Abstract
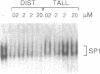
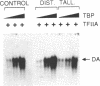
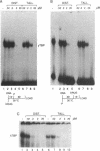
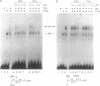
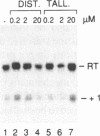
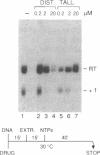
The antibiotic distamycin A is a DNA minor groove binding drug (MGB) that recognizes a stretch of at least four ATs. The alkylating benzoyl mustard derivative tallimustine (FCE 24517) has powerful anti-tumor activity. Using the electrophoretic mobility shift assay (EMSA) we determined that both compounds can prevent binding of TBP and, with 10-fold higher concentration, TBP-TFIIA (DA) and TBP-TFIIA-TFIIB (DAB) to a TATA box. Once formed, the DA and DAB complexes are more resistant to MGB challenge. Both drugs can inhibit basal in vitro transcription of a minimal TATA-containing promoter and similar concentrations are necessary for binding and transcriptional inhibition. Tallimustine shows strong selectivity by decreasing only correctly initiated transcripts. Even at high doses (20 microM), however, they cannot disturb a competent pre-initiation complex or Pol II progression. This functional in vitro model will provide a way to investigate the activity of sequence-specific DNA binding drugs with potential anti-viral and anti-tumour activity and to develop novel more selective compounds.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcamone F. M., Animati F., Barbieri B., Configliacchi E., D'Alessio R., Geroni C., Giuliani F. C., Lazzari E., Menozzi M., Mongelli N. Synthesis, DNA-binding properties, and antitumor activity of novel distamycin derivatives. J Med Chem. 1989 Apr;32(4):774–778. doi: 10.1021/jm00124a008. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Broggini M., Coley H. M., Mongelli N., Pesenti E., Wyatt M. D., Hartley J. A., D'Incalci M. DNA sequence-specific adenine alkylation by the novel antitumor drug tallimustine (FCE 24517), a benzoyl nitrogen mustard derivative of distamycin. Nucleic Acids Res. 1995 Jan 11;23(1):81–87. doi: 10.1093/nar/23.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broggini M., Erba E., Ponti M., Ballinari D., Geroni C., Spreafico F., D'Incalci M. Selective DNA interaction of the novel distamycin derivative FCE 24517. Cancer Res. 1991 Jan 1;51(1):199–204. [PubMed] [Google Scholar]
- Broggini M., Ponti M., Ottolenghi S., D'Incalci M., Mongelli N., Mantovani R. Distamycins inhibit the binding of OTF-1 and NFE-1 transfactors to their conserved DNA elements. Nucleic Acids Res. 1989 Feb 11;17(3):1051–1059. doi: 10.1093/nar/17.3.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brou C., Wu J., Ali S., Scheer E., Lang C., Davidson I., Chambon P., Tora L. Different TBP-associated factors are required for mediating the stimulation of transcription in vitro by the acidic transactivator GAL-VP16 and the two nonacidic activation functions of the estrogen receptor. Nucleic Acids Res. 1993 Jan 11;21(1):5–12. doi: 10.1093/nar/21.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher P. Weight matrix descriptions of four eukaryotic RNA polymerase II promoter elements derived from 502 unrelated promoter sequences. J Mol Biol. 1990 Apr 20;212(4):563–578. doi: 10.1016/0022-2836(90)90223-9. [DOI] [PubMed] [Google Scholar]
- Burton N., Cavallini B., Kanno M., Moncollin V., Egly J. M. Expression in Escherichia coli: purification and properties of the yeast general transcription factor TFIID. Protein Expr Purif. 1991 Oct-Dec;2(5-6):432–441. doi: 10.1016/1046-5928(91)90105-r. [DOI] [PubMed] [Google Scholar]
- Chasman D. I., Flaherty K. M., Sharp P. A., Kornberg R. D. Crystal structure of yeast TATA-binding protein and model for interaction with DNA. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8174–8178. doi: 10.1073/pnas.90.17.8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang S. Y., Welch J., Rauscher F. J., 3rd, Beerman T. A. Effects of minor groove binding drugs on the interaction of TATA box binding protein and TFIIA with DNA. Biochemistry. 1994 Jun 14;33(23):7033–7040. doi: 10.1021/bi00189a003. [DOI] [PubMed] [Google Scholar]
- Coll M., Aymami J., van der Marel G. A., van Boom J. H., Rich A., Wang A. H. Molecular structure of the netropsin-d(CGCGATATCGCG) complex: DNA conformation in an alternating AT segment. Biochemistry. 1989 Jan 10;28(1):310–320. doi: 10.1021/bi00427a042. [DOI] [PubMed] [Google Scholar]
