Abstract
It is now well established that neurogenesis in the rodent subgranular zone of the hippocampal dentate gyrus continues throughout adulthood. Neuroblasts born in the dentate subgranular zone migrate into the granule cell layer, where they differentiate into neurons known as dentate granule cells. Suppression of neurogenesis by irradiation or genetic ablation has been shown to disrupt synaptic plasticity in the dentate gyrus and impair some forms of hippocampus-dependent learning and memory. Using a recently developed transgenic mouse model for suppressing neurogenesis, we sought to determine the long-term impact of ablating neurogenesis on synaptic plasticity in young-adult mice. Consistent with previous reports, we found that ablation of neurogenesis resulted in significant deficits in dentate gyrus long-term potentiation (LTP) when examined at a time proximal to the ablation. However, the observed deficits in LTP were not permanent. LTP in the dentate gyrus was restored within 6 wk and this recovery occurred in the complete absence of neurogenesis. The recovery in LTP was accompanied by prominent changes within the dentate gyrus, including an increase in the survival rate of newborn cells that were proliferating just before the ablation and a reduction in inhibitory input to the granule cells of the dentate gyrus. These findings suggest that prolonged suppression of neurogenesis in young-adult mice results in wide-ranging compensatory changes in the structure and dynamics of the dentate gyrus that function to restore plasticity.
Keywords: adult neurogenesis, thymidine kinase, metaplasticity, miniature inhibitory postsynaptic currents
Forebrain neurogenesis persists into adulthood in the subgranular zone (SGZ) of the hippocampal dentate gyrus in rodents (1–4). Under normal conditions (i.e., in the absence of overt pathology) neuroblasts that arise in the SGZ migrate a short distance into the dentate granule cell layer (GCL) and differentiate into dentate granule cells (DGCs), where they subsequently reach functional maturity (5, 6). The birth, integration, and survival of DGCs are modulated by environmental enrichment (7), exercise (8), stress (9, 10), hippocampus-dependent learning (11), and direct manipulation of neuronal activity (12, 13). In addition, adult-born DGCs respond preferentially in hippocampus-dependent memory tasks (14) and display increased synaptic plasticity relative to mature DGCs (15, 16).
The correlation of increased DGC neurogenesis with cognitively demanding tasks has led to the hypothesis that adult-born neurons are integral participants in hippocampus-dependent memory processing and behavior. The role of adult-born DGCs in hippocampal function has been studied at the behavioral level in rodents after suppressing neurogenesis with antimitotic agents (17, 18), radiation (19), or genetic targeting (19–22). These studies indicate that adult-born DGCs are necessary for some hippocampus-dependent tasks, although results have been inconsistent and vary by rodent species and strain, behavioral paradigm, and ablation method (reviewed in ref. 23). Deficits in fear conditioning (19) and spatial memory (22) correlate in these models with impaired perforant path (PP)-dentate gyrus long-term potentiation (LTP). Conversely, conditions that stimulate adult neurogenesis in mice also increase LTP (8, 24). Immature DGCs generated in the adult exhibit a period of enhanced synaptic plasticity (15, 16), suggesting that this small cell cohort contributes significantly to dentate gyrus function.
Two forms of DGC LTP can be induced by PP stimulation (25). When GABAA receptors are blocked, PP stimulation greatly potentiates DGC postsynaptic responses. With GABAA receptor-mediated neurotransmission intact, PP stimulation induces a lower-amplitude, NR2B-dependent LTP. This LTP is blocked by manipulations that acutely suppress adult neurogenesis and is thought to be mediated by young granule neurons that are under less resting inhibitory tone than mature DGCs (19, 25).
However, the long-term impact of suppressing neurogenesis, as it relates to synaptic plasticity, has yet to be investigated. To address this question, we have examined LTP in the nestin-thymidine kinase (nestin-tk) mouse model, in which herpes simplex virus (HSV) thymidine kinase expression is regulated by the nestin enhancer, enabling the specific and temporally controlled ablation of neurogenesis after the intracerebroventricular administration of ganciclovir (GCV). In this model, treatment with GCV for 28 d results in nearly complete suppression of neurogenesis in young-adult mice (26). Consistent with previous results, we found that LTP was significantly disrupted when assessed immediately following ablation. This deficit in LTP was in marked contrast to the normal levels of LTP observed in GCV-treated nestin-tk mice 42 d following the ablation. This restoration of LTP occurred in the complete absence of neurogenesis, which failed to recover. Interestingly, recovery of LTP was associated with an increase in survival of DGCs that were born immediately before the ablation. Moreover, 42 d following the ablation there was a marked reduction in expression of the inhibitory synaptic marker vesicular GABA transporter (VGAT) within the dentate gyrus. The decrease in VGAT staining was concomitant with a reduction in overall inhibitory tone in the dentate gyrus, as assessed by measuring miniature inhibitory postsynaptic currents (mIPSCs) in DGCs. These findings suggest that the ablation of neurogenesis in young-adult mice results in wide-ranging compensatory changes to the structure and dynamics of the dentate gyrus.
