Abstract
Environmental factors interact with the genome throughout life to determine gene expression and, consequently, tissue function and disease risk. One such factor that is known to play an important role in determining long-term metabolic health is diet during critical periods of development. Epigenetic regulation of gene expression has been implicated in mediating these programming effects of early diet. The precise epigenetic mechanisms that underlie these effects remain largely unknown. Here, we show that the transcription factor Hnf4a, which has been implicated in the etiology of type 2 diabetes (T2D), is epigenetically regulated by maternal diet and aging in rat islets. Transcriptional activity of Hnf4a in islets is restricted to the distal P2 promoter through its open chromatin configuration and an islet-specific interaction between the P2 promoter and a downstream enhancer. Exposure to suboptimal nutrition during early development leads to epigenetic silencing at the enhancer region, which weakens the P2 promoter–enhancer interaction and results in a permanent reduction in Hnf4a expression. Aging leads to progressive epigenetic silencing of the entire Hnf4a locus in islets, an effect that is more pronounced in rats exposed to a poor maternal diet. Our findings provide evidence for environmentally induced epigenetic changes at the Hnf4a enhancer that alter its interaction with the P2 promoter, and consequently determine T2D risk. We therefore propose that environmentally induced changes in promoter-enhancer interactions represent a fundamental epigenetic mechanism by which nutrition and aging can influence long-term health.
Keywords: maternal nutrition, developmental programming, DNA methylation, histone modifications, diet–gene interactions
Environmental signals can alter the epigenetic state of specific genes and modulate their activity (1). Such signals can therefore influence cell function, tissue physiology, and metabolic health (2). Important environmental factors that have been demonstrated to modulate DNA methylation and histone modifications include nutrition, radiation, and chemical toxins (3). Although it is well established that current diet can influence the epigenotype, there is emerging evidence that diet during critical periods of development can have a permanent effect on the epigenetic modification of specific gene promoters to influence transcriptional activity (4). Modulation of epigenetic states therefore provides a plausible mechanism by which maternal diet can mediate known long-term effects on risk for diseases such as cardiovascular disease and type 2 diabetes (T2D), a concept referred to as nutritional programming (5, 6).
Development of the adverse metabolic phenotype resulting from exposure to a suboptimal maternal diet is generally dependent on the accumulation of additional detrimental events that occur during the aging process (7). Little is known about the molecular mechanisms underlying the interaction between maternal diet and the aging trajectory. There are a limited number of studies demonstrating that the epigenetic modification of specific genes alters during the life course (8). Nevertheless, how, and if, these age-associated changes interact dynamically with epigenetic alterations established in early life is unknown.
We have previously used a well-characterized model for nutritional programming of T2D resulting from maternal protein restriction during fetal and early postnatal life (9). In this model, rat dams are fed a low-protein diet (LP; 8% wt/wt protein) compared with an isocaloric control diet (C; 20% wt/wt protein) during pregnancy and lactation, and all offspring are weaned onto a standard diet. The LP offspring are born smaller but have normal glucose tolerance in young adult life. They undergo an age-dependent loss of glucose tolerance (7), however, and develop a phenotype similar to human T2D by 17 mo of age (10). Therefore, as in humans, aging is critical for the development of the phenotype. The maternal LP model shares striking similarities with human low-birth-weight individuals (11), and therefore is an ideal system to define the molecular mechanisms that link poor early growth to T2D.
Most studies to date aimed at identifying genes that mediate the effects of maternal diet on long-term health have adopted a candidate approach. We have focused on hepatocyte nuclear factor 4-α (HNF-4α), a transcription factor from the nuclear hormone receptor superfamily, which is required for pancreatic β-cell differentiation and glucose homeostasis (12). HNF-4α has been linked to the development of a number of forms of T2D (13–17). Several HNF-4α protein variants arise from mRNAs transcribed from two promoters, proximal P1 and distal P2 (18) (Fig. 1A). The P2 promoter is important in early development and adult islets, whereas P1 is the main promoter in adult liver, kidney, and intestine (19, 20). Activation of the P1 promoter has been shown to involve a physical interaction with an upstream enhancer (21).
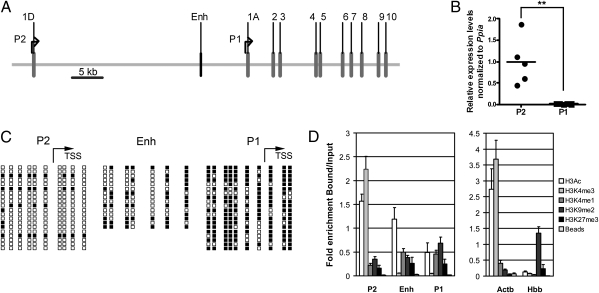
Fig. 1.
