Abstract
Articular cartilage is a highly efficacious water-based tribological system that is optimized to provide low friction and wear protection at both low and high loads (pressures) and sliding velocities that must last over a lifetime. Although many different lubrication mechanisms have been proposed, it is becoming increasingly apparent that the tribological performance of cartilage cannot be attributed to a single mechanism acting alone but on the synergistic action of multiple “modes” of lubrication that are adapted to provide optimum lubrication as the normal loads, shear stresses, and rates change. Hyaluronic acid (HA) is abundant in cartilage and synovial fluid and widely thought to play a principal role in joint lubrication although this role remains unclear. HA is also known to complex readily with the glycoprotein lubricin (LUB) to form a cross-linked network that has also been shown to be critical to the wear prevention mechanism of joints. Friction experiments on porcine cartilage using the surface forces apparatus, and enzymatic digestion, reveal an “adaptive” role for an HA-LUB complex whereby, under compression, nominally free HA diffusing out of the cartilage becomes mechanically, i.e., physically, trapped at the interface by the increasingly constricted collagen pore network. The mechanically trapped HA-LUB complex now acts as an effective (chemically bound) “boundary lubricant”—reducing the friction force slightly but, more importantly, eliminating wear damage to the rubbing/shearing surfaces. This paper focuses on the contribution of HA in cartilage lubrication; however, the system as a whole requires both HA and LUB to function optimally under all conditions.
Keywords: arthritis, mechanical trapping, elastohydrodynamic lubrication, biointerface, biolubrication
Articular joints are almost completely sealed from their surroundings—by the synovial membrane around the joint and by cartilage and bone above and below the joint (1, 2). These barriers restrict rapid chemical transport into and out of joints, making it difficult to replace or repair damaged internal tissue or macromolecules, particularly those molecules that are covalently attached (bound) to the internal cartilage surfaces (1–3). Thus, it is no surprise that the major molecules involved in joint lubrication [lubricin and hyaluronic acid (HA)] are noncovalently bound and yet—to function as effective “boundary lubricants” that exhibit low friction and protect surfaces from wear—they need to act as if they are chemically bound to the surfaces.
Hyaluronic acid has long been considered a potential boundary lubricant for cartilage (3–6), although numerous friction experiments have shown that solutions of free HA exhibit little lubrication activity (4, 5). However, surface forces apparatus (SFA) experiments (4) on chemically grafted and cross-linked HA layers demonstrated that such HA provide excellent wear protection for surfaces shearing at high pressures (200 atm), even though high friction coefficients (μ = 0.15 - 0.3) were measured. These results imply that friction and wear are not necessarily correlated and that a layer of strongly immobilized HA could protect a cartilage surface from wear (damage) if not necessarily contributing to low friction.
Various studies have found that under constant load and shearing the friction coefficient measured for cartilage increases monotonically over time from an initially low value of μ ≤ 0.01 - 0.005 to a considerably higher steady-state value in the range of μ ≤ 0.09–0.21 for cartilage against glass (7–13). The equilibrium values reported correspond to the measured friction coefficient after the cartilage has undergone considerable deformation and are similar to values reported by Benz et al. (4) for grafted HA gels, raising the possibility that the characteristic increasing μ of cartilage with increasing deformation (and time) arises from a gradual transition to boundary lubrication provided by a surface-immobilized HA layer.
Previous work has postulated that a lubricating benefit could be derived from a layer of HA aggregates that are anchored to the surface through partial entanglement within the collagen network of the outermost superficial zone (1). However, in order for partially entangled HA to function as an effective boundary lubricant under large normal loads and shear stresses, it has to be strongly immobilized at the surface. Because HA is known not to form any physical or covalent bonds or exhibit any adhesive interactions with cartilage’s surface or internal collagen fibril pore network, the mechanism through which the necessary immobilization of HA chains is achieved is not immediately obvious. Recent reports describing the anisotropic changes to cartilage’s pore network microstructure under compression suggests the HA immobilization could be achieved through a “mechanical trapping” mechanism by which entangled HA chains become trapped in the collapsing pore network of the deforming cartilage (14, 15).
