Abstract
Animal behaviors are generated by well-coordinated activation of neural circuits. In zebrafish, embryos start to show spontaneous muscle contractions at 17 to 19 h postfertilization. To visualize how motor circuits in the spinal cord are activated during this behavior, we developed GCaMP-HS (GCaMP-hyper sensitive), an improved version of the genetically encoded calcium indicator GCaMP, and created transgenic zebrafish carrying the GCaMP-HS gene downstream of the Gal4-recognition sequence, UAS (upstream activation sequence). Then we performed a gene-trap screen and identified the SAIGFF213A transgenic fish that expressed Gal4FF, a modified version of Gal4, in a subset of spinal neurons including the caudal primary (CaP) motor neurons. We conducted calcium imaging using the SAIGFF213A; UAS:GCaMP-HS double transgenic embryos during the spontaneous contractions. We demonstrated periodic and synchronized activation of a set of ipsilateral motor neurons located on the right and left trunk in accordance with actual muscle movements. The synchronized activation of contralateral motor neurons occurred alternately with a regular interval. Furthermore, a detailed analysis revealed rostral-to-caudal propagation of activation of the ipsilateral motor neuron, which is similar to but much slower than the rostrocaudal delay observed during swimming in later stages. Our study thus demonstrated coordinated activities of the motor neurons during the first behavior in a vertebrate. We propose the GCaMP technology combined with the Gal4FF-UAS system is a powerful tool to study functional neural circuits in zebrafish.
Keywords: neuronal activity, calcium transient, transgenesis, Tol2, gene trapping
Vertebrate behaviors are generated by coordinated activation of neural networks. The zebrafish provides an excellent system to study the neural activities of vertebrates because of its transparency and external development. It has been reported that zebrafish embryos show a first locomotive activity as early as 17 h postfertilization (hpf), which is called spontaneous contraction or coiling; namely, they spontaneously contract the left and right trunk muscles alternatively without any external stimulus. This behavior precedes touch-evoked escape response and swimming, which emerge in later developmental stages (1). The neuronal activities during the spontaneous contractions were analyzed by electrophysiological approaches, and periodic and synchronized depolarization of the spinal neurons has been observed (2, 3). However, it is still challenging to analyze the activity of a neural network by electrophysiology because it requires recording from multiple neurons by using multiple electrodes. To understand how functional neural circuits are formed and operated, a new technology to monitor the activity of multiple neurons is desired.
An alternative approach to record activities from multiple neurons is to detect Ca2+ influx associated with generation of action potentials. For this purpose, fluorescent calcium-indicator dyes, which should be introduced into cells by microinjection or by bulk-loading using their acetoxymethyl ester forms (4, 5), or genetically encoded calcium indicators (GECIs) have been used (6, 7). Previously we created a fusion of the calmodulin-binding domain from the myosin light-chain kinase (which is called an M13 peptide), permutated EGFP, and the calmodulin, and named it GCaMP (7). GCaMP increases its intensity of green fluorescence upon binding to calcium ions. Since the first version was created, efforts have been made to generate brighter and more sensitive versions of GCaMP by introducing amino acid substitutions (8–10).
In this study, we aim to visualize a motor network during the first behavior in zebrafish. For this purpose, we used the GCaMP technology and the Gal4FF-UAS (upstream activator sequences) system, which also was described recently in zebrafish (11). First, we constructed an improved version of GCaMP, GCaMP-HS (GCaMP-hyper sensitive), which is brighter at the resting level and more sensitive to the change of cellular Ca2+ concentrations than the previous version. Second, to express GCaMP-HS under the control of the Gal4FF transcription activator, we constructed an effector fish line that carried the GCaMP-HS gene downstream of the Gal4 recognition sequence UAS. Third, we performed gene-trap screens and isolated a driver line that expressed Gal4FF in a specific subset of motor neurons. Here we demonstrate successful imaging of the activity of the motor neurons during the spontaneous contractions in the vertebrate embryos.
Results
Development of GCaMP-HS.
Previously, it was shown that amino acid substitutions in GFP could enhance the folding activity and thereby increase its fluorescence. This more recent version of GFP was called “superfolder GFP” (12). To develop a better GECI, we tested if the substitutions described in the superfolder GFP can improve GCaMP in terms of the brightness and the signal amplitude. We introduced four of the six superfolder mutations, S30R, Y39N, N105T in the N-terminus half of GFP, and A206V in the C-terminus half of GFP, which were shown to have more effects in folding (12), into the previous version of GCaMP, GCaMP2 (9), and named the new version of GCaMP GCaMP-HS (Fig. 1A).
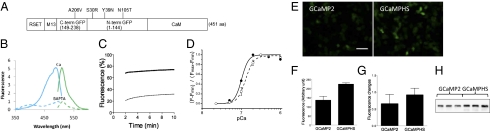
Fig. 1.