DNA methylation and histone modification patterns at the Hnf4a locus in rat islets. (A) Schematic representation of the gene. Enh, enhancer region; 1D–10, coding exons. (B) mRNA expression analysis by qRT-PCR. Data were normalized against cyclophilin A (Ppia) and presented relative to the mean level of P2 transcripts, arbitrarily set to 1 (n = 5). **P < 0.01. (C) Bisulphite sequencing analysis of DNA methylation (n = 2). Filled squares represent methylated CpG dinucleotides. TSS, transcription start site. (D) Native ChIP analysis of histone marks. Actb and Hbb promoter regions were used as controls (n = 7).
In this study, we demonstrate that maternal diet, through epigenetic mechanisms, modifies the interaction between the active P2 promoter and the enhancer region in pancreatic islets. In addition, maternal diet amplifies the age-associated epigenetic silencing of this locus. These findings provide mechanistic insights by which maternal diet can influence expression of a key developmental transcription factor and, consequently, metabolic health. We propose that environmentally induced changes in promoter–enhancer interactions represent a general epigenetic mechanism by which nutrition and aging can influence long-term health.
Results
Transcriptional Activity of Hnf4a Correlates with Epigenetic Marks at Regulatory Regions.
Promoter-specific quantitative RT-PCR (qRT-PCR) showed that P2 is the main active promoter in both rat (Fig. 1B) and human islets (Fig. S1A). The high transcriptional activity of P2 was associated with low levels of CpG methylation at this promoter (Fig. 1C and Fig. S1B). Conversely, the low transcriptional activity of the P1 promoter was correlated with higher levels of CpG methylation (Fig. 1C and Fig. S1B). Further evidence for a role of methylation in the regulation of this locus was the observation that the P2 promoter was highly methylated in two tissues (human blood and rat kidney) that do not express transcripts from this promoter (Fig. S1 C–E).
We also characterized histone modifications by ChIP of marks associated with open chromatin [Acetyl-Histone H3 (H3Ac; Upstate–Millipore) and Histone 3H (trimethyl K4) (H3K4me3; Abcam)], transcriptional repression/closed chromatin [Histone 3H (dimethyl K9) (H3K9me2; Abcam) and Histone 3H (trimethyl K27) (H3K27me3; Abcam)], and active enhancers [Histone 3H (monomethyl K4) (H3K4me1; Abcam)] (22, 23). The active P2 promoter showed high levels of H3Ac and H3K4me3 in rat and human islets, similar to the active housekeeping gene Actb (Fig. 1D and Fig. S1F). The enhancer region also had an open chromatin pattern (i.e., enriched for H3Ac and H3K4me1) (Fig. 1D and Fig. S1F). The inactive P1 promoter had less H3Ac than P2, lacked H3K4me3 and was enriched in H3K9me2 in rat islets (Fig. 1D), and had marks of both open and closed chromatin in human islets (Fig. S1F), consistent with its heterogeneous expression (Fig. S1A). To assess the correlation between chromatin configuration and promoter activity further, we analyzed the pattern of histone marks in rat liver, which expresses low levels of P2 transcripts and high levels of P1 transcripts (Fig. S1G). The chromatin configuration at P2 was similar to the inactive Hbb promoter, whereas P1 was enriched for open chromatin marks (Fig. S1H).
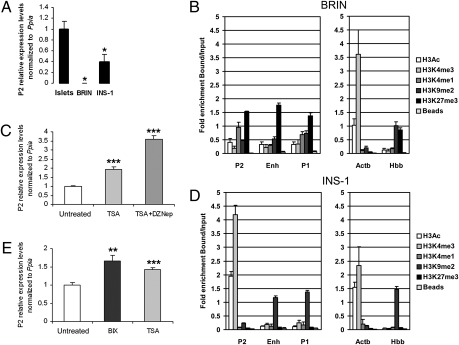
DNA Methylation and Histone Modifications Regulate Hnf4a Transcription in Insulin-Secreting Cell Lines.
The correlation between promoter use and epigenetic marking described above led us to assess the effects of manipulating levels of CpG methylation and histone marks in insulin-secreting cell lines on promoter activity. BRIN, a rat insulinoma cell line that we found to express very low levels of Hnf4a mRNA, was hypermethylated at the P2 promoter (Fig. S2A). Treatment of these cells with the demethylating agent 5-AzaC led to a dose-dependent increase in expression of P2 transcripts that was paralleled by significant demethylation (Fig. S2A). To provide a more direct link, we induced CpG methylation within the 299-bp P2 minimal promoter (Fig. S2B) using SssI and HpaII methylases in a CpG-free luciferase reporter vector (24), which resulted in a reduction of luciferase activity (Fig. S2C).