In previous studies, lubricin (LUB) has been shown to adsorb to a wide variety of charged, uncharged, and hydrophobic surfaces and substrates (16) and to form aggregate complexes both with itself (17) and with free HA chains in solution (18) acting as a type of physical “cross-linking” agent*. Lubricin is thus expected to form cross-links with the surface-imobilized, partially entangled HA layer forming a cross-linked “HA-LUB complex” that is also expected to, under certain conditions, improve the HA layer’s ability to resist wear damage. However, LUB has been shown to form nonspecific and relatively weak physical bonds with anionic substrates (16) (e.g., HA, mica) making the disassociation of HA-LUB cross-link bonds and/or redistribution of LUB between the shearing surfaces more likely when the normal, friction, and/or shear forces are large.
Results and Discussion
A series of SFA friction experiments (for details see Materials and Methods) investigating the effects of enzymatic digestion of HA with hyaluronidase on the dynamically changing and equilibrium friction forces in sheared cartilage against glass in PBS reveals an adaptive lubrication mechanism mediated by an interfacial HA-LUB complex layer and regulated by the mechanical deformation response of the cartilage under different loading conditions. Because the interfacial HA layers at the cartilage surface are cross-linked and complexed with molecules of LUB, digestion of this layer also removes LUB molecules from the cartilage surface† in addition to removing the bulk of HA from the surface, thus decreasing the molecular weight of HA chains in the remaining layer (19). In the first series of “step-load” experiments, described below, the surfaces were not rinsed with fresh PBS following digestion, leaving the HA fragments and associated LUB in the solution between the surfaces (there is no evidence that LUB itself is affected by hyaluronidase). Given the physical nature of the HA-LUB bond, the high affinity of LUB to the cartilage surface (19), and the long time periods (approximately 45 min) over which the friction forces were measured after HA digestion, one may presume that any “liberated” LUB had sufficient time to find its way back and readsorb to the cartilage surface or become trapped between the two shearing surfaces. In the second series of “dynamic-loading” experiments, described below, the cartilage surfaces were rinsed with fresh PBS after HA digestion, thereby removing both HA and liberated LUB from the surface and the solution. The results (described below) showed that the presence or absence of LUB at the surface of HA-digested cartilage did not significantly affect the friction or wear properties observed (under the conditions and time scales of our experiments). These experiments show that the ability of partially entangled (and LUB cross-linked) HA chains to function as a boundary lubricant is intimately connected to and controlled by the structural and mechanical deformation response of the cartilage under compressive loading.
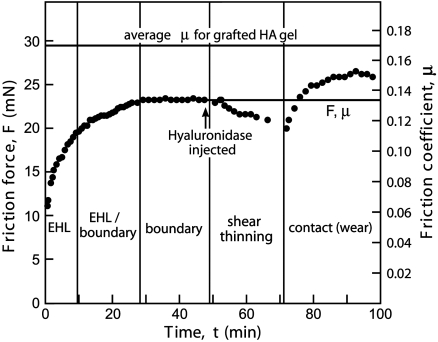
In the first series of experiments termed “step-load” experiments (shown in Fig. 1), the cartilage was compressed with a large instantaneous step load L and the friction force F measured under continuous shearing as the cartilage deformed. Consistent with previous reports (3, 7, 9, 12, 13, 20), an initially low, but temporally increasing friction force characteristic of elastohydrodynamic lubrication (EHL) maintained by a fluid pressurization mechanism was observed. As the pore fluid is driven out and gradually becomes depleted in the increasingly deformed cartilage, the ability of the cartilage to support EHL via the secretion of interstitial fluid diminishes and a “mixed” or “transition” lubrication regime is entered involving a combination of EHL and boundary lubrication (BL) processes (i.e., EHL + BL). At longer loading times, the lubrication of the surfaces shifts more and more toward BL causing the friction force to rise until eventually plateauing at a stable equilibrium value (μeq ≈ 0.13) signifying steady-state sliding in a purely BL regime. Without removing the load, hyaluronidase was injected into the PBS reservoir, initially causing a slow decrease in the friction force lasting approximately 20 min. This decrease is due to the lower molecular weight of digested HA decreasing the effective viscosity and giving rise to shear thinning behavior. After this decline, a sudden and rapid increase in the friction force and the appearance of wear were observed that eventually plateaus at a second equilibrium value (μeq ≈ 0.15) 15% higher than before digestion, indicating a transition into a contact lubrication regime. Contrary to previous reports (4, 5, 21, 22), this increase in the equilibrium friction coefficient, we believe, demonstrates that HA is able to act as a boundary lubricant in cartilage, at least under severe deformation conditions as indicated by the increased friction (and as we will show later, damage) as HA is removed through digestion.