Results
Suppressing Neurogenesis in Nestin-tk Mice Disrupts PP-Dentate Gyrus LTP.
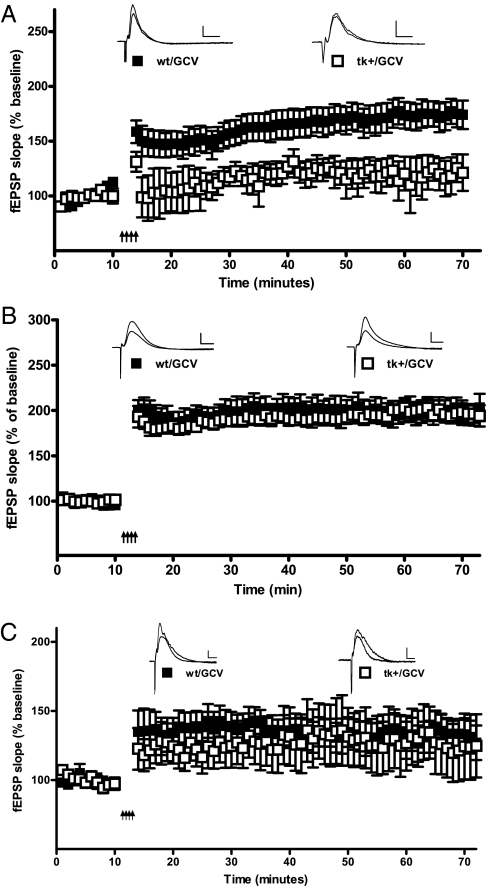
We first examined how suppression of neurogenesis for 4 wk in young-adult nestin-tk mice influenced PP-dentate gyrus LTP. Field potential excitatory postsynaptic potentials (fEPSPs) in response to medial PP stimulation were recorded adjacent to the GCL in hippocampal slices from 2- to 4-mo-old nestin-tk mice (tk+/GCV; 17 slices/nine mice) or wild-type littermate controls (WT/GCV; 15 slices/eight mice) after 28 d of GCV treatment. As shown in Fig. 1A, a tetanus consisting of four bursts (500 ms) of 100-Hz stimulation with a 30-s interburst interval was used to induce LTP after 10 min of baseline recording. A two-way repeated-measures ANOVA revealed that the tetanus significantly increased the normalized fEPSP slope [F(69, 1035) = 26.4; P < 0.001]. However, slices prepared from nestin-tk mice treated with 28 d of GCV exhibited significantly less LTP than those from wild-type mice [F(1,15) = 5.6; P = 0.014 for main effect of genotype]. In fact, comparison of the average fEPSP slope for the last 10 min (minutes 60–70) of the experiment with the average fEPSP slope of the 10 min before the tetanus yielded no significant difference for the GCV-treated nestin-tk group (P = 0.2). This LTP deficit was not a result of insertion of the tk transgene or a disruption in basal synaptic transmission at medial PP-DGC synapses (Fig. S1). These results demonstrate that 28 d of GCV treatment is sufficient to disrupt LTP in 2- to 4-mo-old nestin-tk mice.
Fig. 1.
Suppression of neurogenesis disrupts LTP in the dentate gyrus of young adult mice. (A) After 10 min of baseline recording, a tetanus of four bouts of 100-Hz stimulation (500 ms) separated by 30 s was delivered (arrows). Following the tetanus, the average fEPSP slope (as a percentage of baseline) was significantly enhanced in slices from wild-type mice treated for 28 d with GCV (wt/GCV). In contrast, slices from nestin-tk mice treated with GCV (tk+/GCV) exhibited no significant increase in fEPSP slope after the tetanus. (B) LTP in response to a high-frequency tetanus was examined in the presence of artificial cerebrospinal fluid that contained 50 μM picrotoxin. Slices from wild-type mice (wt/GCV) and nestin-tk mice (tk+/GCV) treated with 28 d of GCV both exhibited robust and comparable LTP. All data are mean ± SEM. (C) LTP is restored 42 d after termination of GCV treatment. Wild-type mice and nestin-tk mice were treated with 28 d of GCV (wt/GCV and tk+/GCV, respectively), and hippocampal slices prepared 42 d later. Slices from both groups exhibited robust LTP following the high-frequency tetanus. All data are mean ± SEM. Insets present representative extracellular fEPSP recordings made before (average of first 10 min) and after (average of last 10 min) the tetanus. (Scale bars, 0.25 mV/5 ms.)
