Abstract
Recent thymic emigrants (RTEs) are the youngest subset of peripheral T cells, and they differ functionally and phenotypically from the rest of the naïve T-cell pool. RTEs are present in the peripheral T-cell pool throughout life but are the most common subset of T cells in neonates and adults recovering from lymphoablation. Using a murine model to study the homeostasis of RTEs, we show that under lymphoreplete conditions, RTEs are at a competitive disadvantage to already established mature naïve (MN) T cells. This disadvantage may be caused by a defect in survival, because RTEs may transduce homeostatic signals inefficiently, and their ability to survive is enhanced with increased expression of IL-7 receptor or B-cell lymphoma 2 (Bcl-2). Conversely, under lymphopenic conditions, enhanced proliferation by RTEs allows them to out-compete their MN T-cell counterparts. These results suggest that in times of need, such as in neonates or lymphopenic adults, RTEs perform well to fill the gaps in the peripheral T-cell pool, but when the periphery already is full, many RTEs are not incorporated into the pool of recirculating lymphocytes.
Recent thymic emigrants (RTEs) are the youngest subset of naïve T cells, those that recently have entered the lymphoid periphery after development in the thymus. RTEs maintain T-cell diversity in the periphery (1), a particularly important contribution in the very young and in adults recovering from lymphopenia.
The original paradigm held that the thymus minted fully functional T cells. However, it is becoming clear that RTEs in both humans and mice undergo postthymic maturation in the lymphoid periphery (2–6) before joining the mature naïve (MN) T-cell pool. RTEs and MN T cells differ in surface phenotype, and, as compared with MN T cells, stimulated RTEs generally proliferate less and secrete less cytokine. Studying RTEs has been facilitated by the characterization of a transgenic (Tg) model system (7) that allows unambiguous identification and live-cell purification of this subset. In mice Tg for GFP under control of the recombination activating gene 2 promoter (RAG2p-GFP Tg mice), RTEs are GFP+ peripheral T cells (2). The GFP label is brightest in the youngest RTEs (8) and decays over time until it can no longer be detected on T cells that have been in the lymphoid periphery for more than 3 wk (2).
Throughout life, the peripheral T-cell pool is maintained at a relatively constant size despite continuous turnover and thymic export of about 1 × 106 cells per day (9, 10). The T-cell pool is maintained at a size large enough to protect adequately against pathogens, with estimated 1 × 106 antigen specificities (11), but small enough to avoid taxing the host's resources. Homeostasis is maintained by competition for two limiting factors, IL-7 and MHC loaded with self-peptides (12, 13).
Lymphopenia occurs in humans in a variety of clinical and disease settings, including after stem cell transplantation, chemotherapy, and HIV infection. Under lymphopenic conditions, the remaining peripheral T cells proliferate, replenishing the peripheral space and gradually acquiring an effector/memory phenotype. Murine models have demonstrated that lymphopenia can induce slower turnover driven by IL-7 and MHC (12, 13) and faster division driven by commensal gut antigens or IL-2/IL-15 (reviewed in ref. 12).
Because RTEs provide the only source of new T-cell diversity for the naïve peripheral T-cell pool, their contribution is clearly desirable. It has been suggested that RTEs populate a distinct niche and are preferentially accepted into the naïve T-cell pool (14, 15). However, RTEs have slightly reduced IL-7 receptor (IL-7R) expression levels (2), suggesting they may not compete as efficiently as MN T cells for this survival factor. Using the RAG2p-GFP Tg RTE reporter system, we assessed the comparative homeostasis of RTEs and MN T cells and found that when the lymphoid periphery is full, RTEs are not accepted preferentially into the periphery and persist less well than MN T cells. However, under lymphopenic conditions, when RTEs would be particularly important for replenishing T-cell receptor (TCR) diversity, RTEs are able to fill the void effectively.
Results
Under Lymphoreplete Conditions, MN T Cells Out-Persist RTEs.