To provide functional evidence that Hnf4a transcription is regulated by histone modifications, we performed pharmacological studies in two rat insulinoma cell lines: the nonexpressing BRIN cells and INS-1 cells that express P2 transcripts (around 40% of the levels observed in primary rat islets) (Fig. 2A). The epigenetic state of BRIN cells was consistent with their transcriptional inactivity, with depletion of active marks and enrichment of repressive marks (Fig. 2B) as well as CpG methylation (Figs. S2A and S3A) at both promoter regions. Treatment of BRIN cells with trichostatin A [TSA, a histone deacetylase inhibitor (25)] and TSA plus DZNep [an S-adenosyl-homocysteine hydrolase inhibitor (26)] resulted in increased mRNA levels from both promoters (Fig. 2C and Fig. S3 B–D). We found that the epigenetic profile of INS-1 cells closely mirrored that of islets, with the P2 promoter being unmethylated (Fig. S3E) and enriched in H3Ac and H3K4me3 (Fig. 2D). Nevertheless, and importantly, the enhancer region was enriched in H3K9me2, depleted in H3Ac (Fig. 2D), and hypermethylated (Fig. S3F). This less open chromatin configuration at the enhancer could explain the lower levels of Hnf4a P2 transcripts in INS-1 cells compared with adult islets (60% less). In support of this hypothesis, we found that transfecting INS-1 cells with a construct containing the enhancer region (27) cloned upstream of the P2 promoter resulted in a 60% increase in luciferase activity compared with a vector containing only the P2 promoter, whereas the enhancer alone lacked any promoter activity (Fig. S3G). Treatment of INS-1 cells with the G9a histone methyltransferase inhibitor BIX-01294 (BIX) (28) reduced the levels of H3K9me2 at the Hnf4a locus, more prominently at the enhancer region (Fig. S3H), and led to an increase in P2 (Fig. 2E) but not P1 (Fig. S3I) mRNA levels. Treatment of INS-1 cells with TSA increased the levels of H3Ac throughout the Hnf4a locus, but mostly at P2 (Fig. S3J), and increased P2 (Fig. 2E) and P1 (Fig. S3I) mRNA levels.
Fig. 2.
Role of histone modifications in controlling Hnf4a P2 expression. (A) mRNA analysis by qRT-PCR in adult rat islets, BRIN cells, and INS-1 cells, normalized against Ppia (n = 3 per group). Data are shown relative to islets, arbitrarily normalized to 1. Native ChIP analysis of histone marks in BRIN (B) and INS-1 (D) cells (n = 4 per group). qRT-PCR analysis in BRIN- (C) and INS-1–treated (E) cells. Data were normalized against Ppia and are shown relative to levels in untreated cells, arbitrarily set to 1 (n = 4 per group). Error bars indicate SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
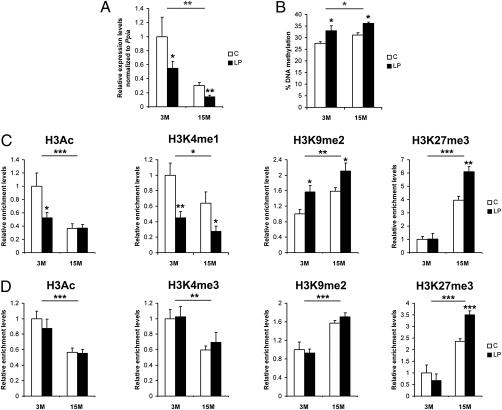
Maternal Diet and Aging Influence Transcriptional Activity and Epigenetic Regulation of the Hnf4a Locus.
We performed qRT-PCR measurements in islets collected from 3-mo-old (3M) and 15-mo-old (15M) rats exposed to a C or LP diet during fetal and early postnatal life. We found that Hnf4a mRNA levels were significantly reduced in LP offspring compared with age-matched controls at both ages (Fig. 3A). There was also a significant reduction of Hnf4a mRNA as result of aging in both groups (Fig. 3A). Western blotting performed on islets collected from 3M C and LP rats demonstrated a reduction of HNF-4α protein levels by 60% in the LP offspring (Fig. S4A).
Fig. 3.