Newborn DGCs are minimally affected by feed-forward inhibition. Thus, immature DGCs are thought to selectively depolarize after medial PP stimulation and preferentially express LTP. In contrast, when inhibition is blocked, mature DGCs are significantly depolarized by medial PP stimulation and ablation of newborn granule cells is thought to have minimal impact (19, 25). To examine the effects of complete disinhibition, LTP was induced using the same stimulation paradigm (four 500-ms bursts at 100-Hz stimulation; 30-s interburst interval) in the presence of artificial cerebrospinal fluid containing the GABAA receptor antagonist picrotoxin (50 μM). Results are presented in Fig. 1B. As in the previous experiment, mice were chronically treated with GCV for 28 d and then acute hippocampal slices were prepared from nestin-tk (tk+/GCV; six slices/five mice) or wild-type (WT/GCV; six slices/five mice) animals. Tetanic stimulation of the medial PP in the presence of picrotoxin substantially increased the normalized fEPSP slope for both groups [F(69, 483) = 138.3; P < 0.001 two-way repeated-measures ANOVA], and no significant difference in LTP was observed between groups [F(1,7) = 1.4; P = 0.27 for main effect of genotype]. These results suggest that the LTP deficit seen in nestin-tk mice treated with GCV is specific to the loss of newborn DGCs. Recent work suggests that 28 d of GCV treatment in nestin-tk mice results in a loss of newborn DGCs that persists for at least 42 d (6 wk) after the discontinuation of the GCV infusion (26). To determine the impact of persistently suppressed neurogenesis on LTP, experiments similar to those described above (Fig. 1A) were performed on slices from nestin-tk and wild-type mice treated for 28 d with GCV and then allowed to remain drug free for 42 d. As shown in Fig. 1C, robust LTP was observed in wild-type (WT/GCV; 18 slices/nine mice) and nestin-tk (tk+/GCV; 13 slices/seven mice) groups [F(69, 966) = 138.3; P < 0.001 two-way repeated-measures ANOVA]. In contrast to the LTP that was observed immediately following 28 d of GCV treatment, there was no significant difference in the magnitude of LTP between groups after 42 d of recovery [F(1,14) = 0.175; P = 0.68]. Importantly, the recovery of LTP occurred in the absence of ongoing neurogenesis as indicated by the loss of immunostaining for doublecortin after 28 d of treatment or 28 d plus 42 d of recovery (Fig. 2). The 28-d GCV infusion significantly reduced the number of doublecortin-immunoreactive neurons in the dentate gyrus of nestin-tk mice (Fig. 2 A and B). Consistent with previous findings (26), very few doublecortin-positive neuroblasts were observed 42 d after the termination of GCV treatment (Fig. 2 C and D). Similar results were obtained when slices used in the LTP experiments were fixed, resectioned, and immunostained (Fig. 2 E–H). It seems unlikely that the deficits in LTP that we observed immediately following GCV treatment were a result of nonspecific effects, such as inflammation, as immunostaining with ED1 (a marker of microglial activation) was minimal and indistinguishable between groups (Fig. S2). Taken collectively, these findings suggest that LTP of medial PP-DGC synapses is disrupted by the loss of newborn granule cells, but that LTP recovers despite the prolonged absence of neurogenesis.
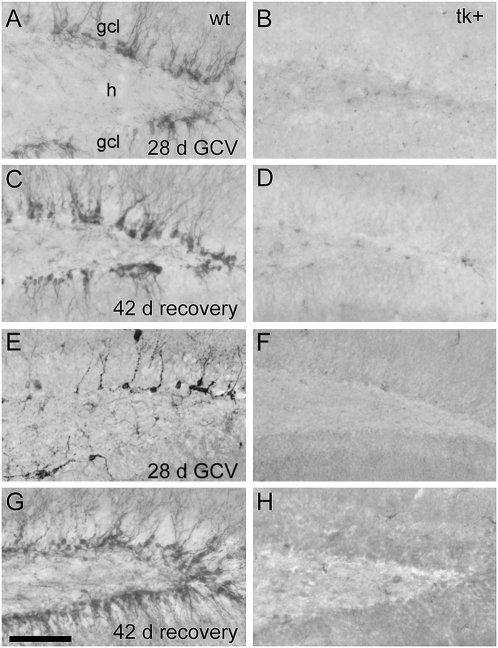
Fig. 2.