Construction and analysis of GCaMP-HS. (A) The structure of GCaMP-HS. The positions of the amino acid substitutions are indicated. (B) Excitation (blue) and emission (green) spectra of GCaMP-HS in Ca2+-chelated (20 mM BAPTA; dotted lines) and saturated (300 nM Ca2+; solid lines) solutions. Excitation and emission are maximum at 488 nm and 509 nm, respectively. (C) Restoration of the fluorescence of GCaMP-HS (solid line) and GCaMP2 (dotted line) after heat-denaturation. (D) Titration assay with defined Ca2+concentrations. Comparison of GCaMP-HS (solid line) and GCaMP2 (dotted line). Means and SD are shown (n = 3). (E) GFP fluorescence in HEK cells transfected with pN1-GCaMP2 (Left) and pN1-GCaMP-HS (Right). (Scale bar, 30 μm.) (F) The basal fluorescence intensity in HEK cells transfected with the GCaMP2 and GCaMP-HS plasmids. (G) The fluorescence changes upon addition of 100 μM carbachol to the GCaMP2- and GCaMP-HS–transfected cells. Means and SD from three independent transfection experiments. (H) Western blot analysis using an anticalmodulin antibody in three independent transfection experiments. Protein levels are increased in the GCaMP-HS–transfected cells.
First, we purified the GCaMP-HS protein produced in bacteria and characterized it in vitro. Spectrum analysis of GCaMP-HS showed maximal excitation at 488 nm and maximal emission at 509 nm in the presence of 300 nM Ca2+, which were similar to those of GCaMP2 (Fig. 1B). To test the effect of the superfolder mutations on the folding activity, we analyzed restoration of the fluorescence after heat-denaturation of the GCaMP proteins. The GCaMP-HS showed higher efficiencies of restoration (Fig. 1C), indicating that the substitutions introduced in GCaMP-HS increased the refolding activity. Then we measured their fluorescence intensities at various Ca2+ concentrations. From this Ca2+ titration assay, we calculated that Kd and Hill coefficient of GCaMP-HS were 102 nM and 5.0, respectively (Fig. 1D). Kd and Hill coefficient of GCaMP2 were 146 nM and 3.8, respectively, indicating that GCaMP-HS has a higher affinity to Ca2+ ions and a higher cooperativity in binding to Ca2+. The actual values of (Fmax − Fmin)/Fmin (maximal fluorescence minus minimal fluorescence per minimal fluorescence) were comparable: 4.1 in GCaMP-HS and 3.9 in GCaMP2. These findings suggested that GCaMP-HS may be used to detect Ca2+ transient where Ca2+ concentrations are low.
Second, we tested the cellular performance of GCaMP-HS. The expression plasmids pN1-GCaMP-HS and pN1-GCaMP2 were used to transfect HEK293 cells. The cells transfected with pN1-GCaMP-HS showed ∼1.6-fold higher fluorescence at the resting levels (Fig. 1 E and F), and 1.4-fold intensity changes when activated with 100 μM carbachol (Fig. 1G). We analyzed the cellular protein levels by Western blotting and found that the levels of the GCaMP-HS protein were constantly 1.7-fold higher than those of the GCaMP2 protein (Fig. 1H), suggesting that the higher folding activity increased the cellular expression level, and hence increased the basal fluorescence of GCaMP-HS.
Construction of the UAS:GCaMP-HS Transgenic Zebrafish.
We aimed to apply the new GCaMP-HS to in vivo imaging of neuronal activities in zebrafish. To take advantage of the Gal4FF-UAS system we described previously (11), we constructed the T2RUASGCaMP-HS plasmid that carried the GCaMP-HS gene downstream of the upstream activation sequence (UAS). The T2RUASGCaMP-HS plasmid was injected with the transposase mRNA to fertilized eggs, and the injected fish were crossed with the hspGGFF27A fish that expressed the Gal4FF-GFP fusion protein in the central nervous system or with the SAGFF73A fish that expressed Gal4FF ubiquitously (11, 13). All of four injected fish used for the mating produced transgenic F1 embryos that carried the T2RUASGCaMP-HS transgene in their offspring. We analyzed the F1 fish by Southern blot hybridization and determined the number of insertions carried by these fish. Then we crossed the F1 fish further with wild-type fish, and finally identified transgenic fish that carried a single transposon insertion and showed the brightest fluorescence when crossed with the Gal4FF-expressing fish lines. We named the fish line and the insertion UAS:GCaMPHS4A. The SAGFF73A;UAS:GCaMPHS4A double-transgenic embryo was embedded in agarose at 5 d postfertilization (dpf) and observed under a fluorescence microscope. We could detect the fluorescence changes in the trunk muscles in accordance with twitching (Movie S1). Therefore, the UAS:GCaMPHS4A transgenic fish was used for further studies.
Identification of a Driver That Expressed Gal4FF in a Subset of the Primary Motor Neurons in the Spinal Cord.
Spontaneous contraction is the first behavior of a zebrafish embryo. It can be observed as early as 17 hpf, reaches the maximum frequency at 19 hpf, and gradually declines by 26 hpf (1). We aimed to visualize the activity of the motor circuit during the spontaneous contraction. To isolate a driver line that expresses Gal4FF in the primary motor neurons, we performed genetic screens using the Tol2-based gene-trap constructs T2KSAGFF and T2TSAIGFF. We observed offspring from ∼500 fish injected with these constructs and identified the SAIGFF213A fish. In the SAIGFF213A;UAS:GFP double-transgenic embryos, GFP was expressed rather ubiquitously in the central nervous system at 24 hpf (Fig. 2A). In the spinal cord, several different types of neurons, including Rohon-Beard neurons, subpopulations of interneurons, and a set of neurons that were located in the ventral spinal cord, one per each spinal segment, that extended their axons to the ventral muscles were identifiable (Fig. 2 B and C). The zebrafish embryos have three types of the primary motor neurons in the spinal cord, middle primary neurons (MiP), rostral primary neurons (RoP), and caudal primary neurons (CaP). Among them, only the CaP motor neurons extend their axons to the ventral muscles (14). From the location and the morphology, we concluded that the SAIGFF213A;UAS:GFP expressed GFP in the CaP motor neurons, and MiP and RoP, which should be detected adjacent to CaP, did not express GFP at the detectable levels. In some cases, two CaP neurons were observed in one segment, as reported previously (15) (Fig. 2B). The GFP expressing CaP motor neurons were detectable as early as 17 hpf (the 16-somites stage) and observable at 2 dpf, and then the expression was gradually decreased and hardly detected at 7 dpf, although GFP expression in the other neurons were still observable (Fig. S1). We analyzed the Tol2 insertion in the SAIGFF213A line and found that the insertion was mapped close to the prdm14 gene on the chromosome 24.