Early diet- and aging-associated effects on transcriptional activity and epigenetic regulation at the Hnf4a locus in rat islets. (A) mRNA analysis by qRT-PCR in 3M and 15M C and LP samples (3M, n = 10 per group; 15M, n = 8 per group). Data were normalized against Ppia and are shown relative to 3M C, arbitrarily set to 1. (B) MassArray analysis of DNA methylation at the P2 promoter in 3M and 15M C and LP samples (n = 6 per group). Native ChIP analysis of histone marks at the enhancer (C) and P2 promoter (D) in 3M and 15M C and LP samples (3M, n = 7 per group; 15M, n = 6 per group). Note that the levels of H3K4me3 were not compared between C and LP islets at the enhancer because of its very low levels at this region (Fig. 1D). H3K4me1 was not compared at the P2 promoter because it is a mark for active enhancers (23). Data are shown relative to the 3M C samples, arbitrarily normalized to 1. Error bars indicate SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
We first assessed if the reduction in Hnf4a mRNA in rats exposed to the LP during early development, or as result of aging, is mediated by changes in DNA methylation. Islets from both 3M and 15M LP offspring had a small (∼5%) but statistically significant increase in DNA methylation at the P2 promoter compared with age-matched C rats (Fig. 3B). There was also a small (∼3.5%) but statistically significant increase in DNA methylation at the P2 promoter associated with aging (Fig. 3B). Neither exposure to the LP diet nor the aging had any statistically significant effect on DNA methylation at the Hnf4a enhancer (Fig. S4B). We conclude that the small differences in DNA methylation at the P2 promoter cannot fully explain the magnitudes of Hnf4a mRNA changes induced by exposure to the LP diet or by aging and that additional epigenetic mechanisms play a role.
We then used native ChIP to measure the levels of histone modifications quantitatively at the Hnf4a locus in 3M C and LP islets. At the enhancer region, 3M LP islets had significant depletion of the active marks H3Ac and H3K4me1, as well as enrichment of the repressive mark H3K9me2, compared with age-matched C islets (Fig. 3C). At this age, there were no differences in histone modifications at the P2 promoter region (Fig. 3D). Maternal diet also led to extensive changes in histone marks at 15M. In 15M LP islets, the enhancer region was depleted in H3K4me1 and enriched in H3K9me2 and H3K27me3 (Fig. 3C). In addition, there was increased H3K27me3 at the P2 promoter region in LP islets at this age (Fig. 3D). We did not observe any significant differences in diet-induced histone modifications at the P1 promoter (Fig. S4C) or at the two control genes, Actb (Fig. S4D) and Hbb (Fig. S4E), at either age.
An effect of age on levels of histone marks was observed across the Hnf4a locus in a manner consistent with an age-dependent reduction of its transcription. With age, the enhancer was depleted in H3Ac and H3K4me1 and enriched in H3K9me2 and H3K27me3 (Fig. 3C) and the P2 promoter was depleted in H3Ac and H3K4me3 and enriched in H3K9me2 and H3K27me3 (Fig. 3D). There was no significant age-associated change at the Actb (Fig. S4D) or Hbb (Fig. S4E) promoter.
Exposure to the LP diet during early development influenced the dynamics of the age-associated changes for several histone marks. Age-associated enrichment of H3K27me3 was enhanced in the LP offspring at both the P2 promoter (interaction, P = 0.009) and enhancer (interaction, P = 0.006) regions (Fig. 3 C and D).
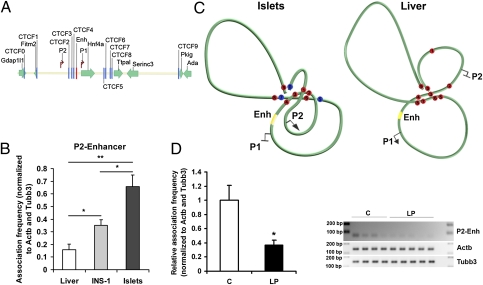
Maternal Diet Modifies the P2 Promoter–Enhancer Interaction in Rat Islets.
To provide mechanistic insight into the effects of maternal diet on high-order chromatin organization, we used quantitative chromatin conformation capture (q3C) to measure association frequencies between CTCF [CCCTC-binding factor (zinc finger protein)]/cohesin-binding sites, P2 and P1 promoters, and enhancer fragments (Fig. 4A and Figs. S5–S7). In rat islets, we found evidence for a P2-enhancer interaction that was CTCF/cohesin-independent. Importantly, the association frequency of this interaction was fourfold lower in liver, which does not express P2 transcripts, and twofold lower in INS-1 cells, which express 60% less P2 transcripts (Fig. 4B). The full q3C dataset shown in Figs. S6 and S7 supports a model in which differential chromatin looping brings P2 closer to the enhancer in islets and keeps it away in liver (Fig. 4C).
Fig. 4.