Fig. 1.
The effect of HA digestion on the friction force between cartilage and glass in PBS. Step-load experiment showing how the friction force, F, and the friction coefficient, μ, changes with time, t, and following the in situ digestion of HA under a normal load of 170 mN, sliding velocity of approximately 50 μm/s, and peak-to-peak sliding amplitude of approximately 500 μm. The solid line at the top is the average friction coefficient of grafted HA gel on mica surfaces under similar load and shear conditions reported in ref. 4, shown for comparison. Solid lines indicate equilibrium values.
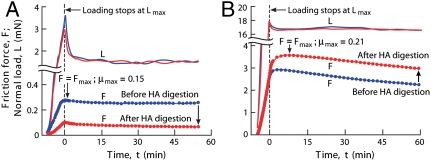
Further insight into how mechanical deformation influences the lubrication behavior of HA was found in a second series of experiments, termed “dynamic-loading” experiments, that compared the frictional response of cartilage samples before and after being digested under different “high” and “low” dynamic loads (see Fig. 2 A and B). For normal loads less than approximately 15 mN (estimated pressure > 1 kPa), HA digestion caused the measured maximum friction force F to decrease relative to that measured before digestion as illustrated in the example shown in Fig. 2A for L ∼ 1.8 mN. However, for higher loads, HA digestion had the opposite effect giving rise to an increase in the friction force relative to that before digestion as shown in Fig. 2B.
Fig. 2.
Normal load, L, and friction force, F, as a function of time, t, for cartilage samples measured before and after digestion of HA under low (A) and high (B) dynamically applied normal loads.
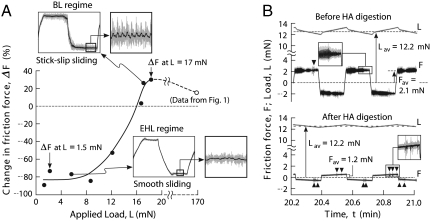
The load dependence of the HA lubrication mechanism can clearly be seen in Fig. 3A, which shows the percent change in the maximum friction force ΔF after HA digestion under different applied loads. For all loads less than approximately 8 mN, HA digestion led to a large and essentially identical negative change (decrease) in F. At these low loads, friction force traces before digestion showed smooth sliding behavior characteristic of EHL, determined by the rheological properties of the confined film. The decrease in F following HA digestion is consistent with the reduced viscosity in an EHL contact. Indeed, the reduction in the effective viscosity of the confined fluid/polymer film is expected to be even more pronounced due to the double effect of the HA digestion that both decreases the chain length of interfacial HA chains and diminishes the LUB cross-link density of the interfacial HA layer.
Fig. 3.
(A) The effect of the load on the change in friction force, ΔF = (Fmax ,undigested-Fmax ,digested)/Fmax ,undigested, and observed sliding behavior. (A, Inset) The sliding behavior of the cartilage before digestion. (B, Inset) Normal load, L, and friction force, F, before and after HA digestion in a cartilage sample under a normal load of approximately 12 mN. Before digestion, the cartilage exhibits stick–slip behavior and high friction force but little evidence of surface wear or damage. After HA digestion, stick–slip behavior disappears and the friction force decreases, but frequent “wear spikes” (darts) indicate significant wear and damage.
At higher loads, ΔF due to HA digestion becomes increasingly less negative and eventually becomes positive as the load exceeds approximately 15 mN. This transition load regime is associated with a gradual transition from EHL to BL and marked by the appearance of stick–slip in the predigestion friction trace that becomes more pronounced with increasing normal load. During the transition from EHL to BL the load both modulates the mechanism of lubrication and changes the functional role of HA in the lubrication process from a bulk viscosity modifier to a surface anchored protective layer (Fig. 3B). Significantly, in the low load EHL regime, friction force traces before and after digestion both show smooth sliding and little or no indication of damage even after several hours of continuous shearing. In contrast, in both the transition and BL load regimes, the friction force trace before digestion showed no evidence of damage in any experiment; however, after digestion, damage became visible in the friction force traces after just a few tens of cycles, becoming more severe with time. As the load increases during this transition regime, the ability of LUB cross-links to maintain the integrity of the cross-linked network of HA chains and support a sufficiently thick lubricating HA/fluid interfacial film becomes increasingly compromised. The breaking of LUB cross-links under larger normal and shear forces leads to the breakdown of the EHL mode and the gradual transition to a BL mechanism mediated by a layer of HA molecules that has now become mechanically trapped in the collapsing pores of the deformed surface.