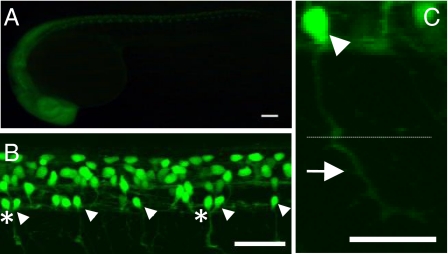
Fig. 2.
GFP expression in the SAIGFF213A;UAS:GFP double transgenic embryos. (A) A side view at 24 hpf. GFP is expressed in the central nervous system. (Scale bar, 100 μm.) (B) A side view of the spinal cord at 24 hpf observed with a confocal microscope. Arrowheads indicate CaP motor neurons. In some cases, two CaP neurons are found in one spinal segment (asterisks). (Scale bar, 100 μm.) (C) The soma (arrowhead) and axon of the GFP-expressing single CaP neuron at 24 hpf. The CaP neurons project their axons to the ventral muscle (arrow). Dotted line shows the boundary between dorsal and ventral muscles. (Scale bar, 50 μm.)
Spontaneous Contractions and Detection of the Activity of the CaP Primary Motor Neurons.
We observed the spontaneous contractions in our laboratory conditions (∼28 °C). The spontaneous contractions started around 19 hpf, showed the maximum frequency (0.6 Hz) at 21 hpf, and gradually ceased by 27 hpf (Fig. 3A and Movie S2). The 2-h delay of the start time in comparison with the previous work (1) may be a result of slightly lower temperatures in our laboratory condition or to a difference in the fish line we used. To analyze how motor circuits are activated during this behavior, we crossed the UAS:GCaMPHS4A fish with the SAIGFF213A fish and created double-transgenic embryos. Then we carried out imaging of the Ca2+ transient in the soma of the CaP neurons between 21 and 27 hpf. We found that the maximal changes of the fluorescence increased from 53% (21 hpf) to 400% (27 hpf) as the motor neurons matured; the basal fluorescence was nearly constant (Fig. 3B).
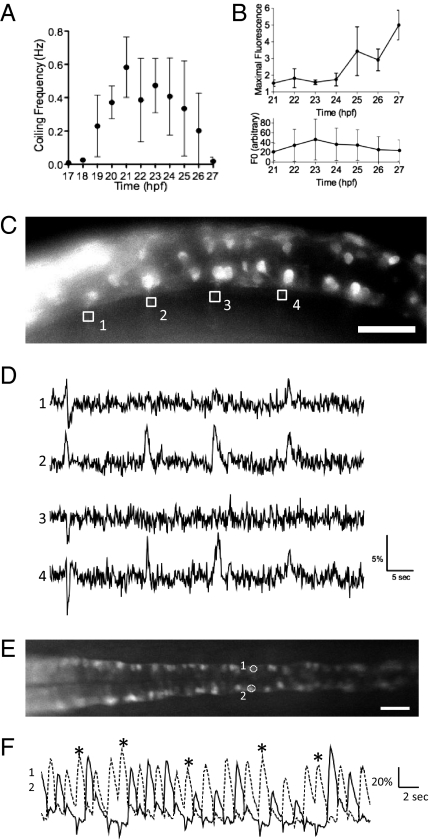
Fig. 3.
Imaging of the activity of CaP motor neurons in the SAIGFF213A;UAS:GCaMPHS double-transgenic embryo during spontaneous contractions. (A) Time course of the frequency of spontaneous contractions between 17 and 27 hpf. (B) The increase of the maximal fluorescence changes (Upper) and the fluorescence at the resting state (Lower) of GCaMP-HS in the soma of the CaP motor neuron between 21 and 27 hpf. Means and SD are shown (n = 5). (C) A side view of the double-transgenic embryo at 18.5 hpf. Anterior to the left. GCaMP fluorescence was detected in the CaP neurons and some other neurons. The axons of the CaP neurons were used as ROI (1–4). (Scale bar, 50 μm.) (D) The fluorescence changes in the ROI-1, -2, -3, and -4 are shown as graphs. Downward peaks at the left ends of ROI-1, -2, and -3 are artifacts caused by the movement of the embryo. (E) A dorsal view of the SAIGFF213A;UAS:GCaMPHS4A embryo at 24 hpf. Anterior to the left. The CaP neurons that are circled and numbered were used as ROIs. (Scale bar, 200 μm.) (F) The fluorescence changes in the ROI-1 (dotted line) and ROI-2 (solid line). Two consecutive peaks detected in ROI-2 are marked with asterisks.