- Coll M., Frederick C. A., Wang A. H., Rich A. A bifurcated hydrogen-bonded conformation in the d(A.T) base pairs of the DNA dodecamer d(CGCAAATTTGCG) and its complex with distamycin. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8385–8389. doi: 10.1073/pnas.84.23.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn A., Affolter M., Müller M., Gehring W. J., Leupin W. Distamycin-induced inhibition of homeodomain-DNA complexes. EMBO J. 1992 Jan;11(1):279–286. doi: 10.1002/j.1460-2075.1992.tb05050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesen M., Pommier Y. Mammalian topoisomerase II activity is modulated by the DNA minor groove binder distamycin in simian virus 40 DNA. J Biol Chem. 1989 Jul 5;264(19):11354–11359. [PubMed] [Google Scholar]
- Hahn S., Buratowski S., Sharp P. A., Guarente L. Yeast TATA-binding protein TFIID binds to TATA elements with both consensus and nonconsensus DNA sequences. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5718–5722. doi: 10.1073/pnas.86.15.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., Roeder R. G. Separation and partial characterization of three functional steps in transcription initiation by human RNA polymerase II. J Biol Chem. 1985 Jul 5;260(13):8163–8172. [PubMed] [Google Scholar]
- Hoopes B. C., LeBlanc J. F., Hawley D. K. Kinetic analysis of yeast TFIID-TATA box complex formation suggests a multi-step pathway. J Biol Chem. 1992 Jun 5;267(16):11539–11547. [PubMed] [Google Scholar]
- Horikoshi M., Hai T., Lin Y. S., Green M. R., Roeder R. G. Transcription factor ATF interacts with the TATA factor to facilitate establishment of a preinitiation complex. Cell. 1988 Sep 23;54(7):1033–1042. doi: 10.1016/0092-8674(88)90118-3. [DOI] [PubMed] [Google Scholar]
- Kim J. L., Nikolov D. B., Burley S. K. Co-crystal structure of TBP recognizing the minor groove of a TATA element. Nature. 1993 Oct 7;365(6446):520–527. doi: 10.1038/365520a0. [DOI] [PubMed] [Google Scholar]
- Kim Y., Geiger J. H., Hahn S., Sigler P. B. Crystal structure of a yeast TBP/TATA-box complex. Nature. 1993 Oct 7;365(6446):512–520. doi: 10.1038/365512a0. [DOI] [PubMed] [Google Scholar]
- Kopka M. L., Yoon C., Goodsell D., Pjura P., Dickerson R. E. The molecular origin of DNA-drug specificity in netropsin and distamycin. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1376–1380. doi: 10.1073/pnas.82.5.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käs E., Izaurralde E., Laemmli U. K. Specific inhibition of DNA binding to nuclear scaffolds and histone H1 by distamycin. The role of oligo(dA).oligo(dT) tracts. J Mol Biol. 1989 Dec 5;210(3):587–599. doi: 10.1016/0022-2836(89)90134-4. [DOI] [PubMed] [Google Scholar]
- Lee D. K., DeJong J., Hashimoto S., Horikoshi M., Roeder R. G. TFIIA induces conformational changes in TFIID via interactions with the basic repeat. Mol Cell Biol. 1992 Nov;12(11):5189–5196. doi: 10.1128/mcb.12.11.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. K., Horikoshi M., Roeder R. G. Interaction of TFIID in the minor groove of the TATA element. Cell. 1991 Dec 20;67(6):1241–1250. doi: 10.1016/0092-8674(91)90300-n. [DOI] [PubMed] [Google Scholar]
- Lescure A., Lutz Y., Eberhard D., Jacq X., Krol A., Grummt I., Davidson I., Chambon P., Tora L. The N-terminal domain of the human TATA-binding protein plays a role in transcription from TATA-containing RNA polymerase II and III promoters. EMBO J. 1994 Mar 1;13(5):1166–1175. doi: 10.1002/j.1460-2075.1994.tb06366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A., Weisman-Shomer P., Fry M. Distamycin paradoxically stimulates the copying of oligo(dA).poly(dT) by DNA polymerases. Biochemistry. 1989 Sep 5;28(18):7262–7267. doi: 10.1021/bi00444a018. [DOI] [PubMed] [Google Scholar]
- Mantovani R. An RNA polymerase II in vitro transcription system. Methods Mol Biol. 1994;31:289–298. doi: 10.1385/0-89603-258-2:289. [DOI] [PubMed] [Google Scholar]