At very high loads (L≥15 mN) an upper plateau is reached where the positive change (increase) in F following digestion is no longer affected by further increasing the load, marking an end to the transition regime. In this high load “BL regime,” the physical bonds between HA chains and LUB are too weak to avoid breaking under the large normal and shear forces. The weakness and ultimate disassociation of HA-LUB under large loads/shears may have important implications for the adaptive lubrication of articular cartilage surfaces, the details of which are discussed below.
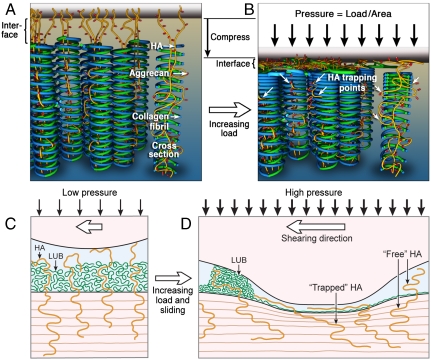
When these experiments are taken in the context of previous experimental results reported by others, in particular studies on the friction of HA gels (4, 5), lubricin protein (16, 17, 23), and cartilage under static loading conditions (7–9, 11, 13, 20), a more complete picture of cartilage lubrication and wear protection emerges. Fig. 4 illustrates how the cartilage’s deformation response to variable normal and shear stresses effectively functions as an adaptive mechanical control, utilizing a combination of BL and EHL processes.
Fig. 4.
Schematic illustration of the HA “mechanical trapping? mechanism. (A) The pore structure of cartilage is formed by counter spiraling collagen fibril coils (blue and green). When undeformed, the lateral pores (void space between fibril “coils”) are open and the entangled HA molecules (yellow), with attached Aggrecans (red) are nominally “free.” (B) In compression, fibril realignment mechanically traps (white arrows) the HA/Aggrecan complexes in the collapsing lateral pores, maintaining a layer of immobilized HA between the collagen and top surface. (C and D) Schematic representation of HA trapping mechanism in the cartilage contact during physiological sliding under low (C) and high (D) loads. For clarity, only the HA-LUB layer from the lower surface was shown.
At low loads and shear velocities, a surface layer of physically adsorbed lubricin and partially entangled and cross-linked HA-LUB complex provides a source of BL that keeps the surfaces well separated and prevents adhesion. As shear velocities increase, a transition from BL to EHL occurs, maintaining surface separation and low friction forces.
At high loads or pressures, interfacial fluid gets squeezed out from the gap between the surfaces; fluid lost from the interface is partially offset by interstitial fluid expelled from the deforming cartilage. Without sufficient recovery time, static or cyclic loading will starve the contact and lead to the failure of the EHL. Upon the failure of the EHL mode, a second “emergency BL mode” is entered whereby lubrication and wear prevention of the cartilage surface rests, primarily, on a layer of “mechanically trapped” HA. Under the large pressures and shear forces that have so severely deformed the cartilage, physically adsorbed molecules such as lubricin bind too weakly to the surface to provide effective boundary lubrication to the shearing surfaces. The relatively weak attachment of lubricin to the cartilage surface, however, allows it to be dragged along with the sheared surfaces leading to a concentration of lubricin within the shearing junction after only a few reciprocating cycles—a process that has been directly observed experimentally (16). In this way, shearing acts to redistribute and concentrate lubricin within the surface regions that have been subjected to the highest shear forces and normal pressures, i.e., where lubricin is needed most.
It is important to note that, in the transition regime, HA digestion significantly lowers the friction coefficient but, at the same time, fails to make the surface less resistant to wear. This apparent contradiction highlights two important points: (i) the primary role of HA in joint lubrication is to protect shearing cartilage surfaces against wear rather than providing a low friction coefficient and (ii) contrary to the belief widely held in the field of joint lubrication and the greater tribology community, low friction does not necessarily imply better wear resistance, and friction and wear should be considered unrelated processes.