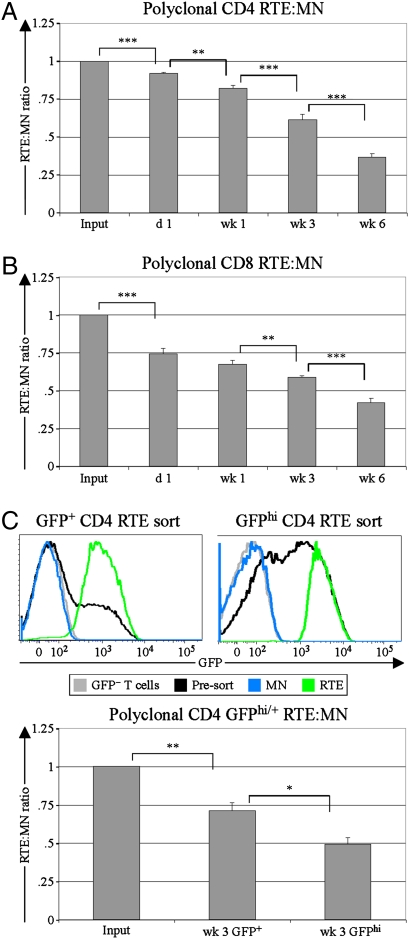
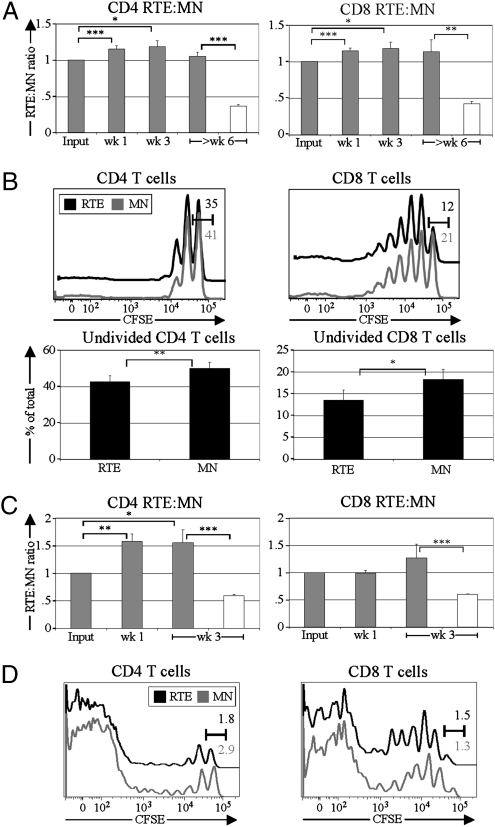
To compare RTEs and MN T cells under lymphoreplete conditions, sorted populations of congenically marked polyclonal RTEs and MN T cells were mixed at a 1:1 ratio and transferred into unmanipulated recipients (Fig. S1). This approach allowed a precise determination of the input RTE:MN ratio for comparison with the RTE:MN ratio of donor cells recovered at various time points after transfer and across multiple experiments. Compared with their counterpart MN T cells, both CD4 (Fig. 1A) and CD8 (Fig. 1B) RTEs were out-competed over the entire time course, with less than half as many RTE-derived cells remaining 6 wk posttransfer.
Fig. 1.
RTEs decline relative to MN T cells in lymphoreplete environments. At the indicated time points following injection of CD4 (A) or CD8 (B) RTEs and MN T cells, the splenic donor RTE:MN ratio was calculated and normalized to the injected (Input) RTE:MN ratio (n = 5–19 mice, from two to five independent experiments per time point). Similar results were obtained for mesenteric and peripheral lymph nodes, and no differences in CD44 or CD62L expression were noted between RTEs and MN T cells. (C) From enriched CD4 T-cell preparations (presort), GFP+ [median fluorescence Intensity (MFI) 1,878; Upper Left] or GFPhi (MFI 2,930; Upper Right) naïve CD4 RTEs were sorted and coinjected with congenic GFP− MN T cells. T cells from WT mice (GFP− T cells) were used to determine background (denoted by gray lines). The presort population (denoted by black lines) for the GFPhi sort contains a larger proportion of younger RTEs because 5-wk-old donors were used to facilitate collection of sufficient numbers of GFPhi cells for injection. The splenic RTE:MN ratio at 3 wk was normalized to the input ratio for each competition (n = 3 mice per group). Error bars represent SEM. *P < 0.05, **P < 0.01, and ***P < 0.001, using an unpaired Student's t test.
Because the GFP signal decays over time (8), the GFP brightness in RTEs correlates inversely with residence time in the periphery. We compared younger GFPhi RTEs and bulk total GFP+ RTEs in separate competitions with cotransferred MN T cells (Fig. 1C Upper Right and Upper Left, respectively). Younger RTEs fared worse than total RTEs relative to MN T cells (Fig. 1C Lower), demonstrating that the newest cells in the lymphoid periphery have the shortest persistence.
GFP Transgene Does Not Affect Development or Cause Rejection.
Because high levels of transgene expression can cause cellular cytotoxicity in Tg systems, we assessed the size of thymocyte and peripheral T-cell compartments in RAG2p-GFP Tg mice, which express relatively low levels of GFP in two transient bursts in the thymus. There was no difference between age- and sex-matched WT and RAG2p-GFP Tg mice in percent distribution or total cellularity of any thymocyte (Fig. S2) or peripheral T-cell (Fig. S3) compartment.
To test the possibility that GFP can serve as a transplantation antigen (16, 17), we assessed the relative persistence of a mix of RTEs and MN T cells cotransferred both to GFP+ recipients and to recipients that were GFP− but were primed with GFP+ cells. Regardless of whether the recipients expressed or were primed to GFP, CD4 and CD8 RTEs declined to the same degree relative to their MN counterparts 3 wk after transfer (Fig. S4), showing that GFP does not serve as a transplantation antigen in this system. Moreover, the kinetics of the decline in RTEs is gradual, not fitting well with rejection, and continues past 3 wk (Fig. 1 A and B), when GFP is undetectable in RTEs by flow cytometry (2). The residual low levels of GFP in RTEs also are unlikely to impact cell fitness, because T cells with a 10-fold brighter GFP signal are functionally unimpaired (2). Therefore, we conclude that the preferential decline of RTEs is unlikely to be an artifact of the RAG2p-GFP Tg system used in these studies.
RTEs Persist Less Well than MN T Cells in Lymphoreplete Hosts, a Disadvantage That Is Corrected by Enhanced Homeostatic Signals.