GCV treatment persistently disrupts neurogenesis. Immunoreactivity for doublecortin was examined in both frozen-brain sections (A–D) and in dentate gyrus slices used for electrophysiological recordings (E–H). Intracerebroventricular infusion of GCV for 28 d dramatically reduces immature neurons in nestin-tk (tk+) mice, shown here by absence of doublecortin immunoreactivity (B and F) vs. controls receiving GCV (A and E). No recovery of doublecortin expression was seen after 28 d of GCV administration plus a 42-d recovery (D and H) vs. similarly treated controls (C and G). (Scale bar, 100 μm.)
Enhanced Survival of Cells Born Immediately Before Suppressing Neurogenesis.
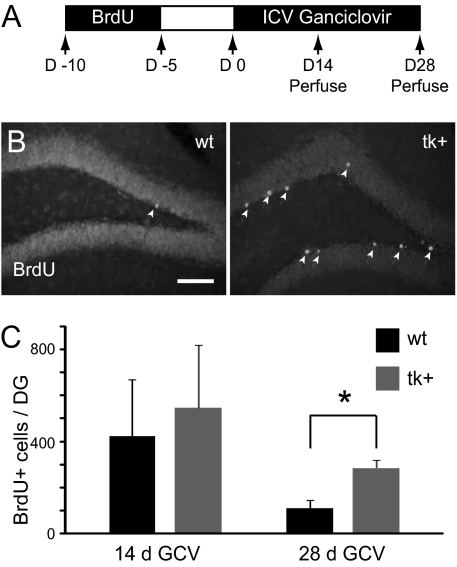
The abolition and restoration of LTP over 10 wk during and after GCV treatment could also be explained by altered survival, maturation, or integration of a cohort of DGCs born before suppression of neurogenesis. To explore the possibility that the survival of newborn DGCs is changed by the absence of continued neurogenesis, we labeled dividing cells by administering BrdU 5 to 10 d before the initiation of GCV infusion (Fig. 3A) such that the cells would be postmitotic at the time of GCV infusion and therefore not be susceptible to GCV-induced death. BrdU-labeled cells were then examined after 14 or 28 d of GCV administration. Basal rates of neurogenesis did not differ between nestin-tk and wild-type mice (26). After 14 d of GCV infusion, there was no significant difference in BrdU-labeled cell numbers in wild-type and nestin-tk mice (Fig. 3C) (P > 0.3, n = 4 per group). After 28 d of GCV, BrdU+ cells in both groups (n = 4 mice per group) decreased compared with the 14-d time point, as expected. The number of surviving BrdU-labeled cells in nestin-tk mice, however, was double the number seen in controls (Fig. 3 B and C) (P < 0.02). This finding suggests that suppression of DGC neurogenesis leads to feedback signals that stimulate the survival of previously generated DGCs that are still developing (i.e., < 5 wk old) at the time of ablation.
Fig. 3.
Ablation of newborn neurons increases survival of cells born before starting the GCV infusion. (A) A cohort of proliferating cells labeled with BrdU 5 to 10 d before GCV infusion was examined after 14 or 28 d of GCV infusion. (B) After 28-d GCV infusion, more BrdU-labeled cells were evident in the SGZ and GCL of tk+ mice than WT controls (arrowheads). (C) Quantification of BrdU labeling revealed no difference after 14 d of GCV treatment, but a significantly greater number of surviving BrdU+ cells in tk+ mice after a 28-d GCV infusion. Data presented as mean ± SEM. *P < 0.02. (Scale bar, 100 μm.)
Immunoreactivity for VGAT Is Reduced in the Dentate Gyrus Following Chronic Suppression of Neurogenesis.