Long-range interactions at the rat Hnf4a locus. (A) Schematic representation of the 300-kb region on rat chromosome 3 containing the Hnf4a locus, analyzed by q3C. Green arrows indicate the transcriptional orientation of the genes within this region. Blue vertical bars indicate CTCF/cohesin-binding sites validated by ChIP in rat liver. (B) Comparative P2-enhancer association frequency in adult liver (n = 4), INS-1 cells (n = 5), and adult islets (n = 3). (C) Proposed looping models that summarize the long-range interactions in islets and liver. Red circles indicate CTCF/cohesin-binding sites, and blue circles indicate sites not bound by CTCF/cohesin. (D) P2-enhancer association frequency in 3M C (n = 4) and LP samples (n = 5). Data are shown relative to the 3M C samples, arbitrarily normalized to 1. 3M C products after 33 PCR cycles are shown. Error bars represent SEM. *P < 0.05; **P < 0.01.
Next, we quantified the association frequencies of the interactions in 3M C and LP islets. We found a significant reduction in the P2-enhancer interaction in 3M LP islets compared with age-matched C islets (Fig. 4D). No effect was observed for the other two islet-“specific” CTCF/cohesin-mediated interactions (Fig. S7C).
Altogether, the in vitro luciferase assays and the in vivo q3C assays demonstrate that the Hnf4a enhancer interacts directly with and controls the activity of the P2 promoter in insulin-secreting cells. These data also suggest that the epigenetic changes induced by maternal diet modify the P2-enhancer interaction.
Discussion
This study has identified a fundamental mechanism by which diet, during critical periods of development, interacts with the genome to influence long-term health. We found that suboptimal nutritional during early life modifies a promoter–enhancer interaction at the Hnf4a locus through alterations in histone marks. Our study also provides molecular insight into how diet and aging can interact over the life course to determine gene expression and, consequently, tissue function and disease risk.
HNF-4α plays a pivotal role in development of the fetal endocrine pancreas, adult pancreatic function, and, consequently, glucose tolerance. In human and rat islets, Hnf4a mRNA is mainly transcribed from the upstream P2 promoter. In both species, the P2 promoter was marked by DNA hypomethylation, H3 acetylation, and H3K4me3 (an active mark). The enhancer region, located 40 kb downstream of P2, was also characterized by open chromatin. By mapping DNA methylation and histone marks in other tissues and cell lines, we found evidence for differential tissue-specific epigenetic regulation of the P2 and enhancer region. Parallel in vitro studies supported the hypothesis that epigenetic marking controls transcriptional states at the Hnf4a locus in islets. Demethylation by aza-C reactivated Hnf4a expression in BRIN cells, and in vitro methylation of the P2 promoter in reporter assays reduced the luciferase activity, thus demonstrating a direct functional link between DNA methylation and transcriptional activity. In INS-1 cells, the enhancer was enriched in H3K9me2 (a repressive mark) and its pharmacological reduction led to reactivation of the P2 promoter, suggesting that H3K9me2 contributes to down-regulation of P2 activity through “repression” of the enhancer element. In BRIN cells, the entire Hnf4a locus was marked with the repressive histone mark H3K27me3 and its pharmacological reduction, in combination with an increase of H3Ac, led to partial reactivation of Hnf4a expression, suggesting that this mark contributes, together with DNA methylation, to the locking of Hnf4a in a silent state. Importantly, we also demonstrated that the Hnf4a enhancer regulates the activity of the P2 promoter. Using luciferase assays, we showed that the enhancer can augment the activity of the P2 promoter in insulin-secreting cell lines, and, by q3C, we demonstrated a direct P2–enhancer interaction in isolated islets.
In light of these findings demonstrating that Hnf4a is epigenetically regulated, we addressed the hypothesis that its transcriptional activity is responsive to environmental factors through changes to the epigenotype. We demonstrated that maternal protein restriction leads to a “programmed” reduction of Hnf4a mRNA in islets of LP offspring in adulthood (young 3M and old 15M rats). Transcriptional down-regulation of Hnf4a was paralleled by a minimal increase in DNA methylation at the P2 promoter and, more notably, by substantial changes in histone marks specifically at the enhancer region. LP islets were characterized by relative excess of the repressive mark H3K9me2 and loss of the active mark H3K4me1 at the enhancer region in both young and old rats. Consistent with these epigenetic changes, we observed a significant reduction of the P2–enhancer interaction in 3M LP offspring islets. These observations strongly suggest that the epigenetic changes at the enhancer region are responsible for the programmed down-regulation of Hnf4a expression and provide a mechanistic basis for the cellular “memory” linking maternal diet to the development of T2D in the offspring. Pancreatic islets from patients who have T2D have reduced HNF4A expression (29) of a magnitude similar to that observed in LP islets, and common variants at the HNF4A locus show association with T2D (13, 16). We believe that the epigenetic mechanisms described in this study may contribute to the development of pancreatic β-cell dysfunction and the subsequent development of T2D.