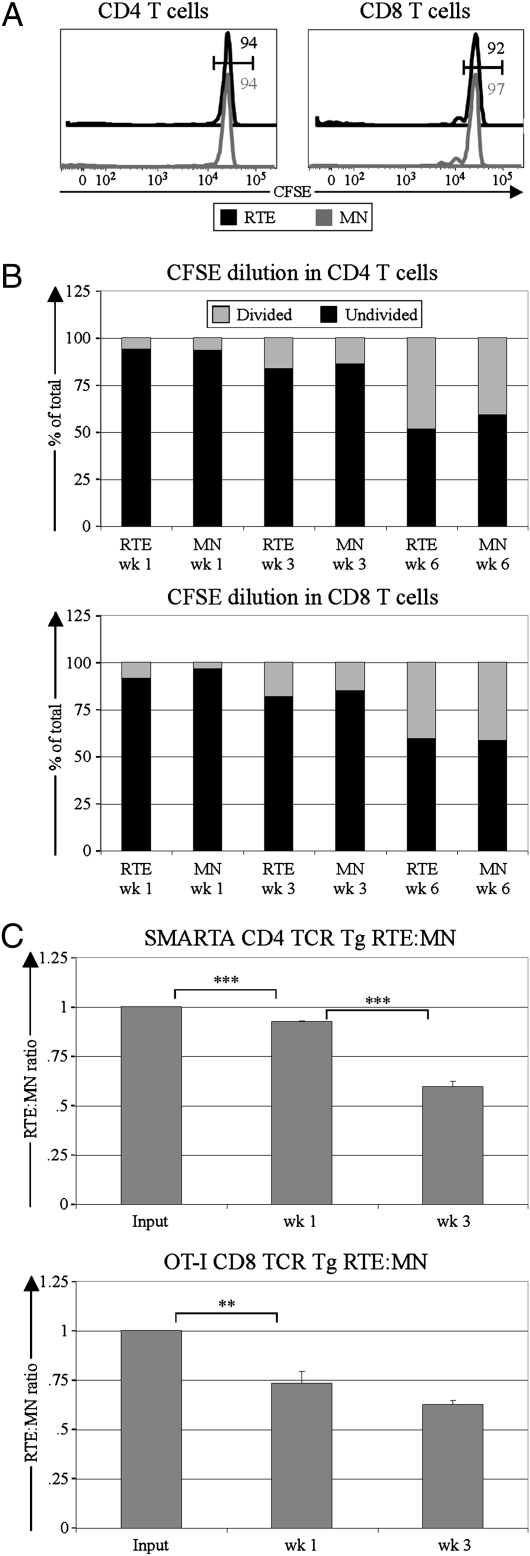
To determine whether differential rates of proliferation account for the relative loss of RTEs in lymphoreplete hosts, rates of division in cotransferred carboxyfluorescein succinimidyl ester (CFSE)-labeled RTEs and MN T cells were assessed over time. At 3 wk posttransfer, RTEs and MN T cells had proliferated little (Fig. 2A), making it unlikely that proliferative differences could drive the advantage of MN T cells over RTEs. Furthermore, MN T cells did not have a higher fraction of divided cells than RTEs at any time point analyzed (Fig. 2B).
Fig. 2.
RTE decline relative to MN T cells is not caused by proliferative differences but is influenced by intrinsic differences. CFSE-labeled naïve CD4 or CD8 RTEs and MN T cells were coinjected into lymphoreplete recipients, and CFSE dilution was measured at various time points following injection. (A) Representative CFSE profiles from donor cells 3 wk posttransfer are shown for CD4 (Left) and CD8 (Right) RTEs and MN T cells. Numbers (black for RTEs and gray for MN T cells) represent the percentage of cells falling into the undivided gate. (B) Degree of CFSE dilution is shown for splenic CD4 (Upper) and CD8 (Lower) RTEs and MN T cells at the indicated times posttransfer. Data are representative of two independent experiments, analyzing a total of 10 mice. (C) CD4 (SMARTA; Upper) and CD8 (OT-I; Lower) TCR Tg RTEs and MN T cells were coinjected into lymphoreplete recipients, and the splenic RTE:MN ratio relative to input was calculated at the indicated time points after transfer. Data represent three or four mice per time point from one or two independent experiments, and error bars represent SEM. **P < 0.01 and ***P < 0.001, using an unpaired student's t test.
To investigate whether the preferential persistence of MN T cells was a result of differences in TCR specificity, competitions between RTEs and MN T cells with a uniform TCR were set up. Both CD4+ SMARTA and CD8+ OT-I TCR Tg T cells declined relative to their MN counterparts (Fig. 2C), suggesting that even with a uniform TCR, RTEs decline relative to MN T cells in lymphoreplete hosts.
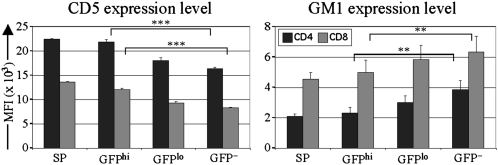
CD5 and monosialotetrahexosylganglioside (GM1) negatively regulate TCR signaling and positively regulate responsiveness to homeostatic cytokines, respectively (18). RTEs on average expressed more CD5 and less GM1 than their MN T-cell counterparts, and this trend was more pronounced in the youngest (GFPhi) RTEs and even more so in their immediate thymic precursors (Fig. 3). These data suggest that, compared with MN T cells, RTEs inefficiently transduce basal homeostatic signals from self-MHC+ peptide and IL-7.