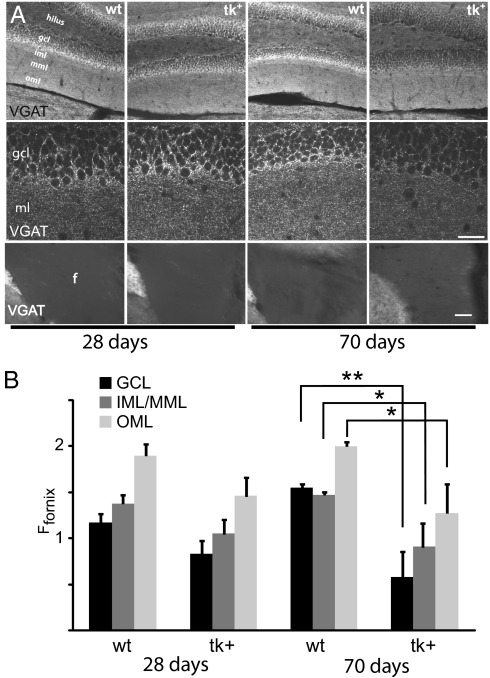
The restoration of basal LTP (i.e., LTP evoked without GABAA receptor blockade) after 42 d of recovery may have also resulted from changes in the mature dentate gyrus network that normally expresses LTP only in the presence of picrotoxin. Such network changes involving mature DGCs could arise either from disinhibition by decreased GABAergic input or increased excitation via excessive glutamatergic input. To assess these possibilities, we examined immunoreactivity for the GABAergic presynaptic marker VGAT and the glutamatergic presynaptic marker vesicular glutamate transporter-1 (VGLUT-1) (27, 28) in hippocampal sections prepared from WT and tk+ mice. In all groups, VGAT immunostaining was most intense (brightest) in the outer GCL and outer molecular layer (OML) of the dentate, with less labeling in the inner and middle molecular layers (IML and MML, respectively) (Fig. 4A, Top and Middle). There was a uniform lack of labeling in the white matter tracts of the fornix (Fig. 4A, Bottom). Although VGAT immunoreactivity in the GCL of nestin-tk mice was slightly decreased relative to controls after 28 d of GCV treatment, it was profoundly decreased after 70 d (28 d of GCV + 42 d of recovery) (Fig. 4A, Top and Middle). Quantitative immunofluorescence microscopy using the fornix for normalization showed no significant difference in VGAT labeling between nesin-tk (six mice) and wild-type (six mice) groups after 28 d of GCV (P > 0.1 for all layers). After 42 d of recovery, however, VGAT immunoreactivity in the GCL was 65% less in nestin-tk animals (three mice) vs. controls (six mice) (Fig. 4B) (P < 0.001). VGAT labeling was also decreased, but less robustly, in the IML/MML and OML (Fig. 4B) (P < 0.05). Note that VGAT labeling is heterogeneous within dentate gyrus such that the normalized optical density is not directly comparable between layers (e.g., the outer GCL is heavily labeled, but the overall optical density of the GCL is lower than the OML because of the absence of reactivity in the inner GCL).
Fig. 4.
Ablation of neurogenesis in young adult mice decreases inhibitory innervation in the dentate gyrus. (A) Staining for VGAT in the dentate gyrus (Top row at 20× and Middle row at 63×) and fornix (Bottom row). The left two columns are immediately after 28-d GCV treatment, and the right two columns are 70 d after initiating GCV infusion (28 d + 42 d recovery). Immunoreactivity for VGAT (lighter staining) is maximal in the outermost portion of the GCL and in the OML, and is significantly decreased in tk+ mice only after treatment with GCV for 28 d followed by a 42-d recovery (70 d; right column of Top and Middle). The white matter tracts of the fornix (Bottom) show minimal VGAT immunoreactivity. [Scale bars: 100 μm (Top and Bottom), 25 μm (Middle.)] (B) Quantification of the optical densities (normalized to the white matter of the fornix). Although a trend toward lower normalized optical density was evident after 28 d of GCV treatment, VGAT immunoreactivity was significantly decreased after 42 d of recovery from GCV treatment. Data presented as mean ± SEM. *P < 0.05, **P < 0.001.
Although normalization of the optical density to the fornix controls for nonspecific overlabeling, it is possible that the observed differences in VGAT labeling were a result of nonspecific globally decreased immunoreactivity because, for example, of tissue-processing artifacts. To control for these artifacts, we examined VGAT labeling in the cingulate cortex, which would not be expected to be affected by the experimental protocol. When dentate gyrus VGAT optical density was normalized using nonspecific changes in cortical VGAT immunoreactivity among animals, VGAT labeling in nestin-tk mice was specifically decreased in the GCL (Fig. S3) [F(1,7) = 184.7, P < 0.001] and IML/MML [F(1,7) = 73.6, P < 0.001]. Thus, the difference in VGAT optical density observed in the dentate gyrus was robust regardless of whether the measurement was controlled for processing artifacts resulting in over- or underimmunoreactivity.