Links between environmentally induced epigenetic modifications and metabolic health are poorly understood. Studies focused on DNA methylation have identified small changes in the PPAR-α (Ppara) promoter in liver from offspring of protein-restricted rats (30). Genome-wide profiling in islets of rats with intrauterine growth restriction (IUGR) following bilateral uterine artery ligation led to discovery of ∼1,400 loci with altered DNA methylation (31). In the same model of uterine artery ligation, it has been found that Pdx1 has reduced expression in islets, which is associated with loss of histone acetylation and H3K4me3, increased levels of H3K9me2, and no major changes in DNA methylation until late adulthood (32). Increased levels of repressive histone modifications without altered DNA methylation have also been associated with reduced expression of Slc2a4 (encoding the GLUT4 glucose transporter) in skeletal muscle of rats exposed to maternal calorie restriction (33). Our study provides an example of nutritionally induced histone modification changes specific to an enhancer region that have an impact on high-order chromatin organization and transcriptional activity of a gene.
It remains to be established when the chromatin changes that we observed at the Hnf4a locus in 3M and 15M LP islets initially appear. In a previous study performed in islets of rats with IUGR following uterine artery ligation (32), however, the authors found altered histone modifications at the Pdx1 promoter only days after induction of the reduced blood flow. These data suggest that the epigenome is directly influenced by environmental factors. The signaling pathways that link altered maternal diet and chromatin changes in the fetus are currently unknown. There is, however, some evidence that several chromatin-modifying enzymes (e.g., histone-deacetylases) are particularly susceptible to intracellular fluctuations in NAD/NADH (34). These data suggest that the mechanisms of action of some histone-modifying enzymes have evolved to provide a link between intermediary metabolism, or environmental components, and gene function (34).
Our data provide the most compelling evidence for age-associated epigenetic silencing in control islets, at a specific locus, that may lead to increased risk for T2D with age. The P2 and the enhancer chromatin regions were enriched in repressive marks, most prominently the Polycomb-dependent histone mark H3K27me3, and depleted in active marks with age. We propose that H3K27me3 contributes to the observed reduction in mRNA levels with age. This hypothesis is supported by our functional assays in BRIN cells showing that loss of H3K27me3 leads to reactivation of the silent state. A previous study reported reduction of H3K27me3 repressive mark at the Ink4a/Arf locus in mouse β-cells, with age, that leads to increased expression (35). This was associated with a decline of the histone methyltransferase Ezh2 with age (35). Notably, our study demonstrates Polycomb-dependent histone modification of a gene implicated in T2D susceptibility that could contribute to the age-associated decline in function of the endocrine pancreas. Another important finding of our study was the influence that maternal diet had on the dynamics of age-associated epigenetic changes. The gain of H3K27me3 at the P2 promoter and enhancer region with age was more pronounced in LP vs. C islets. This suggests that an interaction between the effects of maternal diet and age on epigenetic changes at the Hnf4a gene has a direct role in the development of T2D in old LP rats (10).
In conclusion, our study demonstrates that Hnf4a expression in islets is under tight epigenetic control that is dynamically modulated by maternal diet during early development and by aging. In particular, we demonstrated that the enhancer region is especially susceptible to epigenetic changes resulting from alterations in early nutrition and during the aging process. We propose that changes in promoter–enhancer interactions represent a more general epigenetic mechanism by which early nutrition interacts with the genome to influence gene expression and metabolic health.
Methods
mRNA Analysis.
mRNA levels were measured by qRT-PCR using TaqMan probes or primers for SYBR-Green (Table S1). Additional details are provided in SI Methods.
DNA Methylation Analysis.
Genomic DNA (1 μg) was treated with sodium bisulphite (EpiTect kit; Qiagen). Primer pairs were designed using MethPrimer (Table S1). For bisulphite sequencing, PCR products were separated by agarose gel electrophoresis, purified by gel extraction, and cloned into TOPO-TA vector (Invitrogen). A total of 10–20 individual clones were sequenced per sample. MassArray analysis was performed according to manufacturer’s instructions (Sequenom).
Pharmacological Treatments.
INS-1 and BRIN cells were seeded in six-well plates 24 h before the start of each treatment. Daily 5-AzaC treatment (0.05 μM or 0.5 μM) was performed for 3 d until cells reached confluence [5-AzaC refers to an equimolar mix of 5-azacytidine + 5-aza-2-deoxycytidine (Sigma)]. TSA (Sigma) was used at a concentration of 100 nM for 24 h, BIX (Sigma) was used at a concentration of 2 μM for 24 h, and DZNep was used at a concentration of 5 μM for 24 h.
ChIP.