Fig. 3.
RTEs express more CD5 and less GM1 than MN T cells. CD5 and GM1 expression levels were determined on GFP+ CD4 and CD8 single-positive (SP) TCRβ+ thymocytes and naïve (CD44lo) GFPhi young RTEs, GFPlo older RTEs, and GFP− MN T cells from RAG2p-GFP Tg mice. Bars represent mean MFI from three or four mice per group. Error bars represent SEM. **P < 0.01 and ***P < 0.001, using a paired Student‘s t test.
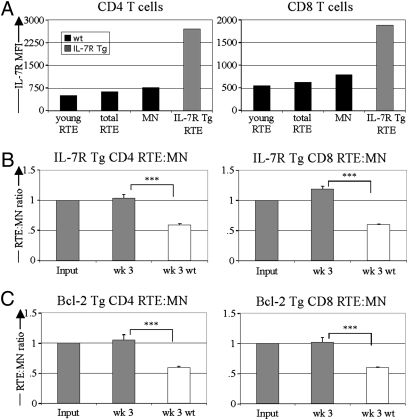
RTEs express lower levels of surface IL-7R than do MN T cells (Fig. 4A and ref. 2). To determine if forced up-regulation of IL-7R expression on RTEs reverses their preferential loss, we cotransferred WT MN T cells and IL-7R overexpressing RTEs from IL-7R Tg mice into lymphoreplete mice (Fig. 4A and ref. 19). Recovery of IL-7R Tg RTEs was comparable to or slightly increased relative to that of WT MN T cells 3 wk after transfer (Fig. 4B). To determine if increasing levels of the antiapoptotic molecule B-cell lymphoma 2 (Bcl-2) also could rescue the loss of RTEs, we competed Bcl-2 Tg RTEs with WT MN T cells. Bcl-2hi RTEs no longer declined relative to WT MN T cells (Fig. 4C). The RTE:MN ratios for both IL-7R and Bcl-2 Tg RTEs were much higher than for WT RTEs. Collectively, these data suggest that in lymphoreplete hosts, WT RTEs persist less well than MN T cells because of survival defects stemming from less robust response to survival factors.
Fig. 4.
RTE decline is rescued by IL-7R and Bcl-2 overexpression. (A) Representative IL-7Rα expression by IL-7R Tg RTEs and WT MN T cells and the youngest (brightest GFP) RTEs. (B) Sorted WT MN T cells and IL-7R Tg RTEs were cotransferred into lymphoreplete recipients, and the splenic donor RTE:MN ratio relative to input was determined 3 wk after transfer. Solid bars depict mean ± SEM from six mice analyzed in two independent experiments. (C) Sorted WT MN T cells and Bcl-2 Tg RTEs were cotransferred into lymphoreplete recipients, and the splenic donor RTE:MN ratio relative to input was determined 3 wk after transfer. Solid bars depict mean ± SEM from a total of five mice. Open bars in B and C denote RTE:MN ratios from Fig. 1 A and B, analyzing WT RTE:MN transfers into lymphoreplete recipients. ***P < 0.001 using an unpaired Student's t test.
RTEs and MN T Cells Occupy Largely Overlapping Niches.
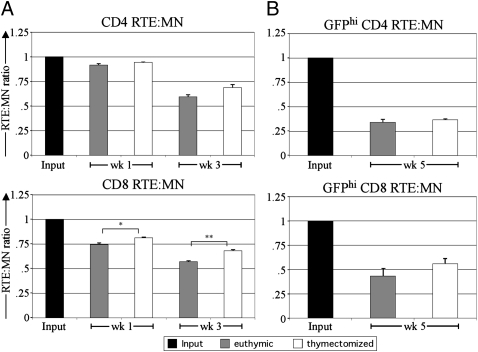
To test whether RTEs and MN T cells occupy separate niches (14, 15), these cells were cotransferred to recipients that had been selectively depleted for RTEs by thymectomy 3 wk previously. At all analysis time points, both CD4 and CD8 RTEs fared slightly worse than MN T cells in both euthymic and thymectomized recipients (Fig. 5A). For CD8 T cells, RTEs did slightly better when transferred into thymectomized recipients, and the trend was similar in CD4 T cells. Thus, RTEs and MN T cells share a largely but not completely overlapping niche. Although total splenic cellularity was similar (P = 0.86; Student's t test) in euthymic (93 ± 5 × 106) and thymectomized (91 ± 6 × 106) mice (n = 11 mice per group), the thymectomized mice were slightly lymphopenic (P < 0.001; Student's t test) for naïve CD44lo/mid T cells (6.0 ± 0.7 × 106 for CD4 and 5.5 ± 0.5 × 106 for CD8) compared with euthymic mice (12.7 ± 0.9 × 106 for CD4 and 8.1 ± 0.5 × 106 for CD8). However, at all analysis time points, 70–90% of transferred RTE and MN T cells were CD44lo/mid in both thymectomized and euthymic mice, suggesting very little homeostatic proliferation.
Fig. 5.