Although decreased inhibitory innervation of mature DGCs would be most concordant with changes in basal (picrotoxin-independent) LTP, an increased excitatory drive would also be expected to enhance LTP in mature DGCs. We therefore examined immunolabeling for VGLUT-1 as a marker for glutamatergic presynaptic terminals in the dentate molecular layer (Fig. S4). VGLUT-1 expression did not differ between nestin-tk and wild-type mice at either the 28-d or 6-wk recovery time points (Fig. S4 A and B) (28-d recovery P > 0.80; 6-wk recovery, P > 0.90). The preserved VGLUT-1 immunoreactivity supports the specificity of reduced VGAT expression in nestin-tk mice after 42 d of recovery from GCV treatment.
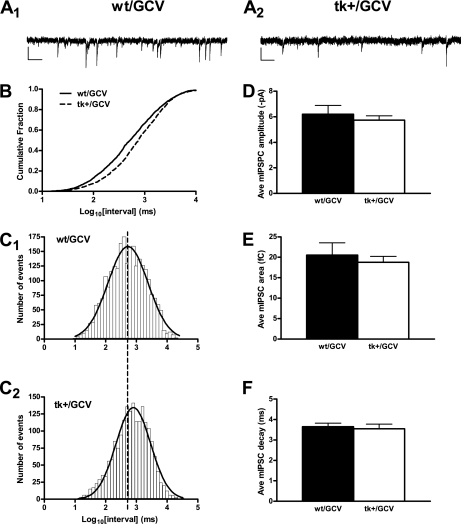
To investigate the functional consequence of the observed decrease in VGAT staining, we recorded mIPSCs in DGCs in hippocampal slices prepared from wild-type and tk+ mice treated with GCV for 28 d, followed by 42 d of recovery (Fig. 5A). Compared with slices prepared from wild-type mice treated with GCV, slices prepared from tk+ GCV-treated mice exhibited a significant reduction in mIPSC frequency (indicated by a rightward shift in the log interevent interval) (Fig. 5 B and C). Conversely, we did not find any significant differences in the average mIPSC amplitude (Fig. 5D), area (Fig. 5E), or decay (Fig. 5F) when we compared wild-type GCV-treated mice with tk+-treated mice. Our observation of a reduction in mIPSC frequency in the absence of altered mIPSC amplitude would suggest an alteration in presynaptic release (29). Taken together with our results from the VGAT immunostaining experiments, these results strongly suggest that there is a reduction in inhibitory tone in the GCL following prolonged loss of neurogenesis in young-adult mice.
Fig. 5.
Miniature IPSC frequency is reduced in tk+ mice 42 d after treatment with GCV. (A) Representative whole-cell voltage clamp recordings from wild-type (A1) and tk+ (A2) mice 42 d after termination of GCV treatment. (B) Plot of cumulative fraction of the log10 interevent intervals revealed an increase in time between events as evidenced by a significant rightward shift in the tk+ curve (Kolmogorov-Smirnov, P < 0.0001). (C) Histograms of the number of events observed in recordings from slices prepared from wild-type mice (C1) and tk+ mice (C2). Log10 (interval) bins were set to 0.1 ms. Average mIPSC amplitude (D), area (E), and decay (F) were not significantly different 42 d after treatment with GCV (P > 0.5, Student t test). Data in D to F presented as mean ± SEM. (Scale bars, 10 pA/200 ms.)
Discussion
Our results indicate that administration of GCV to young-adult nestin-tk mice for 28 d leads to suppression of neurogenesis and the concomitant loss of picrotoxin-independent PP-dentate gyrus LTP in young-adult mice. This result is consistent with previous reports describing cranial irradiation, genetic ablation (19, 25), or knockdown of growth factor signaling to reduce integration of newborn DGCs (30). We have also now investigated the long-term consequences of suppressing neurogenesis in young-adult mice. We found that 42 d after the cessation of GCV administration (10 wk from starting GCV treatment), LTP recovered to control levels. Importantly, this LTP recovery occurred in the complete absence of neurogenesis. Although we previously reported that sparse doublecortin-labeled neurons may appear in the dentate 42 d after the cessation of GCV in this model (26), we did not find any doublecortin-immunoreactive cells in the hippocampal slices used for in vitro recordings in this study. Even if the dentate begins to generate new neurons during the recovery phase after the GCV infusion ended, most would likely be less than 4 wk old by the end of our experimental recovery period, and thus would have not yet entered the critical period of enhanced synaptic plasticity (15, 26). Thus, we sought alternative explanations for the apparent dissociation between picrotoxin-independent LTP and neurogenesis.
When neurogenesis is suppressed by cranial irradiation, neither neurogenesis nor LTP recover 3 mo later (19). However, irradiation alters the neurogenic microenvironment and induces inflammation in addition to directly affecting dividing cells (31, 32), and may also have off-target effects on the hippocampus. Genetically specified ablation of neurogenesis should have fewer off-target effects than radiation, and we found no evidence of microglial activation in our model. Thus, it is unlikely that our results represent a nonspecific suppression of LTP because of transient inflammation.