First, we analyzed the activity of the CaP neurons at early developmental stages. At 18.5 hpf, an embryo contracted its trunk muscles at a frequency less than 0. 1 Hz (Fig. 3A). We performed imaging of the SAIGFF213A;UAS:GCaMPHS4A double transgenic embryo embedded in agarose at 18.5 hpf (Fig. 3C and Movie S3). We used axons of the CaP neurons as ROIs (regions of interest) because the fluorescence changes were obvious in the axons but were hardly detected in the soma at this stage. Periodic fluorescence changes at a frequency of 0.03 Hz were detected in about half of single CaP neurons observed (ROI-2 and ROI-4 in Fig. 3 C and D). The fluorescence changes in the other half of CaP neurons were rarely detected (ROI-1 and ROI-3 in Fig. 3 C and D). We found that the periodic fluorescence changes in different ipsilateral CaP neurons were synchronized at the temporal resolution of 10 fps (frames per second) (ROI-2 and ROI-4 in Fig. 3D). Moreover, nonperiodic activation of a CaP neuron also occurred simultaneously with activation of the other synchronized CaP neurons (ROI-1 in Fig. 3D), suggesting these neurons are physically connected or receiving common inputs at this stage. It should be noted that actual muscle contractions were not associated with 46% (18 of 39) of the cases where the fluorescence increases were detected in 10 different CaP neurons. These findings indicate that the motor network to generate periodic and synchronized activation starts to form before the muscle contractions are fully coupled with the activity of the motor network.
Second, we carried out imaging at 10 fps using the same SAIGFF213A;UAS:GCaMPHS4A double-transgenic embryo at 24 hpf. The periodic synchronized fluorescence changes in the ipsilateral CaP neurons were detected in the embryos embedded in agarose with a frequency of 0.41 ± 0.24 Hz (n = 20 embryos) (ROI-1 and -2 in Fig. 3 E and F, and Movie S4). It should be noted that the muscle contractions occurred concomitantly with the fluorescence increases of GCaMP-HS at this stage. Occasionally, two consecutive contractions occurred at the same side. In such cases, we observed two consecutive peaks in the ipsilateral CaP neurons (asterisks in Fig. 3F). The synchronized activation of contralateral motor neurons occurred alternately with approximately regular intervals (Fig. 3F).
Spatiotemporal Activation of the Motor Network: Synchronization, Alternation, and Propagation.
As it was difficult to calculate the brightness of ROIs precisely in embryos that moved in accordance with muscle contractions, we paralyzed the embryo with a neuromuscular junction blocker, d-tubocurarine, and performed imaging at 10 fps. Because it is not cell-permeable, the embryos were soaked in 100 μM d-tubocurarine and their tail tips were cut. The periodic and synchronized fluorescence changes with a frequency of 0.67 ± 0.11 Hz (n = 5 embryos) were detected in the tubocurarine-treated embryos, which was comparable but slightly faster than the frequencies detected in the untreated embryos (ROI-1, -2, -3, and -4 in Fig. 4 A and B and Movie S5). We also imaged embryos whose tails were cut but not treated with d-tubocurarine as a control, and detected the fluorescence change with a frequency of 0.39 ± 0.10 Hz (n = 6) (0.41 ± 0.24 Hz in uncut embryos), suggesting that the surgery had little effect on the frequency.
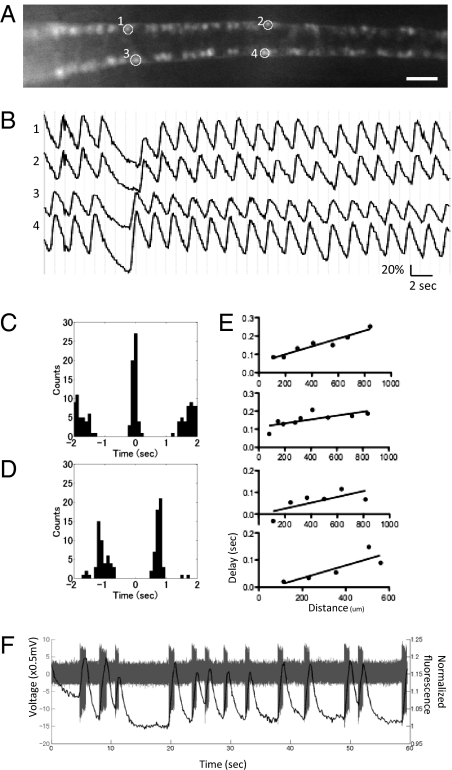
Fig. 4.
Spatiotemporal patterns of activation of the CaP motor neurons. (A) A dorsal view of the SAIGFF213A;UAS:GCaMPHS4A embryo at 24 hpf treated with 100 μM d-tubocurarine. (Scale bar, 200 μm.) (B) The fluorescence changes in ROIs in A. (C) Cross-correlogram of the ipsilateral CaP neurons. (D) Cross-correlogram of the contralateral CaP neurons. (E) The averaged timing of the peaks of the ipsilateral CaP neurons located in a rostral (Left)-to-caudal (Right) sequence. Data from four embryos are shown. (F) A whole cell-patch recording and simultaneous calcium imaging of the CaP neurons during spontaneous contractions. Action potentials (gray) and calcium signals (black) are shown.