Histone modification levels were analyzed by native ChIP (SI Methods). Quantification was performed by qPCR (SYBR-Green) (Table S1) using 5 ng of DNA from each fraction. Bound/input (for in vivo measurements) or bound/unbound (for pharmacological treatments) ratios were calculated for each sample. Actb and Hbb (and the corresponding genes in humans) were used as controls for active and inactive promoters, respectively.
ChIP for CTCF and Rad21 was performed on formaldehyde-fixed chromatin (SI Methods). Input and bound fractions were measured by Picogreen (Invitrogen), and quantification was performed by qPCR (SYBR-Green) (Table S1).
q3C.
Details of q3C are provided in SI Methods. Briefly, cells were cross-linked in 1% formaldehyde, washed in cold PBS, lysed, and then digested overnight with HindIII. Ligation with T4 ligase was carried out at 16 °C overnight using 2.5 ng/μL digested chromatin. A second digestion step with BglII was used before reversal of cross-links, followed by phenol chloroform purification and ethanol precipitation. qPCR was performed using primers for SYBR-Green (Tables S1 and S2). All interaction frequencies were normalized to the circularization frequency of the Actb and Tubb3 fragments, as internal digestion-ligation controls.
Statistical Analysis.
Statistical significance of differences between groups was tested by two-tailed Student t tests or by two-way repeated-measures ANOVA with Bonferroni posttests. All statistical analysis was performed using GraphPad Prism software. Results are represented as mean values ± SEM (*P < 0.05; **P < 0.01; ***P < 0.001).
Supplementary Material
Acknowledgments
We thank N. Wakes, S. Dowd, U. Obi, A. Wayman, and D. Hawkes for technical assistance; F. Reimann for providing INS-1 cells; M. Rehli for supplying the pCpGL vector; and D. Schmidt and D. Odom for access to unpublished data. This work was supported by the Biotechnology and Biological Sciences Research Council, the British Heart Foundation, the FP6 Epigenome Network of Excellence programme, GlaxoSmithKline, the Nuffield Foundation, the Royal Society, the National Institute for Health Research Cambridge Biomedical Research Centre, and the Medical Research Council Centre for Obesity and Related Metabolic Diseases. Studies in Malmö were funded by grants from the Swedish Research Council, Region Skåne, Novo Nordisk, Söderberg, Påhlsson, and Linné (Grant B31 5631/2006). S.E.O. is a British Heart Foundation Senior Fellow. M.A.-J. is supported by a scholarship from the Wellcome Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019007108/-/DCSupplemental.
References
- 1.Jaenisch R, Bird A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 2.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandovici I, Smith NH, Ozanne SE, Constância M. In: Epigenetics. Tost J, editor. Norfolk, UK: Caister Academic; 2008. pp. 343–370. [Google Scholar]
- 4.Burdge GC, Lillycrop KA. Nutrition, epigenetics, and developmental plasticity: Implications for understanding human disease. Annu Rev Nutr. 2010;30:315–339. doi: 10.1146/annurev.nutr.012809.104751. [DOI] [PubMed] [Google Scholar]
- 5.Ozanne SE, Constância M. Mechanisms of disease: The developmental origins of disease and the role of the epigenotype. Nat Clin Pract Endocrinol Metab. 2007;3:539–546. doi: 10.1038/ncpendmet0531. [DOI] [PubMed] [Google Scholar]
- 6.Waterland RA, Michels KB. Epigenetic epidemiology of the developmental origins hypothesis. Annu Rev Nutr. 2007;27:363–388. doi: 10.1146/annurev.nutr.27.061406.093705. [DOI] [PubMed] [Google Scholar]
- 7.Remacle C, et al. Intrauterine programming of the endocrine pancreas. Diabetes Obes Metab. 2007;9(Suppl 2):196–209. doi: 10.1111/j.1463-1326.2007.00790.x. [DOI] [PubMed] [Google Scholar]
- 8.Calvanese V, Lara E, Kahn A, Fraga MF. The role of epigenetics in aging and age-related diseases. Ageing Res Rev. 2009;8:268–276. doi: 10.1016/j.arr.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Snoeck A, Remacle C, Reusens B, Hoet JJ. Effect of a low protein diet during pregnancy on the fetal rat endocrine pancreas. Biol Neonate. 1990;57:107–118. doi: 10.1159/000243170. [DOI] [PubMed] [Google Scholar]
- 10.Petry CJ, Dorling MW, Pawlak DB, Ozanne SE, Hales CN. Diabetes in old male offspring of rat dams fed a reduced protein diet. Int J Exp Diabetes Res. 2001;2:139–143. doi: 10.1155/EDR.2001.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozanne SE, et al. Low birthweight is associated with specific changes in muscle insulin-signalling protein expression. Diabetologia. 2005;48:547–552. doi: 10.1007/s00125-005-1669-7. [DOI] [PubMed] [Google Scholar]