We hypothesize that LTP recovers 42 d after the cessation of GCV via compensatory changes in dentate gyrus networks induced by suppressing neurogenesis. These changes may reflect an altered fate of young DGCs continuing to develop and integrate as successive generations of neurons are ablated. Recent results suggest that the developmental program of adult-born DGCs is plastic and may be altered and even reversed by alterations in neuromodulatory tone (33). Furthermore, survival of immature DGCs is dependent on both GABAergic and glutamatergic input and may be controlled by competition among DGCs for connections within the dentate network (34, 35). Our results indicate that survival of cells born 5 to 10 d before the beginning of the GCV infusion was twofold greater when successive generations of neurons were ablated. These cells were nearly 3 mo old at the time LTP recovered, and under normal circumstances would have passed the window of enhanced plasticity. Given that ablation of neurogenesis so profoundly alters cell survival in this cohort, however, it is possible that their maturation is altered as well, and further studies will be necessary to fully elucidate the physiology of these cells. Nevertheless, their altered survival indicates that the dynamics of newborn DGC integration is influenced by the subsequent ablation of DGC progenitors.
Alternatively, the reemergence of LTP 42 d after the cessation of GCV infusion may not depend on newborn neurons. Instead, “homeostatic-like” changes within mature dentate gyrus networks may restore the balance of inhibition and excitation in the absence of immature DCGs. Indeed, the level of neurogenesis correlates inversely with rates of synaptic turnover under certain conditions (36).
Given that picrotoxin-independent PP-DGC LTP is thought to depend on immature DGCs because they receive less inhibitory input than their mature counterparts (19, 25), we hypothesized that a reduction in the inhibitory innervation of the GCL as a whole may accompany the recovery of LTP. Indeed, we found that VGAT immunoreactivity, a marker of GABAergic synapses, decreased in association with recovery of LTP. In contrast, no significant difference in VGAT density between nestin-tk and wild-type mice was present after 28 d of GCV administration, when LTP was absent.
This concordance suggests that LTP in the dentate gyrus may recover as a function of decreased inhibition over time, as homeostatic-like changes occur within the network. Supporting the specificity of changes in inhibition, we did not find concurrent changes in immunoreactivity for VGLUT-1, a marker of glutamatergic synapses. Furthermore, we observed that the frequency of DGC mIPSCs was significantly decreased in tk+ GCV-treated mice at 70 d after the beginning of the GCV infusion, further suggesting a functional relationship between the loss of VGAT staining and a decrease in inhibitory tone.
Our findings demonstrate that widespread dynamic changes occur in the dentate gyrus following the chronic suppression of neurogenesis in young-adult mice, and that these changes correlate with alterations in network physiology. A number of reports suggest that inhibiting neurogenesis has little or no impact on learning and memory (for review, see ref. 23). It is notable that in many cases the behavioral assessments were made several weeks after ablation of neurogenesis. Although it is unknown if the ablation methods in these experiments resulted in permanent disruption of neurogenesis (as in our experiments), our data raise the intriguing possibility that the absence of behavioral deficits may reflect a compensatory rearrangement within the dentate gyrus. Similarly, recent work suggests that adult DGC neurogenesis is required for the transition of remote contextual fear memories from a hippocampus-dependent to a hippocampus-independent form (37). Interestingly, suppression of adult neurogenesis in these experiments did not completely inhibit fear memories from being converted to a hippocampus-independent form. The authors speculate that this may result from an incomplete suppression of neurogenesis or that perhaps adult neurogenesis plays more of a modulatory role in this process (37). In light of our data, it is possible that the compensatory shift in plasticity that follows disruption of neurogenesis may be sufficient to promote the extrahippocampal consolidation of remote memories. Setting these speculations aside, our findings suggest that chronic disruption of neurogenesis in young-adult mice transiently impairs LTP and that this transient disruption is reversed by compensatory changes that take place at the systems or circuit level.
Materials and Methods
Details of materials and methods are in SI Materials and Methods.
Mice.
The mice used in these experiments have been previously described (26). Briefly, the tk gene from HSV-1, with the DNA sequence of the viral gene modified by humanizing the usage codon and eliminating all of the CpGs, was fused downstream of a minimal tk promoter element followed by a 1.8-kb fragment of the second intronic nestin enhancer. Mice were generated and maintained on a FVB genetic background. All procedures involving animals were approved by the University Committee on the Use and Care of Animals of the University of Michigan.