- Mantovani R., Pessara U., Tronche F., Li X. Y., Knapp A. M., Pasquali J. L., Benoist C., Mathis D. Monoclonal antibodies to NF-Y define its function in MHC class II and albumin gene transcription. EMBO J. 1992 Sep;11(9):3315–3322. doi: 10.1002/j.1460-2075.1992.tb05410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani R., Tora L., Moncollin V., Egly J. M., Benoist C., Mathis D. The major histocompatibility complex (MHC) Ea promoter: sequences and factors at the initiation site. Nucleic Acids Res. 1993 Oct 25;21(21):4873–4878. doi: 10.1093/nar/21.21.4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncollin V., Schaeffer L., Chalut C., Egly J. M. Expression in Escherichia coli: purification and properties of the recombinant human general transcription factor rTFIIB. Protein Expr Purif. 1992 Oct;3(5):374–379. doi: 10.1016/s1046-5928(05)80038-5. [DOI] [PubMed] [Google Scholar]
- Mortensen U. H., Stevnsner T., Krogh S., Olesen K., Westergaard O., Bonven B. J. Distamycin inhibition of topoisomerase I-DNA interaction: a mechanistic analysis. Nucleic Acids Res. 1990 Apr 25;18(8):1983–1989. doi: 10.1093/nar/18.8.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolov D. B., Hu S. H., Lin J., Gasch A., Hoffmann A., Horikoshi M., Chua N. H., Roeder R. G., Burley S. K. Crystal structure of TFIID TATA-box binding protein. Nature. 1992 Nov 5;360(6399):40–46. doi: 10.1038/360040a0. [DOI] [PubMed] [Google Scholar]
- Ozer J., Moore P. A., Bolden A. H., Lee A., Rosen C. A., Lieberman P. M. Molecular cloning of the small (gamma) subunit of human TFIIA reveals functions critical for activated transcription. Genes Dev. 1994 Oct 1;8(19):2324–2335. doi: 10.1101/gad.8.19.2324. [DOI] [PubMed] [Google Scholar]
- Radic M. Z., Saghbini M., Elton T. S., Reeves R., Hamkalo B. A. Hoechst 33258, distamycin A, and high mobility group protein I (HMG-I) compete for binding to mouse satellite DNA. Chromosoma. 1992 Oct;101(10):602–608. doi: 10.1007/BF00360537. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. TATA-binding protein is a classless factor. Cell. 1992 Mar 6;68(5):819–821. doi: 10.1016/0092-8674(92)90023-6. [DOI] [PubMed] [Google Scholar]
- Sun X., Ma D., Sheldon M., Yeung K., Reinberg D. Reconstitution of human TFIIA activity from recombinant polypeptides: a role in TFIID-mediated transcription. Genes Dev. 1994 Oct 1;8(19):2336–2348. doi: 10.1101/gad.8.19.2336. [DOI] [PubMed] [Google Scholar]
- Tjian R., Maniatis T. Transcriptional activation: a complex puzzle with few easy pieces. Cell. 1994 Apr 8;77(1):5–8. doi: 10.1016/0092-8674(94)90227-5. [DOI] [PubMed] [Google Scholar]
- Van Dyke M. W., Hertzberg R. P., Dervan P. B. Map of distamycin, netropsin, and actinomycin binding sites on heterogeneous DNA: DNA cleavage-inhibition patterns with methidiumpropyl-EDTA.Fe(II). Proc Natl Acad Sci U S A. 1982 Sep;79(18):5470–5474. doi: 10.1073/pnas.79.18.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke M. W., Sawadogo M. DNA-binding and transcriptional properties of human transcription factor TFIID after mild proteolysis. Mol Cell Biol. 1990 Jul;10(7):3415–3420. doi: 10.1128/mcb.10.7.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viville S., Mantovani R. S1 mapping using single-stranded DNA probes. Methods Mol Biol. 1994;31:299–305. doi: 10.1385/0-89603-258-2:299. [DOI] [PubMed] [Google Scholar]
- Wobbe C. R., Struhl K. Yeast and human TATA-binding proteins have nearly identical DNA sequence requirements for transcription in vitro. Mol Cell Biol. 1990 Aug;10(8):3859–3867. doi: 10.1128/mcb.10.8.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe A. P. Transcription: in tune with the histones. Cell. 1994 Apr 8;77(1):13–16. doi: 10.1016/0092-8674(94)90229-1. [DOI] [PubMed] [Google Scholar]
- Wong J. M., Bateman E. TBP-DNA interactions in the minor groove discriminate between A:T and T:A base pairs. Nucleic Acids Res. 1994 May 25;22(10):1890–1896. doi: 10.1093/nar/22.10.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomori K., Zeidler M. P., Chen J. L., Verrijzer C. P., Mlodzik M., Tjian R. Drosophila TFIIA directs cooperative DNA binding with TBP and mediates transcriptional activation. Genes Dev. 1994 Oct 1;8(19):2313–2323. doi: 10.1101/gad.8.19.2313. [DOI] [PubMed] [Google Scholar]
- Zawel L., Reinberg D. Initiation of transcription by RNA polymerase II: a multi-step process. Prog Nucleic Acid Res Mol Biol. 1993;44:67–108. doi: 10.1016/s0079-6603(08)60217-2. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Wähnert U. Nonintercalating DNA-binding ligands: specificity of the interaction and their use as tools in biophysical, biochemical and biological investigations of the genetic material. Prog Biophys Mol Biol. 1986;47(1):31–112. doi: 10.1016/0079-6107(86)90005-2. [DOI] [PubMed] [Google Scholar]