RTEs and MN T cells share a largely overlapping niche. (A) Naïve CD4 (Upper) and CD8 (Lower) RTEs and MN T cells were coinjected into recipients that were euthymic or thymectomized 3 wk previously, and the splenic RTE:MN ratio relative to input was determined at the indicated time points after transfer. Each injected cell population contained 1–1.5 × 106 cells. Data depict means of three recipients per time point and are representative of two independent experiments. (B) Low numbers (<2.5 × 105) of naïve CD4 (Upper) and CD8 (Lower) GFPhi young RTEs and MN T cells were coinjected into recipients that were euthymic or thymectomized, and the splenic RTE:MN ratio relative to input was determined at 5 wk after transfer. Data depict means calculated from three recipients. Error bars represent SEM. *P < 0.05 and **P < 0.01, using an unpaired Student's t test.
To test whether a separate RTE niche could be revealed if smaller numbers or only the youngest RTEs were transferred, we sorted GFPhi young RTEs and transferred low numbers (2.5 × 105) of each cell type. Even under these conditions, RTEs fared worse than MN T cells in both euthymic and thymectomized hosts (Fig. 5B).
Under Lymphopenic Conditions, RTEs Out-Compete MN T Cells.
Upon cotransfer of RTEs and MN T cells into mice made acutely lymphopenic by sublethal irradiation, RTE numbers increased slightly relative to MN T cells for both CD4 and CD8 T cells (Fig. 6A). The difference in dynamics in this lymphopenic environment compared with normal homeostatic conditions (open bars in Fig. 6A) is notable. A lower percentage of RTEs remained undivided at 1 wk posttransfer (Fig. 6B Lower), and more CD4 RTEs had undergone two or more divisions (Fig. 6B Upper Left). At 1 wk posttransfer, the bulk of each transferred cell population had not fully diluted CFSE, suggesting these cells were undergoing slower, lymphopenia-induced proliferation rather than faster proliferation driven by commensal microbial antigens or IL-2 and IL-15.
Fig. 6.
RTEs out-compete MN T cells in lymphopenic environments. CFSE-labeled naïve CD4 or CD8 RTEs and MN T cells were coinjected into lymphopenic recipients. (A) At the indicated times after transfer into irradiated recipients, the splenic RTE:MN ratio relative to input was calculated for CD4 (Left) and CD8 (Right) RTE-MN competitions. Solid bars depict means from three or four independent experiments analyzing 4–12 mice per time point. Open bars denote RTE:MN ratios from Fig. 1 A and B, analyzing RTE:MN transfers into lymphoreplete recipients. (B) CFSE profiles from CD4 (Left) and CD8 (Right) RTEs and MN T cells were determined 1 wk after transfer. (Upper) Representative CFSE dilution profiles; numbers (black for RTEs and gray for MN T cells) represent the percentage of cells falling into the undivided gate. (Lower) The mean proportions of undivided cells. n = 4 per sample, combining data from two independent experiments. (C) At the indicated time points after transfer into TCR βδ−/− recipients, the splenic RTE:MN ratio relative to input was calculated for CD4 (Left) and CD8 (Right) RTE-MN competitions. Solid bars depict means from two independent experiments analyzing six or seven mice per time point. Open bars denote the same ratio as in A. (D) CFSE profiles from CD4 (Left) and CD8 (Right) RTEs and MN T cells were determined 1 wk after transfer. CFSE dilution profiles are representative of three recipients. Numbers (black for RTEs and gray for MN T cells) represent the percentage of cells falling into the undivided gate. Error bars represent SEM. *P < 0.05, **P < 0.01, and ***P < 0.001, using a paired Student's t test.
We next performed a similar analysis in chronically T-cell lymphopenic recipients. CD8 and CD4 RTEs persisted better than their MN T-cell counterparts (Fig. 6C), in both cases at notably enhanced levels relative to the lymphoreplete environment (open bars in Fig. 6C). The rate of proliferation by RTEs and MN T cells 1 wk posttransfer (Fig. 6D) was notably faster than that seen in acutely lymphopenic recipients for the same time point (Fig. 6B), as indicated by the frequency of cells that had fully diluted CFSE, which ranged from 10–25% in acutely lymphopenic recipients and from 65–90% in chronically lymphopenic recipients.
Discussion
The work presented here demonstrates that, under lymphoreplete conditions, polyclonal populations of transferred RTEs persist less well than MN T cells. This decline is not caused by the outgrowth of MN T cells, because neither cell type proliferated extensively under these conditions, and their levels of proliferation were comparable. Using cells with uniform TCRs gave similar results, suggesting that all RTEs may enter the lymphoid periphery with a tendency to persist less well than already established MN T cells. IL-7R expression is lower on RTEs, and its overexpression through transgenesis rescued RTE persistence in the periphery, suggesting that RTEs may not compete well initially for IL-7, a key cytokine for controlling the steady-state size of the peripheral T-cell pool. The preferential loss of RTEs also can be rescued by overexpression of the survival factor Bcl-2. Determining whether increasing IL-7R and/or Bcl-2 expression alone can improve RTE relative to MN T-cell persistence would require competing transgenic RTEs against transgenic MN T cells, an experiment that has not been performed. However, the reduced ability of RTEs relative to MN T cells to transduce survival signals from homeostatic factors may help explain why RTEs are less robust under normal homeostatic conditions, because their increased levels of CD5 may inhibit basal signals triggered by self-MHC/peptide, and their reduced GM1 levels may dampen further the already reduced signals through the IL-7R. Taken together, these data suggest that RTEs do not survive as well as MN T cells in lymphoreplete conditions. It also is possible that enhanced avidity for self-MHC/peptide (perhaps because of altered intrathymic selection) contributes to the increased survival of Bcl-2 and IL-7R Tg RTEs. However, autoimmunity has not been reported in these mice, and in our experiments the Tg RTEs did not up-regulate CD44, suggesting little homeostatic proliferation.