We applied the cross-correlogram, which is commonly used to analyze correlation of timing of spike trains (16), to the analysis of the calcium signals (Fig. 4 C and D). The cross-correlogram of two ipsilateral neurons created a peak at zero, indicating that the activation of ipsilateral neurons signals were synchronized (Fig. 4C). The synchronized activation of the right and left side occurred alternately in both tubocurarine-untreated and treated embryos. The cross-correlogram of pairs of contralateral neurons showed the peak was shifted by a 0.46 to 0.53 cycle (n = 6 pairs from 6 embryos), indicating relatively precise alternate activation of the contralateral neurons (Fig. 4D).
Although the imaging at 10 fps demonstrated synchronized activation of ipsilateral CaP neurons, one could imagine activation of motor neurons in a rostral-to-caudal sequence, which was detected during a swimming behavior in later developmental stages (17). To test if propagation of the CaP activation occurs during the spontaneous contractions and if GCaMP-HS can detect such rapid propagation, we performed imaging at 20 fps. Although time resolution of 20 fps was still too low to directly observe the propagation during a single activation event, the averaged timing of the peaks of each CaP neuron during a total of 7-min imaging revealed delayed activation of CaP neurons located caudally (Fig. 4E). With linear regression [the coefficient of determination (R2) was in the range of 0.8–0.85], the velocity of the propagation of CaP activation was calculated as 5.6 μm/ms ± 0.8 (mean ± SE, n = 4 embryos).
Finally, to get insight into correlation between Ca2+ imaging using GCaMP-HS and spikes of the CaP neurons, we performed Ca2+ imaging at 8 fps and electrophysiological recording simultaneously using ∼24-hpf embryos. On average, 53.5 ± 9.0 peaks per burst (n = 20 bursts from two embryos) were detected (Fig. 4F). With linear regression, we calculated a linear relationship (R2 > 0.99) between the fluorescence change and the number of spikes that had occurred to give rise to the change in the rising phase (Fig. S2). The increment of the fluorescence changes per spike was calculated as 0.29% ± 0.019 (mean ± SE, n = 21). Because, in our imaging, a range of the noise or a fluctuation of the base line were typically within 1%, it is reasonable to assume that neuronal activation composed of at least four spikes could be detected by using the current GCaMP technology.
Discussion
In this study, we analyzed spontaneous contractions, the first locomotive behavior in zebrafish, by combination of an improved GCaMP technology and the Gal4FF-UAS system. We successfully imaged Ca2+ signals in the motor circuit during the natural vertebrate behavior and demonstrate how the motor neurons are coordinately activated.
Improvement of GCaMP.
GCaMP-HS was generated by introducing substitutions described in the GFP superfolder mutant (12) into the previous version of GCaMP, GCaMP2. As expected, the substitutions conferred the higher refolding activity after heat-denaturation to GCaMP-HS. Consistent with this, the GCaMP-HS protein became more stable and showed stronger fluorescence than GCaMP2 in transfected cells, suggesting that the substitutions indeed affected in vivo activities. Moreover, GCaMP-HS showed the increased sensitivity to Ca2+ [i.e., the affinity for Ca2+ (Kd = 102 nM) was higher than that of GCaMP2 (Kd = 146 nM) (9)], and Hill coefficient became 5.0 although it is 3.8 in GCaMP2. How is the sensitivity to Ca2+ affected by the “superfolder” substitutions in the GFP domain? It has been shown by X-ray crystallography that Arg-377 (position 74 of the calmodulin domain in Fig. 1A), which is located close to the second EF hand of the calmodulin, is interacting with the GFP chromophore (18). Therefore, we infer that the structural changes in the GFP chromophore affected the Ca2+ interacting part of the calmodulin domain, causing the change in the Ca2+ sensitivity. This Kd value is closer to the resting level of estimated intracellular Ca2+ concentrations (∼100 nM) (19, 20), suggesting that GCaMP-HS is better suited to detect Ca2+ transients in vivo.
Recently, Tian et al. (10) reported construction of GCaMP3 by introducing amino acid substitutions into GCaMP2. None of these substitutions is overlapping with the substitutions introduced in GCaMP-HS. Therefore, it will be interesting to combine these substitutions and test whether they show additive effects in detecting cellular Ca2+ transients.
Alternating and Synchronized Periodic Activation of the Motor Network.
The activity of the primary neurons during the spontaneous contraction has been analyzed by electrophysiology. Periodic depolarization of single primary motor neurons and interneurons, synchronized depolarization of a pair of ipisilateral neurons of one side, and alternating activation of a pair of contralateral neurons at 19 to 24 hpf have been observed (2, 3). However, it has been difficult to analyze multiple neuronal activities by the electrophysiological approach. In this article, we demonstrated the periodic and synchronized activation of the right and left sets of the ipsilateral motor neurons and their alternating activation.