Statistical Analysis.
All data are presented as mean ± SEM. Data from field-potential recordings were analyzed using one-way or two-way repeated-measures ANOVA, as noted. Inter mIPSC interval data were analyzed by subjecting the cumulative fraction of the log10 interevent interval to a Kolmogorov-Smirnov two-sample test. Mean mIPSC amplitude, area, and decay were averaged across neurons and compared using unpaired Student's t tests. Cell counts were compared among nestin-tk and wild-type mice using a Student's t test. Normalized optical densities of each region of interest were compared among nestin-tk and wild-type mice using a general linear model with fixed effects of genotype and time point and random effect of subject, and post hoc comparisons made with Bonferroni-corrected t tests. Statistical analysis was performed in SPSS (SPSS Inc.) or in Statview (SAS Institute Inc.).
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health (NIH) Grants HD044775 (to J.P.) and AG28488 (to G.M.) and NIH/National Institute on Aging Grant T32-AG000114 and NIH/National Institute of Neurological Disorders and Stroke Grant T32 NS007222-30 (to A.E.G. and B.H.S.). S.J.T. is a National Science Foundation Graduate Research Fellow and was also supported by a University of Michigan Rackham Merit Fellowship.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015425108/-/DCSupplemental.
References
- 1.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 4.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 5.Espósito MS, et al. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Overstreet-Wadiche LS, Westbrook GL. Functional maturation of adult-generated granule cells. Hippocampus. 2006;16:208–215. doi: 10.1002/hipo.20152. [DOI] [PubMed] [Google Scholar]
- 7.Brown JP, et al. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- 8.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 9.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gould E, Woolley CS, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. J Comp Neurol. 1991;313:479–485. doi: 10.1002/cne.903130308. [DOI] [PubMed] [Google Scholar]
- 11.Gould E, Beylin A, Tanapat P, Reeves A, Shores TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 12.Bruel-Jungerman E, Davis S, Rampon C, Laroche S. Long-term potentiation enhances neurogenesis in the adult dentate gyrus. J Neurosci. 2006;26:5888–5893. doi: 10.1523/JNEUROSCI.0782-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin CW, et al. Genetically increased cell-intrinsic excitability enhances neuronal integration into adult brain circuits. Neuron. 2010;65:32–39. doi: 10.1016/j.neuron.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 15.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 17.Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS ONE. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saxe MD, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dupret D, et al. Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imayoshi I, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 22.Shimazu K, et al. NT-3 facilitates hippocampal plasticity and learning and memory by regulating neurogenesis. Learn Mem. 2006;13:307–315. doi: 10.1101/lm.76006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng W, Aimone JB, Gage FH. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pujadas L, et al. Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J Neurosci. 2010;30:4636–4649. doi: 10.1523/JNEUROSCI.5284-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 26.Singer BH, et al. Conditional ablation and recovery of forebrain neurogenesis in the mouse. J Comp Neurol. 2009;514:567–582. doi: 10.1002/cne.22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaudhry FA, et al. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura K, Hioki H, Fujiyama F, Kaneko T. Postnatal changes of vesicular glutamate transporter (VGluT)1 and VGluT2 immunoreactivities and their colocalization in the mouse forebrain. J Comp Neurol. 2005;492:263–288. doi: 10.1002/cne.20705. [DOI] [PubMed] [Google Scholar]
- 29.Otis TS, Mody I. Modulation of decay kinetics and frequency of GABAA receptor-mediated spontaneous inhibitory postsynaptic currents in hippocampal neurons. Neuroscience. 1992;49:13–32. doi: 10.1016/0306-4522(92)90073-b. [DOI] [PubMed] [Google Scholar]
- 30.Bergami M, et al. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci USA. 2008;105:15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 32.Monje ML, et al. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62:515–520. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi K, et al. Reversal of hippocampal neuronal maturation by serotonergic antidepressants. Proc Natl Acad Sci USA. 2010;107:8434–8439. doi: 10.1073/pnas.0912690107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li G, Pleasure SJ. Ongoing interplay between the neural network and neurogenesis in the adult hippocampus. Curr Opin Neurobiol. 2010;20:126–133. doi: 10.1016/j.conb.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 36.Butz M, Teuchert-Noodt G, Grafen K, van Ooyen A. Inverse relationship between adult hippocampal cell proliferation and synaptic rewiring in the dentate gyrus. Hippocampus. 2008;18:879–898. doi: 10.1002/hipo.20445. [DOI] [PubMed] [Google Scholar]
- 37.Kitamura T, et al. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.