In further support of an intrinsic defect in RTE survival capacity, our preliminary work suggests that, compared with their MN counterparts, polyclonal WT CD8 RTEs have slightly elevated Annexin V levels directly ex vivo and show diminished survival when cultured for 1 wk in the presence of IL-7. However, the same trends are not seen in CD4 RTEs, a discrepancy that requires further study.
The tendency for RTEs to die probably is tied to the fact that, although the thymus presents an array of tissue-restricted antigens, it does not present all self-antigens (20). Potentially autoreactive T cells do escape the thymus, as evidenced by the many T-cell–mediated autoimmune diseases. Thus, endowing RTEs with a tendency to die before incorporation into the MN T-cell pool may be a sound evolutionary strategy. Consistent with this idea, RTEs exhibit reduced stimulation-induced proliferation and cytokine production under most conditions (2, 21), suggesting that they experience a period of adjustment to the lymphoid periphery before gaining full effector potential. It is possible that some of the cells that die as RTEs are those with inappropriate anti-self specificity, a notion corroborated by evidence that the TCR repertoire is modulated in RTEs (5). The notably reduced CD8 RTE levels seen in our data at early time points after transfer may be attributable to differences in trafficking in addition to differences in survival, because CD8 RTEs may have an increased tendency to home to the gut (22).
Our data suggest that under normal homeostatic conditions, RTEs are not accepted preferentially into the lymphoid periphery. Furthermore, they do not appear to have a separate niche. It is unlikely that the number of RTEs transferred in our experiments would overwhelm such a niche. Our transfers of 1–2 × 106 RTEs should result in an ∼10% engraftment (23) that is well below the ∼1 × 106 RTEs that enter the lymphoid periphery daily (14). Furthermore, a preferential niche was not revealed when lower numbers of RTEs were transferred. Previous thymus graft data suggested that RTEs have a preferential niche in the lymphoid periphery, based on the observation that excess numbers of RTEs take up to 3 wk to die (14, 15). However, it now is clear that this time frame is in line with the kinetics for the disappearance of cells failing to receive homeostatic survival signals (24), eliminating the need to hypothesize an RTE niche. Our studies in which RTEs compete with cotransferred MN T cells directly test the notion of an RTE niche and provide strong data against such a compartment.
In lymphopenic conditions, RTEs compete well with MN T cells, dividing at a slightly faster rate and accumulating to a higher level. This finding is corroborated by the observation that, relative to MN T cells, RTEs express high levels of CD24 (2), a molecule necessary for optimal homeostatic proliferation (25). Because RTEs are the sole source of new repertoire diversity (1), their contribution would be especially important when T cells are underrepresented in the lymphoid periphery. Our results suggest that in these extenuating circumstances, RTEs are capable of filling the peripheral niche effectively.
The preferential RTE expansion under lymphopenic conditions may suggest that RTEs are somewhat self-reactive, given that autoreactive T cells undergo faster lymphopenia-induced proliferation (26, 27). Furthermore, RTEs appear to have the greatest competitive advantage in chronically lymphopenic mice, which have an increased load of gut flora (28), suggesting that RTEs may be more strongly reactive to antigens from commensal bacteria (12) and that this environment may allow RTEs to overcome their reduced ability to compete for IL-7. The TCR repertoire of RTEs differs from that of MN T cells (5), suggesting that RTEs may harbor a greater autoreactive potential that generally is masked by their tendency to respond less robustly upon stimulation.
Recent data suggest that naïve CD4 T cells from mice ≥6 mo of age have increased survival relative to naïve CD4 T cells from younger mice, in part because of reduced expression of the proapoptotic molecule Bcl-2-interacting mediator of cell death (Bim) (29, 30). Our data suggest that the decreased survival of RTEs is caused primarily by the young age of RTEs, although the older age (3–5 mo) of our MN T cells also may contribute to enhanced survival. The youngest CD4 RTEs declined faster than CD4 RTEs that had been out in the periphery longer. In addition, we observed that this decreased persistence occurred for both CD4 and CD8 RTEs, and in our preliminary studies, MN T cells did not have reduced Bim expression relative to RTEs.
The data from lymphoreplete conditions suggest that joining the pool of recirculating lymphocytes may be a nontrivial task, contrary to the historical view that T cells are full-fledged members of the peripheral T-cell pool immediately upon thymic egress. To seed the periphery properly, RTEs must exit the thymus, circulate in the blood, and extravasate across endothelium into secondary lymphoid organs (23). RTEs lost under normal homeostatic conditions may include cells with marginal defects in metabolism or other sustaining properties that affect their ability to populate the lymphoid periphery.