Our present system enabled analysis of the onset of the spontaneous contraction in early stages. We found periodic and synchronized activation of ipsilateral CaP neurons in the very beginning of the spontaneous contractions. Although the soma of CaP neurons can be fluorescently labeled by GCaMP-HS even before 18 hpf in the double-transgenic embryo, the fluorescence changes in the soma were hardly detected at this stage. In contrast, axonal Ca2+ transients were observable at 18.5 hpf. Larger Ca2+ transients in the axons than in the soma were detected in developing Purkinje neurons in the rat by using a calcium-indicator dye (21), and it was shown that Ca2+ channels are functioning in the axons of developing chick motor neurons (22). Although it remains to be proven, these findings suggest that voltage-gated Ca2+ channels may be more abundant in the axonal membranes in developing neurons. We found that the Ca2+ transients in the CaP neurons were not always associated with muscle contractions at this stage, demonstrating that periodic and synchronized activation of the CaP neurons precedes its functional coupling to muscle contractions. The clustering of acetylcholine receptors and formation of neuromuscular junctions proceed as the formation of the myotomes proceeds in a rostral-to-caudal direction between 16 and 20 hpf (23) (Fig. S3). Therefore, in these stages, the connection between the motor circuits and muscle cells may not be complete or the activity of the motor circuit may not be strong enough to reliably contract muscles. It has not been known how the rhythmicity emerges in the early motor circuit. Although it still remains to be elucidated, our present study demonstrated that the periodic and synchronized activity is generated by a network involving a small number of CaP neurons before synchronization of all of the CaP neurons.
Propagation of Activation of CaP Motor Neurons.
We found propagation of activation of the CaP neurons along a rostrocaudal axis in the spinal cord during spontaneous contractions. The calculated velocity was 5.6 μm/ms. A similar activity, the rostrocaudal delay, was detected during a swimming behavior in later stages (17). However, its velocity was much faster (about 20-fold faster) than those in the spontaneous contractions. It will be interesting to disclose what makes this difference. Interestingly, a similar slow rostrocaudal wave has been reported in the neonatal mouse spinal cord (24). The calculated velocity was 13 to 18 μm/ms, which is faster but closer to that we observed for the CaP motor neurons. These findings may suggest the existence of a common mechanism for motor circuits in the vertebrate developing spinal cord.
GCaMP Technology and the Gal4FF-UAS System.
In vivo calcium imaging of neuronal activities has been carried out in zebrafish larvae. Bulk loading of the fluorescent calcium-indicator dyes has been used to record the activity of a neuronal ensemble in the spinal cord (4). However, with this method, neuronal subtypes could not be specified. To overcome this problem, Cameleon, a FRET-based GECI, was used in zebrafish (25). However, until now it has not been widely used probably because it is not easy to analyze the calcium transient with a poor signal-to-noise ratio. GCaMP1.6 and GCaMP2 were successfully used to detect calcium signals in the neuropil of the zebrafish tectum, which consisted of axonal arbors of retinal ganglion cells and dendritic arbors of the tectal cells (26, 27). In contrast to these studies, our present study clearly demonstrated that GCaMP-HS can detect calcium signals in both the cell bodies and neurites. This finding is important because axonal imaging does not immediately allow us to identify neurons that are being activated, especially when multiple neurons are imaged.
It has been shown that GCaMP has faster kinetics in calcium ion binding than other GECIs (28). In our recording of action potentials and simultaneous calcium imaging (at 8 fps), we could detect the fluorescence change even on the same frame as the first spike was generated (Fig. S2). Furthermore, the half-life of the decay of the maximal fluorescence change was 0.92 ± 0.13 second (mean and SD, n = 20 events) (Fig. S2), which was about 0.8 s with a calcium-indicator dye Oregon Green BAPTA (26). From these findings, we think that time resolution of GCaMP-HS in vivo is similar to that of calcium-indicator dyes.
We are currently constructing transgenic fish that express Gal4FF in various types of specific neural circuits (11, 29). By combining these with the present GCaMP technology, we expect that neuronal activities of various neural circuits will be imaged and studies of functional neural circuits in a living vertebrate will be facilitated.
Materials and Methods
Construction and Analysis of GCaMP-HS.
We used pN1-GCaMP2 (9) as a template for mutagenesis. S30R (TCC to CGC), Y39N (TAC to AAC), N105T (AAC to AAC), and A206V (AAA to GTA) were introduced by using QuikChange Multi Site-directed Mutagenesis Kit (Agilent Technologies), giving rise to pN1-GCaMP-HS. The SalI-MluI fragment from pN1-GCaMP-HS was cloned into pRSETb, giving rise to pRSETb-GCaMPHS that was used to produce the His-tagged GCaMP-HS protein in Escherichia coli. For calcium titration assay, the His-tagged GCaMP-HS protein was purified by metal affinity chromatography, and fluorescence (F) of GCaMP-HS was measured in different Ca2+ concentrations. Fmin and Fmax were measured in Ca2+-chelated buffer (20 mM BAPTA) and Ca2+-saturated buffer (300 nM Ca2+), respectively. All of the measurements were carried out in triplicate. (F − Fmin)/(Fmax − Fmin) was plotted against Ca2+ concentrations (X). Kd (dissociation constant) and n (Hill coefficient) were calculated by applying the real data to the Hill equation, Y = Xn/(Kdn + Xn). To test the refolding activity, the purified proteins were denatured at 95 °C for 5 min, cooled down to 25 °C, and measured for restoration of the fluorescence every 17 s in KM buffer (100 mM KCl, 20 mM Mops pH 7.5) in the presence of 1 mM EGTA. The fluorescence was normalized by the initial fluorescence before heat-denaturation. The pN1-GCaMP2 and pN1-GCaMP-HS plasmids were transfected to HEK cells, as described previously (7). In three independent transfection experiments, the fluorescence intensity was calculated by measuring those of 150 cells per dish. For Western blot analysis, anticalmodulin antibody (Upstate #05–173) was used to detect both GCaMP2 and GCaMP-HS.
