scholar]
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug and alcohol dependence. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Storr CL, Chen CY, Anthony JC. “Unequal opportunity”: neighbourhood disadvantage and the chance to buy illegal drugs. Journal of epidemiology and community health. 2004;58:231–237. doi: 10.1136/jech.2003.007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . Tobacco Use Among Racial/Ethnic Minority Groups African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, and Hispanics: A Report of the Surgeon General. US Department of Health and Human Services, Centers of Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 1998. [Google Scholar]
- United Nations Office on Drugs and Crime . World Drug Report 2007. UNODC; Viena: 2007. [Google Scholar]
- van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. Cannabis use and psychosis: a longitudinal population-based study. American journal of epidemiology. 2002;156:319–327. doi: 10.1093/aje/kwf043. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002a;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. Into the world of illegal drug use: exposure opportunity and other mechanisms linking the use of alcohol, tobacco, marijuana, and cocaine. American journal of epidemiology. 2002b;155:918–925. doi: 10.1093/aje/155.10.918. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. Male-female differences in the risk of progression from first use to dependence upon cannabis, cocaine, and alcohol. Drug and alcohol dependence. 2007;86:191–198. doi: 10.1016/j.drugalcdep.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, Graham DL, Green TA, Kirk A, Iniguez SD, Perrotti LI, Barrot M, DiLeone RJ, Nestler EJ, Bolanos-Guzman CA. The influence of DeltaFosB in the nucleus accumbens on natural reward-related behavior. J Neurosci. 2008;28:10272–10277. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner LA, Kessler RC, Hughes M, Anthony JC, Nelson CB. Prevalence and correlates of drug use and dependence in the United States. Results from the National Comorbidity Survey. Archives of general psychiatry. 1995;52:219–229. doi: 10.1001/archpsyc.1995.03950150051010. [DOI] [PubMed] [Google Scholar]
- Whitbeck LB, Hoyt DR, McMorris BJ, Chen X, Stubben JD. Perceived discrimination and early substance abuse among American Indian children. Journal of health and social behavior. 2001;42:405–424. [PubMed] [Google Scholar]
- Wilhelmsen KC, Ehlers C. Heritability of substance dependence in a native American population. Psychiatric genetics. 2005;15:101–107. doi: 10.1097/00041444-200506000-00006. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Frohlich C, Behrendt S, Gunther A, Rehm J, Zimmermann P, Lieb R, Perkonigg A. Cannabis use and cannabis use disorders and their relationship to mental disorders: a 10-year prospective-longitudinal community study in adolescents. Drug and alcohol dependence. 2007;88(Suppl 1):S60–70. doi: 10.1016/j.drugalcdep.2006.12.013. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Health Report 2002: Reducing Risk. Promoting Healthy Life WHO; Geneva: 2002. [Google Scholar]
- Zimmermann P, Wittchen HU, Hofler M, Pfister H, Kessler RC, Lieb R. Primary anxiety disorders and the development of subsequent alcohol use disorders: a 4-year community study of adolescents and young adults. Psychological medicine. 2003;33:1211–1222. doi: 10.1017/s0033291703008158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.