Taken together, our results suggest that RTEs enter the lymphoid periphery in a vulnerable state, with a tendency to be winnowed out of the peripheral T-cell pool. Only some RTEs, perhaps those that receive the strongest survival signals, including cells with useful TCR specificities, are incorporated into the lymphoid periphery. Under lymphopenic conditions, in which RTEs are most needed, these youngest T cells are able to fill the void effectively.
Materials and Methods
Mice.
CD45.2+ and CD45.1+ C57BL/6 (B6), F1 (CD45.1 × CD45.2), Ubiquitin-GFP Tg, and TCR βδ knockout mice were from The Jackson Laboratory or were bred on site. RAG2p-GFP Tg mice (7) were backcrossed in our laboratory at least 10 generations onto the B6 background.
Also used were MHC class I-restricted CD45.1+ OT-I and MHC class II-restricted CD45.2+ SMARTA TCR Tg B6 mice and mice Tg for human CD2 promoter-driven IL-7R (a gift from K. Elkon, University of Washington, Seattle, WA) and human Bcl-2 (a gift from P. Marrack, National Jewish Medical and Research Center, Denver, CO). Each of these lines (19, 31) was maintained as heterozygotes and crossed onto the RAG2p-GFP Tg background. Thy1.1+ SMARTA TCR Tg mice were a gift from M. Bevan (University of Washington, Seattle, WA).
All mice were used at age 5–20 wk. RTEs were sorted from 5- to 9-wk-old RAG2p-GFP Tg mice. MN T cells were from mice thymectomized at least 3 wk previously, from euthymic mice >14 wk old, or sorted from RAG2p-GFP Tg mice >12 wk old. All experiments were performed in compliance with the guidelines of the University of Washington Institutional Animal Care and Use Committee.
Mouse Procedures.
Mice were thymectomized as in ref. 2, and full thymectomy was verified at analysis. Where noted, GFP− recipients were primed intraperitoneally with 15–20 × 106 GFP Tg splenocytes from RAG2p-GFP or Ubiquitin-GFP Tg mice 2 wk before transfer of sorted RTEs and MN T cells.
Adoptive transfers were performed by i.v. injection of congenic RTEs and MN T cells. An aliquot of the injected mix was stained to determine the precise input RTE:MN ratio. Our results represent data combined from separate experiments in which CD45.1+ and CD45.2+ RTEs (and MN T cells) were used, to correct for potential differences in engraftment (32). Sublethally irradiated mice were given 600 rad 1 d before adoptive transfer and were maintained on water containing neomycin sulfate (Mediatech, Inc.) and Polymyxin B (Invitrogen) from 1 d before to 14 d after irradiation.
Cell Preparation, Staining, Enrichment, and Sorting.
Spleen, thymus, and lymph node cell preparation and staining was done as described (23). Where noted, cells were labeled for 10 min at 37 °C with 4 μm CFSE (Molecular Probes), which was quenched with >10× volume of HBSS + 1% BSA. CFSE labeling gave a signal more than an order of magnitude brighter than the GFP signal in RTEs; thus, detection of CFSE dilution was unaffected in GFP+ RTEs (Fig. 2A and ref. 21). Abs included those conjugated to FITC, phycoerythrin, peridinin chlorophyll protein-cyanine 5.5, phycoerythrin-Cy7, allophycocyanin, and allophycocyanin-eFluor 780 against the following molecules: CD4 (RM4-5), CD5 (53-7.3), CD8 (53-6.7), CD25 (PC61), CD44 (Pgp-1), CD45.1 (A20), CD45.2 (104), CD62L (MEL-14), CD69 (H1.2F3), CD127 (A7R34), TCRβ (H57-597), Thy1.1 (HIS51), Vα2 (B20.1), and Vβ5 (MR9-4), all from eBioscience or BD Biosciences. GM1 was detected with cholera toxin B (Sigma). Events were collected on a FACSCanto system (BD Biosciences), and data were analyzed using FlowJo (TreeStar) after excluding doublets from live-gated samples.
For sorting, untouched T cells were enriched with an EasySep kit (StemCell Technologies) and stained to eliminate non–T-cell lineages with phycoerythrin-conjugated anti-CD11b (M1/70), anti-NK1.1 (PK136), anti-B220 (RA3-6B2), and anti-Ter119 (Ly-76), all from eBioscience or BD Biosciences. CD62L and CD44 were used as positive markers for naïve cells. Sorted populations were peripheral naïve (CD62LhiCD44lo/mid) CD4 or CD8 RTEs (GFP+) or MN T cells (GFP−) and were >97% pure. For analysis of time points of TCR Tg RTE:MN competitions, in addition to congenic markers, cells also were stained for the TCR Tg Vα to exclude outgrowth of non–transgene-expressing T cells.
Supplementary Material
Acknowledgments
We thank Drs. M. Nussenzweig, P. Marrack, M. Bevan, and K. Elkon for mice. This work was supported by Grants T32CA0095 (to E.G.H.) and T32CA009537 (to L.E.H.) from the National Cancer Institute and by Grant R01 AI 064318 (to P.J.F) from the National Institutes of Health.
Footnotes
*This Direct Submission article had a prearranged editor.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015286108/-/DCSupplemental.