- 12.Odom DT, et al. Control of pancreas and liver gene expression by HNF transcription factors. Science. 2004;303:1378–1381. doi: 10.1126/science.1089769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silander K, et al. Genetic variation near the hepatocyte nuclear factor-4 alpha gene predicts susceptibility to type 2 diabetes. Diabetes. 2004;53:1141–1149. doi: 10.2337/diabetes.53.4.1141. [DOI] [PubMed] [Google Scholar]
- 14.Gupta RK, et al. The MODY1 gene HNF-4alpha regulates selected genes involved in insulin secretion. J Clin Invest. 2005;115:1006–1015. doi: 10.1172/JCI200522365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta RK, et al. Expansion of adult beta-cell mass in response to increased metabolic demand is dependent on HNF-4alpha. Genes Dev. 2007;21:756–769. doi: 10.1101/gad.1535507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barroso I, et al. Population-specific risk of type 2 diabetes conferred by HNF4A P2 promoter variants: A lesson for replication studies. Diabetes. 2008;57:3161–3165. doi: 10.2337/db08-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamagata K, et al. Mutations in the hepatocyte nuclear factor-4alpha gene in maturity-onset diabetes of the young (MODY1) Nature. 1996;384:458–460. doi: 10.1038/384458a0. [DOI] [PubMed] [Google Scholar]
- 18.Thomas H, et al. A distant upstream promoter of the HNF-4alpha gene connects the transcription factors involved in maturity-onset diabetes of the young. Hum Mol Genet. 2001;10:2089–2097. doi: 10.1093/hmg/10.19.2089. [DOI] [PubMed] [Google Scholar]
- 19.Nakhei H, Lingott A, Lemm I, Ryffel GU. An alternative splice variant of the tissue specific transcription factor HNF4alpha predominates in undifferentiated murine cell types. Nucleic Acids Res. 1998;26:497–504. doi: 10.1093/nar/26.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres-Padilla ME, Fougère-Deschatrette C, Weiss MC. Expression of HNF4alpha isoforms in mouse liver development is regulated by sequential promoter usage and constitutive 3′ end splicing. Mech Dev. 2001;109:183–193. doi: 10.1016/s0925-4773(01)00521-4. [DOI] [PubMed] [Google Scholar]
- 21.Hatzis P, Kyrmizi I, Talianidis I. Mitogen-activated protein kinase-mediated disruption of enhancer-promoter communication inhibits hepatocyte nuclear factor 4alpha expression. Mol Cell Biol. 2006;26:7017–7029. doi: 10.1128/MCB.00297-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 23.Heintzman ND, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39:311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 24.Klug M, Rehli M. Functional analysis of promoter CpG methylation using a CpG-free luciferase reporter vector. Epigenetics. 2006;1:127–130. doi: 10.4161/epi.1.3.3327. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990;265:17174–17179. [PubMed] [Google Scholar]
- 26.Tan J, et al. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007;21:1050–1063. doi: 10.1101/gad.1524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailly A, Torres-Padilla ME, Tinel AP, Weiss MC. An enhancer element 6 kb upstream of the mouse HNF4alpha1 promoter is activated by glucocorticoids and liver-enriched transcription factors. Nucleic Acids Res. 2001;29:3495–3505. doi: 10.1093/nar/29.17.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kubicek S, et al. Reversal of H3K9me2 by a small-molecule inhibitor for the G9a histone methyltransferase. Mol Cell. 2007;25:473–481. doi: 10.1016/j.molcel.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Gunton JE, et al. Loss of ARNT/HIF1β mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell. 2005;122:337–349. doi: 10.1016/j.cell.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 30.Lillycrop KA, et al. Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPAR alpha promoter of the offspring. Br J Nutr. 2008;100:278–282. doi: 10.1017/S0007114507894438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson RF, et al. Experimental intrauterine growth restriction induces alterations in DNA methylation and gene expression in pancreatic islets of rats. J Biol Chem. 2010;285:15111–15118. doi: 10.1074/jbc.M109.095133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park JH, Stoffers DA, Nicholls RD, Simmons RA. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest. 2008;118:2316–2324. doi: 10.1172/JCI33655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raychaudhuri N, Raychaudhuri S, Thamotharan M, Devaskar SU. Histone code modifications repress glucose transporter 4 expression in the intrauterine growth-restricted offspring. J Biol Chem. 2008;283:13611–13626. doi: 10.1074/jbc.M800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner BM. Epigenetic responses to environmental change and their evolutionary implications. Philos Trans R Soc Lond B Biol Sci. 2009;364:3403–3418. doi: 10.1098/rstb.2009.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen H, et al. Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev. 2009;23:975–985. doi: 10.1101/gad.1742509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.