Construction of the UAS:GCaMPHS4A and SAIGFF213A Transgenic Zebrafish.
The GCaMP-HS gene was cloned into the EcoRI site of pT2MUASMCS (11), giving rise to pT2RUASGCaMP-HS. The DNA construct was injected with transposase mRNA into zebrafish embryos at one-cell stage (30). The injected fish were crossed with the hspGGFF27A fish or with the SAGFF73A;UAS:RFP fish (11). Among the offspring, UASGCaMP-HS;hspGGFF27A double-transgenic embryos were identifiable because they showed much brighter green fluorescence than the Gal4FF-GFP transgene alone, and UASGCaMP-HS;SAGFF73A double-transgenic embryos were distinguishable because of the different color of the fluorescence.
For gene trapping, the Tol2 constructs T2KSAGFF (11) and T2TSAIGFF were used. T2TSAIGFF was constructed by cloning a splice acceptor, the Gtx IRES sequence (31) and the Ga4FF gene into a minimal Tol2 vector (32). The gene-trap screens were performed essentially as described previously (33). The Southern blot hybridization, inverse PCR and adaptor-ligation PCR were carried out as described previously (34).
Calcium Imaging and Microscopy.
A Zeiss Axiovert upright microscope with a 10×/NA0.3 lens and a water-immersion 40×/NA0.8 lens, equipped with a high-sensitivity cooled CCD camera (Hamamatsu Photonics, ORCA-R2), was used for calcium imaging. Each frame was taken under 100-ms exposure at 10 fps or 50-ms exposure at 20 fps using the manufacturer's image-acquisition software AquaCosmos. Images were analyzed off-line with custom-made softwares developed on LabVIEW (National Instruments) and Matlab (Mathworks). Zebrafish embryos were embedded in 5 mL of 2% low-melting agarose in a lid of a 6-cm dish covered with 5 mL E3 water with or without 100 μM d-tubocurarine chloride hydrate (Sigma; T2379). Because tubocrarine is not cell-permeable, their tail tips were cut for tubocurarine to permeate throughout the body. The GFP-expressing CaP neurons were observed with a confocal laser microscope Zeiss LSM510Meta.
Electrophysiological Recording.
The SAIG213A;UAS:GCaMPHS embryos at 24 hpf were immobilized by treatment of Tricaine and d-tubocrarine and whole-cell recording of the activity of CaP neurons was performed essentially as described previously (25). Simultaneously, the image of the CaP neurons were taken under 100-ms exposure at 8 fps by a cooled CCD camera (ORCA-R2) with a software Metamorph (Molecular Devices). The TTL pulses from the camera for each frame were recorded in the electrophysiology measurements.
Supplementary Material
Acknowledgments
We thank M. Suster for helpful discussion; N. Mouri, M. Mizushina, M. Suzuki, Y. Kanebako, and A. Ito for fish room works; and M. Itoh-Hiratani and H. Takakubo for technical assistance. This work was supported in part by the Mitsubishi Foundation (A.M.) and the National BioResource Project, and grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000887108/-/DCSupplemental.
References
- 1.Saint-Amant L, Drapeau P. Time course of the development of motor behaviors in the zebrafish embryo. J Neurobiol. 1998;37:622–632. doi: 10.1002/(sici)1097-4695(199812)37:4<622::aid-neu10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 2.Saint-Amant L, Drapeau P. Synchronization of an embryonic network of identified spinal interneurons solely by electrical coupling. Neuron. 2001;31:1035–1046. doi: 10.1016/s0896-6273(01)00416-0. [DOI] [PubMed] [Google Scholar]
- 3.Saint-Amant L, Drapeau P. Motoneuron activity patterns related to the earliest behavior of the zebrafish embryo. J Neurosci. 2000;20:3964–3972. doi: 10.1523/JNEUROSCI.20-11-03964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fetcho JR, O'Malley DM. Visualization of active neural circuitry in the spinal cord of intact zebrafish. J Neurophysiol. 1995;73:399–406. doi: 10.1152/jn.1995.73.1.399. [DOI] [PubMed] [Google Scholar]
- 5.Smetters D, Majewska A, Yuste R. Detecting action potentials in neuronal populations with calcium imaging. Methods. 1999;18:215–221. doi: 10.1006/meth.1999.0774. [DOI] [PubMed] [Google Scholar]
- 6.Miyawaki A, et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 7.Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;19(2):137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- 8.Ohkura M, Matsuzaki M, Kasai H, Imoto K, Nakai J. Genetically encoded bright Ca2+ probe applicable for dynamic Ca2+ imaging of dendritic spines. Anal Chem. 2005;77:5861–5869. doi: 10.1021/ac0506837. [DOI] [PubMed] [Google Scholar]
- 9.Tallini YN, et al. Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proc Natl Acad Sci USA. 2006;103:4753–4758. doi: 10.1073/pnas.0509378103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian L, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asakawa K, et al. Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc Natl Acad Sci USA. 2008;105:1255–1260. doi: 10.1073/pnas.0704963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pédelacq JD, Cabantous S, Tran T, Terwilliger TC, Waldo GS. Engineering and characterization of a superfolder green fluorescent protein. Nat Biotechnol. 2006;24(1):79–88. doi: 10.1038/nbt1172. [DOI] [PubMed] [Google Scholar]