References
- 1.Yager EJ, et al. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 3.Haines CJ, et al. Human CD4+ T cell recent thymic emigrants are identified by protein tyrosine kinase 7 and have reduced immune function. J Exp Med. 2009;206:275–285. doi: 10.1084/jem.20080996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci USA. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houston EG, Jr, Fink PJ. MHC drives TCR repertoire shaping, but not maturation, in recent thymic emigrants. J Immunol. 2009;183:7244–7249. doi: 10.4049/jimmunol.0902313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Opiela SJ, Koru-Sengul T, Adkins B. Murine neonatal recent thymic emigrants are phenotypically and functionally distinct from adult recent thymic emigrants. Blood. 2009;113:5635–5643. doi: 10.1182/blood-2008-08-173658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu W, et al. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 1999;400:682–687. doi: 10.1038/23287. [DOI] [PubMed] [Google Scholar]
- 8.McCaughtry TM, Wilken MS, Hogquist KA. Thymic emigration revisited. J Exp Med. 2007;204:2513–2520. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scollay RG, Butcher EC, Weissman IL. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10:210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 10.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moon JJ, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Takada K, Jameson SC. Naive T cell homeostasis: From awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–832. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- 14.Berzins SP, Boyd RL, Miller JFAP. The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool. J Exp Med. 1998;187:1839–1848. doi: 10.1084/jem.187.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berzins SP, Godfrey DI, Miller JFAP, Boyd RL. A central role for thymic emigrants in peripheral T cell homeostasis. Proc Natl Acad Sci USA. 1999;96:9787–9791. doi: 10.1073/pnas.96.17.9787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bubnic SJ, Nagy A, Keating A. Donor hematopoietic cells from transgenic mice that express GFP are immunogenic in immunocompetent recipients. Hematology. 2005;10:289–295. doi: 10.1080/10245330500093468. [DOI] [PubMed] [Google Scholar]
- 17.Han WG, Unger WW, Wauben MH. Identification of the immunodominant CTL epitope of EGFP in C57BL/6 mice. Gene Ther. 2008;15:700–701. doi: 10.1038/sj.gt.3303104. [DOI] [PubMed] [Google Scholar]
- 18.Cho JH, Kim HO, Surh CD, Sprent J. T cell receptor-dependent regulation of lipid rafts controls naive CD8+ T cell homeostasis. Immunity. 2010;32:214–226. doi: 10.1016/j.immuni.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu Q, Erman B, Park JH, Feigenbaum L, Singer A. IL-7 receptor signals inhibit expression of transcription factors TCF-1, LEF-1, and RORγt: Impact on thymocyte development. J Exp Med. 2004;200:797–803. doi: 10.1084/jem.20032183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallegos AM, Bevan MJ. Central tolerance: Good but imperfect. Immunol Rev. 2006;209:290–296. doi: 10.1111/j.0105-2896.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 21.Hendricks DW, Fink PJ. Recent thymic emigrants are biased against the Th1 and toward the Th2 effector lineage. Blood. 2011;117:1239–1249. doi: 10.1182/blood-2010-07-299263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staton TL, et al. CD8+ recent thymic emigrants home to and efficiently repopulate the small intestine epithelium. Nat Immunol. 2006;7:482–488. doi: 10.1038/ni1319. [DOI] [PubMed] [Google Scholar]
- 23.Houston EG, Jr, Nechanitzky R, Fink PJ. Cutting edge: Contact with secondary lymphoid organs drives postthymic T cell maturation. J Immunol. 2008;181:5213–5217. doi: 10.4049/jimmunol.181.8.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan JT, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li O, Zheng P, Liu Y. CD24 expression on T cells is required for optimal T cell proliferation in lymphopenic host. J Exp Med. 2004;200:1083–1089. doi: 10.1084/jem.20040779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kassiotis G, Zamoyska R, Stockinger B. Involvement of avidity for major histocompatibility complex in homeostasis of naive and memory T cells. J Exp Med. 2003;197:1007–1016. doi: 10.1084/jem.20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kieper WC, Burghardt JT, Surh CD. A role for TCR affinity in regulating naive T cell homeostasis. J Immunol. 2004;172:40–44. doi: 10.4049/jimmunol.172.1.40. [DOI] [PubMed] [Google Scholar]
- 28.Kieper WC, et al. Recent immune status determines the source of antigens that drive homeostatic T cell expansion. J Immunol. 2005;174:3158–3163. doi: 10.4049/jimmunol.174.6.3158. [DOI] [PubMed] [Google Scholar]
- 29.Tsukamoto H, et al. Age-associated increase in lifespan of naive CD4 T cells contributes to T-cell homeostasis but facilitates development of functional defects. Proc Natl Acad Sci USA. 2009;106:18333–18338. doi: 10.1073/pnas.0910139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsukamoto H, Huston GE, Dibble J, Duso DK, Swain SL. Bim dictates naive CD4 T cell lifespan and the development of age-associated functional defects. J Immunol. 2010;185:4535–4544. doi: 10.4049/jimmunol.1001668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strasser A, Harris AW, Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 32.Waterstrat A, Liang Y, Swiderski CF, Shelton BJ, Van Zant G. Congenic interval of CD45/Ly-5 congenic mice contains multiple genes that may influence hematopoietic stem cell engraftment. Blood. 2010;115:408–417. doi: 10.1182/blood-2008-03-143370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.