These experiments demonstrate one way in which nature has developed purely “physical” or “mechanical” control of the lubrication behavior of a major macromolecule, the polysaccharide HA, that allows it to function either as a free or as an (effectively) bound molecule as required at different stages of articulation of joints, employing mechanisms that could be mimicked in artificial joints and nonmedical tribological applications. How cartilage adapts the mechanism of lubrication to suit various loading and shearing conditions is shown schematically in Fig. 4A and illustrates the way the structural rearrangement of the pore matrix in cartilage undergoing deformation activates and modulates the boundary lubrication properties of surface HA molecules. In this model, the unstressed, uncompressed pore structure of cartilage (Fig. 4A) is represented by coils of counter spiraling collagen fibrils arrayed normal to the fluid interface (14, 15, 24). HA molecules, decorated with aggrecans and cross-linked with LUB, are partially entangled in the collagen fibrils and partially extended into the interfacial fluid. Thus, HA molecules are not anchored to the fibrils but become so once a large normal load compresses the cartilage (see Fig. 4B), closing up the lateral pores in the collagen network while preserving the integrity of the axial pores. In this way, the deforming pore network, particularly that of the superficial zone, is able to mechanically “trap” nominally free HA molecules, immobilizing them at the surface and thus enabling an interfacial layer of HA molecules to behave both as an elastohydrodynamic lubricant under low loads and as an “emergency” boundary lubricant when the cartilage has undergone severe deformation, as under high or prolonged static loading.
The HA trapping mechanism serves a vital role in the context of overall joint performance, mainly that of the last line of defense against damage when the primary fluid pressurization-driven EHL (3, 7, 9, 12, 13, 20), HA-LUB complex supported EHL, and lubricin-based BL (16, 17, 22) processes cease to be effective as occurs at high loading pressures or long loading durations. During physiological sliding (see Fig. 4 C and D), elastohydrodynamic deformations to the cartilage surfaces concentrates the normal load and shearing forces (and thus tissue deformation) within a narrow band. The increased deformation leads to increased trapping of HA within this band allowing it to function as a BL and provide additional wear protection where wear is most likely to occur.
Materials and Methods
Cartilage tissue was collected from porcine knee articular joints (Sierra for Medical Science). The knee was dissected no later than 1 d after slaughter and was shipped intact on ice. Full-thickness (1–2 mm) cartilage samples were extracted at room temperature (25 °C) in a dust-free laminar flow hood using a scalpel. The exposed joint was kept moist during dissection by frequent rinsing with Hank’s Balanced Salt Solution. The cartilage samples were stored in Hank’s buffer solution at -50 °C until use (less than 3 mo). Hyaluronidase (Sigma) was diluted with PBS buffer (Sigma; 120 mM NaCl, 10 mM phosphate salt, 2.7 mM KCl, pH 7.2–7.6) to a concentration of 200 units/mL and stored at -50 °C before use. Milli-Q water (Millipore) was used in all buffer solutions.
Normal and friction forces were measured using a SFA 2000 equipped with a friction device attachment or a 3D/XYZ device (25). Prior to use, the cartilage samples were immersed in a PBS buffer for 30 min at 25 °C. Two flat glass disks were soaked in chloroform for 1 d, rinsed with ethanol, wiped with a lint-free cloth, and then rinsed thoroughly with PBS buffer. The back side of the cartilage sample was partially dried by blotting on a lint-free cloth, then glued onto one of the glass disks using a thin layer of poly(cyanoacrylate) adhesive followed by soaking in PBS buffer. The disk containing the cartilage sample was then mounted into the SFA 2000 opposite the second glass disk for friction forces measurement as shown in Fig. S1. A droplet (approximately 100 μL) of fresh PBS buffer was then injected between the surfaces.
For each of the two series of experiments described in this study, the HA digestion was performed in two different ways. In both methods, a full-thickness cartilage sample was mounted into an SFA 2000 opposite a second apposing flat glass disk (Fig. S1A). The cartilage section was then pressed against the apposing disk in an approximately 100-μL droplet of PBS solution.