- 13.Asakawa K, Kawakami K. A transgenic zebrafish for monitoring in vivo microtubule structures. Dev Dyn. 2010;239:2695–2699. doi: 10.1002/dvdy.22400. [DOI] [PubMed] [Google Scholar]
- 14.Myers PZ, Eisen JS, Westerfield M. Development and axonal outgrowth of identified motoneurons in the zebrafish. J Neurosci. 1986;6:2278–2289. doi: 10.1523/JNEUROSCI.06-08-02278.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato-Maeda M, Obinata M, Shoji W. Position fine-tuning of caudal primary motoneurons in the zebrafish spinal cord. Development. 2008;135:323–332. doi: 10.1242/dev.007559. [DOI] [PubMed] [Google Scholar]
- 16.Perkel DH, Gerstein GL, Moore GP. Neuronal spike trains and stochastic point processes. II. Simultaneous spike trains. Biophys J. 1967;7:419–440. doi: 10.1016/S0006-3495(67)86597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masino MA, Fetcho JR. Fictive swimming motor patterns in wild type and mutant larval zebrafish. J Neurophysiol. 2005;93:3177–3188. doi: 10.1152/jn.01248.2004. [DOI] [PubMed] [Google Scholar]
- 18.Akerboom J, et al. Crystal structures of the GCaMP calcium sensor reveal the mechanism of fluorescence signal change and aid rational design. J Biol Chem. 2009;284:6455–6464. doi: 10.1074/jbc.M807657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nohmi M, Hua SY, Kuba K. Intracellular calcium dynamics in response to action potentials in bullfrog sympathetic ganglion cells. J Physiol. 1992;458(1):171–190. doi: 10.1113/jphysiol.1992.sp019412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wanaverbecq N, Marsh SJ, Al-Qatari M, Brown DA. The plasma membrane calcium-ATPase as a major mechanism for intracellular calcium regulation in neurones from the rat superior cervical ganglion. J Physiol. 2003;550(1):83–101. doi: 10.1113/jphysiol.2002.035782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callewaert G, Eilers J, Konnerth A. Axonal calcium entry during fast ‘sodium’ action potentials in rat cerebellar Purkinje neurones. J Physiol. 1996;495:641–647. doi: 10.1113/jphysiol.1996.sp021622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hata K, Polo-Parada L, Landmesser LT. Selective targeting of different neural cell adhesion molecule isoforms during motoneuron myotube synapse formation in culture and the switch from an immature to mature form of synaptic vesicle cycling. J Neurosci. 2007;27:14481–14493. doi: 10.1523/JNEUROSCI.3847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flanagan-Steet H, Fox MA, Meyer D, Sanes JR. Neuromuscular synapses can form in vivo by incorporation of initially aneural postsynaptic specializations. Development. 2005;132:4471–4481. doi: 10.1242/dev.02044. [DOI] [PubMed] [Google Scholar]
- 24.Bonnot A, Whelan PJ, Mentis GZ, O'Donovan MJ. Spatiotemporal pattern of motoneuron activation in the rostral lumbar and the sacral segments during locomotor-like activity in the neonatal mouse spinal cord. J Neurosci. 2002;22:RC203. doi: 10.1523/JNEUROSCI.22-03-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higashijima S, Masino MA, Mandel G, Fetcho JR. Imaging neuronal activity during zebrafish behavior with a genetically encoded calcium indicator. J Neurophysiol. 2003;90:3986–3997. doi: 10.1152/jn.00576.2003. [DOI] [PubMed] [Google Scholar]
- 26.Sumbre G, Muto A, Baier H, Poo MM. Entrained rhythmic activities of neuronal ensembles as perceptual memory of time interval. Nature. 2008;456(7218):102–106. doi: 10.1038/nature07351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dreosti E, Odermatt B, Dorostkar MM, Lagnado L. A genetically encoded reporter of synaptic activity in vivo. Nat Methods. 2009;6:883–889. doi: 10.1038/nmeth.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mank M, et al. A FRET-based calcium biosensor with fast signal kinetics and high fluorescence change. Biophys J. 2006;90:1790–1796. doi: 10.1529/biophysj.105.073536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koide T, et al. Olfactory neural circuitry for attraction to amino acids revealed by transposon-mediated gene trap approach in zebrafish. Proc Natl Acad Sci USA. 2009;106:9884–9889. doi: 10.1073/pnas.0900470106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawakami K, et al. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev Cell. 2004;7(1):133–144. doi: 10.1016/j.devcel.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Chappell SA, Edelman GM, Mauro VP. Biochemical and functional analysis of a 9-nt RNA sequence that affects translation efficiency in eukaryotic cells. Proc Natl Acad Sci USA. 2004;101:9590–9594. doi: 10.1073/pnas.0308759101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urasaki A, Morvan G, Kawakami K. Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics. 2006;174:639–649. doi: 10.1534/genetics.106.060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asakawa K, Kawakami K. The Tol2-mediated Gal4-UAS method for gene and enhancer trapping in zebrafish. Methods. 2009;49:275–281. doi: 10.1016/j.ymeth.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urasaki A, Kawakami K. Analysis of genes and genome by the tol2-mediated gene and enhancer trap methods. Methods Mol Biol. 2009;546(2):85–102. doi: 10.1007/978-1-60327-977-2_6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.