In the step-load experiments (shown in Fig. 1), the sample was compressed by manually applying a normal step load of approximately 0.17 N using the SFA 2000 micrometer. While the cartilage was being compressed under the constant load, the surfaces were continuously sheared laterally at a sliding velocity of approximately 50 μm/s and peak-to-peak sliding amplitude of approximately 500 μm (0.1 Hz) using a friction device attachment that both applies the shearing motion and measures the resulting friction force (25). Once the measured friction force had settled into a stable equilibrium, 5 μL of a 200 units/mL solution of hyaluronidase was added to the PBS droplet (yielding a final working concentration of approximately 10 units/mL) while the cartilage remained under compression (Fig. S1 B and C). The cartilage sample was continuously sheared against the glass surface in the hyaluronidase solution for about 50 min. As the HA is digested, a fraction of the LUB at the cartilage surface may be liberated into the solution as noted in ref. 19. However, as noted earlier (see also footnote*), the surface was not rinsed: The LUB may therefore be assumed to be present at the cartilage surface, but appears to make little difference to the friction and wear properties measured. The results are shown in Fig. 1.
In the dynamic-loading experiments (results shown in Figs. 2 and 3), cartilage samples were first continuously sheared against the glass substrate while the surfaces are brought together from separation to the targeted load using a constant-speed motor. The loading motor was then stopped and the shearing continued up to 100 min at a constant imposed displacement (the load relaxes slightly as the cartilage compresses), while the friction forces were measured as a function of load and time (Fig. S1 A, B′, and C′). After shearing, the cartilage sample and the glass substrate were well separated to let the cartilage recover under zero load. During recovery, HA in the sample was digested by injecting 5 μL of a 200 units/mL solution of hyaluronidase to the PBS droplet (yielding a final working concentration of approximately 10 units/mL) between the two surfaces. After waiting 2 h at 25 °C the surfaces were thoroughly rinsed with PBS buffer to remove the enzyme from the medium. This rinsing step also removes the digested fragments of HA as well as any LUB that may have been liberated during the digestion. The friction experiment of the HA-digested cartilage sample surface was performed with the same procedure mentioned above.
For the dynamic-loading experiments, the 3D/XYZ device was used inorder to monitor the normal load as well as the friction forces. The 3D/XYZ device can actuate in the lateral direction with a travel distance of about 60 μm and measure the normal and friction forces at the same time. For all dynamic experiments a sliding velocity of 12 μm/s (0.2 Hz) was used.
Supplementary Material
Acknowledgments.
This work was funded by the McCutchen Foundation.
Footnotes
The authors declare no conflict of interest.
*The notion that LUB “cross-links” HA chains comes from a previous report (18) that shows that the addition of small concentrations of LUB alters the bulk rheological properties of free HA solutions and has been linked to strain energy dissipation in synovial fluid. Recent and continuing SFA studies, which will be the subject of a separate report, also support the existence of strong (possibly cross-linking) interactions between LUB and HA. Though the exact nature of the interactions has yet to be determined, the cross-link “bonds” between LUB and HA are physical (electrostatic, mechanical entanglements, etc.) as opposed to chemical and thus are fully reversible.
†Only the LUB attached to HA protruding into the solution from the cartilage surfaces is expected to be liberated or “removed” from the surface, while maintaining a high affinity for non-HA-covered cartilage (see figure 3 in ref. 19). Also, strictly, it is not established that LUB is naturally liberated from the cartilage surface during HA digestion, only that it can be easily removed by rinsing or washing.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1101002108/-/DCSupplemental.
References
- 1.Huber M, Trattnig S, Lintner F. Anatomy, biochemistry, and physiology of articular cartilage. Invest Radiol. 2000;35:573–580. doi: 10.1097/00004424-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Kuettner KE, Schleyerbach R, Peyron JG, Hascall VC, editors. Articular Cartilage and Osteoarthritis. New York: Raven; 1991. [Google Scholar]
- 3.Klein J. Molecular mechanisms of synovial joint lubrication. Proc Inst Mech Eng, Part J. 2006;220:691–710. [Google Scholar]
- 4.Benz M, Chen NH, Israelachvili J. Lubrication and wear properties of grafted polyelectrolytes, hyaluronan and hylan, measured in the surface forces apparatus. J Biomed Mater Res A. 2004;71A:6–15. doi: 10.1002/jbm.a.30123. [DOI] [PubMed] [Google Scholar]
- 5.Benz M, Chen NH, Jay G, Israelachvili JI. Static forces, structure and flow properties of complex fluids in highly confined geometries. Ann Biomed Eng. 2005;33:39–51. doi: 10.1007/s10439-005-8961-z. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage—Role of synovial fluid constituents. Arthritis Rheum. 2007;56:882–891. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- 7.Ateshian GA, Wang HQ, Lai WM. The role of interstitial fluid pressurization and surface porosities on the boundary friction of articular cartilage. J Tribol-T ASME. 1998;120:241–248. [Google Scholar]
- 8.Carter MJ, Basalo IM, Ateshian GA. The temporal response of the friction coefficient of articular cartilage depends on the contact area. J Biomech. 2007;40:3257–3260. doi: 10.1016/j.jbiomech.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forster HFJ. The influence of loading time and lubricant on the friction of articular cartilage. P I Mech Eng H. 1996;210:109–119. doi: 10.1243/PIME_PROC_1996_210_399_02. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan R, Kopacz M, Ateshian GA. Experimental verification of the role of interstitial fluid pressurization in cartilage lubrication. J Orthopaed Res. 2004;22:565–570. doi: 10.1016/j.orthres.2003.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis PR, Mccutchen CW. Experimental evidence for weeping lubrication in mammalian joints. Nature. 1959;184:1285–1285. doi: 10.1038/1841285a0. [DOI] [PubMed] [Google Scholar]
- 12.McCutchen CW. Sponge-hydrostatic and weeping bearings. Nature. 1959;184:1284–1285. doi: 10.1038/1841284a0. [DOI] [PubMed] [Google Scholar]
- 13.Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927–934. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 14.Greene GW, et al. Anisotropic dynamic changes in the pore network structure, fluid diffusion and fluid flow in articular cartilage under compression. Biomaterials. 2010;31:3117–3128. doi: 10.1016/j.biomaterials.2010.01.102. [DOI] [PubMed] [Google Scholar]
- 15.Greene GW, et al. Changes in pore morphology and fluid transport in compressed articular cartilage and the implications for joint lubrication. Biomaterials. 2008;29:4455–4462. doi: 10.1016/j.biomaterials.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 16.Zappone B, Ruths M, Greene GW, Jay GD, Israelachvili JN. Adsorption, lubrication, and wear of lubricin on model surfaces: Polymer brush-like behavior of a glycoprotein. Biophys J. 2007;92:1693–1708. doi: 10.1529/biophysj.106.088799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zappone B, Greene GW, Oroudjev E, Jay GD, Israelachvili JN. Molecular aspects of boundary lubrication by human lubricin: Effect of disulfide bonds and enzymatic digestion. Langmuir. 2008;24:1495–1508. doi: 10.1021/la702383n. [DOI] [PubMed] [Google Scholar]
- 18.Jay GD, Torres JR, Warman ML, Laderer MC, Breuer KS. The role of lubricin in the mechanical behavior of synovial fluid. Proc Natl Acad Sci USA. 2007;104:6194–6199. doi: 10.1073/pnas.0608558104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nugent-Derfus GE, Chan AH, Schumacher RL, Sah RL. PRG4 exchange between the articular cartilage surface and synovial fluid. J Orthopaed Res. 2007;25:1269–1276. doi: 10.1002/jor.20431. [DOI] [PubMed] [Google Scholar]
- 20.McCutchen CW. The frictional properties of animal joints. Wear. 1962;5:1–17. [Google Scholar]
- 21.Linn FC, Radin EL. Lubrication of animal joints. III. The effect of certain chemical alterations of the cartilage and lubricant. Arthritis Rheum. 1968;11:674–682. doi: 10.1002/art.1780110510. [DOI] [PubMed] [Google Scholar]
- 22.Jay GD, Haberstroh K, Cha CJ. Comparison of the boundary-lubricating ability of bovine synovial fluid, lubricin, and healon. J Biomed Mater Res A. 1998;40:414–418. doi: 10.1002/(sici)1097-4636(19980605)40:3<414::aid-jbm11>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 23.Jay GD. Lubricin and surfacing of articular joints. Curr Opin Orthopaed. 2004;15:355–359. [Google Scholar]
- 24.ap Gwynn I, Wade S, Ito K, Richards G. Novel aspects to the structure of rabbit articular cartilage. Euro Cells Mater. 2002;4:18–29. doi: 10.22203/ecm.v004a02. [DOI] [PubMed] [Google Scholar]
- 25.Israelachvili J, et al. Recent advances in the surface forces apparatus (SFA) technique. Rep Prog Phys. 2010;73:036601. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.